Anemia is very common in chronic kidney disease (CKD) patients,1 and has been associated with adverse cardiovascular outcomes in this population.2 The pathogenesis of CKD-associated anemia is multifactorial, contributed to by relative erythropoietin (Epo) deficiency, absolute and functional iron deficiencies, decreased red blood cell survival, and uremia-induced erythropoiesis inhibition.3 The iron-regulatory hormone hepcidin may provide a link between inflammation and disordered iron homeostasis that contributes to CKD-associated anemia. Moreover, hepcidin-mediated iron restriction may contribute to resistance to recombinant erythropoietin,4 one of the mainstays of CKD-associated anemia treatment. Erythropoietin hyporesponsiveness and the utilization of high-dose erythropoietin have been associated with increased mortality rates in the CKD population.5 As such, possible therapies targeting hepcidin may result in improved erythropoietin responsiveness and improved outcomes. Therefore, elucidation of the role of hepcidin in CKD may allow for a better understanding of the pathogenesis of CKD-associated anemia, as well as insight into optimal anemia treatment strategies in CKD patients.
Hepcidin is secreted by the liver, and regulates iron levels by binding to the cellular iron exporter ferroportin, causing its internalization and degradation, resulting in decreased iron export from cells into plasma.6 Hepcidin affects enterocytes, inhibiting dietary iron absorption; hepatocytes, preventing the mobilization of hepatic iron stores; and hepatic and splenic macrophages, inhibiting the efflux of recycled iron.6 Reticuloendothelial cell sequestration of iron results in a functional iron deficiency and an iron-restricted erythropoiesis. Hepcidin production is induced by iron loading and inflammatory cytokines, and suppressed by iron deficiency and erythropoietic activity.7
Cross-sectional studies have shown that, as glomerular filtration rate declines, circulating hepcidin levels increase in CKD patients.8 Elevated hepcidin in CKD is thought to result from increased inflammation and/or decreased renal function, as kidney excretion represents a major mode of hepcidin clearance.9 However, the assessment of hepcidin levels in CKD patients may be confounded by the use of erythropoietin-stimulating agents (ESAs) and iron therapy, as both erythropoiesis and iron therapy affect hepcidin levels. Importantly, cross-sectional studies demonstrate an association between increased hepcidin levels and worsening kidney function only, without proving that hepcidin actually contributes to CKD-associated anemia. Furthermore, the measurement of serum hepcidin levels in humans is not standardized, and different studies have utilized different assays, including ELISAs, radioimmunoassays, and mass spectrometry-based assays. As absolute hepcidin levels differ based on which assay is used,10 comparing hepcidin measurements among studies is difficult.
We sought to determine the role of hepcidin in CKD-associated anemia using a mouse model, which allowed us to circumvent possible confounding by ESA use and/or iron therapy. We compared hepcidin knockout (HKO) CKD mice to wild-type (WT) CKD mice, allowing us to directly test the hypothesis that hepcidin ablation ameliorates CKD-associated anemia. CKD was induced in WT C57BL/6 mice (n=16) and HKO C57BL/6 mice (n=22) by feeding them a 0.2% adenine diet for eight weeks.11 Corresponding WT (n=16) and HKO (n=20) control groups were placed on identical diets without adenine. The study diets were started when the mice were eight weeks old. To prevent the development of iron overload, the HKO mice were fed a low iron diet containing 4 ppm iron, from weaning at four weeks of age until study diet initiation. WT mice were fed standard mouse chow containing 335 ppm iron until study diet initiation. All study diets (with adenine and without adenine) contained 50 ppm iron, a concentration which provided sufficient iron to the WT mice without overloading the HKO mice. Hematologic parameters were measured at diet initiation and at study conclusion.
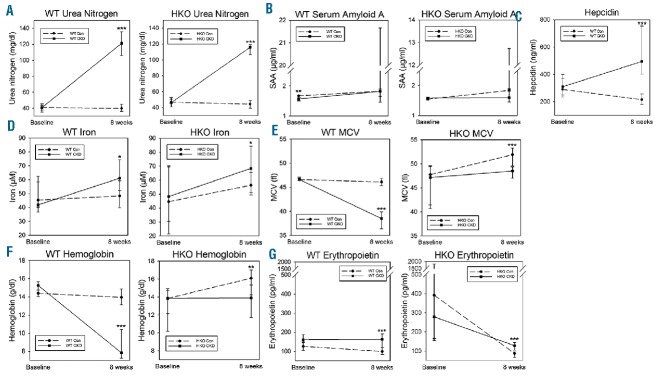
After eight weeks of the adenine-rich diet, the WT CKD and HKO CKD groups developed marked kidney tubulointerstitial damage with leukocytic infiltration and adenine crystal deposition, similar to what has been observed in other C57BL/6 mouse adenine-induced CKD models11 (Figure 1). Correspondingly, the CKD groups had markedly higher blood urea nitrogen (BUN) levels than their control counterparts (Figure 2A). In both the WT and HKO cohorts, levels of serum amyloid A (SAA), an inflammatory marker, did not differ between the control and CKD groups at the study endpoint (Figure 2B). Serum hepcidin levels were higher in the WT CKD group than in the WT control group (Figure 2C), and increased despite anemia, mimicking human CKD. In HKO mice, circulating hepcidin levels have previously been shown to be undetectable.12 Interestingly, serum iron increased in the WT CKD and HKO CKD groups (Figure 2D). This increase in serum iron occurred despite elevated hepcidin levels and anemia in the WT CKD group, suggesting decreased consumption of iron, and less efficient use of iron for erythropoiesis, in the setting of CKD. In addition, serum iron and hepcidin did not correlate, similar to what has been observed in human CKD studies.13
Figure 1.
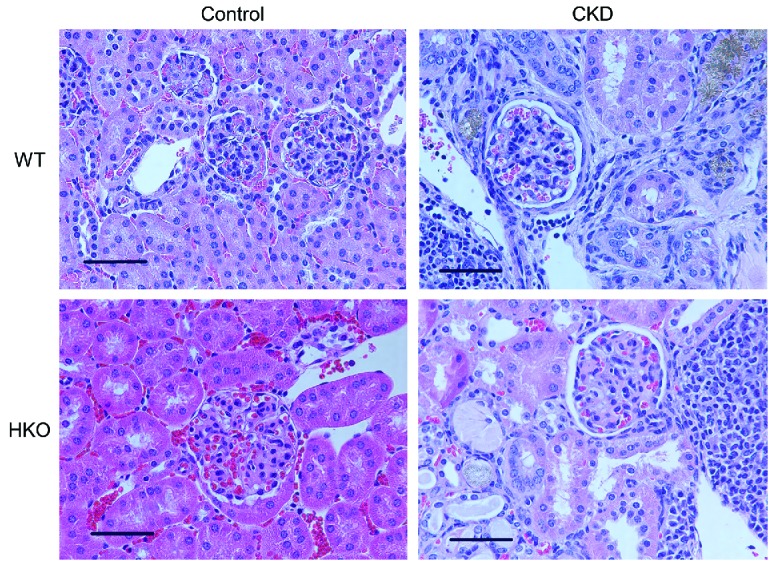
Renal histology in wild-type and hepcidin knockout control and CKD mice. WT and HKO mice fed the adenine diets developed adenine crystal deposition and diffuse tubulointerstitial damage with leukocytic infiltration. 40× magnification, scale bar 50 μm. CKD: chronic kidney disease; WT: wild-type; HKO: hepcidin knockout.
Figure 2.
A–G. Serum parameters in wild-type and hepcidin knockout control and CKD mice. Serum urea nitrogen, amyloid A, hepcidin, iron, MCV, hemoglobin and erythropoietin, measured at baseline and at eight weeks. Data are presented as medians and interquartile ranges. For CKD vs. control at eight weeks, *P<0.05, **P<0.01, ***P<0.001. Although the WT CKD and HKO CKD groups had similar increases in blood urea nitrogen, only the WT CKD group became anemic. WT: wild-type; HKO: hepcidin knockout; MCV: mean corpuscular volume; SAA: serum amyloid A.
Among the WT mice, the WT CKD group experienced marked decreases in mean corpuscular volume (MCV) and hemoglobin (Figure 2E,F). The WT CKD group had only marginally higher serum erythropoietin levels than the WT control group, and the levels remained unchanged from baseline (Figure 2G, P=0.53 vs. baseline), indicating a failure to respond to anemia. In contrast to the WT CKD mice, the HKO CKD mice developed only slightly lower MCV and hemoglobin vs. controls (Figure 2E,F). Indeed, the development of CKD, with similar degrees of uremia, resulted in significantly greater anemia in the WT mice (hemoglobin ~6 g/dl lower than controls) vs. the HKO mice (hemoglobin ~2 g/dl lower than controls) (P=0.048), suggesting the pathogenic role of hepcidin in the anemia of CKD. Compared to baseline values, hemoglobin remained unchanged in the HKO CKD group (P=0.99), but increased from baseline in the HKO control group (P<0.001). This small increase in hemoglobin in the HKO control group was likely due to the different diets that the HKO mice were fed prior to the baseline measurements (four weeks of 4 ppm iron diet) vs. the final measurements (eight weeks of 50 ppm iron diet), a regimen necessary to prevent iron overload in these mice. The higher iron content in the eight-week study diet allowed the HKO control group to develop mild polycythemia, which is commonly observed in HKO mice.14 For the same reason, both the HKO control and CKD groups had increased MCV (Figure 2E) and decreased erythropoietin (Figure 2G) compared to baseline (P<0.05 for both), although the differences were more pronounced in the HKO control group. Also, the slightly lower hemoglobin and MCV in the HKO CKD mice vs. controls suggest that other CKD-associated factors besides hepcidin affect erythropoiesis.
In all WT mice, hepcidin positively correlated with BUN (Figure 3A). The observations that the WT CKD group had higher hepcidin levels than the WT control group, despite similar SAA concentrations, and that hepcidin positively correlated with BUN, suggest that decreased hepcidin clearance may be important in this CKD model. In all WT mice, MCV and hemoglobin inversely correlated with hepcidin (Figure 3B,C).
Figure 3.
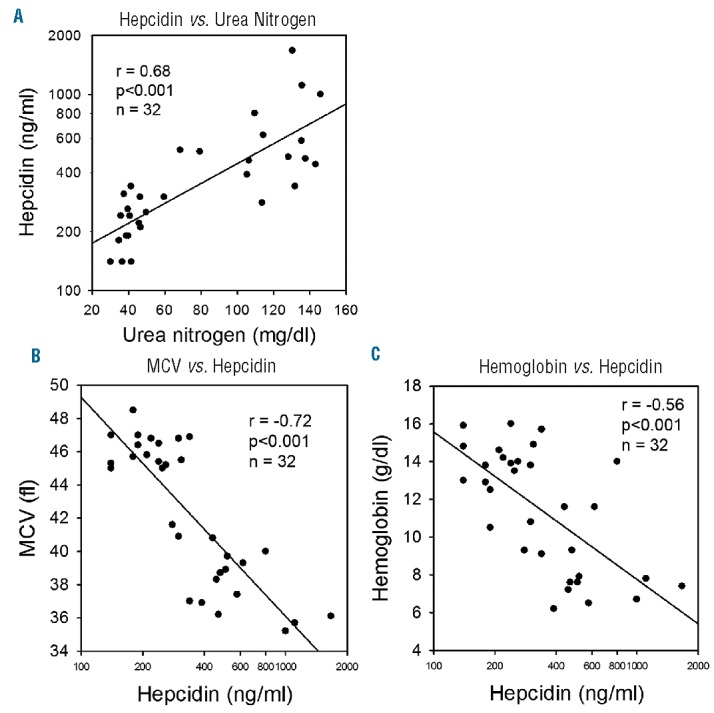
A–C. Serum hepcidin correlations in wild-type mice. Hepcidin vs. urea nitrogen, MCV vs. hepcidin, and hemoglobin vs. hepcidin in the WT group. Hepcidin correlated directly with urea nitrogen, and inversely with MCV and hemoglobin. Pearson product-moment correlation coefficients are presented. MCV: mean corpuscular volume.
In summary, in this murine CKD model, hepcidin ablation clearly ameliorated CKD-associated anemia, demonstrating the importance of hepcidin in this pathologic process. Similar results were recently published by Akchurin et al.,15 demonstrating the reproducibility and robustness of this finding. The significant contribution of hepcidin to the anemia of CKD is an important observation given the potential of anti-hepcidin therapies as possible future treatments of CKD-associated anemia. Furthermore, in the WT CKD group, we observed elevated hepcidin levels despite no difference in inflammatory markers, suggesting that impaired renal clearance may be an important determinant of hepcidin levels in CKD.
There are several limitations to our study, including incomplete biochemical assessment. Given the limited amount of blood available from each mouse, BUN was the only evaluation of renal function. Moreover, to assess iron status, we measured serum iron levels, but did not calculate transferrin saturation or quantitate hepatic iron content, the latter of which may have provided a better estimation of total body iron stores. Still, in a mouse model unburdened by the limitations of human studies, we have demonstrated that serum hepcidin levels increase in CKD and significantly contribute to CKD-associated anemia.
Supplementary Material
Footnotes
Funding: this work was supported in part by USPHS Grants DK-67563, DK-35423, DK-80984; NIH/NCATS/UCLA CTSI Grant UL1 TR-000124; NIH K12 Child Health Research Career Development Award K12-HD-034610; NIH Training Grant T32-DK-07789; and funds from the UCLA Children’s Discovery and Innovation Institute.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13(2):504–510. [DOI] [PubMed] [Google Scholar]
- 2.Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14(11):2919–2925. [DOI] [PubMed] [Google Scholar]
- 3.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109(9):4038–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. [DOI] [PubMed] [Google Scholar]
- 8.Zaritsky J, Young B, Wang HJ, et al. Hepcidin–a potential novel bio-marker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. [DOI] [PubMed] [Google Scholar]
- 10.Macdougall IC, Malyszko J, Hider RC, Bansal SS. Current status of the measurement of blood hepcidin levels in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(9):1681–1689. [DOI] [PubMed] [Google Scholar]
- 11.Jia T, Olauson H, Lindberg K, et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013;14(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zumerle S, Mathieu JR, Delga S, et al. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood. 2014;123(23):3646–3650. [DOI] [PubMed] [Google Scholar]
- 13.Troutt JS, Butterfield AM, Konrad RJ. Hepcidin-25 concentrations are markedly increased in patients with chronic kidney disease and are inversely correlated with estimated glomerular filtration rates. J Clin Lab Anal. 2013;27(6):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCranor BJ, Langdon JM, Prince OD, et al. Investigation of the role of interleukin-6 and hepcidin antimicrobial peptide in the development of anemia with age. Haematologica. 2013;98(10):1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akchurin O, Sureshbabu A, Doty SB, Zhu YS, Patino E, Cunningham-Rundles S, Choi ME, Boskey AL, Rivella S. Lack of Hepcidin Ameliorates Anemia and Improves Growth in an Adenine-induced Mouse Model of Chronic Kidney Disease. Am J Physiol Renal Physiol. 2016. July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.