Abstract
The survival of patients with diffuse large B-cell lymphoma has increased during the last decade as a result of addition of anti-CD20 to anthracycline-based chemotherapy. Although the trend is encouraging, there are persistent differences in survival within and between the USA and European countries suggesting that non-biological factors play a role. Our aim was to investigate the influence of such factors on relative survival of patients with diffuse large B-cell lymphoma. We conducted a retrospective, multicenter, registry-based study in France on 1165 incident cases of diffuse large B-cell lymphoma between 2002 and 2008. Relative survival analyses were performed and missing data were controlled with the multiple imputation method. In a multivariate analysis, adjusted for age, sex and International Prognostic Index, we confirmed that time period was associated with a better 5-year relative survival. The registry area, the medical specialty of the care department (onco-hematology versus other), the time to travel to the nearest teaching hospital, the place of treatment (teaching versus not-teaching hospital -borderline significance), a comorbidity burden and marital status were independently associated with the 5-year relative survival. Adjusted for first-course treatment, inclusion in a clinical trial and treatment discussion in a multidisciplinary meeting were strongly associated with a better survival outcome. In contrast, socio-economic status (determined using the European Deprivation Index) was not associated with outcome. Despite therapeutic advances, various non-biological factors affected the relative survival of patients with diffuse large B-cell lymphoma. The notion of lymphoma-specific expertise seems to be essential to achieve optimal care management and reopens the debate regarding centralization of these patients’ care in hematology/oncology departments.
Introduction
Non-Hodgkin lymphoma (NHL) is the most frequent hematologic malignancy in the world and comprises a heterogeneous group of more than 40 different subtypes.1 Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of NHL, accounting for up to 25–30% of all cases globally, with an age-adjusted incidence rate of 5.0 cases per 100,000 person-years in both sexes worldwide.2–4 Although DLBCL is curable in many cases, it remains an aggressive disease and fatal if left untreated or treated improperly. DLBCL usually affects adults over 60 years old, although it occurs in patients of all ages, including children, and needs many courses of curative treatments (polychemotherapy associated or not with immunotherapy followed by radiotherapy for localized disease). Recent data from the USA5 show a significant reduction in DLBCL mortality, reflecting a better survival. Positive trends in DLBCL survival were also observed in population-based studies in France and Europe beginning in the early 2000s.6–8 However, if these trends in DLBCL survival are due to clinical advances in the treatment of the disease (i.e., the introduction of rituximab), they may not be equally distributed in the population. Indeed, persistent differences in DLBCL survival are observed within and between countries (USA and European countries) suggesting the role of variations in access to/quality of care and availability of new drugs.
Moreover, a growing body of literature suggests a persistent relationship between non-biological factors such as socio-economic status (SES) and health status that may influence survival of patients with various common cancers. Individual characteristics (e.g., age, sex, marital status),9,10 contextual data such as a high Deprivation Index (living in a poorer district),11,12 living in a rural area,13,14 living far away from the referral center,15,16 being treated in a community hospital17,18 and low hospital volume19 have been associated with poorer outcome.
However, only a few studies assessing the impact of non-biological factors on NHL survival have been reported and most of them focused on the influence of SES or place of residence on NHL survival20–22 or, more recently, specifically DLBCL survival.23–25 These latest studies took into account, in their analyses, the introduction of rituximab in DLBCL treatment in 2002.
The aim of this study was to investigate the influence of socio-economic determinants, care management and place of care on relative survival of DLBCL patients during the early rituximab era.
Methods
Data source
Our study concerns all DLBCL cases diagnosed between 01/01/2002 and 12/31/2008 and collected in three population-based registries of hematologic malignancies in France (Basse-Normandie, Côte d’Or and Gironde). The cases were classified according to the International Classification of Diseases for Oncology 3rd edition using morphology codes: 9678/3, 9679/3, 9680/3, and 9684/3.26,27 All pathology reports were reviewed to ascertain the diagnosis of DLBCL. The study was approved by the French national consultative committee.
Individual data of the study population
We collected socio-demographic details, medical data and information about care management. Place of care was classified as the reference center, being either a “teaching hospital” (university or specialized oncology hospital) or “not-teaching hospital” (private clinic or community hospital). First medical contact (general practitioner or specialist) and medical specialty (hematology/oncology versus other specialties) for care management were also noted. Distances between the place of residence and the nearest reference care center were calculated with ArcGis10© combined with a roadmap database (Multinet TéléAtlas©), and expressed as travel time in minutes.
Vital status was determined from the date of diagnosis to the death or until 30th June, 2013 using the Repertoire National d’Identification des Personnes Physiques (RNIPP). Loss to follow-up was <2%.
Aggregate data of the study population
Residential address at diagnosis was geocoded and allocated to an Ilôts Regroupés pour l’Information Statistique (IRIS) the smallest geographical area for which census data are available. We used the French ecological European Deprivation Index (EDI),28 which attributes a social deprivation score to each IRIS,29 as a proxy measure of individual SES at the time of diagnosis. Residential address at diagnosis was defined according to the rural or urban commuting area code. Care provision was determined by the density of general practitioners per IRIS.
Statistical analysis
Patients’ characteristics were compared with the t test or χ2 test, as appropriate. The percentage of missing values was also provided.
We first estimated net survival using the Pohar Perme unbiased method for descriptive analyses.30 To evaluate the impact of prognostic factors on relative survival, we used the Esteve approach.31 Data were missing for a few variables in our dataset, with more than 10% of values missing for three variables. In this context, a complete-case method multivariate analysis would have dropped 35% of the subjects from the dataset. We, therefore, used a multiple imputations by chained equations (MICE) method to estimate supplementary variability of the Esteve model parameters due to the missing data.32–34
Finally, we fitted two multivariate analyses: in model A, the date of origin was the date of diagnosis; in model B, the date of origin was the date of the first treatment and care management variables were added into the model.
The stability of the results regarding SES was also tested by conducting the same analyses using the Townsend score.35 Finally, we described the proportion of patients treated with anthracycline-based chemotherapy (ABC) and immunotherapy over time by study region. To comfort assumptions regarding type of hospital and medical specialty, we conducted two sensitive analyses on survival with different criteria to characterize the level of lymphoma-specific expertise. One analysis involved the case volume as the total number of DLBCL treated by each center during the period of the study with three cutoffs determined by fitting segmented regression. The second analysis was based on combinations of two variables “type of hospital” and “medical specialties” (4 modalities).
As we were using ecological data, we used a hierarchical model. However, since the number of patients per IRIS was 1.7 on average, hierarchical models did not bring any supplementary information to residual variability (data not shown).
The statistical analyses were performed with Stata® 14 and R® (version 3.2.2).
Results
Patients’ characteristics
A total of 1,312 DLBCL were diagnosed between 2002 and 2008 in the three regions (Figure 1). After applying exclusion criteria, the population analyzed was composed of 1,165 subjects. Table 1 shows the distribution of patients’ characteristics according to the period of diagnosis (2002–2005 versus 2006–2008). Eighty-seven percent of patients (1017/1165) were treated in oncology/hematology departments. Fifty-nine percent of DLBCL patients (690/1165) were referred to teaching hospitals for their management.
Figure 1.
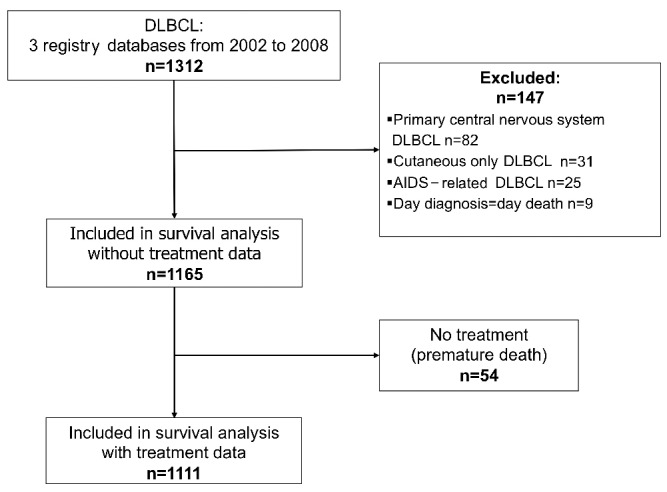
Flowchart of the survival analysis of patients with diffuse large B-cell lymphoma (2002–2008). DLBCL: diffuse large B-cell lymphoma; AIDS: acquired immunodeficiency syndrome.
Table 1.
Socio-demographics and clinical characteristics of diffuse large B-cell lymphoma patients at diagnosis, divided by study period.
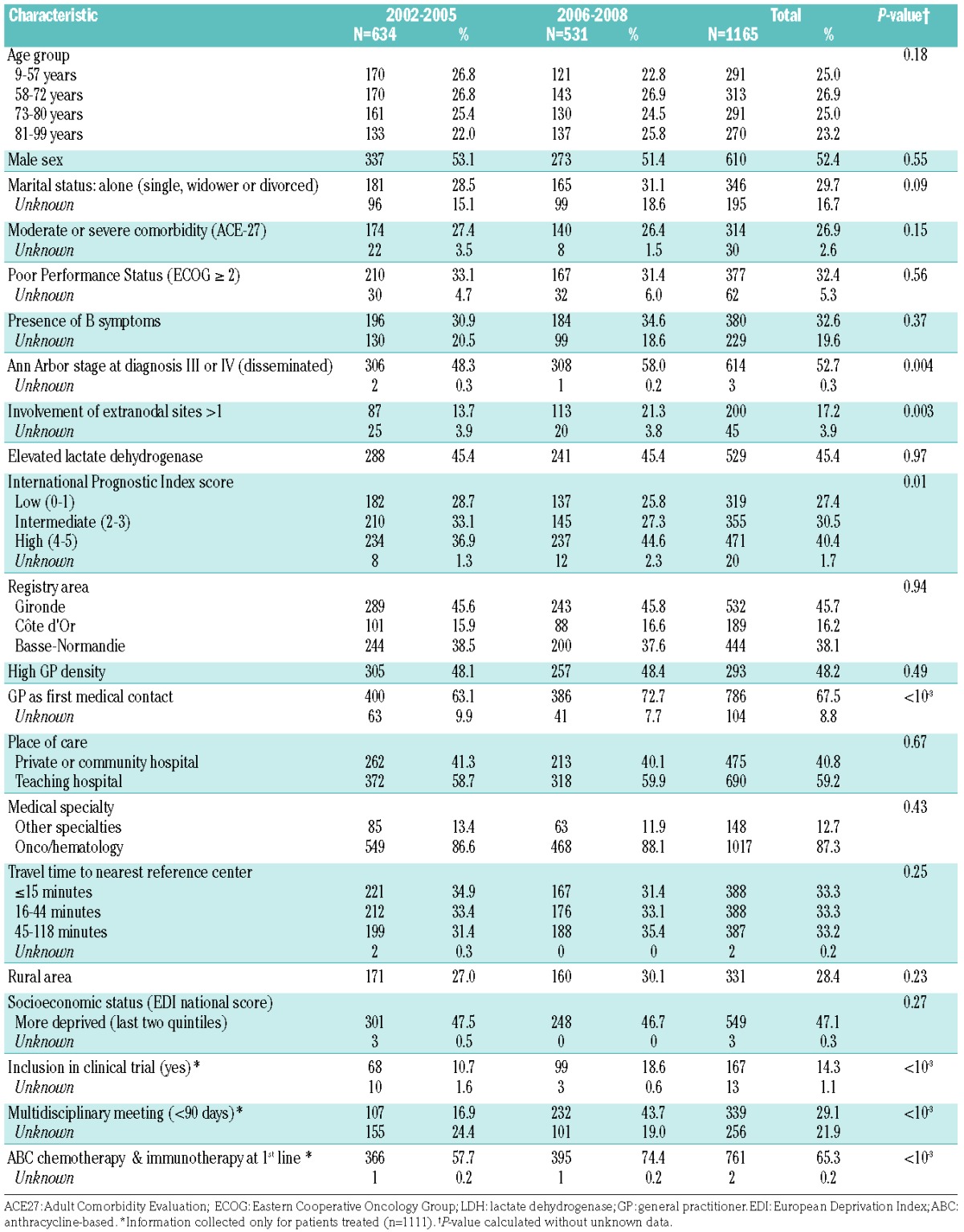
The median of age at diagnosis was 72 years (range, 9 – 99) and the sex ratio (male/female) was 1.1 (610/556).
Clinical outcome and factors associated with relative survival of patients with diffuse large B-cell lymphoma
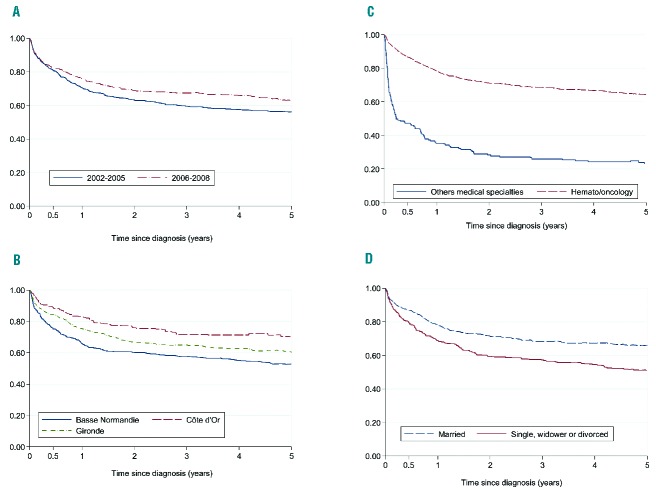
The 5-year net survival for the entire cohort was 59%. Figure 2 shows the plots of net survival probability according to groups of patients divided by period of diagnosis, marital status, medical specialty, and registry area.
Figure 2.
Unadjusted net survival in the 5 years after diagnosis for patients with diffuse large B-cell lymphoma (2002–2008). Patients are divided by: (A) period of diagnosis; (B) registry area; (C) treatment department (oncohematology vs. other medical specialities); and (D) marital status.
The final models are shown in Tables 2 and 3. Adjusted for age, sex and International Prognostic Index (IPI) score, living alone [adjusted excess hazard ratio (adjusted EHR): 1.41; 95% confidence interval (95% CI): 1.01–1.99], having mild or severe comorbidity (adjusted EHR: 1.64; 95% CI: 1.22–2.22) and having been diagnosed and treated in Basse-Normandie county (adjusted EHR: 1.49; 95% CI: 1.19–1.85) were independently associated with unfavorable relative survival in model A (Table 2). Conversely, having been diagnosed during the later study period (2006–2008 compared to 2002–2005) (adjusted EHR: 0.71; 95% CI: 0.58–0.88) and treated in an oncohematology department (adjusted EHR: 0.34; 95% CI: 0.27–0.43) were factors independently associated with a favorable relative survival in model A. The results were comparable in the final multivariate model B, with treatment information incorporated, as reported in Table 3, except for the period of diagnosis that was no longer associated with relative survival. First-course treatment with a combination of ABC and rituximab was strongly associated with a better survival outcome (adjusted EHR: 0.32; 95% CI: 0.25–0.41), together with inclusion in a clinical trial (adjusted EHR: 0.44; 95% CI: 0.28–0.69), or having treatment discussed in a multidisciplinary meeting (adjusted EHR: 0.69; 95% CI: 0.50–0.96). Patients treated in a teaching hospital had a better outcome (adjusted EHR: 0.82; 95% CI: 0.66–1.01) although the association was of borderline statistical significance (P=0.05) in model A, and no longer statistically significant after introduction of treatment information (model B, Table 3). SES variables (French EDI, urban/rural area also used as means of considering issues of accessibility or medical density) were not associated with relative survival of DLBCL patients in either model A or B.
Table 2.
Factors related to the relative survival of diffuse large B-cell lymphoma patients in the 5 years following diagnosis in a multivariate Esteve model with MICE; model A (no information on treatment) (n=1165).
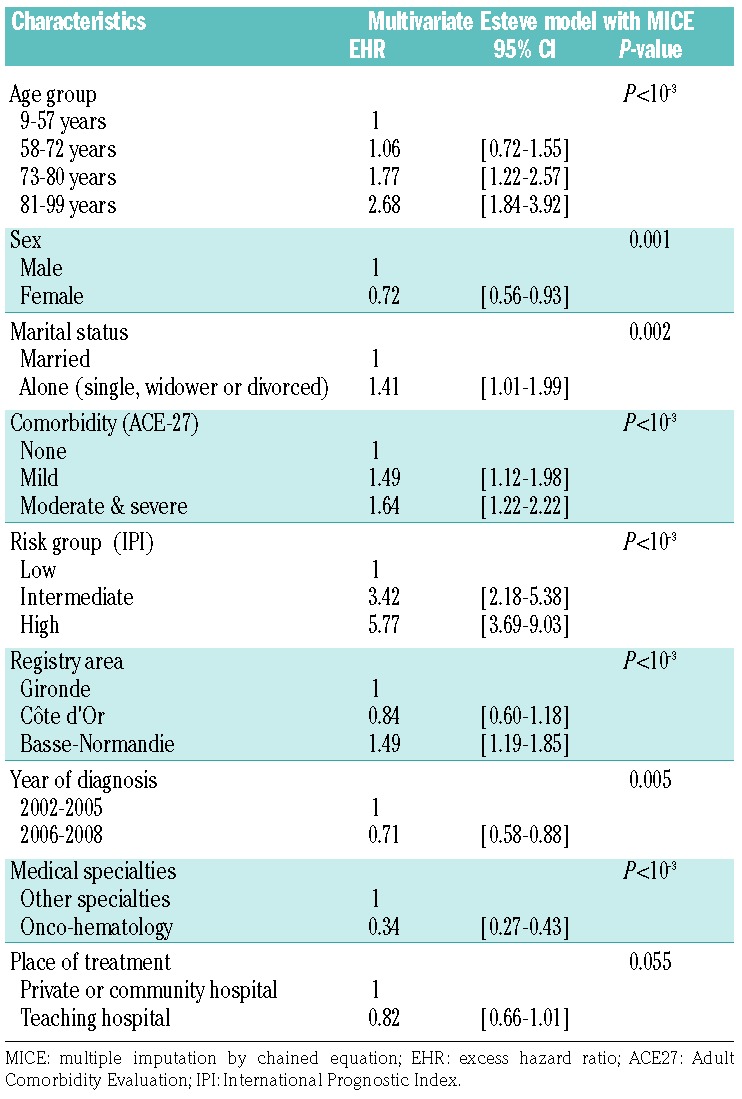
Table 3.
Factors related to the relative survival of diffuse large B-cell lymphoma patients in the 5 years following diagnosis in a multivariate Esteve model with MICE; model B (information on treatment) (n=1111).
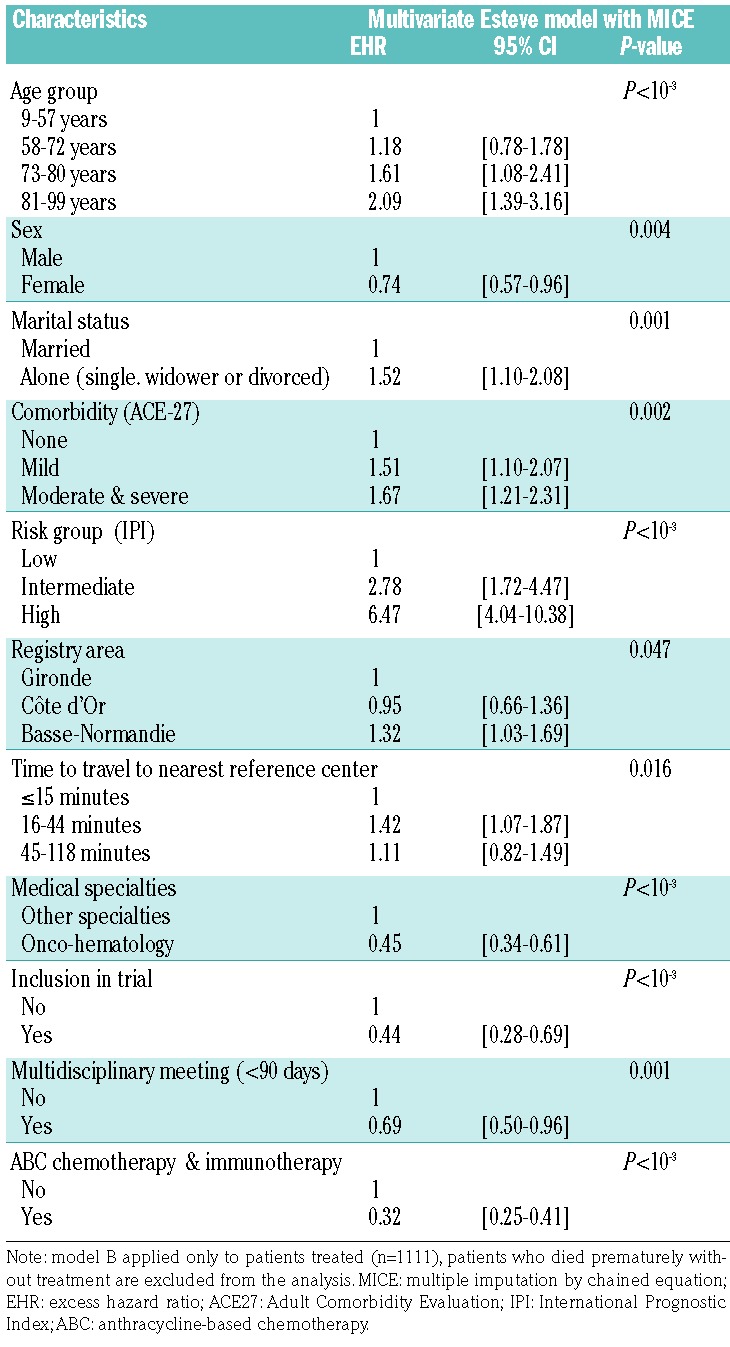
Using other cutoff points of EDI, splitting in quintiles or as a continuous variable did not change the results. We observed comparable results with the introduction of the Townsend index (a common deprivation measure used in the UK) in the place of the EDI. We refined our adjustment on prognostic factors by using each item of information contained in the IPI score (i.e., Eastern Cooperative Oncology Group Performance Status, age, Ann Arbor stage, serum lactate dehydrogenase level, number of extra-nodal sites of disease) rather than the score itself and found comparable results. Adjusting for age as a continuous variable also gave similar results. Our results were stable when each center was excluded in turn from the analyses, except for the association with marital status that became non-significant after exclusion of the Gironde center.
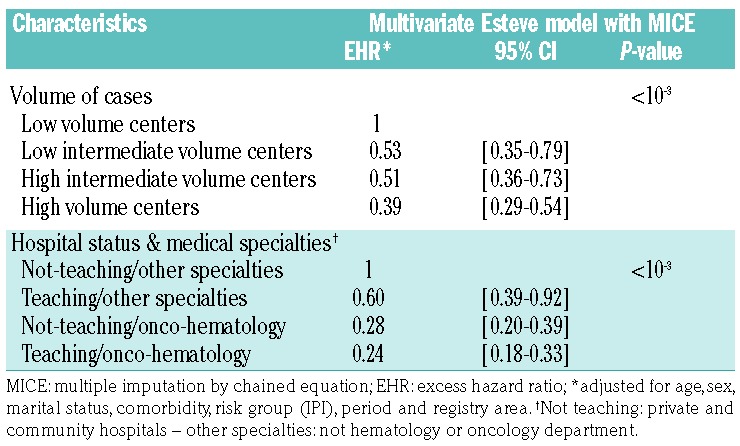
Our sensitive analysis on lymphoma-specific expertise showed that, adjusted for age, sex, IPI, living alone, comorbidity, registry area and period, patients treated in “high-volume centers” had a better relative survival (adjusted EHR: 0.42; 95% CI: 0.31–0.57) than those treated in “low-volume centers” with a trend between the four modalities. Moreover, a multivariate survival analysis including a variable mixing the medical specialties and types of hospital suggested that specialty was more important than type of hospital: patients treated in hematology/oncology departments had a better survival than patients treated in other specialty departments whether or not these were in teaching hospitals (adjusted EHR: 0.28; 95% CI 0.20–0.39 for treatment in a hematology/oncology department in a not-teaching hospital, and adjusted EHR: 0.24; 95% CI 0.18–0.33 for treatment in a hematology/oncology department in a teaching hospital) versus adjusted EHR: 0.60; 95% CI: 0.39–0.92 for patients treated in teaching hospitals in other specialty departments (Table 4).
Table 4.
Effect of volume of cases and diffuse large B-cell lymphoma expertise on relative survival in the 5 years following diagnosis in a multivariate Esteve model with MICE.
Regarding the use of rituximab, and more specifically ABC with immunotherapy, as first-line treatment, we observed that the implementation of this regimen was less frequent in Basse-Normandie than in the other two regions: 78.9% of DLBCL patients in Basse-Normandie were given ABC with immunotherapy during the study period 2006–2008, compared with 92.2% of the patients in the two other regions in the same time period. This difference is comparable to that during the first study period (2002–2005) although the proportions were lower (i.e., 60.3% versus 70.2%).
Discussion
To our knowledge, this study assessing the impact of social disparities, care management and place of care on DLBCL patients’ survival is the first performed in France to date. After adjusting for age, sex and IPI, our results suggest that SES (measured by the EDI) is not associated with relative survival of DLBCL patients. However, we observed a better survival during the later study period. This positive trend in survival is likely to be explained by the addition of immunotherapy to ABC regimens in front-line therapy (official agreement in France in 2002): study period was no longer associated with DLBCL survival when treatment variables were entered into the model. Moreover, the area in which a patient is diagnosed, his or her medical care team (onco-hematology versus other) and to a lesser extent the type of treatment center (teaching versus not-teaching hospital) are independently associated with better 5-year relative survival. Lastly, a higher comorbidity burden and being single are independently associated with poorer survival.
The result on SES and DLBCL survival is consistent with a French study on mortality which did not find any statistically significant relative indices of inequality related to education for NHL mortality, although the study was conducted in the pre-rituximab era.36,37 Only one study published in 2014 evaluated the role of SES in specific DLBCL survival.23 In contrast to our results, the authors reported lower DLBCL survival in patients with a lower SES, with the association being more pronounced in the modern treatment era after the introduction of rituximab and in younger patients. As the authors stated, inadequate insurance coverage with additional financial burden due to modern treatments may be associated with increased DLBCL mortality. Differences in health care systems could, therefore, explain our different results, as the French universal health care system may be better able to obviate barriers to access necessary NHL care, as demonstrated in Canada by Darmawilarta et al.38 and more recently, in Germany for patients with acute myeloid leukemia.39 A Scandinavian study also found a relationship between low SES (specifically, educational level) and poor relative survival outcome of NHL patients overall, without any subtype analysis.40 In this study, performed in the pre-rituximab era, the authors observed a difference in survival mainly due to differences in excess mortality rates within the first year after diagnosis of NHL. They also observed a relationship between comorbidity and poor survival but did not analyze variables simultaneously, although the latter could partly explain the association between education and DLBCL survival.
Our result suggesting that unmarried people have lower DLBCL relative survival than married people, independently of other prognostic factors, is in agreement with findings of other recent studies.41 Kravdal et al. also reported a 15% excess of all-cause mortality in never-married men or women and divorced male cancer patients, compared to married people of the same sex.42 These results highlight the potentially significant impact that social support can have on cancer detection, treatment, and survival.
However, our observation of a persistent “registry area effect” on DLBCL survival could be interpreted as a residual indirect effect of patients’ SES on survival since patients living in the Basse-Normandie area were more deprived, more likely to live in rural areas, and further from the regional hospital compared to patients living in the other regions investigated. These patients were older (median age: 73.5 years), more frequently had a Performance Status ≥ 2, and more frequently had disseminated disease at diagnosis (i.e. Ann Arbor stage III/IV) than those in other registry areas. On the other hand, the fact that patients from this area were less frequently treated with rituximab or ABC with rituximab (whatever the study period), and less frequently included in clinical trials compared to patients in the other areas also suggests a delay in the implementation of these treatments in this specific region, as shown by Flowers et al.43
Thus, lower survival in Basse-Normadie than in other areas could be interpreted as a consequence of a poorer care provision in hematology. This interpretation is in agreement with the lower number of hematologists per inhabitant in Basse-Normadie and may have contributed to the creation in 2016 of a hematology Institute in Caen to reinforce care provision in this region.
Another explanation of DLBCL survival disparities would be the existence of discrepancies in other non-measured risk factors linked to poorer survival such as cigarette smoking or other risk factors with a higher prevalence in Basse-Normandie than in the other areas.44
Beside the benefit of new treatments, other improvements in patients’ management, such as better work-up, better prognostication, better treatment decisions that could be aggregated in concepts such as “included in a clinical trial” or “treatment discussed by a multidisciplinary team”, could also have led to better survival. Prior reports suggest that both factors are related to better survival outcomes.45
In our study, we found a borderline association with teaching/not-teaching hospital. It has been demonstrated, in a large panel of cancer survival studies, that patients treated in teaching hospitals have a better survival than patients treated in community hospitals. The effect of centralizing operative treatment might improve survival but these results always concerned solid tumors treated first by surgery and were correlated with increased hospital procedure volume.19 Regarding hematologic malignancies, few studies have explored the role of the care provider in a universal health care system setting in which each patient can choose his/her place of care management. The type of care center has been linked to survival, with a benefit being shown for patients with acute myeloid leukemia and DLBCL being treated in high volume hospitals.37,46 More recently, Lamy et al.47 suggested that even in a universal health care system, disparities in the management of DLBCL patients still exist depending on the type of care center, even after adjustment for differences between patients.
The first explanation of such a small difference that we found in survival between patients treated in teaching or not-teaching hospitals could be related to training and the hematology/oncology network: all hematologists/oncologists are trained in teaching hospitals and they continue to maintain strong relationships and collaborate together after their training when they work in private clinics through multidisciplinary discussion of treatment decisions and clinical trial participation. In France, there is a single cooperative group, named the Lymphoma Study Association (LYSA), which has the mission of bringing together professionals specializing in the field of lymphoma in both public and private hospitals in order to promote basic and clinical research, improve prevention, management, and treatment of lymphoma patients, and share their knowledge on lymphoma. This collaborative system works well in hematology/oncology and created a strong network all over the country. However, this type of cooperation could be reinforced between hematology/oncology and other departments (mostly internal medicine) that also treat NHL patients in teaching or private hospitals.
More broadly speaking, this result could be organizational and may also be an effect of the last two French cancer plans (2003–2013) that implemented several actions aimed to reinforce the quality of care throughout the country. Several measures were taken to ensure all patients access to medical expertise whatever his/her place of treatment (better access to clinical trials and innovative treatment, panel review of tumor samples by expert pathologists and systematic discussion of medical records by clinical experts thereby promoting multidisciplinary management). Each NHL patient’s treatment must be discussed in a multidisciplinary meeting with the strict application of latest guidelines regardless of whether the patient is being treated in a teaching or not-teaching hospital and all patients must have better access to targeted treatment and clinical trials.
The major strengths of our study are related to its population-based design that limits selection bias making the results generalizable to a larger DLBCL patient population. In our study, the 5-year net survival was 58%, which is very close to the rate observed at the national level with data from all cancer registries in a recent population-based study (57%).48 Secondly, we simultaneously analyzed a large set of variables in relation to DLBCL survival, adjusting for major known prognostic factors (age, sex and IPI). Moreover, a senior registrar performed a systematic centralized review of all the pathology reports with particular attention to cases of ‘not otherwise specified’ NHL and transformation from follicular lymphoma.
This study had some limitations: we did not have access to information regarding individual risk factors, biomarkers that could be used for prognostication or official thresholds of hospitals’ expertise as for solid tumors. The last lack may explain why we did not find a clear relationship between the type of the hospital and survival outcomes. However, we did bring to light the importance of being treated in a specialized hematology or oncology department rather than in another type of department (internal medicine, polyvalent medicine, pneumology, etc.). This issue regarding the importance of medical specialty as a factor influencing survival of cancer patients has already been shown by other authors.49
As we mentioned above, we observed differences in the characteristics of patients treated in teaching hospitals and those treated in not-teaching hospitals and between those treated in onco-hematology departments and those treated in other departments (e.g., internal medicine). However, these characteristics (age, sex, Performance Status, comorbidity, etc.) were taken into account in the multivariate survival models.
This study suggests that type of specialty care is more important than type of hospital. All our findings, together with the need to treat patients (at least the elderly) near their place of residence and the observation of a recent increase in the number of survivors with NHL (positive trends in survival), reinforce the authors’ opinion that the implementation of hematology networks (combining routine, guidelines and clinical research) is a better solution than centralizing the care of NHL patients in teaching hospitals. The organization of different levels of expertise over the territory (whatever the hospital status) is helpful to prevent delays and give access to the cutting edge of hematology instead of triggering the bottleneck that would be created in teaching hospitals if all NHL patients were to be referred to such structures.
In the near future, we may expect an increase in elderly NHL cases and hematology/oncology departments will not be able to take care all these new patients. Alternatively, cooperation could be reinforced between hematology/oncology departments and other departments taking care of NHL patients, in all types of hospital. The onco-geriatric units that have been created all over France during the last 5 years are a step in that direction.
Since information was missing for a limited but not negligible number of cases regarding marital status, B symptoms, multidisciplinary teamwork and other health care behaviors, we used the MICE method, based on a Monte Carlo Markov chain algorithm under a missing random data hypothesis. This method is used to manage incomplete observations, avoiding biased estimates and improving their precision.50
The social welfare system in France is intended to give free access to all types of hospital and innovative treatments. Our results showing no association between an aggregate SES index and survival outcome do not call into question this organization. However, heterogeneity in care management and later introduction of the use of innovative drugs in some regions could explain survival differences between registry areas, as could other non-measured risk factors that should be prospectively collected in new cohorts, together with genetic factors.
The notion of lymphoma-specific expertise seems to be essential for the best DLBCL management and raises the question of centralization of NHL patients’ care in hematology/oncology departments. However, the expected number of new NHL cases for the next 20 years (due to demographic variations in the elderly and improved NHL survival) argues more for the implementation of collaborative networks with close communication between hematology/oncology departments and other medical departments rather than centralization of NHL care.
Supplementary Material
Acknowledgments
The authors would like to thank all those who contributed to recording cancer data in the registries, in particular the laboratories and departments of anatomy, cytology, and pathology; the medical informatics departments of the public and private hospitals and general practitioners and specialists. The authors also express their gratitude to JM. Poncet, Dr. A. Collignon, S. Boulanger, H. Rachou and A. Viale for collecting data; L. Launay of INSERM U1086 for geocoding addresses and applying the EDI and Dr. R. Nookala of the Institut Bergonié for the medical writing service.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/3/584
Funding
This work was supported by the French “Institut National du Cancer” (INCa).
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on October 10th, 2016. [Google Scholar]
- 2.Stewart B, Wild CP. (eds.), International Agency for Research on Cancer, WHO. (2014) World Cancer Report 2014. [Online]. Available at: http://www.thehealthwell.info/node/725845, accessed on 10th October 2016.
- 3.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–3734. [DOI] [PubMed] [Google Scholar]
- 4.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Morton LM, Feuer EJ, Besson C, Engels EA. Contributions of subtypes of non-Hodgkin lymphoma to mortality Trends. Cancer Epidemiol Biomarkers Prev. 2016;25(1):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sant M, Minicozzi P, Mounier M, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15(9): 931–942. [DOI] [PubMed] [Google Scholar]
- 7.Mounier M, Bossard N, Belot A, et al. Trends in excess mortality in follicular lymphoma at a population level. Eur J Haematol. 2015;94(2):120–129. [DOI] [PubMed] [Google Scholar]
- 8.Monnereau A, Troussard X, Belot A, et al. Unbiased estimates of long-term net survival of hematological malignancy patients detailed by major subtypes in France. Int J Cancer. 2013;132(10):2378–2387. [DOI] [PubMed] [Google Scholar]
- 9.Faggiano F, Partanen T, Kogevinas M, Boffetta P. Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ. 1997;138:65–176. [PubMed] [Google Scholar]
- 10.Borate UM, Mineishi S, Costa LJ. Non biological factors affecting survival in younger patients with acute myeloid leukemia. Cancer. 2015;121(21):3877–3884. [DOI] [PubMed] [Google Scholar]
- 11.Coleman MP, Rachet B, Woods LM, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales to 2001. Br J Cancer. 2004;90(7):1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggleston KS, Coker AL, Williams M, Tortolero-Luna G, Martin JB, Tortolero SR. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995–2001. J Women Health. 2006;15(8):941–945. [DOI] [PubMed] [Google Scholar]
- 13.Meilleur A, Subramanian SV, Plascak J, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the US: issues and challenges. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988–2000. Cancer. 2006;107(5 Suppl):1189–1195. [DOI] [PubMed] [Google Scholar]
- 15.Dejardin O, Jones AP, Rachet B, et al. The influence of geographical access to health care and material deprivation on colorectal cancer survival: evidence from France and England. Health Place. 2014;30:36–44. [DOI] [PubMed] [Google Scholar]
- 16.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12): 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103(3):435–441. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry R, Goel V, Sawka C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ. 2001;164(2):183–188. [PMC free article] [PubMed] [Google Scholar]
- 19.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284(23):3028–3035. [DOI] [PubMed] [Google Scholar]
- 20.Keegan TH, McLure LA, Foran JM, Clarke CA. Improvements in survival after follicular lymphoma by race/ethnicity and socioeconomic status: a population-based study. J Clin Oncol. 2009;27(18):3044–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keegan TH, Clarke CA, Chang ET, Shema SJ, Glaser SL. Disparities in survival after Hodgkin lymphoma: a population-based study. Cancer Causes Control. 2009;20(10): 1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loberiza FR, Cannon AJ, Weisenburger DD, et al. Survival disparities in patients with lymphoma according to place of residence and treatment provider: a population-based study. J Clin Oncol. 2009;27(32):5376–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. 2014;123(23):3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee B, Goktepe O, Hay H, et al. Effect of place of residence and treatment on survival outcomes in patients with diffuse large B-cell lymphoma in British Columbia. Oncologist. 2014;19(3):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flowers CR, Nastoupil LJ. Socioeconomic disparities in lymphoma. Blood. 2014;123(23):3530–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;110(2):695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology, 3rd edition. World Health Organisation, 2000. [Google Scholar]
- 28.Pornet C, Delpierre C, Dejardin O, et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66(11):982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods LM, Rachet B, Coleman MP. Choice of geographic unit influences socioeconomic inequalities in breast cancer survival. Br J Cancer. 2005;92(7):1279–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohar Perme M, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68(1):113–120. [DOI] [PubMed] [Google Scholar]
- 31.Estève J, Benhamou E, Croasdale M, Raymond L. Relative survival and the estimation of net survival. Stat Med. 1990;9(5): 529–538. [DOI] [PubMed] [Google Scholar]
- 32.Giorgi R, Belot A, Gaudart J, Launoy G, French Network of Cancer Registries FRANCIM. The performance of multiple imputation for missing covariate data within the context of regression relative survival analysis. Stat Med. 2008;27(30):6310–6331. [DOI] [PubMed] [Google Scholar]
- 33.White IR, Royston P, Wood A. Tutorial in biostatistics: multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 34.Nur U, Shack LG, Rachet B, Carpenter JR, Coleman MP. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiology. 2010;39(1):118–128. [DOI] [PubMed] [Google Scholar]
- 35.Townsend P. Deprivation. J Soc Policy. 1987;16:125–146. [Google Scholar]
- 36.Menvielle G, Chastang JF, Luce D, Leclerc A, Groupe EDISC. Changing social disparities and mortality in France (1968–1996): cause of death analysis by educational level. Rev Epidemiol Sante Publique. 2007;55(2):97–105. [DOI] [PubMed] [Google Scholar]
- 37.Borel C, Lamy S, Compaci G, et al. A longitudinal study of non-medical determinants of adherence to R-CHOP therapy for diffuse large B-cell lymphoma: implication for survival. BMC Cancer. 2015;15:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darmawikarta D, Pole JD, Greenberg M. The association between socioeconomic status and survival among children with Hodgkin and non-Hodgkin lymphomas in a universal health care system. Pediatr Blood Cancer. 2013; 60(7):1171–1177. [DOI] [PubMed] [Google Scholar]
- 39.Erdmann F, Kaatsch P, Zeeb H, Roman E, Lightfoot T, Schüz J. Survival from childhood acute lymphoblastic leukaemia in West Germany: does socio-demographic background matter? Eur J Cancer. 2014; 50(7):1345–1353. [DOI] [PubMed] [Google Scholar]
- 40.Roswall N, Olsen A, Christensen J, Rugbjerg K, Mellemkjaer L. Social inequality and incidence of and survival from Hodgkin lymphoma, non-Hodgkin lymphoma and leukaemia in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44(14):2058–2073. [DOI] [PubMed] [Google Scholar]
- 41.Aizer A, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med. 2001;52(3): 357–368. [DOI] [PubMed] [Google Scholar]
- 43.Flowers CR, Fedewa SA, Chen AY, et al. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(9): 1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battaglioli T, Gorini G, Costantini AS, et al. Cigarette smoking and alcohol consumption as determinants of survival in non-Hodgkin’s lymphoma: a population-based study. Ann Oncol. 2006;17(8):1283–1289. [DOI] [PubMed] [Google Scholar]
- 45.Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. [DOI] [PubMed] [Google Scholar]
- 46.Giri S, Pathak R, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood. 2015;125(21):3359–3360. [DOI] [PubMed] [Google Scholar]
- 47.Lamy S, Bettiol C, Grosclaude P, et al. The care center influences the management of lymphoma patients in a universal health care system: an observational cohort study. BMC Health Serv Res. 2016;16(a):336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monnereau A, Urhy Z, Bossard N, et al. Survie des personnes atteintes de cancer en France métropolitaine, 1989–2013. Partie 2 – Hémopathies malignes. Saint-Maurice: Institut de veille sanitaire; 2015. Available at: http://www.invs.sante.fr or http://www.e-cancer.fr Accessed on 10th October 2016. [Google Scholar]
- 49.Engelen MJ, Kos HE, Willemse PH, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106(3):589–598. [DOI] [PubMed] [Google Scholar]
- 50.Falcaro M, Nur U, Rachet B, Carpenter JR. Estimating excess hazard ratios and net survival when covariate data are missing: strategies for multiple imputation. Epidemiology. 2015;26(3):421–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.