Invasive fungal infections (IFIs) are serious, life-threatening complications frequently observed in hematological malignancies, in particular in patients affected by acute myeloid leukemia (AML) or in allogeneic hematopoietic stem cell transplantation (HSCT) recipients.1 Epidemiological data on patients with AML have been updated during the last few years, while on the contrary little information is available on the incidence of IFIs in chronic lymphoproliferative disorders (CLDs).2,3 Treatment strategies for CLD have changed rapidly over the past years, and new drugs with profound effects on the immune system have been introduced. This new scenario also requires a revision of the epidemiology and specific risk factors for IFIs among patients with CLD.
In this monocentric retrospective analysis all consecutive patients diagnosed and treated for CLDs [Chronic lymphocytic leukemia (CLL); Hodgkin lymphoma (HL); aggressive non-Hodgkin lymphoma (aNHL); indolent non-Hodgkin lymphoma (iNHL) and multiple myeloma (MM)] admitted to our hospital between 2006 and 2014 were censored with the aim to identify the incidence and the kind of IFIs, risk factors, prognosis and outcome of IFIs.
For each patient, baseline data were recorded at the time of admission (age, gender, hematological malignancy subtype and treatments). Underlying CLD characteristics were also considered, with particular attention paid to the onset, the status of the disease and the number of lines of chemotherapy or HSCT and the drugs administered. Patients who underwent allogenic HSCT were excluded from the study at the time of transplantation.
Clinical data were matched with microbiological (fungal culture or serology) and radiological data. All patients suspected of having an IFI underwent a common diagnostic workup that included blood cultures and chest X-rays, galactomannan (GM) and β-glucan assays tests, performed for 3 consecutive days, and a chest computed tomography (CT) scan. Additional examinations (e.g., an abdominal ultrasound scan, a sinus or brain CT, a skin biopsy, bronchoalveolar lavage, fundus examination) were performed as required.
Only probable or proven IFI, according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria, were considered.4
Mortality due to IFIs (IFI-attributable mortality) was considered when patients died within 12 weeks after the onset of fever and had microbiological, histological or clinical evidence of an active IFI, if other potential causes of death could be excluded by the physician.
Potential risk factors (age, sex, previous treatment and phase of treatment) predicting the outcome of IFIs were analyzed using the Fisher’s exact test. The two-tailed significance test at P<0.05 was used to determine statistical significance. The measure of association was expressed by the odds ratio. Statistical analyses were performed using the STATA 10 software.
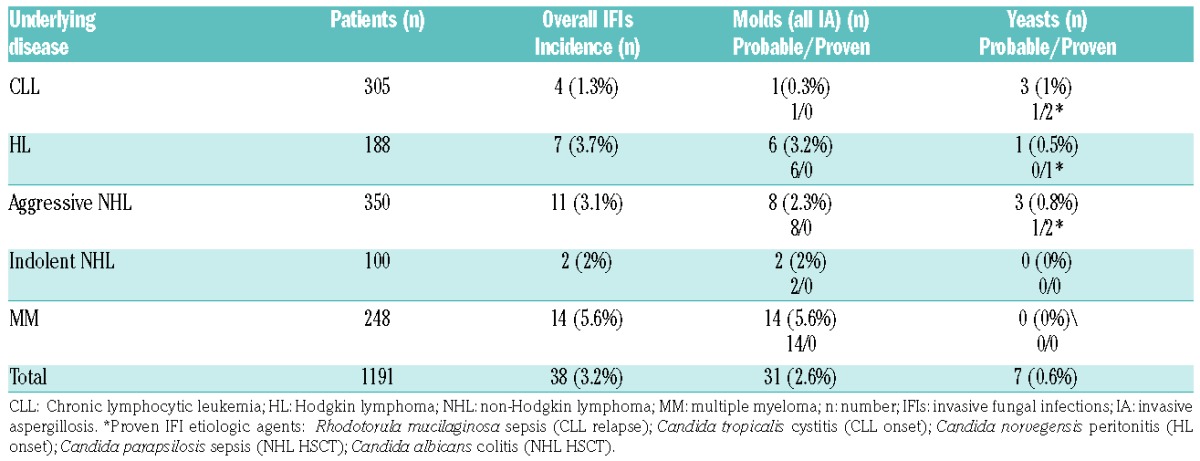
A total of 1,191 adult patients affected by CLD were collected [CLL n=305; HL n=188; aggressive NHL n=350; indolent NHL n=100; MM n=248]. Only 38 patients (3.2%) presented clinical signs and symptomatology compatible with a proven/probable IFI.
Invasive aspergillosis (IA) was the most frequent fungal infection observed (31 cases, 2.6%), all probable, while the other 7 infections, 2 probable and 5 proven, were caused by yeasts (0.6%), Table 1. The prevalence of IFIs was highest in patients with MM (5.6%), followed by HL (3.7%) and aggressive NHL (3.1%). Patients with indolent NHL and CLL were at a lower risk for IFIs (2% and 1.3%, respectively).
Table 1.
Incidence of fungal infections in different lymphoproliferative disorders.
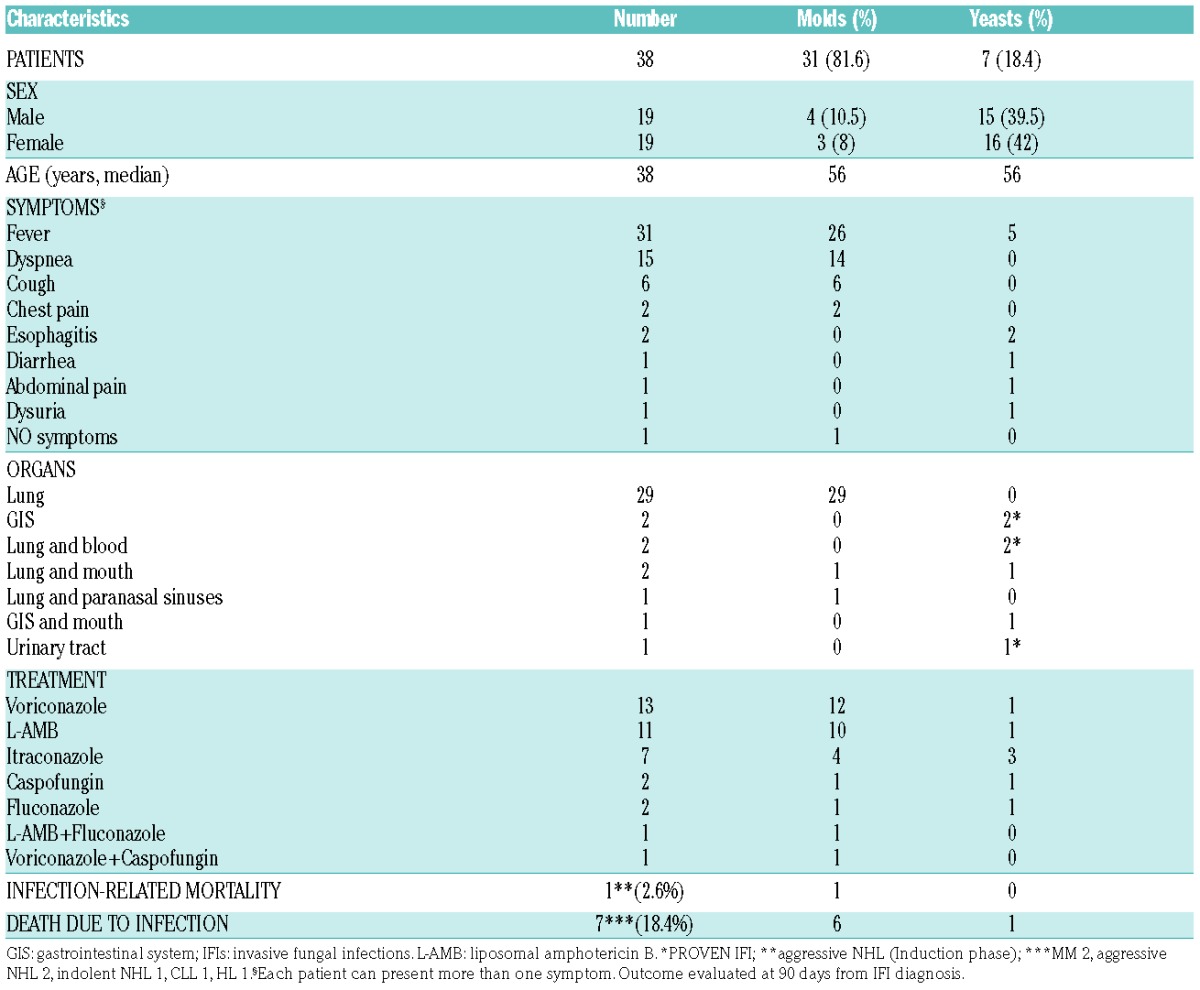
With respect to symptoms that were present during IFIs, 82% of patients presented with fever and 55% with dyspnea or a cough. The lungs were the most frequent organs involved (76%). We recorded 5 proven infections. Clinical characteristics are detailed in Table 2. In 22/38 cases (58%), IFIs were documented on CT scans. The GM test was positive in 55% (21/38) of patients on blood samples (index cutoff for a positive GM test: one test with 0.7 or two tests with 0.5), and in 24% (9/38) of patients on bronchoalveolar fluid (index cutoff >1.5). The treatment of IFIs varied widely, as shown in Table 2, and IFI was the cause of death in only one patient (2.6%), while 7 patients died from the hematological disease with a concomitant IFI. The outcome was evaluated at 90 days from IFI diagnosis, and 37/38 patients were still alive at that time point.
Table 2.
IFIs: clinical and microbiological characteristics.
Treatment-related factors that were frequently present in patients with IFIs were: HSCT within 100 days (18/38, 47% of patients), steroid treatments (prednisone equivalent >20 mg per day for >1 month, 19/38, 50% of patients) and neutropenia (PMN <500/mmc at the time of infection, 18/38, 47% of patients). All 18 patients who developed IFIs during first-line therapy that included HSCT, had IFIs after HSCT: 12/18 (67%) in the first 30 days after HSCT, and 6/18 (33%) after 30 days. No patients had received a previous prophylaxis. We observed 25 IFIs during first-line therapy (2.1%), while 13 IFIs occurred out of 398 patients with relapsed disease (3.3%) (P=0.2).
Restricting the analysis to patients with aggressive NHL, we observed that the majority of patients (7/11) who developed IFIs had a high-risk international prognostic index (IPI), and developed IFIs during the consolidation phase that followed induction treatment with rituximab- cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), consisting in the rituximab-mitoxantrone, carboplatin, cytosine arabinoside, and methyl-prednisolone (R-MICMA) regimen and the subsequent HSCT, as part of first-line treatment.5 This group of patients (young high-risk diffuse large B-cell lymhpoma (DLBCL)) had a 6.1-fold risk (odds ratio (OR); 95% confidence interval (CI), 1.1– 60.1, P=0.03) for IFIs with respect to young standard-risk DLBCL (<65 years, treated with R-CHOP without consolidation phase and subsequent HSCT).
The risk for IFIs was not increased in older DLBCL patients (>65 years), who did not receive intensive consolidation treatment.
IFIs in HL occurred in 5/188 (2.7%) patients during first-line treatment, and in 2/47 (4.3%) patients with relapsed disease. There was a trend for association between age >35 years and risk for IFIs in HL: 6/96 (6.3%) versus 1/92 (1%) in patients age >35 years and <35 years, respectively, (P=0.1). Patients with indolent NHL and CLL developed IFIs more often during salvage treatment for relapsed/progressive disease.
According to current guidelines, in MM patients first-line treatment includes VTD (bortezomib, thalidomide, and dexamethasone) followed by HSCT in patients <65 years or VMP (bortezomib, melphalan, and prednisone) in patients >65 years; patients with relapsed/refractory disease receive lenalidomide and dexamethasone. IFIs in MM patients appeared to be equally distributed between first-line treatment including VTD + HSCT or VMP and treatment for relapsed/refractory disease including lenalidomide (8/248 vs. 6/98, OR 0.5; 95% CI, 0.2–1.6). Patients undergoing HSCT were at higher risk for IFIs than non-transplanted patients (OR 14.1; 95% CI, 3–131.1, P<0.01).
The median time to the onset of IFIs in the 25 patients who suffered from IFIs in first-line therapy was 7 months from the first day of treatment.
IA was the most frequent fungal infection in our patients (31 cases, 2.6%). The prevalence of IFIs was highest in patients with MM and aggressive NHL, who often received a more aggressive treatment including HSCT as part of induction or consolidation treatment. Young high-risk DLBCL patients had a 6.1-fold risk (OR; 95% CI, 1.1– 60.1, P=0.03) for IFIs with respect to young standard-risk DLBCL (<65 years, treated with R-CHOP); similarly MM patients undergoing HSCT were at a higher risk for IFIs than non-transplanted patients (OR, 14.1; 95% CI, 3–131.1, P<0.01), whereas no difference was found between first-line treatments containing bortezomib and second-line treatments containing lenalidomide.
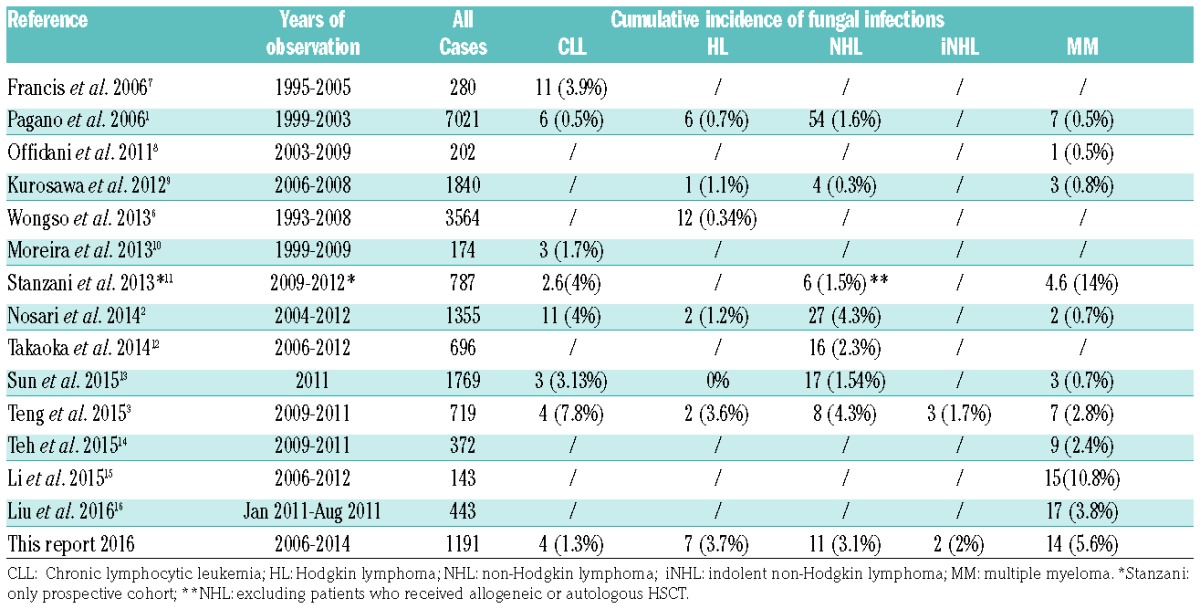
The vast majority of studies reported in the literature are retrospective and the analysis performed are extremely heterogeneous (Table 3). An increased risk of severe infection has been described in MM patients treated with bortezomib and in advanced stage HL treated with the intensified bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) regimen.6 The 3.2% incidence of IFIs in our series is in line with recent reports from the literature: 3% in the study by Nosari et al.2 and 3.8% in the study from Teng et al.,3 even if in the latter study both possible IFIs and precursor lymphoid neoplasms were included. This incidence is higher compared to the report of SEIFEM-2004 (NHL 1.6%, HL 0.7%, MM 0.5%, CLL 0.5%),1 which registered IFIs in the years from 1999 to 2003. However, it should be underlined that treatments of CLD have been changed with respect to that period, and new agents have been introduced in first-line treatment causing a modification of infectious epidemiology.
Table 3.
Incidence of fungal infections in lymphoproliferative disorders: literature revision.
The new treatment strategies in lymphoproliferative disorders, that include immunomodulating and immunosuppressive agents in addition to cytotoxic treatments and a wide routinary use of autologous HSCT, have caused an increased risk of IFIs among these patients. In the study herein, it seems particularly evident in MM and aggressive NHL patients, particularly when they underwent a HSCT procedure. These changes in the epidemiology induced us to modify the diagnostic workup for IFIs, now more frequent than in the past, in these patients. Furthermore, more oriented mold-active prophylaxis are taken into consideration, particularly for these latter patients categories. Prospective studies on this topic are required.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 2.Nosari AM, Pioltelli ML, Riva M, et al. Invasive fungal infections in lymphoproliferative disorders: a monocentric retrospective experience. Leuk Lymphoma. 2014;55(8):1844–1848. [DOI] [PubMed] [Google Scholar]
- 3.Teng JC, Slavin MA, The BW, et al. Epidemiology of invasive fungal disease in lymphoproliferative disorders. Haematologica. 2015;100(11):462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisi MC, Maiolo E, D’Alò F, et al. Fist line treatment for high-risk DLBCL in the rituximab era: high-dose chemotherapy with autologous stem cell transplantation, an intention to treat-analysys. Haematologica. 2014;99(S2):S1–S113. [Google Scholar]
- 6.Wongso D, Fuchs M, Plütschow A, et al. Treatment-related mortality in patients with advanced-stage hodgkin lymphoma: an analysis of the german hodgkin study group. J Clin Oncol. 2013;31(22):2819–2824. [DOI] [PubMed] [Google Scholar]
- 7.Francis S, Karanth M, Pratt G, et al. The effect of immunoglobulin VH gene mutation status and other prognostic factors on the incidence of major infections in patients with chronic lymphocytic leukemia. Cancer. 2006;107(5):1023–1033. [DOI] [PubMed] [Google Scholar]
- 8.Offidani M, Corvatta L, Polloni C, et al. Infectious complications in patients with multiple myeloma treated with new drug combinations containing thalidomide. Leuk Lymphoma. 2011;52(5):776–785. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa M, Yonezumi M, Hashino S, et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2012;96(6):748–757. [DOI] [PubMed] [Google Scholar]
- 10.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27(1):136–141. [DOI] [PubMed] [Google Scholar]
- 11.Stanzani M, Lewis RE, Fiacchini M, et al. A risk prediction score for invasive mold disease in patients with hematological malignancies. PLoS One. 2013;8(9):e75531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaoka K, Nannya Y, Shinohara A, Arai S, Nakamura F, Kurokawa M. A novel scoring system to predict the incidence of invasive fungal disease in salvage chemotherapies for malignant lymphoma. Ann Hematol. 2014;93(10):1637–1644. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Huang H, Chen J, et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China. Tumour Biol. 2015; 36(2):757–767. [DOI] [PubMed] [Google Scholar]
- 14.The BW, Teng JC, Urbancic K. Invasive fungal infections in patients with multiple myeloma: a multi-center study in the era of novel myeloma therapies. Haematologica. 2015;100(1):e28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Li Y, Huang B, Zheng D, Chen M, Zhou Z. Drug-induced modulation of T lymphocytes as a potential mechanism of susceptibility to infections in patients with multiple myeloma during bortezomib therapy. Cell Biochem Biophys. 2015;71(1):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Huang H, Li Y, et al. Epidemiology and treatment of invasive, fungal diseases in patients with multiple myeloma: findings from a multicenter prospective study from China. Tumour Biol. 2016; 37(6):7893–7900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


