Abstract
The European Conference on Infections in Leukemia (ECIL) provides recommendations for diagnostic strategies and prophylactic, pre-emptive or targeted therapy strategies for various types of infection in patients with hematologic malignancies or hematopoietic stem cell transplantation recipients. Meetings are held every two years since 2005 and evidence-based recommendations are elaborated after evaluation of the literature and discussion among specialists of nearly all European countries. In this manuscript, the ECIL group presents the 2015-update of the recommendations for the targeted treatment of invasive candidiasis, aspergillosis and mucormycosis. Current data now allow a very strong recommendation in favor of echinocandins for first-line therapy of candidemia irrespective of the underlying predisposing factors. Anidulafungin has been given the same grading as the other echinocandins for hemato-oncological patients. The beneficial role of catheter removal in candidemia is strengthened. Aspergillus guidelines now recommend the use of either voriconazole or isavuconazole for first-line treatment of invasive aspergillosis, while first-line combination antifungal therapy is not routinely recommended. As only few new data were published since the last ECIL guidelines, no major changes were made to mucormycosis recommendations.
Introduction
The European Conference on Infections in Leukemia (ECIL) is the result of a collaboration between the European Organization for Research and Treatment of Cancer (EORTC), the European Society for Blood and Marrow Transplantation (EBMT), the European Leukemia Net (ELN), and the International Immunocompromised Host Society (ICSH). First recommendations for the treatment of Candida and Aspergillus infections in hematologic patients were published in 2007 after the first conference (ECIL-1) and have then been updated at ECIL-2 and ECIL-3.1,2 First recommendations for the diagnosis and treatment of mucormycosis have been published after ECIL-3.3 ECIL-4 updates for antifungal therapy were only available as slides on the websites of these participating societies without publication of a manuscript in consideration of the lack of substantial new data and the limited modifications compared to the latest publication.
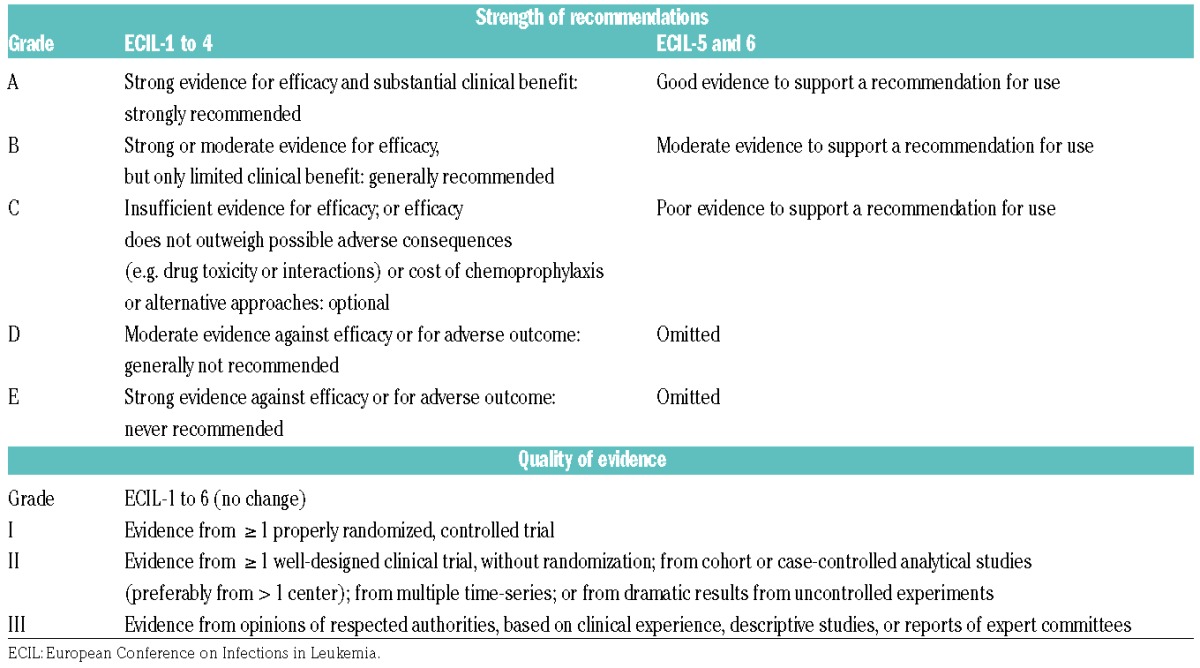
With respect to the targeted treatment of fungal infections, the goals for ECIL-5 were to update the recommendations with analysis of the new data for invasive candidiasis, aspergillosis and mucormycosis in hematologic patients. The update was also necessary to change the prior 5-level grading (A to E) used during the ECILs 1 to 4 for the strength of recommendations for Candida and Aspergillus infections into the 3-level grading (A to C) already used during ECIL-3 for the first recommendation for mucormycosis (Table 1).1–3 The grading for quality of evidence has not been modified.
Table 1.
Evolution over time of the grading system used for treatment of invasive Candida and Aspergillus infections.
Methods
The ECIL-5 meeting was held in September 2013 and involved 57 experts from 21 countries, including 3 non-European countries. Slides of the conclusions of the ECIL-5 were made available on the websites of the EORTC, EBMT, ELN, and ICHS. The ECIL-6 meeting was held in September 2015 with the presence of 55 experts from 24 countries, including 4 non-European countries (see list of collaborators at the end of this Review).
At both the ECIL-5 and the ECIL-6 meetings, the antifungal therapy working group made a search for new publications regarding treatment of invasive candidiasis, aspergillosis and mucormycosis. The group was divided into three subgroups, each being responsible for one of each fungal infection type. The literature search was performed in Pubmed and Cochrane databases. Abstracts presented at major congresses during the previous two years were also retrieved and integrated into the ECIL recommendation. All recommendations referring to an abstract, however, were classified as provisional until the publication of the final manuscript.
The working group presented its recommendations during the plenary session at the ECIL-5 meeting and then incorporated the suggestions coming from the assembly. In cases in which full consensus was not obtained, the decision was put to the vote, and the final decision was based on a majority of votes from the full ECIL-5 assembly. The updated recommendations were presented on the next day during a second plenary session for final approval. Recommendations were graded on the basis of the strength of recommendations (3-level scale: A, B, or C) and quality of evidence (3-level scale: I, II, or III), as detailed in Table 1.
The manuscript of the ECIL-5 was put on hold after a debate arose on differences between ECIL and European Society for Clinical Microbiology and Infectious Diseases (ESCMID) / European Confederation of Medical Mycology (ECMM) recommendations on guidelines for prophylaxis and treatment of invasive aspergillosis (draft presented at the ECCMID 2014).4 Two joint meetings were subsequently held (December 2014 and April 2015) to identify the differences and the exact reasons for these differences. The aim was not to modify the recommendations made by each of the two groups but rather to add explanations on the differences in the manuscript. For further clarification, a joint presentation was given at the ECIL-6 by members of the ECIL group and of the ESCMID/ECMM group. This resulted in a delay in publication of the ECIL-5 recommendations and during the ECIL-6 plenary session, the ECIL assembly approved a new search for publications or abstracts until September 2015 with inclusion of all relevant data on aspergillosis, candidiasis and mucormycosis for a full update of the guidelines. Final approval by the majority of the members of the group was obtained in Autumn 2015. The current manuscript includes updates from both the ECIL-5 and the ECIL-6 and is called “ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients”.
Invasive candidiasis
Like previous ECIL recommendations, the current guidelines for invasive candidiasis cover the hematologic population as well as the general population of patients. Although hematologic patients are the main focus of the recommendation, this distinction is maintained because available data from the original randomized controlled trials mainly include non-neutropenic patients. Chronic infections are not considered. Twenty-two major publications were identified (Tables 2 and 3).5–26 Fifteen reported primary results from clinical trials.5–11,13–17,19,20 One publication analyzed results of a subgroup of cancer patients from a previously published trial.12 One publication reported the analysis of pooled data from 2 trials previously published with a focus on patients with an underlying malignancy.21 All these studies were published before the ECIL-4. Since then, 5 studies have been identified, including one patient-level quantitative review of 7 published trials on invasive candidiasis, one pooled patient-level data analysis from 5 prospective trials on anidulafungin, one systematic review of 17 randomized clinical trials focusing on invasive candidiasis in neutropenic patients, one prospective non-comparative trial evaluating a strategy of early oral switch from anidulafungin for invasive candidiasis, and one observational study comparing the initial use of echinocandin-based versus azole-based regimen for C. parapsilosis candidemia.22–26 These publications were the reasons for the change in guidelines. Characteristics of these studies and main results are shown in Tables 2 and 3.
Table 2.
Trials for first-line therapy of invasive candidiasis: critical inclusion and exclusion criteria, treatment and relevant characteristics of the patients.
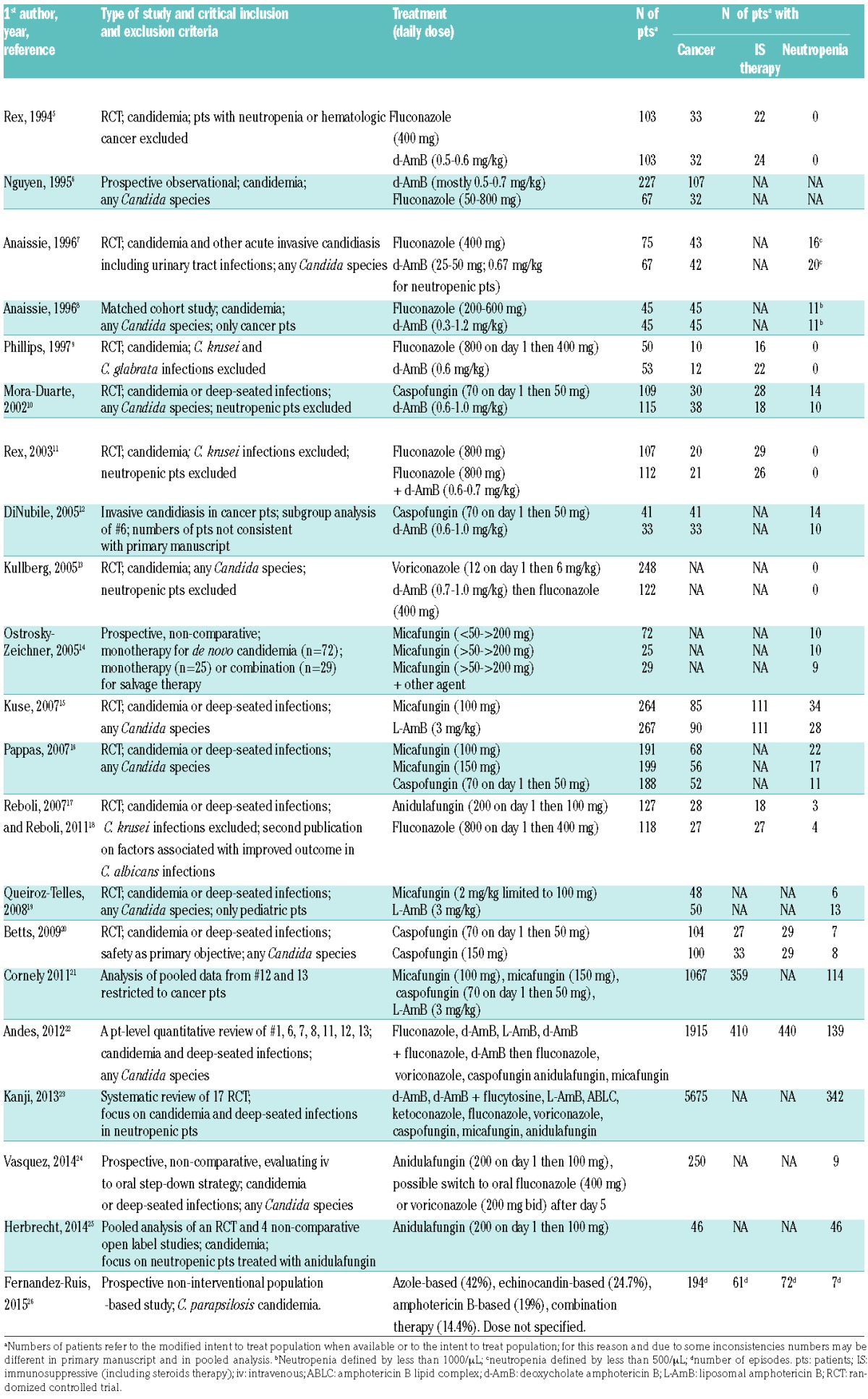
Table 3.
Trials for first-line therapy of invasive candidiasis: outcomes
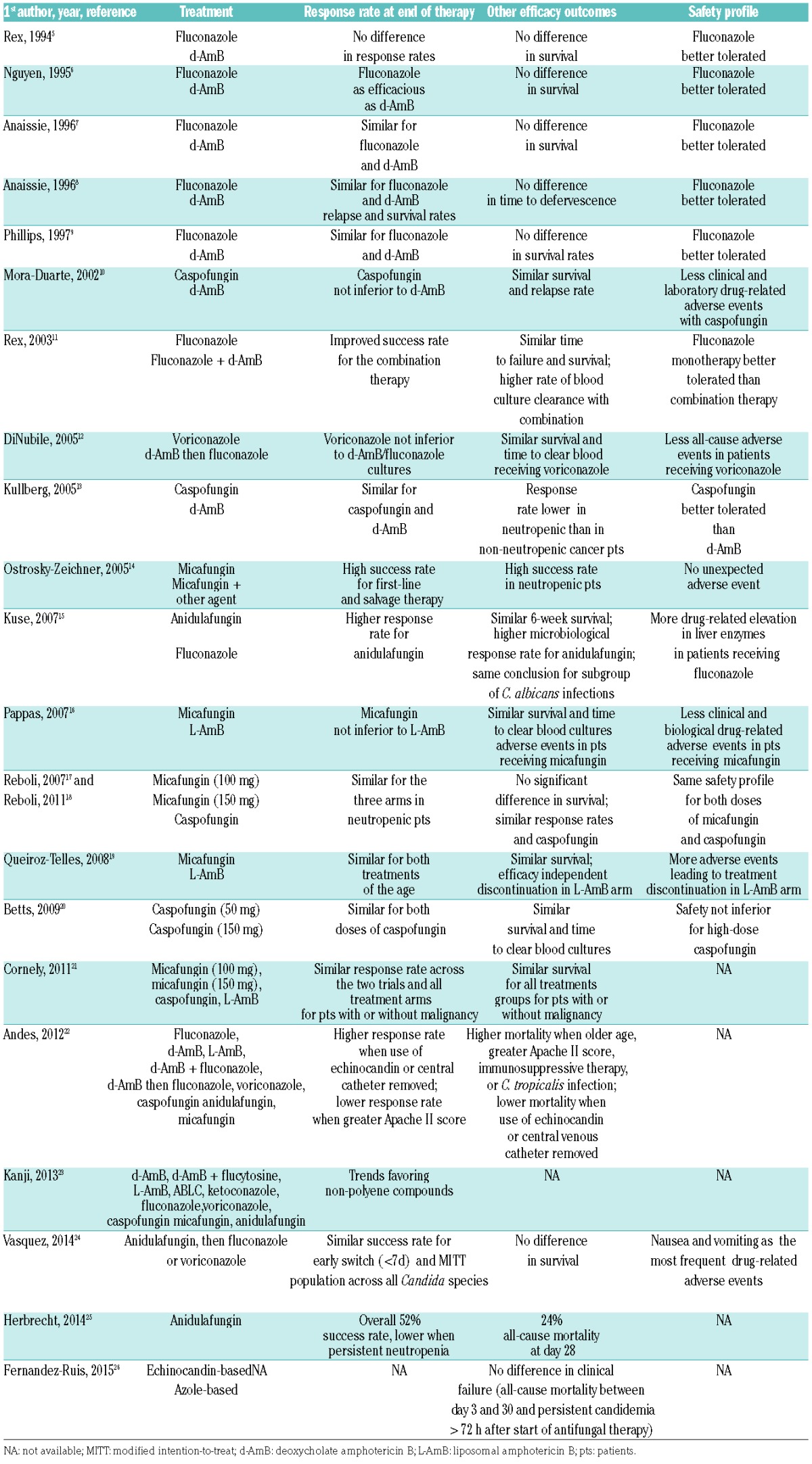
The number of neutropenic patients included in each of these studies was low and limited the level of evidence of the recommendation for this group of patients. The review published by Andes et al. showed that, in the univariate analysis, neutropenia was one of the factors significantly and negatively associated both with clinical outcome and with survival.22 In the multivariate analysis, however, the effect of neutropenia disappeared, but there was a significant association of immunosuppressive therapy (including steroids) with lower survival. Other factors significantly associated with lower survival were the APACHE score, infection by C. tropicalis and age, while treatment with an echinocandin [Odds Ratio (OR) 0.65, 95%CI: 0.45–0.94; P=0.02] and catheter removal were both significantly associated with better survival (OR 0.50, 95%CI: 0.35–0.72; P=0.0001).
Based on the patient-level quantitative analysis by Andes et al., echinocandins must be considered as first-line choice for invasive Candida infections before species identification (Table 4).22 The strength of recommendation is the same (A) for anidulafungin, caspofungin and micafungin and is also the same for the overall and the hematologic population. However, the quality of evidence is lower for hematologic patients (II) compared to the overall population, as the number of neutropenic patients recruited in the clinical trials was low. A recent communication on a patient-level pooled analysis of one randomized clinical trial and 4 open label studies focusing on anidulafungin in 46 neutropenic patients with candidemia showed comparable response and survival rates to those observed with caspofungin and micafungin in other studies.25 Therefore, the grading is now similar (A II) for all three echinocandins for the treatment of invasive candidiasis in hematologic patients.
Table 4.
ECIL-6 recommendations for initial first-line treatment of candidemia.
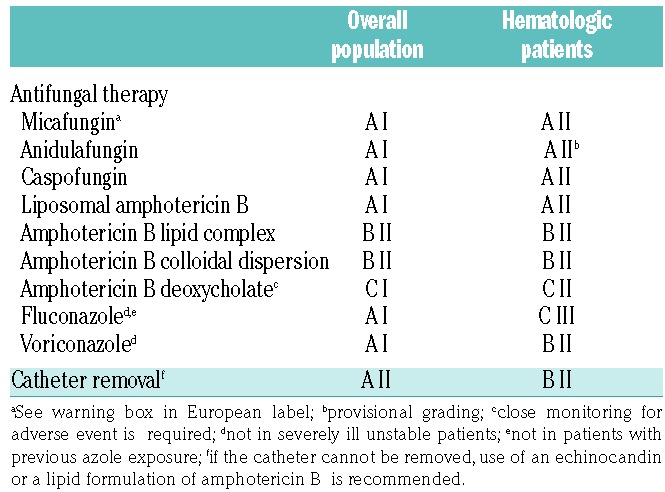
Liposomal amphotericin B has also been graded A I for the overall population and A II for hematologic patients due to similar efficacy in comparison to micafungin.15,21 However, its safety profile is less favorable and therefore liposomal amphotericin B should be considered as an alternative in case of contraindication to echinocandins. Fluconazole and voriconazole are potential alternatives for first-line treatment in the overall population provided there is no previous exposure to azoles and the infection is not severe (fluconazole).
After species identification, susceptibility testing should guide the treatment. In general, echinocandins remain the drug of choice, except for C. parapsilosis where fluconazole is more appropriate (Table 5). However, a recent observational study reported no difference in 30-day mortality and persistent candidemia at 72 hours of an echinocandin-based regimen compared to an azole-based therapy for patients with C. parapsilosis candidemia.26 Therefore, the continuing use of echinocandins might be considered in patients with a clinical and microbiological response. When Candida species is azole-susceptible, step-down to fluconazole can be considered in stable patients after five days of intravenous (iv) therapy.24 In patients with Candida krusei infection, switch to oral voriconazole is an option.
Table 5.
ECIL-6 recommendations for first-line treatment of candidemia after species identification.
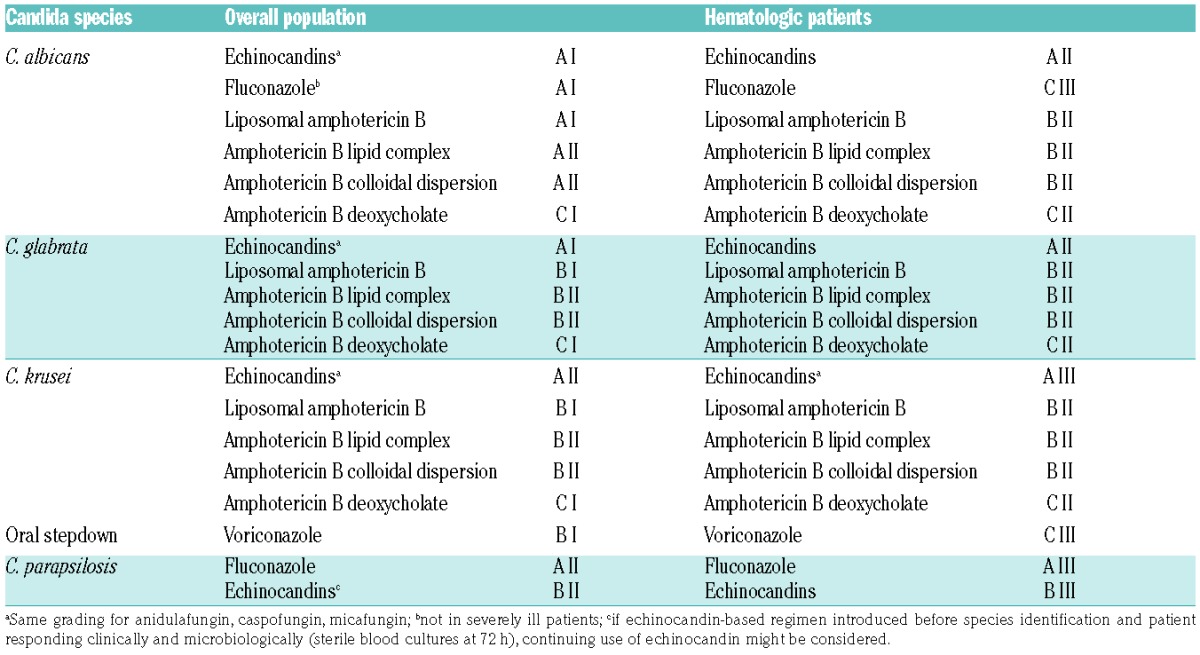
Although the role of catheter removal in the management of candidemia has long been controversial, most recent studies suggest a beneficial effect on outcome. 6–8,10,11,15,16,20,26–33 Garnacho-Montero et al. showed in a large number of candidemia that early adequate therapy and removal of central venous line were independently associated with lower mortality.34 The patient-level quantitative analysis by Andes et al. also demonstrated in a multivariate analysis that removal of catheter was associated with a decreased mortality (OR 0.50; 95%CI: 0.35–0.72; P=0.0001).22 The recommendation is, therefore, to rapidly remove the catheter in the overall population (grade A II) as well as in hematologic patients (grade B II) irrespective of the Candida species. If central venous catheter cannot be removed, treatment should include an echinocandin or a lipid formulation of amphotericin B due to their better activity on Candida biofilms.35–37
Invasive Aspergillus infections
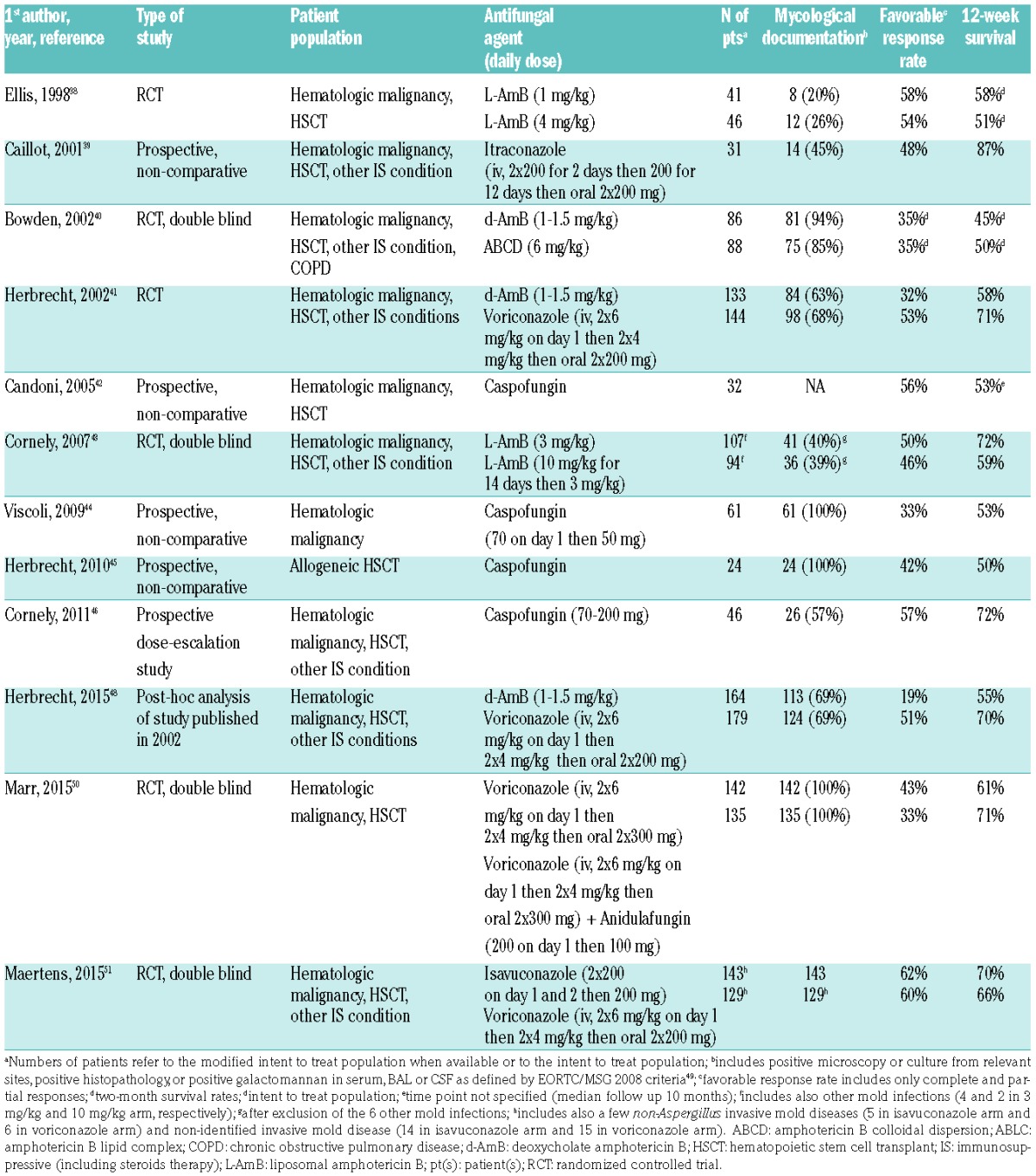
Nine prospective trials (only 4 being randomized comparative trials) had been published before the ECIL-4 and provided the basis of the previous guidelines for first-line therapy in invasive aspergillosis (Table 6).38–46 An additional paper reported a post-hoc analysis of the trial comparing standard dose of liposomal amphotericin B to high-dose liposomal amphotericin B.47 This post-hoc analysis comparing outcome in possible versus mycologically documented aspergillosis underscored the limited number of mycologically documented infections but did not lead to any change in the grading for liposomal amphotericin B. A second post-hoc analysis was performed on the voriconazole versus amphotericin B deoxycholate trial.48 Integration of the results of baseline galactomannan detection tests performed after primary analysis and re-categorization according to the 2008 EORTC/MSG definition criteria allowed more mycologically documented cases of invasive aspergillosis to be identified.49 Conclusions of this post-hoc analysis were similar to those of the primary analysis and therefore its results did not affect the grading for voriconazole and for amphotericin B deoxycholate.
Table 6.
Trials for first-line therapy of invasive aspergillosis: main characteristics and outcome.
At the time of the ECIL-5, results from the comparative study of voriconazole plus anidulafungin versus voriconazole plus placebo were only available in abstract form. The results have been discussed with a provisional grading that could be transformed in a definite grading, as no additional data available in the full paper suggested a need for change in provisional recommendations.50 This study failed to reach the primary endpoint of decreased all-cause mortality at week 6 (difference of −8.2% in favor of combination; P=0.087). However, in a subgroup of patients with an invasive aspergillosis documented by positive galactomannan in either serum or bronchoalveolar lavage, 6-week all-cause mortality was lower in patients receiving combination therapy (difference of −11.6% in favor of combination; P=0.037). A large majority of the ECIL members felt that this subgroup analysis, that had not been originally planned, was not sufficient to give a stronger recommendation although this subgroup included 80% of the modified intent-to-treat population. Therefore, the combination of voriconazole plus anidulafungin was graded C I for primary therapy of invasive aspergillosis while all other combinations were graded C III in the absence of well-designed studies for first-line therapy.
Table 6 summarizes the main characteristics and results of the various studies. Importantly, very few studies had a large number of patients with a mycological documentation.40,41,50 As shown by the 2 post-hoc analyses, survival was substantially lower in mycologically-documented infections compared to possible cases.47,48 Therefore, studies with a limited number of documented cases cannot lead to the strongest recommendations. As no study specifically addressed management of breakthrough aspergillosis after failure of posaconazole or voriconazole prophylaxis, no recommendation could be made on this issue.
The clinical trial comparing the new triazole isavuconazole versus voriconazole for primary therapy of invasive aspergillosis could not be discussed during the ECIL-5 as results were only presented as an abstract in 2014. However, the group could review the data from these abstracts during the ECIL-6 meeting. Isavuconazole appears to be as effective as voriconazole for the treatment of invasive aspergillosis and has a better safety profile. Therefore, a grade A I similar to the grading for voriconazole has been given to isavuconazole (Table 7). As the full paper was published shortly after the meeting, and confirms the results, the provisional grading attributed during the meeting has been transformed into a definite grading in this manuscript.51
Table 7.
ECIL-6 recommendations for first-line treatment of invasive aspergillosis.
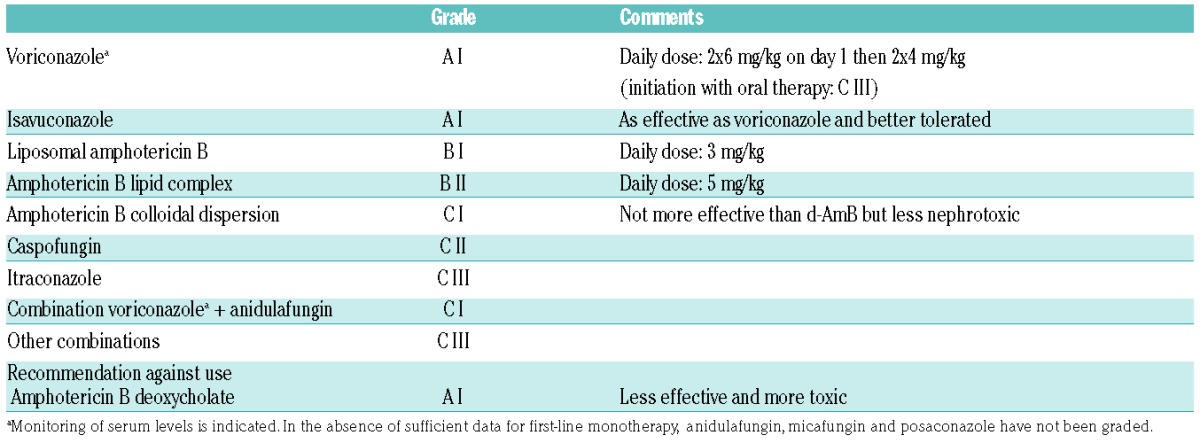
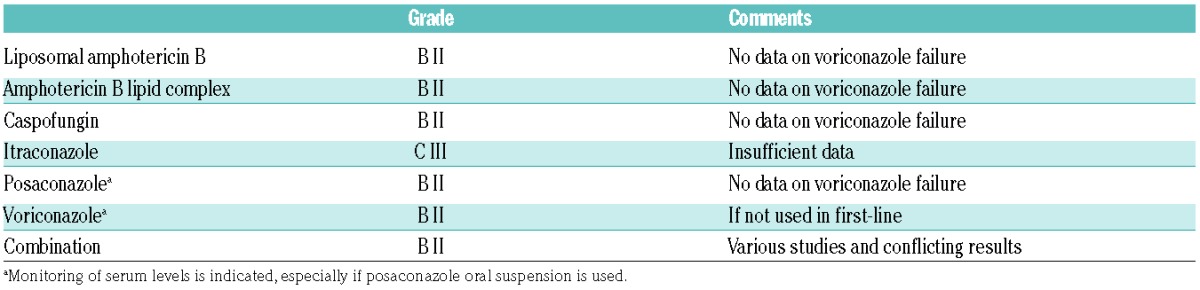
Currently, amphotericin B deoxycholate is considered to have no role in the treatment of invasive aspergillosis when more effective and less toxic agents are available. Its limited efficacy and its poor safety profile led to a recommendation against its use. No substantial change has been made for second-line therapy in the absence of new data (Table 8).
Table 8.
ECIL-6 recommendations for salvage therapy of invasive aspergillosis.
Mucormycosis
Diagnostic and therapeutic strategies were discussed during the ECIL-5 and the ECIL-6. Rhizopus, Mucor, Lichtheimia (previously classified as Absidia), Cunninghamella, Rhizomucor, Apophysomyces, and Saksenaea are the genera most frequently involved in human disease.52 Cunninghamella species is more virulent in experimental models and may be associated with a higher mortality rate in patients.53 So far, there has not been enough evidence that identification of mucormycosis to the genus and/or species level helps guide antifungal treatment.54,55 Species identification remains, nevertheless, important for outbreak investigations.56 However, the differentiation between mucormycosis and other invasive mold infection is of critical importance as it has major therapeutic implications.
While epidemiological aspects and some clinical (sinus disease, concomitant diabetes, occurrence under voriconazole therapy) and radiological (reverse halo sign on chest CT-scan) factors may help to suspect mucormycosis, the diagnosis remains difficult and biopsy of the lesion is often required. Identification of the pathogen most often comes from microscopic, culture and/or histopathological examination of relevant samples. New diagnostic approaches include molecular testing on serum and various other clinical samples including formalin-fixed tissues, MALDI-TOF and Mucorales-specific T-cell detection.57–64 Although these new approaches are very promising for an earlier diagnosis, no grading for their use can be given yet due to the lack of data.
Amphotericin B, posaconazole and isavuconazole are the most potent agents in vitro.65–67 Currently, no validated minimum inhibitory concentration breakpoints for any of the drugs are available and thus determination of susceptibility categories is not possible for the agents of mucormycosis. The ECIL-3 recommendations for the treatment of mucormycosis were mostly based on retrospective studies, registry data and small prospective non-controlled studies.3,68–77 Few new data are available for the treatment of mucormycosis since the ECIL 4 and, therefore, the current recommendations are very similar (Table 9).
Table 9.
ECIL-6 recommendations for first-line therapy of mucormycosis.
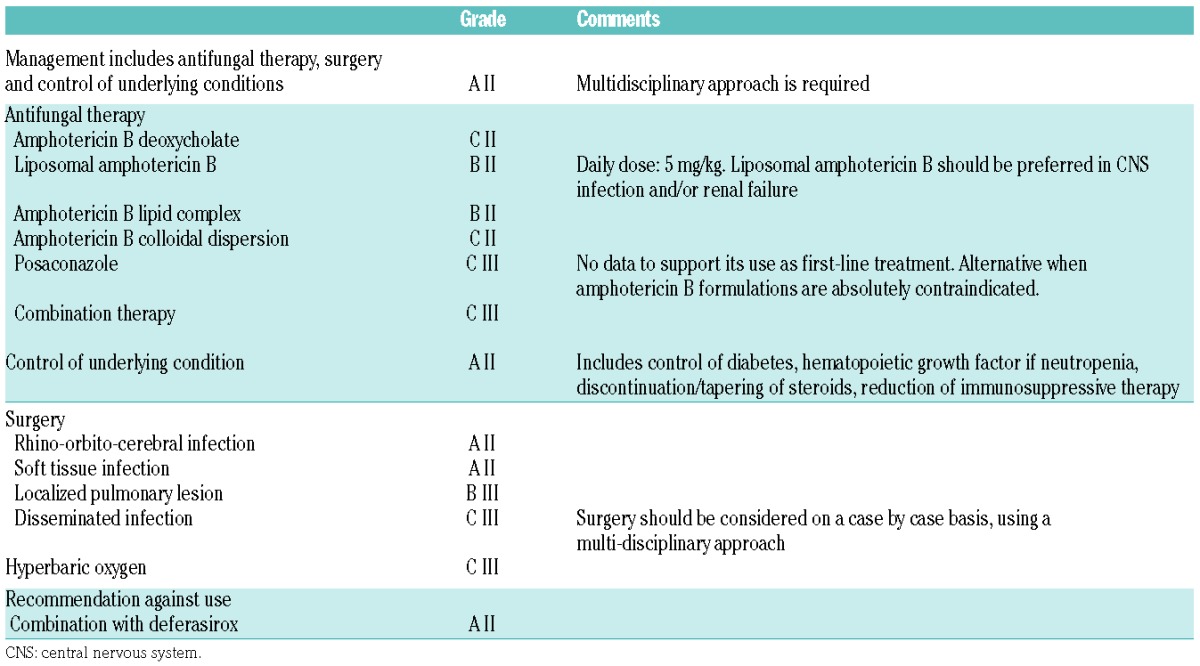
A prospective non-comparative trial assessed the efficacy and safety of first-line therapy with high-dose liposomal amphotericin B given at 10 mg/kg/day combined with surgery when appropriate.78 This trial demonstrated efficacy of high-dose liposomal amphotericin B plus surgery in mucormycosis with a survival rate of 62% at week 12. The only factor associated with mortality was the presence of hematologic malignancy or cancer (HR: 3.15; 95%CI: 1.12–8.91; P=0.02). Renal impairment of any degree was observed in 40% of the patients but was transient in most of them. These results confirm the beneficial role of liposomal amphotericin B but do not yet allow any recommendation for the administration of such a high dose of 10 mg/kg/day.
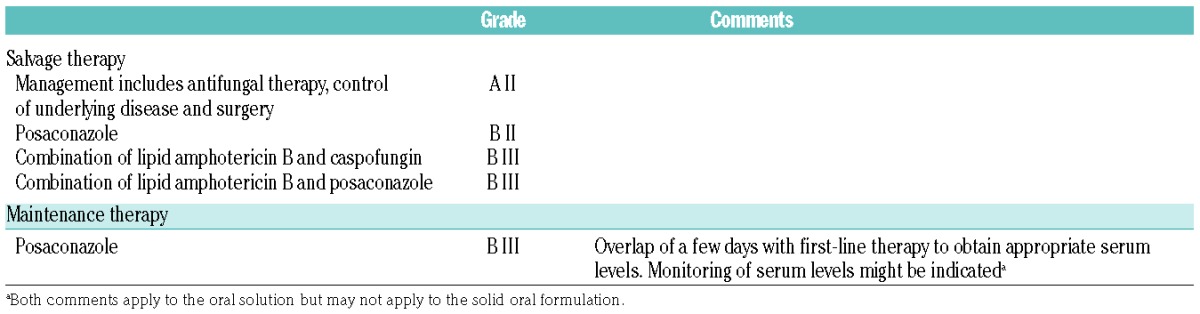
A short paper presented data from a retrospective analysis of a combination of posaconazole and a lipid formulation of amphotericin B.79 Thirty-two patients received this combination of posaconazole with liposomal amphotericin B (n=27) or amphotericin B lipid complex (n=5). Only 3 of them were treated with this combination in first line. Overall response rate was 56% but a large proportion of patients (59%) died before day 90. The low number of patients, the retrospective nature of the study, and the high mortality rate at day 90 only allowed for a B III recommendation for this combination for salvage therapy of mucormycosis (Table 10).
Table 10.
ECIL-6 recommendations for salvage and maintenance therapy of mucormycosis.
Discussion and conclusions
An update of the ECIL antifungal treatment recommendations was needed as there were important new data, and also because of necessary changes in the ECIL grading system so as to be in harmony with other ECIL recommendations. The most important data for invasive candidiasis came from a large review of patients included in 7 major trials.22 The multivariate analysis now allows a very strong recommendation in favor of an echinocandin for the first-line therapy of candidemia irrespective of the underlying predisposing factors. The controversy on the beneficial role of catheter removal can now be considered to be resolved. The most interesting new data were the publication of a first-line combination study in invasive aspergillosis and the results of a randomized comparative trial comparing isavuconazole to voriconazole. Aspergillus guidelines now include the results of these 2 clinical trials and should help clinicians in their treatment decision making. Since few new data have been published since the last ECIL guidelines, no major changes were made to mucormycosis management. Importantly, the posology and indication of antifungal agents reported in the current guidelines do not necessarily reflect those licensed by the European Medicines Agency (EMA), but are the result of a consensus-based analysis of available literature within the ECIL group.
There has been controversy about some discrepancies between the ECIL-5 and the ESCMID recommendations for invasive aspergillosis in hematologic patients. These differences were identified during a joint meeting and an ESCMID representative was invited to discuss them at the ECIL-6 meeting. Most differences were minor and mostly reflected a difference in grading system. The ECIL Aspergillus recommendations are restricted to hematologic patients who represent more than 90% of the patients included in the major clinical trials.41,43,50,51 No subgroup of hematologic patients deserving specific recommendation for Aspergillus infection treatment has been identified by the ECIL group. In contrast, the ESCMID group had a broader approach considering all other conditions predisposing to invasive aspergillosis, grading the diagnostic procedures, and including environmental measures in the prevention, also providing a grade for specific infection sites. In addition, the ESCMID group also segregated the hematologic patients into subgroups and provided specific grading for each of them, with usually weaker recommendations when there was not a sufficient number of patients with these specific underlying conditions included in the clinical studies. Finally, and importantly, some data were not available at the time of the ECIL-5 meeting but were in the public domain when the ESCMID group met. In September 2015, the ECIL-6 group was able to incorporate the new data, and this has helped to reduce the apparent differences with the ESCMID guidelines. Therefore, neither the ECIL group nor the ESCMID group felt any change other than this update was required.
Supplementary Material
Acknowledgments
The authors and contributors thank the group GL-Events, Lyon, France, for the organization of the meeting. They also thank Valérie Rizzi-Puechal (Pfizer), France; Markus Rupp (MSD), Germany; Sonia Sanchez (Gilead Sciences), UK; Anne-Therese Witschi (Basilea), Switzerland; Lorraine Tweddle (Astellas), UK.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/3/433
Collaborators: Agrawal Samir, United Kingdom; Akova Murat, Turkey; Alanio Alexandre, France; Aljurf Mahmoud, Saudi Arabia; Averbukh Dina, Israel; Barnes Rosemary, United Kingdom; Blennow Ola, Sweden; Bochud Pierre Yves, Switzerland; Bouza Emilio, Spain; Bretagne Stephane, France; Bruggemann Roger, Netherlands; Calandra Thierry, Switzerland; Carratalà Jordi, Spain; Castagnola Elio, Italy; Cesaro Simone, Italy; Cordonnier Catherine, France; Cornely Oliver, Germany; Dalianis Tina, Sweden; De La Camara Rafael, Spain; Donnelly Peter Joseph, Netherlands; Drgona Lubos, Slovakia; Duarte Rafael, Spain; Einsele Hermann, Germany; Engelhard Dan, Israel; Ferreira Isabelina, Portugal; Fox Christopher, United Kingdom; Girmenia Corrado, Italy; Groll Andreas, Germany; Hauser Philippe, Switzerland; Heldal Dag, Norway; Helweg-Larsen Jannik, Denmark; Herbrecht Raoul, France; Hirsch Hans, Switzerland; Hubacek Petr, Czech Republic; Johnson Elizabeth M, United Kingdom; Kibbler Christopher, United Kingdom; Klyasova Galina, Russian Federation; Koskenvuo Minna, Finland; Kouba Michal, Czech Republic; Kullberg Bart-Jan, Netherlands; Lagrou Katrien, Belgium; Lewis Russel, Italy; Ljungman Per, Sweden; Maertens Johan, Belgium; Mallet Vincent, France; Marchetti Oscar, Switzerland; Martino Rodrigo, Spain; Maschmeyer Georg, Germany; Matos Olga, Portugal; Melchers Willem, Netherlands; Mikulska Malgorzata, Italy; Munoz Patricia, Spain; Nucci Marcio, Brazil; Orasch Christina, Switzerland; Padoin Christophe, France; Pagano Livio, Italy; Pagliuca Antonio, United Kingdom; Penack Olaf, Germany; Petrikkos Georgios, Greece; Racil Zdenek, Czech Republic; Ribaud Patricia, France; Rinaldo Christine H, Norway; Robin Christine, France; Roilides Emmanuel, Greece; Rovira Montserrat, Spain; Schellongowski Peter, Austria; Sedlacek Petr, Czech Republic; Sinko Janos, Hungary; Skiada Anna, Greece; Slavin Monica, Australia; Styczynski Jan, Poland; Tissot Frédéric, Switzerland; Van Boemmel Florian, Germany; Viscoli Claudio, Italy; Von Lilienfeld-Toal Marie, Germany; Ward Katherine, United Kingdom.
References
- 1.Herbrecht R, Flückiger U, Gachot B, Ribaud P, Thiebaut A, Cordonnier C. Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients. Eur J Cancer Suppl. 2007;5(2):49–59. [Google Scholar]
- 2.Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant. 2011;46(5):709–718. [DOI] [PubMed] [Google Scholar]
- 3.Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98(4):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullmann AJ. ESCMID guidelines for the diagnosis and treatment of Aspergillus diseases. Invasive aspergillosis in haematology and oncology. European Congress of Clinical Microbiology and Infectious Diseases Barcelona, Spain, 2014: Abstract EW081. [Google Scholar]
- 5.Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331(20):1325–1330. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen MH, Peacock JE, Jr, Tanner DC, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Arch Intern Med. 1995;155(22):2429–2435. [PubMed] [Google Scholar]
- 7.Anaissie EJ, Darouiche RO, Abi-Said D, et al. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23(5):964–972. [DOI] [PubMed] [Google Scholar]
- 8.Anaissie EJ, Vartivarian SE, Abi-Said D, et al. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. Am J Med. 1996; 101(2):170–176. [DOI] [PubMed] [Google Scholar]
- 9.Phillips P, Shafran S, Garber G, et al. Multicenter randomized trial of fluconazole versus amphotericin B for treatment of candidemia in non-neutropenic patients. Canadian Candidemia Study Group. Eur J Clin Microbiol Infect Dis. 1997;16(5):337–345. [DOI] [PubMed] [Google Scholar]
- 10.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–2029. [DOI] [PubMed] [Google Scholar]
- 11.Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36(10):1221–1228. [DOI] [PubMed] [Google Scholar]
- 12.DiNubile MJ, Hille D, Sable CA, Kartsonis NA. Invasive candidiasis in cancer patients: observations from a randomized clinical trial. J Infect. 2005;50(5):443–449. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–1442. [DOI] [PubMed] [Google Scholar]
- 14.Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis. 2005;24(10):654–661. [DOI] [PubMed] [Google Scholar]
- 15.Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527. [DOI] [PubMed] [Google Scholar]
- 16.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–893. [DOI] [PubMed] [Google Scholar]
- 17.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–2482. [DOI] [PubMed] [Google Scholar]
- 18.Reboli AC, Shorr AF, Rotstein C, et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis. 2011;11:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27(9):820–826. [DOI] [PubMed] [Google Scholar]
- 20.Betts RF, Nucci M, Talwar D, et al. A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009;48(12):1676–1684. [DOI] [PubMed] [Google Scholar]
- 21.Cornely OA, Marty FM, Stucker F, Pappas PG, Ullmann AJ. Efficacy and safety of micafungin for treatment of serious Candida infections in patients with or without malignant disease. Mycoses. 2011;54(6):e838–847. [DOI] [PubMed] [Google Scholar]
- 22.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122. [DOI] [PubMed] [Google Scholar]
- 23.Kanji JN, Laverdiere M, Rotstein C, Walsh TJ, Shah PS, Haider S. Treatment of invasive candidiasis in neutropenic patients: systematic review of randomized controlled treatment trials. Leuk Lymphoma. 2013;54(7):1479–1487. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez J, Reboli AC, Pappas PG, et al. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis. 2014;14:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbrecht R, Conte U, Biswas P, Capparella MR, Aram J. Efficacy of anidulafungin in the treatment of invasive candidiasis in neutropenic patients: analysis of pooled data from five prospective studies. European Conference on Clinical Microbiology and Infectious Diseases Barcelona, Spain, 2014: Abstract R692. [Google Scholar]
- 26.Fernandez-Ruiz M, Aguado JM, Almirante B, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58(10):1413–1421. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers C, Gaur S, Chew J, et al. Epidemiology and management of candidaemia–a retrospective, multicentre study in five hospitals in the UK. Mycoses. 2011;54(6):e795–800. [DOI] [PubMed] [Google Scholar]
- 28.Horn DL, Ostrosky-Zeichner L, Morris MI, et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis. 2010;29(2):223–229. [DOI] [PubMed] [Google Scholar]
- 29.Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia¿ An evidence-based review. Clin Infect Dis. 2002;34(5):591–599. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez D, Park BJ, Almirante B, et al. Impact of early central venous catheter removal on outcome in patients with candidaemia. Clin Microbiol Infect. 2007;13(8):788–793. [DOI] [PubMed] [Google Scholar]
- 31.Velasco E, Portugal RD. Factors prompting early central venous catheter removal from cancer patients with candidaemia. Scand J Infect Dis. 2011;43(1):27–31. [DOI] [PubMed] [Google Scholar]
- 32.Rex JH, Bennett JE, Sugar AM, et al. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis. 1995;21(4):994–996. [DOI] [PubMed] [Google Scholar]
- 33.Raad I, Hanna H, Boktour M, et al. Management of central venous catheters in patients with cancer and candidemia. Clin Infect Dis. 2004;38(8):1119–1127. [DOI] [PubMed] [Google Scholar]
- 34.Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, Ruiz Perez de Pipaon M, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013;68(1):206–213. [DOI] [PubMed] [Google Scholar]
- 35.Kucharikova S, Sharma N, Spriet I, Maertens J, Van Dijck P, Lagrou K. Activities of systemically administered echinocandins against in vivo mature Candida albicans biofilms developed in a rat subcutaneous model. Antimicrob Agents Chemother. 2013;57(5):2365–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidler M, Salvenmoser S, Muller FM. Liposomal amphotericin B eradicates Candida albicans biofilm in a continuous catheter flow model. FEMS Yeast Res. 2010;10(4):492–495. [DOI] [PubMed] [Google Scholar]
- 37.Shuford JA, Rouse MS, Piper KE, Steckelberg JM, Patel R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis. 2006;194(5):710–713. [DOI] [PubMed] [Google Scholar]
- 38.Ellis M, Spence D, de Pauw B, et al. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis. 1998;27(6):1406–1412. [DOI] [PubMed] [Google Scholar]
- 39.Caillot D, Bassaris H, McGeer A, et al. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis. 2001;33(8):e83–90. [DOI] [PubMed] [Google Scholar]
- 40.Bowden R, Chandrasekar P, White MH, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2002;35(4):359–366. [DOI] [PubMed] [Google Scholar]
- 41.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. [DOI] [PubMed] [Google Scholar]
- 42.Candoni A, Mestroni R, Damiani D, et al. Caspofungin as first line therapy of pulmonary invasive fungal infections in 32 immunocompromised patients with hematologic malignancies. Eur J Haematol. 2005;75(3):227–233. [DOI] [PubMed] [Google Scholar]
- 43.Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007;44(10):1289–1297. [DOI] [PubMed] [Google Scholar]
- 44.Viscoli C, Herbrecht R, Akan H, et al. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J Antimicrob Chemother. 2009;64(6):1274–1281. [DOI] [PubMed] [Google Scholar]
- 45.Herbrecht R, Maertens J, Baila L, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: an European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant. 2010;45(7):1227–1233. [DOI] [PubMed] [Google Scholar]
- 46.Cornely OA, Vehreschild JJ, Vehreschild MJ, et al. Phase II dose escalation study of caspofungin for invasive Aspergillosis. Antimicrob Agents Chemother. 2011; 55(12):5798–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornely OA, Maertens J, Bresnik M, et al. Efficacy outcomes in a randomised trial of liposomal amphotericin B based on revised EORTC/MSG 2008 definitions of invasive mould disease. Mycoses. 2011;54(5):e449–455. [DOI] [PubMed] [Google Scholar]
- 48.Herbrecht R, Patterson TF, Slavin MA, et al. Application of the 2008 Definitions for Invasive Fungal Diseases to the Trial Comparing Voriconazole Versus Amphotericin B for Therapy of Invasive Aspergillosis: A Collaborative Study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin Infect Dis. 2015;60(5):713–720. [DOI] [PubMed] [Google Scholar]
- 49.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–89. [DOI] [PubMed] [Google Scholar]
- 51.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–769. [DOI] [PubMed] [Google Scholar]
- 52.Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(Suppl 1):S8–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petraitis V, Petraitiene R, Antachopoulos C, et al. Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med Mycol. 2013;51(1):72–82. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez MM, Pastor FJ, Sutton DA, et al. Correlation between in vitro activity of posaconazole and in vivo efficacy against Rhizopus oryzae infection in mice. Antimicrob Agents Chemother. 2010;54(5):1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salas V, Pastor FJ, Calvo E, et al. In vitro and in vivo activities of posaconazole and amphotericin B in a murine invasive infection by Mucor circinelloides: poor efficacy of posaconazole. Antimicrob Agents Chemother. 2012;56(5):2246–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S44–54. [DOI] [PubMed] [Google Scholar]
- 57.Bernal-Martinez L, Buitrago MJ, Castelli MV, Rodriguez-Tudela JL, Cuenca-Estrella M. Development of a single tube multiplex real-time PCR to detect the most clinically relevant Mucormycetes species. Clin Microbiol Infect. 2013;19(1):E1–7. [DOI] [PubMed] [Google Scholar]
- 58.Buitrago MJ, Aguado JM, Ballen A, et al. Efficacy of DNA amplification in tissue biopsy samples to improve the detection of invasive fungal disease. Clin Microbiol Infect. 2013;19(6):E271–277. [DOI] [PubMed] [Google Scholar]
- 59.Millon L, Herbrecht R, Grenouillet F, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect. 2016;22(9):810.e1–810.e8. [DOI] [PubMed] [Google Scholar]
- 60.Lass-Florl C, Mutschlechner W, Aigner M, et al. Utility of PCR in diagnosis of invasive fungal infections: real-life data from a multicenter study. J Clin Microbiol. 2013; 51(3):863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walther G, Pawlowska J, Alastruey-Izquierdo A, et al. DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia. 2013;30:11–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Carolis E, Posteraro B, Lass-Florl C, et al. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect. 2012; 18(5):475–484. [DOI] [PubMed] [Google Scholar]
- 63.Schrodl W, Heydel T, Schwartze VU, et al. Direct analysis and identification of pathogenic Lichtheimia species by matrix-assisted laser desorption ionization-time of flight analyzer-mediated mass spectrometry. J Clin Microbiol. 2012;50(2):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potenza L, Vallerini D, Barozzi P, et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011; 118(20):5416–5419. [DOI] [PubMed] [Google Scholar]
- 65.Verweij PE, Gonzalez GM, Wiedrhold NP, et al. In vitro antifungal activity of isavuconazole against 345 mucorales isolates collected at study centers in eight countries. J Chemother. 2009;21(3):272–281. [DOI] [PubMed] [Google Scholar]
- 66.Vitale RG, de Hoog GS, Schwarz P, et al. Antifungal susceptibility and phylogeny of opportunistic members of the order mucorales. J Clin Microbiol. 2012;50(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drogari-Apiranthitou M, Mantopoulou FD, Skiada A, et al. In vitro antifungal susceptibility of filamentous fungi causing rare infections: synergy testing of amphotericin B, posaconazole and anidulafungin in pairs. J Antimicrob Chemother. 2012;67(8):1937–1940. [DOI] [PubMed] [Google Scholar]
- 68.Greenberg RN, Mullane K, van Burik JAH, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herbrecht R, Letscher-Bru V, Bowden RA, et al. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion. Eur J Clin Microbiol Infect Dis. 2001;20(7):460–466. [DOI] [PubMed] [Google Scholar]
- 70.Oppenheim BA, Herbrecht R, Kusne S. The safety and efficacy of amphotericin B colloidal dispersion in the treatment of invasive mycoses. Clin Infect Dis. 1995; 21(5):1145–1153. [DOI] [PubMed] [Google Scholar]
- 71.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. [DOI] [PubMed] [Google Scholar]
- 73.Ruping MJ, Heinz WJ, Kindo AJ, et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother. 2010;65(2):296–302. [DOI] [PubMed] [Google Scholar]
- 74.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012; 67(3):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–65. [DOI] [PubMed] [Google Scholar]
- 76.Xhaard A, Lanternier F, Porcher R, et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: a French Multicentre Cohort Study (2003–2008). Clin Microbiol Infect. 2012; 18(10):E396–400. [DOI] [PubMed] [Google Scholar]
- 77.Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39(1):3–22. [DOI] [PubMed] [Google Scholar]
- 78.Lanternier F, Poiree S, Elie C, et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (LAMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015; 70(11):3116–3123. [DOI] [PubMed] [Google Scholar]
- 79.Pagano L, Cornely OA, Busca A, et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: a report from the SEIFEM and FUNGISCOPE registries. Haematologica. 2013;98(10):e127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


