Abstract
The forkhead transcription factor FOXP1 is generally regarded as an oncogene in activated B cell-like diffuse large B-cell lymphoma. Previous studies have suggested that a small isoform of FOXP1 rather than full-length FOXP1, may possess this oncogenic activity. Corroborating those studies, we herein show that activated B cell-like diffuse large B-cell lymphoma cell lines and primary activated B cell-like diffuse large B-cell lymphoma cells predominantly express a small FOXP1 isoform, and that the 5′-end of the Foxp1 gene is a common insertion site in murine lymphomas in leukemia virus- and transposon-mediated insertional mutagenesis screens. By combined mass spectrometry, (quantative) reverse transcription polymerase chain reaction/sequencing, and small interfering ribonucleic acid-mediated gene silencing, we determined that the small FOXP1 isoform predominantly expressed in activated B cell-like diffuse large B-cell lymphoma lacks the N-terminal 100 amino acids of full-length FOXP1. Aberrant overexpression of this FOXP1 isoform (ΔN100) in primary human B cells revealed its oncogenic capacity; it repressed apoptosis and plasma cell differentiation. However, no difference in potency was found between this small FOXP1 isoform and full-length FOXP1. Furthermore, overexpression of full-length FOXP1 or this small FOXP1 isoform in primary B cells and diffuse large B-cell lymphoma cell lines resulted in similar gene regulation. Taken together, our data indicate that this small FOXP1 isoform and full-length FOXP1 have comparable oncogenic and transcriptional activity in human B cells, suggesting that aberrant expression or overexpression of FOXP1, irrespective of the specific isoform, contributes to lymphomagenesis. These novel insights further enhance the value of FOXP1 for the diagnostics, prognostics, and treatment of diffuse large B-cell lymphoma patients.
Introduction
The forkhead transcription factor FOXP1 plays an important role in a wide variety of biological processes, including T- and B-cell development and function.1–5 Furthermore, FOXP1 has been recognized as a potential oncogene in hepatocellular carcinoma, pancreatic cancer, and various types of B-cell non-Hodgkin lymphomas.1–4 In hepatocellular carcinoma, diffuse large B-cell lymphoma (DLBCL), and mucosa-associated lymphoid tissue (MALT) lymphoma, overexpression of FOXP1, by chromosomal translocations, copy number alterations, or other means, is associated with poor prognosis and transformation to aggressive lymphoma.3,5,6 Rare but recurrent chromosomal translocations affecting FOXP1 have been found in activated B-cell (ABC)-DLBCL and MALT lymphoma. The majority of these translocations involve FOXP1 and the immunoglobulin heavy chain (IgH) enhancer (t(3;14)(p13;q32)).7–10 These FOXP1-IgH rearrangements mostly affect the 5′ untranslated region of FOXP1 and result in overexpression of full-length FOXP1.11 Non-IG/FOXP1 rearrangements have also been described, and these often target FOXP1 downstream of its first coding exon, resulting in increased expression of N-terminally truncated FOXP1 isoforms.12 In addition, expression levels of FOXP1 can be used as a discriminator between the ABC and germinal center (GC) subtypes of DLBCL, which are biologically distinct disease entities. ABC-DLBCL combines high FOXP1 expression with an unfavorable prognosis, supporting an oncogenic role of FOXP1.13,14 Paradoxically, FOXP1 is located on a chromosomal region that is associated with a loss of heterozygosity and deletions in a number of solid tumors.1,15 In line with this, FOXP1 transcriptional activity is inhibited in a large number of epithelial malignancies by either a decrease in FOXP1 messenger ribonucleic acid (mRNA), a decrease in FOXP1 protein levels, or by aberrant cytoplasmic localization of FOXP1.16 Moreover, high FOXP1 expression is associated with favorable prognosis in breast cancer, lung cancer, epithelial ovarian carcinoma and peripheral T–cell lymphoma.17–22
A possible explanation for the above-mentioned apparently contradictory role of FOXP1 as either an oncogene or a tumor suppressor gene was presented with the identification of smaller FOXP1 isoforms (encoding proteins with N-terminal deletions), that are preferentially expressed in ABC-DLBCL.23,24 It was proposed that these smaller FOXP1 isoforms might have oncogenic potential in B-cell non-Hodgkin lymphomas, whereas the full-length protein might function as a tumor suppressor.23,25 The hypothesis that loss of the FOXP1 N-terminus might be linked to malignancy is further supported by a study in which Foxp1 was identified as the second most frequent viral integration sites that results in avian nephroblastoma.26 These insertions clustered within the second coding exon of Foxp1, but did not affect mRNA expression levels,26 suggesting that they might result in expression of an N-terminally truncated Foxp1 protein. Moreover, in contrast to FOXP1-IGH translocations, non-IG/FOXP1 rearrangements, which cause increased expression of N-terminally truncated FOXP1 isoforms, are found as secondary genetic hits acquired during the clinical course of various B-cell neoplasms, suggesting that these smaller isoforms might be involved in disease progression.12
Thus, several lines of evidence suggest that the smaller FOXP1 isoforms, rather than full-length FOXP1 (FOXP1-FL), might act as oncogenes in B-cell malignancies. However, functional studies with these smaller FOXP1 isoforms in B cells, including a direct comparison with the actions of FOXP1-FL, are lacking. This became even more relevant as recent studies by our own and other laboratories have shown that high FOXP1 expression can contribute to B-cell lymphomagenesis by promoting B-cell survival,27–29 inhibiting plasma cell differentiation,30,31 potentiating Wnt/β-catenin signaling,32 and suppressing major histocompatibility complex (MHC) class II expression.28,33 Therefore, we herein determined the identity of the small FOXP1 isoform (FOXP1-iso) predominantly expressed in ABC-DLBCL and studied its oncogenic potential and transcriptional activity, in direct comparison to FOXP1-FL in DLBCL cell lines and primary human B cells.
Methods
Constructs
LZRS-FOXP1-IRES and LZRS-BCL6-IRES-GFP were generated as previously described.27,30 A FOXP1-iso construct that starts translation from the second coding ATG (in exon 6; M101), encoding a 100 AA N-terminally deleted FOXP1, was PCR-cloned and subcloned into LZRS.
B-cell cultures, cell-lines, retroviral transduction and siRNA-mediated knockdown
With informed consent and approval of the Academic Medical Center review board, human B cells were isolated, cultured, and retrovirally transduced, followed by 3 day recovery, after which cells were passaged and cultured with or (for plasma cell differentiation experiments, or as indicated) without CD40L-L cells, essentially as described.30 For microarray analysis, after transduction cells were cultured without cytokines for three days.
DLBCL cell lines OCI-Ly1, OCI-Ly3, OCI-Ly7 and OCI-Ly10 were obtained and cultured as described.34 HBL1, TMD8 and RIVA were kindly provided by Dr. G. Lenz (Munster, Germany). Retroviral transduction and small interfering ribonucleic acid (siRNA)-mediated knockdown were performed as described.27
Immunoblotting
10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with rabbit polyclonal anti-FOXP1 ab16645 (Abcam) was performed as described.30
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Quantitative PCR was conducted exactly as described.27
Mass spectrometry
FOXP1 protein was immunoprecipitated from OCI-Ly10 cells using anti-FOXP1 antibody (JC12) and analyzed by SDS-PAGE and silver stained. Protein bands corresponding to FOXP1 full-length and the smaller isoform were trypsin-digested as described35 and analyzed by nano-LC-ESI-ion trap MS/MS and by MALDI-ToF MS. For details, see the Online Supplementary Information. FOXP1 peptides identified by both methods in the FOXP1 full-length and isoform protein bands, respectively, were combined to get the overall picture of exons covered by these peptides.
Flow cytometry, proliferation and cell cycle analysis, and Caspase-Glo 3/7 assay
For identification of plasma cells, cells were stained with anti-human CD38-APC and CD20-PE and analyzed on a FACSCanto. Flow cytometry of green fluorescent protein (GFP) and/or yellow fluorescent protein (YFP) fluorescence, proliferation and cell-cycle analysis by eFluor 670 and propidium iodide, and the Caspase-Glo 3/7 assay (Promega, Madison, WI, USA), was performed as described.27
Enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISPOT) assay
Human immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA) and IgG isotypes ELISA, and IgG and IgM ELISPOTs were performed as described.30
Microarray analysis
Microarray analysis was performed essentially as described.27,36 Data were analyzed and heatmaps were generated using the microarray analysis and visualization platform R2.
Results
A small FOXP1 isoform is highly expressed in ABC-DLBCL
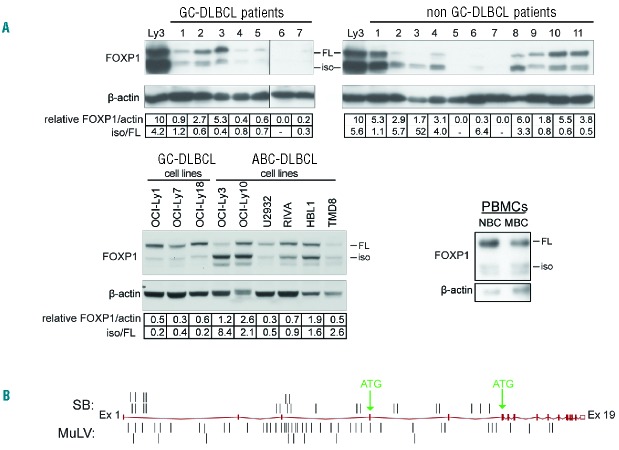
FOXP1 is a discriminator (between the ABC and GC subtype), prognosticator, and putative oncogene in DLBCL.5,13,14,37–40 To study the expression of FOXP1 in DLBCL, we analyzed a panel of DLBCL patient biopsies (n=18) and the ABC-DLBCL cell lines OCI-Ly3, OCI-Ly10, U2932, RIVA, HBL1, and TMD8, and the GC-DLBCL cell lines OCI-Ly-1, OCI-Ly7, and OCI-Ly18, by immunoblotting. We observed that the majority of non-GC-DLBCL patients (7/11) and ABC-DLBCL cell lines (4/6) predominantly express the smaller FOXP1 protein species, whereas all GC-DLBCL patients, GC-DLBCL cell lines, and peripheral blood B cells from healthy donors predominantly express FOXP1-FL protein (Figure 1A). These results corroborate a previous study by Brown et al.,23 suggesting that a small isoform of FOXP1 rather than FOXP1-FL, might have oncogenic potential in ABC-DLBCL.
Figure 1.
A small FOXP1 isoform is highly expressed in ABC-DLBCL patients, and the 5′end of the Foxp1 gene is a frequent target of insertional mutagenesis in mouse lymphoma models. (A) Western blot analysis for FOXP1 protein expression in tissue samples of non-GC- and GC-DLBCL patients, DLBCL cell lines (i.e., the ABC-DLBCL cell lines OCI-Ly3, OCI-Ly10, U2932, RIVA, HBL1, and TMD8 and the GC-DLBCL cell lines OCI-Ly-1, OCI-Ly7, and OCI-Ly18), and human peripheral blood B cells. NBC: naïve B cell (CD19+CD27−); MBC: memory B cell (CD19+CD27+). Expression of FOXP1 (iso + FL) was relative to β-actin. For comparison, in the blots with DLBCL patient biopsies the FOXP1 levels of OCI-Ly3 were normalized to an arbitrary level of 10 units; please note that shorter exposures were used for accurate quantification of FOXP1 expression in OCI-Ly3. (B) locations of insertions found in the Foxp1 gene in MuLV and sleeping beauty (SB) transposon insertional mutagenesis screens in murine lymphoma models. DLBCL: diffuse large B-cell lymphoma; GC: germinal center; ABC: activated B-cell; iso: isoform; FL: full-length; PBMC: peripheral blood mononuclear cell; MuLV: murine leukemia virus.
The 5′-end of the Foxp1 gene is a frequent target of insertional mutagenesis in murine lymphoma models
Additional support for an oncogenic role of FOXP1, including FOXP1-iso, is provided by insertional mutagenesis screens in murine lymphoma models, using either murine leukemia virus (MuLV) or sleeping beauty (SB) transposon.41,42 In these datasets Foxp1 ranked number 40 and number 13 of the most frequently targeted genes, respectively42 (and unpublished results). Herein, we show that in both models, most insertions are present at the 5′-end of Foxp1 (26/26 and 47/51, respectively), and several insertions are found between the start of the first coding exon (mouse Foxp1 exon 4; corresponding to human FOXP1 exon 6) and the third coding exon (7/26 and 9/51, respectively) (Figure 1B). This is of specific interest because truncations in this region might lead to expression of an N-terminally truncated Foxp1 isoform due to the presence of an in frame ATG site in the third coding exon (Figure 1B).
Taken together, the predominant expression of FOXP1-iso in ABC-DLBCL, and the clustering of insertions at the 5′ end of the Foxp1 gene, in particular between the first and third coding exon in mouse lymphoma models, indicate that deregulated expression of FOXP1 or (N-terminally) truncated FOXP1 is oncogenic in lymphomas.
The major FOXP1 isoform expressed in ABC-DLBCL lacks the N-terminal 100 amino acids
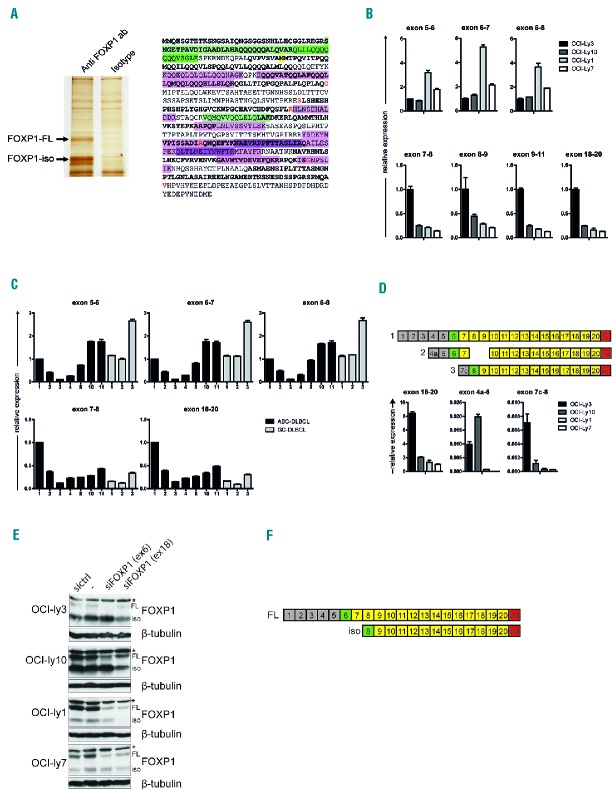
To identify the FOXP1-iso protein specie(s), we isolated FOXP1-FL and the major FOXP1-iso protein from the ABC-DLBCL cell line OCI-Ly10 and performed mass spectrometric analysis of trypsin-digested protein (Figure 2A). Whereas protein fragments encoded by exon 9, 10, and 13 to 19 were found in mass spectrometric analysis of both FOXP1-FL and FOXP1-iso, three fragments were exclusively detected in the full-length protein: one is encoded by exons 13 and 14 and the two others by exons 6 and 7 (Figure 2A). Intriguingly, the absence of exon 6, the first coding exon, would result in translation of a protein that starts at the first available in-frame start codon, being an ATG site in exon 8; the encoded protein would lack the N-terminal 100 amino acids (AAs), which would be in line with the observed difference in molecular weight between FOXP1-iso and FOXP1-FL.
Figure 2.
Identification of the FOXP1-iso transcript and protein. (A) FOXP1 protein was immunoprecipitated from OCI-Ly10 cells and FOXP1-FL and FOXP1-iso proteins were isolated from a silverstained gel (left). The proteins were trypsin-digested and fragments were subsequently analyzed by mass spectrometry. Fragments found in both proteins are indicated in purple, fragments exclusively found in the FOXP1-FL protein are indicated in green. Different color intensities are used to discriminate individual fragments (right). Alternating exons are indicated by regular and bold font. Amino acids overlapping exon boundaries are indicated in red. Methionine-101 is indicated by yellow shading. (B,C) qRT-PCR analysis for the levels of 5′FOXP1 exons in cell lines with high levels of FOXP1-FL (OCI-Ly1 and OCI-Ly7) or of FOXP1-iso (OCI-Ly3 and OCI-Ly10) (B), and in ABC-DLBCL and GC-DLBCL patient biopsies. Numbers correspond to numbers of biopsies in Figure 1A (C). Expression levels were normalized for HPRT expression levels and to expression levels in OCI-Ly3 (B) or in ABC-DLBCL patient 1 (C). (D) qRT-PCR analysis for the levels of mRNA transcripts containing alternative exons 4a and 7c. Upper panel: diagram illustrating the exon structure of alternative spliced isoforms that encode for FOXP1-FL (1), or that have been previously described by Brown et al. to encode for the most prominently expressed isoforms (2,3).23 Lower panel: qRT-PCR analysis for the levels of expression of transcript 1, 2, and 3 in cell lines with high levels of FOXP1-FL (OCI-ly1 and OCI-ly7) or of FOXP1-iso (OCI-ly3 and OCI-ly10). Please note the difference in y-axis values. (E) DLBCL cell lines were transfected with siRNA against FOXP1 exon 6, siRNA against FOXP1 exon 18, control siRNA, or were treated in the same way without addition of siRNA (−). Two days after nucleofection with siRNA against FOXP1, cell lysates were harvested and immunoblotted for FOXP1. β-tubulin was used as a loading control. *indicates a non-specific background band. (F) Diagram illustrating the exon structure of FOXP1-FL and FOXP1-iso. FL: full-length; iso: isoform; sictrl: si control; siFOXP1: small interfering RNA directed against FOXP1; ab: antibody.
Next, to investigate the potential presence of internal deletions in FOXP1-iso transcripts, we conducted RT-PCR analysis of the GC-DLBCL cell line OCI-Ly1 and the ABC-DLBCL cell line OCI-Ly3, the latter expressing high levels of FOXP1-iso (Figure 1A). Employing primer pairs spanning multiple exons (Online Supplementary Figure S1A), we did not detect any shorter products (Online Supplementary Figure S1B). Indeed, sequencing of these RT-PCR products, as well as additional RT-PCR products covering single exons 3 to 21, confirmed the lack of deletions, insertions or mutations in FOXP1 encoding exons in these cell lines. These data demonstrate that the major isoform does not contain any “gaps” of 1 or 2 consecutive internal exons in the region of exons 4 to 19. Notably, any protein product of a transcript that lacks more than 2 exons would be too small in size to represent the major FOXP1 isoform. Thus, our combined spectrometry and PCR/sequencing data indicate that the FOXP1-iso transcript lacks mRNA encoded by exons preceding exon 8.
The previous non-quantitative PCR setup may not reveal the absence of multiple 5′ or 3’ exon-encoded sequences in the FOXP1-iso transcript: primers against these regions will bind the transcript encoding FOXP1-FL. Therefore, by quantitative RT-PCR, we compared expression of several 5′ versus 3’ exon-encoded sequences in cell lines which predominantly express either FOXP1-iso (OCI-Ly3 and OCI-Ly10), or FOXP1-FL (OCI-Ly1 and OCI-Ly7) (Figure 2B), and in ABC- and GC-DLBCL patient biopsies (Figure 2C). Interestingly, whereas ABC-DLBCL cell lines and patient biopsies show relatively high expression of the FOXP1 C-terminal region (exons 18–20), they show relatively low expression of regions upstream of exon 7 (Figure 2B,C). In addition, we also performed qRT-PCRs with primers targeting alternative exons 4a and 7c, previously described to be present in transcripts encoding small isoforms of FOXP1 (Figure 2D).23 Although mRNA containing these alternative exons could be detected, expression levels were very low and did not correlate with expression of FOXP1-iso. Together with the lack of internal exon deletions, these results strongly suggest that exon 6 and preceding exons are absent in the FOXP1-iso transcript.
Finally, to confirm the absence of exon 6 in the transcript encoding FOXP1-iso, we specifically targeted FOXP1 transcripts containing exon 6 with an exon 6 specific siRNA pool (Figure 2E). Whereas nucleofection of DLBCL cell lines with an siRNA that targets exon 18 silenced expression of both FOXP1-FL and FOXP1-iso, the exon 6 siRNA exclusively reduced expression of FOXP1-FL, demonstrating that the FOXP1-iso transcript indeed does not contain exon 6 (Figure 2E).
Combined, our mass spectrometry, RT-PCR, and knockdown data firmly demonstrated the absence of any internal deletions in FOXP1-iso, that FOXP1-iso is encoded by an mRNA that lacks exon 6, and that translation starts from exon 8 resulting in a protein product that lacks the 100 N-terminal AAs (Figure 2F).
FOXP1-iso and FOXP1-FL display similar effects on expansion and survival of primary human B cells
We have previously shown that overexpression of FOXP1-FL in primary human B cells directly represses a panel of pro-apoptotic genes, and promotes the expansion of these cells by inhibiting caspase-dependent apoptosis, without affecting B-cell proliferation.27 These data indicate that high FOXP1 expression might contribute to lymphomagenesis by promoting B-cell survival. To investigate the effect of FOXP1-iso on B-cell survival and proliferation, we generated a retroviral expression vector encoding a FOXP1 protein lacking the first 100 AAs (“FOXP1-iso”), followed by IRES-YFP (FOXP1-iso-IRES-YFP). To compare the effects of FOXP1-FL versus FOXP1-iso overexpression, primary human B cells were retrovirally transduced with FOXP1-FL-IRES-YFP, FOXP1-iso-IRES-YFP or control-IRES-YFP (as a negative control) (Figure 3A), and cultured on L cells expressing CD40 ligand (CD40L-L cells), in the presence of interleukin (IL)-21 and IL-2. Consistent with our previous study, the percentage of YFP positive cells in FOXP1-FL transduced cultures rapidly increased with time (Figure 3B). Interestingly, a similar increase in the percentage of YFP positive cells was observed in cultures transduced with FOXP1-iso, indicating that FOXP1–FL and FOXP1-iso are equally potent in promoting selective outgrowth of primary human B cells (Figure 3B).
Figure 3.
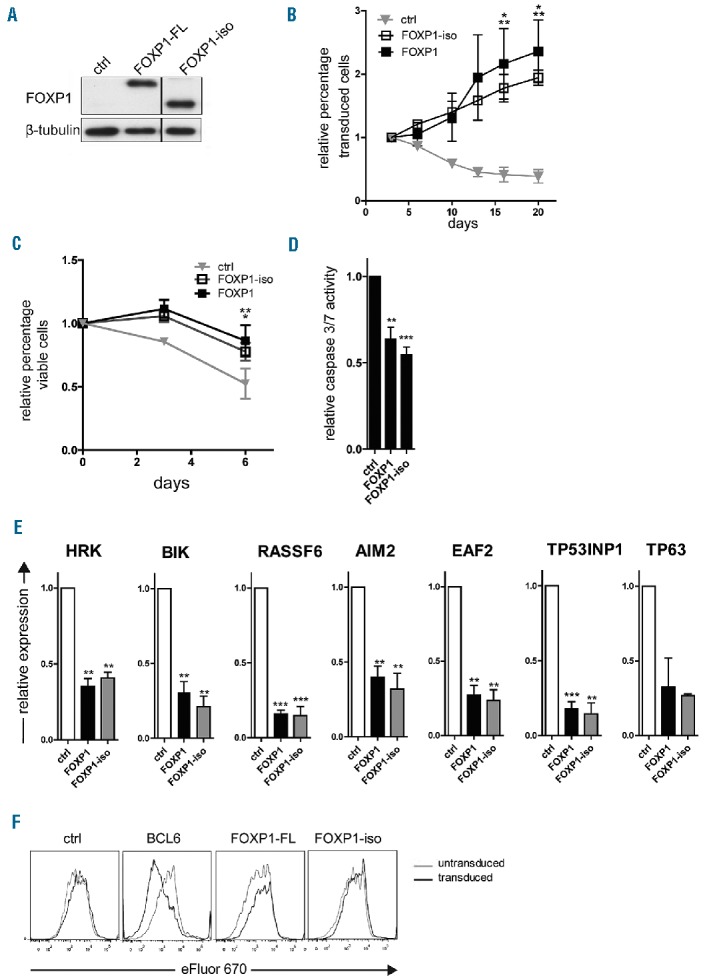
FOXP1-iso exerts similar effects on B-cell survival as FOXP1-FL. Memory B cells were sorted from human peripheral blood and transduced with either FOXP1-IRES-YFP, FOXP1-iso-IRES-YFP, BCL6-IRES-GFP (F) or ctrl-IRES-YFP, and cultured with CD40L-L cells, IL-21 and IL-2 (A-D,F) or without CD40L-L cells as of day 3 after transduction (E). (A) Representative example of FOXP1 and FOXP1-iso overexpression in primary YFP+ B cells. 6 days after transduction YFP+ cells were sorted and analyzed by immunoblotting. β-tubulin was used as loading control. (B) FOXP1-IRES-YFP, FOXP1-iso-IRES-YFP, and ctrl-IRES-YFP transduced B cells were continuously cultured with IL-21 and IL-2 and CD40L-L cells. The percentage of transduced cells in each culture was followed over time by FACS analysis and normalized to the percentage of transduced cells at day 3 after transduction. Mean ± SD of three independent experiments are shown. Significant differences were observed at day 15 and 20 after transduction between FOXP1 transduced cells vs. ctrl transduced cells (*P<0.05) and between FOXP1-iso transduced and control transduced cells (**P<0.01). (C) A total of 6–7 days after transduction, live YFP positive and negative fractions of FOXP1 and control vector single transduced cultures were sorted. After a recovery period of 4–5 days, the percentage of cells in the FSC/SCC live gate was determined by flow cytometry at three consecutive time points. The data were normalized to the percentage of living cells measured at the first time point. Mean ± SEM of three independent experiments are shown. Significant differences were observed at day 6 after transduction between FOXP1 transduced cells vs. ctrl transduced cells (**P<0.01) and between FOXP1-iso transduced and control transduced cells (*P<0.05). (D) Six days after transduction, the YFP positive fractions of FOXP1-FL, FOXP1-iso, and control vector-transduced cultures were sorted. After a recovery period of 5–7 days, caspase 3/7 activity was determined by the caspase glo3/7 assay. Values were corrected for the number of living cells as determined by FACS analysis. Mean ± SD of five independent experiments are shown (t-test **P<0.01, *** P<0.001). (E) Six days after transduction YFP positive cells were sorted. Gene expression levels of FOXP1 repressed pro-apoptotic genes were analyzed by quantitative RT-PCR. Expression levels were normalized to expression levels in control transduced cells. Mean ± SEM of at least three independent experiments (except for TP63) are shown (one sample t-test, **P<0.01, ***P<0.001). (F) 7 days after transduction, cells were labeled with eFluor 670 and cultured for four days, after which the eFluor 670 intensity was determined by flow cytometry. Representative graphs of two independent experiments are shown. FL: full-length; iso: isoform; ctrl: control.
To assess whether FOXP1-iso also promotes B-cell survival, viable cells of the YFP positive and negative fractions of FOXP1-FL, FOXP1-iso or empty vector transduced cells were sorted, and the percentage of live cells was monitored by flow cytometry during 1 week of subsequent culturing. A clear, and similar, survival benefit was observed in cells transduced with either FOXP1-FL or FOXP1-iso (Figure 3C). Moreover, caspase 3/7 activity was significantly and similarly reduced in FOXP1-FL and FOXP1-iso transduced cells (Figure 3D). Furthermore, expression of a panel of 7 pro-apoptotic genes (HRK, BIK, RASSF6, AIM2, EAF2, TP53INP1 and TP63), previously shown to be repressed by FOXP1-FL, was repressed to a similar extent by FOXP1-iso overexpression (Figure 3E). Together, these results indicate that FOXP1-iso promotes the same survival pathways with a similar potency as FOXP1-FL in primary human B cells.
Whereas FOXP1-FL promotes selective outgrowth and inhibits apoptosis of primary human B cells, it does not affect proliferation in these cells.27 To investigate whether FOXP1-iso would have the additional capacity to affect proliferation of primary human B cells, we analyzed proliferation in FOXP1-iso, FOXP1-FL, BCL6 (as a positive control), or empty vector (as a negative control) transduced cultures, by labeling the cells with the proliferation dye eFluor 670. After four days of culturing, eFluor 670 dilution was not increased in FOXP1-FL nor in FOXP1-isotransduced cells (Figure 3F). In accordance, cell cycle analysis indicated no major changes in cell cycle distribution upon FOXP1-FL or FOXP1-iso overexpression (Online Supplementary Figure S2). BCL6 overexpression, on the other hand, did result in increased eFluor 670 dilution and a higher percentage of cells in synthesis (S) phase (Figure 3F and Online Supplementary Figure S2).
Combined, these results show that FOXP1-iso overexpression has a very similar effect on B-cell outgrowth as that observed with FOXP1-FL, both qualitatively and quantitatively. Like FOXP1-FL, overexpression of FOXP1-iso represses a panel of 7 pro-apoptotic genes, promotes survival, and prevents caspase-dependent apoptosis, but does not affect B-cell proliferation.
FOXP1-iso and FOXP1-FL display similar effects on plasma cell differentiation of primary human memory B cells
We have previously demonstrated that overexpression of FOXP1-FL in primary human B cells also represses plasma cell differentiation by inhibiting the expression of master regulators of plasma cell differentiation.30 These results indicate that the oncogenic potential of FOXP1 in B cells not only lies in its ability to promote cell survival but also in its ability to block terminal differentiation. Therefore, sorted CD27+ memory B cells from human peripheral blood were transduced with FOXP1-FL, FOXP1-iso or control empty vector, and cultured under conditions that drive their differentiation towards cells that have acquired a plasma cell phenotype (CD20−CD38+), display strongly increased expression of the master regulators of plasma cell differentiation PRDM1, IRF4 and XBP1, and secrete immunoglobulins.30
Overexpression of FOXP1-FL or FOXP1-iso resulted in a strong, similar repression of PRDM1, IRF4, and XBP1 in these cells, as determined by qRT-PCRs analysis (Figure 4A). Furthermore, flow cytometry analysis indicated an equal reduction in the formation of CD20−CD38+ plasma cells upon overexpression of either FOXP1-FL or FOXP1-iso (Figure 4B). Moreover, the secretion of IgGs, as determined by ELISA (Figure 4C), and the formation of IgG secreting plasma cells, as determined by ELISPOT (Figure 4D), were reduced to a similar extent upon overexpression of either FOXP1 isoform. These results indicate that FOXP1-FL and FOXP1-iso are equally potent in repressing plasma cell differentiation of primary human B cells.
Figure 4.
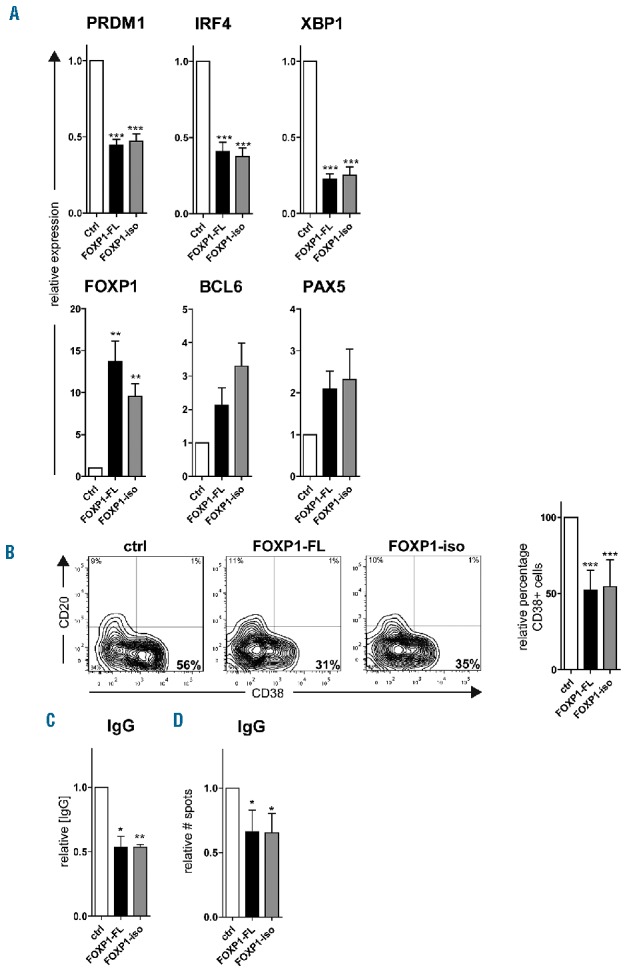
FOXP1-iso exerts similar effects on plasma cell differentiation as FOXP1-FL. CD19+CD27+ MBCs were sorted from human peripheral blood and transduced with FOXP1-FL-IRES-YFP, FOXP1-iso-IRES-YFP, or control empty vector and cultured under conditions that promote PC differentiation. Six days after transduction YFP positive cells were sorted (A,C,D). (A) Gene expression levels of PRDM1, IRF4, XBP1, FOXP1, BCL6 and PAX5 were analyzed in sorted cells by qRT-PCR. Expression levels in FOXP1-FL and FOXP1-iso- and control-transduced cells were normalized to β-actin and then to expression levels in control transduced cells. Mean ± SEM of five independent experiments are shown. (**P<0.01; ***P<0.001). (B) Six days after transduction, YFP positive cells were analyzed for CD20 and CD38 surface expression by flow cytometry. Representative dot plots of one out of 10 independent experiments are shown (left panel). Percentages of CD38+ cells in FOXP1-FL and FOXP1-iso-transduced cultures were normalized to control cultures. Mean ± SD values of 10 independent experiments are shown (right panel) (one sample t-test, ***P<0.001). (C) Equal numbers of sorted cells (50000) were cultured with IL-21 and IL-2 for an additional 24 hours. Thereafter, the supernatants were collected, and IgG protein levels were analyzed by ELISA. Levels were normalized to levels in control transduced cells. Mean ± SD of three independent experiments are shown (one sample t-test, *P<0.05, **P<0.01). (D) Equal numbers of sorted cells were plated onto membranes in serial dilutions and cultured with IL-21 and IL-2 for an additional 18 hours, after which numbers of IgM or IgG secreting cells were determined by ELISPOT. Spot numbers were normalized to numbers in stimulated, control transduced cells. Mean ± SD of four independent experiments are shown. (one sample t-test, *P<0.01). FL: full-length; iso: isoform; IgG: immunoglobulin G; ctrl: control.
FOXP1-iso and FOXP1-FL show similar gene expression regulation in DLBCL cell-lines
Thus far, our results revealed that the previously described effects of aberrant overexpression of FOXP1-FL in primary human B cells, i.e., promotion of B-cell survival and inhibition of plasma cell differentiation, are similarly and equally executed by FOXP1-iso. To obtain a broader insight into potential differences in the functions of FOXP1-iso and FOXP1-FL, we performed gene expression microarray analysis of the GC-DLBCL OCI-Ly1 and OCI-Ly7 (which predominantly express FOXP1-FL) and the ABC-DLBCL cell-line OCI-Ly10 (which predominantly expresses FOXP1-iso) (Figure 1A), retrovirally transduced with FOXP1-IRES-YFP, FOXP1-iso-IRES-YFP or control empty vector (Figure 5). Upon overexpression of either FOXP1-FL or FOXP1-iso the expression of 79 genes was changed by at least 1.5-fold in at least two cell lines. Chromatin immunoprecipitation sequencing (ChIP-Seq) analysis established that the majority of these genes were also directly bound within 20 kb of the transcription start site by FOXP1 in at least 2 DLBCL cell lines (Figure 5). Among the directly bound and repressed genes are AICDA, encoding activation-induced cytidine deaminase (AID), and several genes characteristic for GC-DLBCL, i.e., MYBL1, MME and LPP,43–45 which, like FOXP1, are also important prognosticators for DLBCL patient survival (high expression of those three genes and low expression of FOXP1 being correlated with better prognosis),45 indicating an important role for FOXP1 in defining the discriminatory gene signatures of ABC vs. GC-DLBCL. Importantly, however, all FOXP1-FL-regulated genes were also regulated by FOXP1-iso overexpression and vice versa, and always in the same direction. Thus, no functional differences in gene regulation were observed between FOPX1-FL and FOXP1-iso in DLBCL cell lines.
Figure 5.
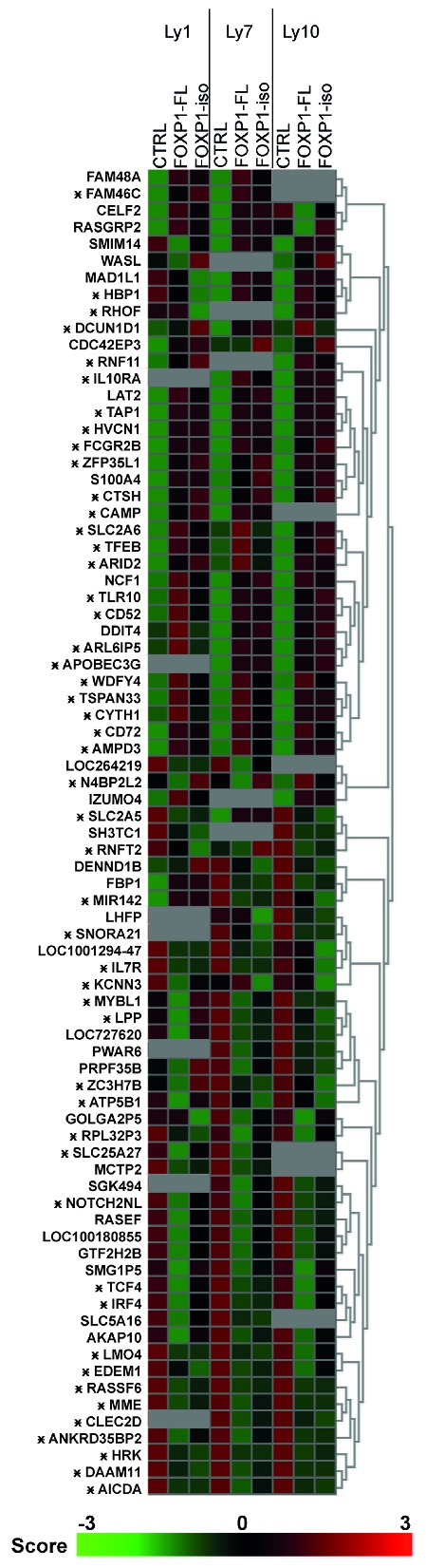
FOXP1-iso and FOXP1-FL regulate the same genes in DLBCL cell lines. DLBCL cell lines were retrovirally transduced with FOXP1-FL-IRES-YFP, FOXP1-iso-IRES-YFP, or control-IRES-YFP, and YFP positive cells were sorted. Gene expression microarray analysis was performed on these samples. All genes of which the expression was changed by either FOXP1-FL or FOXP1-iso overexpression by at least 1.5-fold in at least two cell lines are shown. Data are represented as z-scores calculated within samples of each cell line. Genes that were identified by ChIP-seq analysis to be bound by FOXP1 within 20kb of their TSS in at least 2 DLBCL cell lines are indicated by an asterisk. The gray squares indicate expression beneath the threshold value (= no expression). FL: full-length; iso: isoform: CTRL: control.
Discussion
FOXP1 has long been recognized as a putative oncogene in ABC-DLBCL, but molecular mechanisms underlying its oncogenic potential, i.e., promotion of B-cell survival, inhibition of plasma cell differentiation, potentiation of Wnt signaling, and suppression of MHC class II expression have only recently been described.27–33 Previous studies have suggested that smaller FOXP1 isoforms, which are preferentially expressed in ABC-DLBCLs, rather than FOXP1-FL protein, might exert oncogenic roles.1,23 It has been proposed that these smaller isoforms may either act as a dominant negative over FOXP-FL or may have a deregulated and/or distinct function.1,23,26 However, until now, functional studies, directly addressing the activity of these smaller FOXP1 isoforms and comparing the actions of these isoforms with full-length FOXP1 in B cells, have not been performed. Herein, we established that the major isoform of FOXP1 expressed in ABC-DLBCL, FOXP1-iso, lacks the N-terminal 100 AAs, and that aberrant overexpression of this FOXP1-iso (ΔN100) in primary human B cells results in a similar and equally strong promotion of B-cell survival and inhibition of plasma cell differentiation as overexpression of FOXP1-FL. Moreover, gene expression microarray analysis in DLBCL cell lines and qRT-PCR analysis in primary human B cells did not reveal any differences in regulation of gene expression by these different protein species.
Foxp1 has previously been identified as the most frequent integration site in a piggyBac transposon screen in a pancreatic tumorigenesis model, and the second most frequent viral integration site in an avian nephroblastoma model.4,26 The insertions in the latter model were clustered around the second coding exon of Foxp1 (corresponding to human FOXP1 exon 7), which may lead to expression of an N-terminally truncated Foxp1 isoform due to the presence of an in-frame ATG site in the third coding exon (Figure 1B). Herein, we show that Foxp1 insertions in insertional mutagenesis screens in murine lymphoma models, using either MuLV or SB transposon, cluster at the 5′ end of Foxp1, upstream of the third coding exon. Therefore, these insertions might also lead to expression of an N-terminally truncated Foxp1 isoform. Together these insertional mutagenesis screens support the hypothesis that specifically N-terminally truncated Foxp1 isoforms possess oncogenic function.
The exact identity of the major FOXP1-iso protein expressed in ABC-DLBCL has not been completely clarified before. Using semi-quantitative RT-PCR analysis, Brown et al. proposed that smaller isoforms were encoded by two alternative FOXP1 splice variants, one of which might start translation in exon 8 (isoform 2), resulting in a protein that lacks the N-terminal 100 AAs, whereas the other starts translation in exon 6, but does not contain exons 8 and 9, resulting in an internal N-terminal deletion (isoform 3).23 By mass spectrometry, (q)RT-PCR, sequencing, and siRNA-mediated knockdown studies, we established that the major FOXP1-iso expressed in ABC-DLBCL cell lines is encoded by a transcript that lacks exon 6, but does contain exons 7–21, resulting in expression of a protein that lacks the first 100 N-terminal AAs. During the preparation of this manuscript, essentially similar results were reported by Banham’s group.46 Since we found evidence for only one transcript encoding for the major isoform, the detection of two FOXP1-iso protein species by western blotting suggests that these two protein species may have undergone different post-translational protein modifications. A previous study by Wang et al. showed that the murine Foxp1D isoform, which lacks the N-terminus of the protein, exerts stronger repression of an SV40 promoter-driven luciferase construct in transfected HEK293T cells, compared to Foxp1-FL.47 However, we could not find any differences in gene repression strength between FOXP1-FL and FOXP1-iso upon overexpression in primary human B cells (Figures 3E and 4A), suggesting that at least these human isoforms do not differ in their strength of transcriptional regulation. Moreover, the functional consequences of overexpression of these two protein species, at least in the responses studied by us, i.e., survival, proliferation and plasma cell differentiation, were equally affected by both. Notably, a recent study by Walker et al. showed that FOXP1-FL and N-terminal truncated FOXP1 proteins show similar effects on Wnt reporter activity in HEK293T cells, indicating that these two isoforms might also equally potentiate Wnt signaling in DLBCL.32 The differences with the study by Wang et al. might be explained by dissimilarities in species (murine versus human FOXP1), cell types (HEK293T versus DLBCL cell lines) or the assay system being used (artificial versus endogenous promoters). Together, these results suggests that, at least in B cells and DLBCL cell lines, the effect of FOXP1-FL and FOXP1-iso on gene expression and cellular responses seem to be similar.
Our results imply that expression of FOXP1-iso in B-cell non-Hodgkin lymphomas versus expression of FOXP1-FL in epithelial tissues cannot explain its paradoxical actions as an oncogene in the former and as a tumor suppressor in the latter tissues. These opposing functions of FOXP1 in different cell types are therefore more likely to be due to tissue-specific expression of transcriptional co-regulators or regulation of transcriptional targets, an intriguing and important aspect that warrants future studies. By ChIP-seq analysis of FOXP1 in DLBCL cell lines, we previously established that FOXP1 binding sites in these cells are enriched for binding sites and consensus motifs for other transcription factors that regulate ABC-DLBCL pathogenesis.27 By analogy to its family member FOXP3,48,49 these results suggest that FOXP1 might preferentially bind sites co-occupied by other transcription factors and regulate gene expression through physical or functional interaction with those factors.27 Therefore, the transcriptional outcome of high FOXP1 expression in different cell types might be dictated by expression levels of other transcriptional co-regulators in these cells.
Combined, our results indicate that FOXP1-FL and FOXP1-iso do not differ in terms of oncogenic activity and gene regulation in B cells and DLBCLs, and that total levels of FOXP1 expression, rather than the relative expression of specific isoforms, contributes to the oncogenic potential of FOXP1 in B-cell lymphomas. The specific mechanisms underlying transcriptional regulation of FOXP1-FL versus FOXP1-iso expression remain to be established. Interestingly, however, a previous study showed that activation of the B-cell receptor in primary B cells resulted in specific induction of FOXP1-iso expression.23 Since ABC-DLBCLs are characterized by chronic active B-cell-receptor signaling,50 this might explain the relatively high expression of FOXP1-iso in these lymphomas. Importantly, however, since the total levels of FOXP1 are also higher in ABC- as compared to GC-DLBCL,13,14,38–40 deregulated expression of FOXP1, irrespective of the isoform, may contribute to the worse prognosis of ABC-DLBCL patients,5,38–40 thereby reflecting its role in lymphomagenesis. Taken together, these novel insights into the function of FOXP1 isoforms in controlling the transcriptional program, survival and differentiation of B cells advance our understanding of the role of FOXP1 in lymphomagenesis and further enhance the value of FOXP1 for diagnostics, prognostics, and treatment of DLBCL patients.
Supplementary Material
Acknowledgments
The authors thank Berend Hooibrink and Toni van Capel for FACS sorting, and Jan Koster and Richard Volckman for providing help with microarray analysis.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/3/573
Funding
This study was supported by a grant from the Dutch Cancer Society.
References
- 1.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11(7):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328(2):198–206. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang S, Wang X, et al. Prognostic significance of FOXP1 as an oncogene in hepatocellular carcinoma. J Clin Pathol. 2012;65(6):528–533. [DOI] [PubMed] [Google Scholar]
- 4.Rad R, Rad L, Wang W, et al. A conditional piggyBac transposition system for genetic screening in mice identifies oncogenic networks in pancreatic cancer. Nat Genet. 2015;47:47–56. [DOI] [PubMed] [Google Scholar]
- 5.Banham AH, Connors JM, Brown PJ, et al. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11(3): 1065–1072. [PubMed] [Google Scholar]
- 6.Sagaert X, de Paepe P, Libbrecht L, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(16):2490–2497. [DOI] [PubMed] [Google Scholar]
- 7.Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19(4):652–658. [DOI] [PubMed] [Google Scholar]
- 8.Fenton JA, Schuuring E, Barrans SL, et al. t(3;14)(p14;q32) Results in aberrant expression of FOXP1 in a case of diffuse large B-cell lymphoma. Genes Chromosom Cancer. 2006;45(2):164–168. [DOI] [PubMed] [Google Scholar]
- 9.Wlodarska I, Veyt E, de Paepe P, et al. FOXP1, a gene highly expressed in a subset of diffuse large B-cell lymphoma, is recurrently targeted by genomic aberrations. Leukemia. 2005;19(8):1299–1305. [DOI] [PubMed] [Google Scholar]
- 10.Haralambieva E, Adam P, Ventura R, et al. Genetic rearrangement of FOXP1 is predominantly detected in a subset of diffuse large B-cell lymphomas with extranodal presentation. Leukemia. 2006;20(7):1300–1303. [DOI] [PubMed] [Google Scholar]
- 11.Goatly A, Bacon CM, Nakamura S, et al. FOXP1 abnormalities in lymphoma: translocation breakpoint mapping reveals insights into deregulated transcriptional control. Mod Pathol. 2008;21(7):902–911. [DOI] [PubMed] [Google Scholar]
- 12.Rouhigharabaei L, Finalet Ferreiro J, Tousseyn T, et al. Non-IG aberrations of FOXP1 in B-Cell malignancies lead to an aberrant expression of N-truncated isoforms of FOXP1. PLoS ONE. 2014;9(1): e85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 14.Staudt LM, Dave S. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol. 2005;87:163–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krohn A, Seidel A, Burkhardt L, et al. Recurrent deletion of 3p13 targets multiple tumour suppressor genes and defines a distinct subgroup of aggressive ERG fusion-positive prostate cancers. J Pathol. 2013;231(1):130–141. [DOI] [PubMed] [Google Scholar]
- 16.Banham AH, Beasley N, Campo E, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61(24):8820–8829. [PubMed] [Google Scholar]
- 17.Fox SB, Brown P, Han C, et al. Expression of the forkhead transcription Factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10(10):3521–3527. [DOI] [PubMed] [Google Scholar]
- 18.Shigekawa T, Ijichi N, Ikeda K, et al. FOXP1, an estrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer. Horm Cancer. 2011;2(5):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayoo M, Yan M, Takano EA, et al. Expression of the forkhead box transcription factor FOXP1 is associated with oestrogen receptor alpha, oestrogen receptor beta and improved survival in familial breast cancers. J Clin Pathol. 2009;62(10): 896–902. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Sato F, Xia H, et al. Forkhead box P1 overexpression and its clinicopathologic significance in peripheral T-cell lymphoma, not otherwise specified. Human Pathol. 2012;43(8):1322–1327. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Hu Z, Liu J, Gao J, Lin B. Gene expression profile analysis identifies metastasis and chemoresistance-associated genes in epithelial ovarian carcinoma cells. Med Oncol. 2014;32(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J. High expression of FoxP1 is associated with improved survival in patients with non-small cell lung cancer. Am J Clin Pathol. 2012;138(2):230–235. [DOI] [PubMed] [Google Scholar]
- 23.Brown PJ, Ashe SL, Leich E, et al. Potentially oncogenic B-cell activation-induced smaller isoforms of FOXP1 are highly expressed in the activated B cell-like subtype of DLBCL. Blood. 2008;111(5): 2816–2824. [DOI] [PubMed] [Google Scholar]
- 24.Courts C, Brunn A, Montesinos-Rongen M, et al. Preferential Expression of Truncated Isoforms of FOXP1 in Primary Central Nervous System Lymphoma. J Neuropathol Exp Neurol. 2009;68(9):727–741. [DOI] [PubMed] [Google Scholar]
- 25.Green MR, Gandhi MK, Courtney MJ, Marlton P, Griffiths L. Relative abundance of full-length and truncated FOXP1 isoforms is associated with differential NFkappaB activity in Follicular Lymphoma. Leuk Res. 2009;33(12):1699–1702. [DOI] [PubMed] [Google Scholar]
- 26.Pajer P, Pecenka V, Kralova J, et al. Identification of potential human oncogenes by mapping the common viral integration sites in avian nephroblastoma. Cancer Res. 2006;66(1):78–86. [DOI] [PubMed] [Google Scholar]
- 27.van Keimpema M, Gruneberg LJ, Mokry M, et al. FOXP1 directly represses transcription of pro-apoptotic genes and cooperates with NF-kappaB to promote survival of human B-cells. Blood. 2014;124(23):3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dekker JD, Park D, Shaffer AL, et al. Subtype-specific addiction of the activated B-cell subset of diffuse large B-cell lymphoma to FOXP1. Proc Natl Acad Sci. 2016;113(5):E577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flori M, Schmid CA, Sumrall ET, et al. The hematopoietic oncoprotein FOXP1 promotes tumor cell survival in diffuse large B-cell lymphoma by repressing S1PR2 signaling. Blood. 2016;127(11):1438–1448. [DOI] [PubMed] [Google Scholar]
- 30.van Keimpema M, Grüneberg LJ, Mokry M, et al. The forkhead transcription factor FOXP1 represses human plasma cell differentiation. Blood. 2015;126(18):2098–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai DY, Hung KH, Lin IY, et al. Uncovering MicroRNA regulatory hubs that modulate plasma Cell differentiation. Sci Rep. 2015;11(5):17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker MP, Stopford CM, Cederlund M, et al. FOXP1 potentiates Wnt/betacatenin signaling in diffuse large B cell lymphoma. Sci Signal. 2015;8(362):ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown PJ, Wong KK, Felce SL, et al. FOXP1 suppresses immune response signatures and MHC class II expression in activated B-cell-like diffuse large B-cell lymphomas. Leukemia. 2015;30(3):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjin EPM, Groen RWJ, Vogelzang I, et al. Functional analysis of HGF/MET signaling and aberrant HGF-activator expression in diffuse large B-cell lymphoma. Blood. 2006;107(2):760–768. [DOI] [PubMed] [Google Scholar]
- 35.Zauner G, Hoffmann M, Rapp E, et al. Glycoproteomic analysis of human fibrogen reveals novel regions of O-glycosylation. J Proteome Res. 2012;11(12):5804–5814. [DOI] [PubMed] [Google Scholar]
- 36.Kocemba KA, van Andel H, de Haan-Kramer A, et al. The hypoxia target adrenomedullin is aberrantly expressed in multiple myeloma and promotes angiogenesis. Leukemia. 2013; 27(8):1729–1737. [DOI] [PubMed] [Google Scholar]
- 37.Hu CR, Wang JH, Wang R, Sun Q, Chen LB. Both FOXP1 and p65 expression are adverse risk factors in diffuse large B-cell lymphoma: A retrospective study in China. Acta Histochem. 2013;115(2):137–143. [DOI] [PubMed] [Google Scholar]
- 38.Hoeller S, Schneider A, Haralambieva E, Dirnhofer S, Tzankov A. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-germinal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57(1):73–80. [DOI] [PubMed] [Google Scholar]
- 39.Barrans SL, Fenton JA, Banham A, Owen RG, Jack AS. Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma (DLBCL) patients with poor outcome. Blood. 2004;104(9):2933–2935. [DOI] [PubMed] [Google Scholar]
- 40.Yu B, Zhou X, Li B, Xiao X, Yan S, Shi D. FOXP1 expression and its clinicopathologic significance in nodal and extranodal diffuse large B-cell lymphoma. Ann Hematol. 2011;90(6):701–708. [DOI] [PubMed] [Google Scholar]
- 41.Uren AG, Kool J, Matentzoglu K, et al. Large-scale mutagenesis in p19ARF- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell. 2008;133(4):727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jong J, de Ridder J, van der Weyden L, et al. Computational identification of insertional mutagenesis targets for cancer gene discovery. Nucleic Acids Res. 2011;39(15): e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 44.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100(17):9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jais JP, Haioun C, Molina TJ, et al. The expression of 16 genes related to the cell of origin and immune response predicts survival in elderly patients with diffuse large B-cell lymphoma treated with CHOP and rituximab. Leukemia. 2008;22(10):1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown PJ, Gascoyne DM, Lyne L, et al. N-terminally truncated FOXP1 protein expression and alternate internal FOXP1 promoter usage in normal and malignant B cells. Haematologica. 2016;101(7):861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B, Lin D, Li C, Tucker P. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem. 2003; 278(27):24259–24268. [DOI] [PubMed] [Google Scholar]
- 48.Rudra D, deRoos P, Chaudhry A, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13(10):1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samstein RM, Arvey A, Josefowicz SZ, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151(1):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.