Abstract
Uterine labor requires the conversion of a quiescent (propregnancy) uterus into an activated (prolabor) uterus, with increased sensitivity to endogenous uterotonic molecules. This activation is induced by stressors, particularly inflammation in term and preterm labor. Neuromedin U (NmU) is a neuropeptide known for its uterocontractile effects in rodents. The objective of the study was to assess the expression and function of neuromedin U receptor 2 (NmU-R2) and its ligands NmU and the more potent neuromedin S (NmS) in gestational tissues, and the possible implication of inflammatory stressors in triggering this system. Our data show that NmU and NmS are uterotonic ex vivo in murine tissue, and they dose-dependently trigger labor by acting specifically via NmU-R2. Expression of NmU-R2, NmU, and NmS is detected in murine and human gestational tissues by immunoblot, and the expression of NmS in placenta and of NmU-R2 in uterus increases considerably with gestation age and labor, which is associated with amplified NmU-induced uterocontractile response in mice. NmU- and NmS-induced contraction is associated with increased NmU-R2-coupled Ca++ transients, and Akt and Erk activation in murine primary myometrial smooth muscle cells (mSMCs), which are potentiated with gestational age. NmU-R2 is upregulated in vitro in mSMCs and in vivo in uterus in response to proinflammatory interleukin 1beta (IL1beta), which is associated with increased NmU-induced uterocontractile response and Ca++ transients in murine and human mSMCs; additionally, placental NmS is markedly upregulated in vivo in response to IL1beta. In human placenta at term, immunohistological analysis revealed NmS expression primarily in cytotrophoblasts; furthermore, stimulation with lipopolysaccharide (LPS; Gram-negative endotoxin) markedly upregulates NmS expression in primary human cytotrophoblasts isolated from term placentas. Correspondingly, decidua of women with clinical signs of infection who delivered preterm display significantly higher expression of NmS compared with those without infection. Importantly, in vivo knockdown of NmU-R2 prevents LPS-triggered preterm birth in mice and the associated neonatal mortality. Altogether, our data suggest a critical role for NmU-R2 and its ligands NmU and NmS in preterm labor triggered by infection. We hereby identify NmU-R2 as a relevant target for preterm birth.
Keywords: calcium, contraction, infection, inflammation, neuromedin S, neuromedin U, NmU-R2, preterm birth, preterm labor, uterine labor
INTRODUCTION
Uterine labor is characterized by vigorous myometrial contractions required to expulse the fetus from the uterus. A number of genes and their translated proteins, referred to as uterine activation proteins (UAPs), stimulate and coordinate myometrial contractions during labor. The expression of UAPs is induced by uterotrophins (uterine activators) [1]. As pregnancy nears the end, uterotrophins (such as estrogen, CRH, and proinflammatory cytokines [e.g., interleukin 1β {IL1β}, IL6, and tumor necrosis factor α {TNFα}]) induce the expression of many UAPs in the uterus, including oxytocin receptor (OXR), prostaglandin F2α receptor (FP), connexin-43, prostaglandin-endoperoxide synthase 2 (COX-2) [1–5], and many others [6]. The uterus then becomes increasingly sensitive to uterotonins, which are proteins responsible for inducing uterine contractions, including oxytocin (OT) and prostaglandin F2α (PGF2α). Preterm labor results from the unscheduled induction of UAPs by stressors, including inflammation with or without infection. Hence, UAPs are interesting targets to arrest preterm labor.
Neuromedin U (NmU; U for uterus) was named for its ability to induce contractions on ex vivo uterine strips; yet, there is a growing list of functions associated with NmU that includes regulation of appetite, diminution of insulin secretion, release of different hormones, and smooth muscle contraction (of various organs: blood vessels, gut, and uterus) [7, 8]. NmU exerts its actions by binding to two G protein-coupled receptors: neuromedin U receptor 1 (NmU-R1; FM-3 or GPR66) and NmU-R2 (FM-4) [8–12]. Both NmU receptors are coupled to Gi and Gq/11-phospholipase Cβ [13]. The activation of the latter leads to the intracellular release of calcium and induces contractions of smooth muscle cells. Another ligand of NmU receptors, neuromedin S (NmS), is expressed in the hypothalamus, spleen, and testis, and has been described to be more specific and exhibit higher affinity to NmU-R2 [14]. NmU has been extensively shown to exert pleiotropic effects in the brain. Notwithstanding its known procontractile effects on rodent uterus [15], which have been suggested to be mediated by NmU-R2 [16], little is known regarding its mechanism of action in uterus and potential role in labor, but it may be relevant in the context that neuromedin B, a neuropeptide of the same family, was recently shown to induce labor in mice via its receptor NmBR [17].
We therefore studied the effects and mode of action of NmU and NmS, and their cognate receptor NmU-R2, in uterus, and their implication in term and preterm labor. Our findings reveal expression of NmU and NmS in human and murine placenta, and of their receptor NmU-R2 in human and murine myometrium, with a significant increase in the expression of NmU-R2 and NmS near the end of gestation and during labor. Studies in mice suggest that NmU-R2, once activated by its ligand NmU or NmS, shares all major characteristics that are common to UAPs, by 1) exhibiting increased expression with onset of labor, 2) exerting gestational age-dependent uterocontractile effects, 3) contributing endogenously to inflammation-triggered preterm birth (PTB), and 4) being induced by uterotrophins [1, 4, 5, 18–20]; the latter was again corroborated on decidual biopsies of women with clinical evidence of infection. More importantly, NmU and NmS administration in pregnant mice was found to shorten gestation, and Nmur2 knocked-down mice had significantly lower PTB induced by lipopolysaccharide (LPS). Hence, NmU-R2 is a potential new UAP that appears important for PTB associated with infection.
MATERIALS AND METHODS
Ethics Approval
Approval was obtained from North West Research Ethics Committee in Manchester, United Kingdom (ref.: 08/H1010/55), for decidual samples, provided by Dr. Rebecca L. Jones, and the Sainte-Justine Hospital Ethics Board (ref.: 4058 and 3988) for placental samples and placenta from uncomplicated (normal) term pregnancies for cytotrophoblast isolation (see below). Myometrium tissue biopsies were collected in part from women undergoing cesarean delivery at the Royal Alexandra Hospital in Edmonton, Alberta, with ethics approval received from the University of Alberta Research Ethics board, and in part collected from women undergoing cesarean delivery at the MacDonald Women's Hospital, University Hospitals, Cleveland, with Institutional Review Board approval (no. 11-04-06), provided by Dr. Sam Mesiano. All participants provided written informed consent.
Animals
Timed-pregnant CD-1 mice were obtained from Charles River Inc. at different gestational ages and were allowed to acclimatize for 4 days prior to experiments. Animal studies were approved by the Animal Care Committee of Hôpital Sainte-Justine along the principles of the Guide for the Care and Use of Experimental Animals of the Canadian Council on Animal Care. The animals were maintained on standard laboratory chow under a 12L:12D cycle and were allowed free access to chow and water.
Chemicals
Chemicals were purchased from the following manufacturers: rhIL1β (no. 200-01B; PeproTech), Neuromedin U-23 (no. NMU72-P; Alpha Diagnostic International), Neuromedin S (no. 045-88; Phoenix Pharmaceuticals), Neuromedin U-25 (no. 17617; Cayman Chemical), Prostaglandin F2α (no. 16010; Cayman Chemical), Oxytocin (no. 66-0-52; American Peptide), W-7 (no. A3281; Sigma), U73122 (no. U6756; Sigma), and LPS from Escherichia coli 0111:B4 (L2630; Sigma).
Protein Extraction from Human Myometrial Biopsies
Myometrial biopsies were flash frozen in liquid nitrogen and stored at −80°C. Frozen myometrial tissues wrapped in aluminum foil were later crushed using a mortar and pestle in liquid nitrogen, and 0.1–0.2 g of myometrial tissue was placed in a round-bottomed tube with a 7-mm bead and 0.5 ml of lysis buffer containing 0.05% Tris, 0.01% ethylene diamine tetraacetic acid, 0.001% Triton X-100, 0.005% PMSF, and 0.1% protease inhibitor. Tissues were then lysed by shaking the tubes at a high speed (frequency 25/sec) using the TissueLyser II (Qiagen). Tissue lysates were centrifuged at 12 000 × g for 10 min at 4°C, and the supernatants were collected for Western blot analysis.
Primary Murine Myometrial Smooth Muscle Cell Isolation and Culture
Primary murine myometrial smooth muscle cells (mSMCs) were isolated as previously described [21] and used at fewer than three passages. Briefly, pregnant mice (at Gestational Day 10 [G10] or 19) were killed by cervical dislocation and sprayed with 70% ethanol. The whole uterus was excised under sterile hood and placed in buffer A (Hanks balanced salt solution [HBSS], pH 7.4; 0.098 g/L magnesium sulfate; 0.185 g/L calcium chloride; 2.25 mmol/L I-HEPES [N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid]; 100 U/ml penicillin-streptomycin [Gibco]; and 2.5 μg/ml amphotericin B [Sigma]). Placentas, fetal membranes, and products of conception were discarded and the uterine horns were cleansed of fat and vessels and then transferred into buffer B (buffer A without magnesium sulfate or calcium chloride) for several washes by gentle flushing. Afterward, the uterine horns were cut into 1-mm-wide fragments and transferred into a volume of 10 ml/g of tissue of digestion buffer (1 mg/ml collagenase type II [Sigma], 0.15 mg/ml deoxyribonuclease I [Roche Diagnostics GmbH], 0.1 mg/ml soybean trypsin inhibitor [Sigma], 10% fetal bovine serum [FBS], and 1 mg/ml bovine serum albumin [Sigma] in buffer B). Enzymatic digestion was performed at 37°C with agitation (100 revolutions per minute) for 30 min. The homogenate (still containing undigested myometrium fragments) was then poured through a 100-μm cell strainer. The resulting filtered solution was centrifuged at 200 × g for 10 min, and the pellet was resuspended in complete Dulbecco modified Eagle medium (DMEM) and plated in a T-75 dish. The remaining myometrium fragments were reused in an enzymatic digestion, and the whole digestion-centrifugation process was repeated for a total of five times. First, two digestion results were discarded because they mostly contained fibroblasts. The other three SMC-containing dishes were subjected to a differential adhesion technique to selectively enrich for uterine myocytes. Briefly, 30–45 min after the cells were first plated, the medium was removed and dispensed in another T-75 culture dish to separate quickly adhering fibroblasts from slowly adhering myocytes. Purity of the cells was assessed by immunohistochemistry using the smooth muscle cell marker α-actin and was always maintained above 95%.
Cell Culture
Primary murine mSMCs or human mSMCs (hTERT cell line) were cultured in DMEM growth medium supplemented with 10% serum, 50 U/ml penicillin, and 50 mg/ml streptomycin. Cells were propagated in regular conditions (37°C, 5% CO2). For immunoblotting and PCR experiments, cells were serum starved overnight and treated with various concentrations of NmU or 5 ng/ml IL1β for 10 min. Cells were collected in ice-cold RIPA buffer containing a cocktail of protease/phosphatase inhibitors and cleared from debris by centrifugation. Samples were stored in Laemmli buffer at −20°C or used fresh for Western blotting.
Induction of Birth
Timed-pregnant mice were injected intraperitoneally with NmU, NmS, PGF2α, or oxytocin twice a day for 2 consecutive days. Injections were made at G13–G14, G15–G16, or G17–G18 twice a day for a total of four injections. Doses used for NmU and NmS correspond to those previously used to induce labor in mice with neuromedin B [17]. Mice were checked every 2 h for any signs of labor/delivery, such as vaginal bleeding or delivery of at least one pup.
Intrauterine IL1β and LPS Injection
Timed-pregnant mice at G9 were steadily anesthetized with isoflurane. Body hair was removed and peritoneal skin was sterilized with 70% ethanol and then covered with povidone-iodine 7.5% (Atlas Laboratory). A 1.5-cm-tall median incision was made in the abdominal wall of the lower abdomen. The lower segment of the right uterine horn was then exposed and 1 μg of IL1β, LPS, or an equivalent volume of saline (for sham animals) was injected between two gestational sacs without entering the amniotic cavity. The abdominal muscle layer was sutured and the skin closed with clips. Twenty-four hours after the intrauterine injection (at G10), mice were killed with CO2, and placenta and lower (cervical end) uterus samples were collected and preserved at −80°C for subsequent RNA purification and Western blotting. For contraction experiments ex vivo, fresh uterine fragments were used immediately after killing.
Lentivirus Production
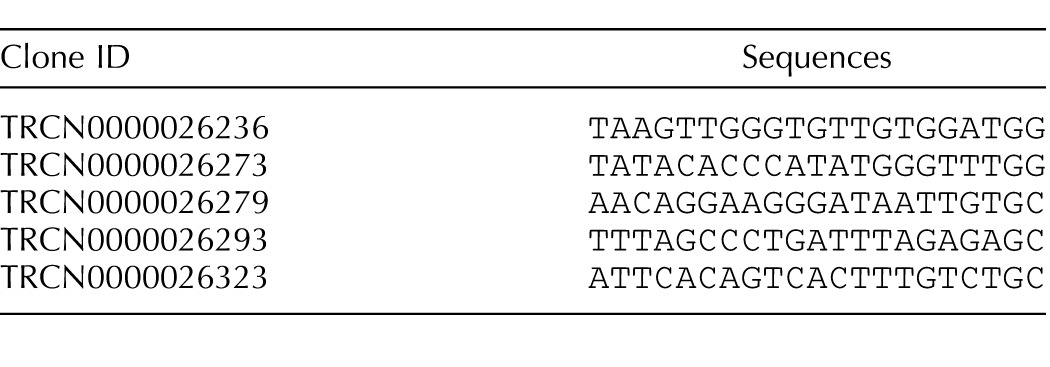
We produced infectious lentivirus (LV) by transiently transfecting lentivector and packaging vectors into 293FT cells (Invitrogen) as previously described [22]. We used five different small-hairpin RNA (shRNA) sequences against Nmur2 (see Table 1 for sequences) and selected the most effective sequence for further experiments (see Supplemental Fig. S1, A and B, for efficacy and variability of NmU-R2 knockdown using LV.shNmU-R2; Supplemental Data are available online at www.biolreprod.org). In vivo infections were performed on G13 or G15 mice via a single intrauterine injection under the same protocol as described above. Lentivirus was allowed to infect cells/tissues for at least 72 h. Lentiviral syringes were coded; hence, the person injecting was blinded to treatment attribution.
TABLE 1.
Sequences used for the design of shRNA against Nmur2.
LPS-Induced Preterm Birth Model
Timed-pregnant mice pretreated for 72 h with an intrauterine injection of lentivirus or saline received 10 μg of intraperitoneal LPS or an equivalent volume of saline at G16. Signs of delivery were assessed every 2 h (as described above). Survival of pups was assessed at the moment of birth (±2 h after completion of delivery).
RNA Extraction and Real-Time Quantitative PCR
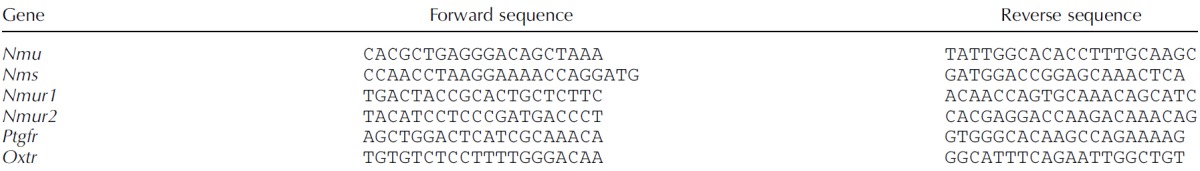
Myometrium fragments were thawed and rapidly preserved in Ribozol (AMRESCO), whereas cells from in vitro experiments were treated for 6 h with 5 ng/ml IL1β and collected directly into Ribozol. RNA was extracted according to the manufacturer's protocol and RNA concentration and integrity were measured with a NanoDrop 1000 spectrophotometer. A total of 500 ng of RNA was used to synthetize cDNA using iScript Reverse Transcription SuperMix (Bio-Rad). Primers were designed using NCBI Primer Blast (Table 2). Quantitative gene expression analysis was performed on Stratagene MXPro3000 (Stratagene) with SYBR Green Master Mix (Bio-Rad). Gene expression levels were normalized to 18S universal primer (Ambion Life Technology). Dissociation curves were also acquired to test primer specificity. Genes analyzed include: Nmu, Nms, Nmur1, Nmur2, Ptgfr (prostaglandin F receptor), and Oxtr (oxytocin receptor). Detailed primer sequences are shown in Table 2.
TABLE 2.
Mouse primers used for real-time quantitative PCR.
Western Blotting
Proteins from homogenized myometrium fragments and cell samples lysed in RIPA buffer were quantified using Bradford method (Bio-Rad). A total of 50 μg of protein sample was loaded onto SDS-PAGE gel and electrotransferred onto PVDF membranes. After blocking, membranes were incubated with either an antibody against NmU (no. sc-368069; Santa Cruz Biotechnology), NmS (no. PAA828Mu01; Cloud-Clone Corp.), NmU-R1 (no. sc-47241; Santa Cruz Biotechnology), NmU-R2 (no. sc-47250; Santa Cruz Biotechnology), cyclophilin B (no. ab16045; Abcam), anti-GAPDH (PA1-987; Pierce Protein Biology, Thermo Scientific), or β-actin (no. sc-47778; Santa Cruz Biotechnology); although NmU and NmS antibodies detect the corresponding propeptides [7], expression patterns were corroborated throughout the study by nearly identical mRNA profiles. Membranes were then washed with PBS containing 0.1% Tween 20 (Sigma-Aldrich) and incubated for 1 h with their respective secondary antibodies conjugated to horseradish peroxidase (HRP; Sigma). For kinases, membranes were incubated with an antibody against either phospho-JNK (no. 9251; Cell Signaling Technology), phospho-c-jun (no. 9261; Cell Signaling Technology), phospho-p38 (no. 4511; Cell Signaling Technology), phospho-Akt (no. 9271; Cell Signaling Technology), phospho-Erk (no. 9101; Cell Signaling Technology), JNK (no. 9252; Cell Signaling Technology), c-jun (no. 9165; Cell Signaling Technology), p38 (no. 9212; Cell Signaling Technology), Akt (no. 9272; Cell Signaling Technology), or Erk (no. 4695; Cell Signaling Technology). Enhanced chemiluminescence (GE Healthcare) was used for detection using the ImageQuant LAS-500 (GE Healthcare), and densitometric analysis was performed using ImageJ (National Institutes of Health). Resulting values were normalized first with the loading controls (β-actin, GAPDH, or cyclophilin B) and then as a ratio of the control samples.
Calcium Assay
A total of 40 000 mSMCs per well were cultured overnight in 96-well clear-bottomed black plates (no. 3603; Corning) prior to the calcium assay performed according to manufacturer's protocol (F36206; Life Technologies). In brief, medium was changed for a probenecid-containing assay buffer, and plates were read using a microplate reader (EnVision Multilabel reader, PerkinElmer), in response to on-time stimulations with NmU, NmS, PGF2α, or OT (using apparatus injectors). Five readings were taken before the injection (basal readings), and 2-sec interval postinjection readings were automatically stopped after 30 sec. Assay buffer was used as a negative control. Values are presented as a ratio between means of readings and means of basal readings.
Ex Vivo Uterine Contraction Experiment
Timed-pregnant mice at different gestational ages were killed with CO2, and uterine tissue fragments were collected. Briefly, a midline abdominal incision was made, and the uterine horns were rapidly excised and carefully cleansed of surrounding connective tissues. Longitudinal myometrial strips (2–3 mm wide and 10 mm long) were dissected free from uterus, mounted isometrically in organ tissue baths, and initial tension was set at 2 g. The tissue baths contained 20 ml of Krebs buffer of the following composition (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 0.9 MgSO4, 1 KH2PO4, 11.1 glucose, and 23 NaHCO3 (pH 7.4). The buffer was equilibrated with 95% oxygen:5% carbon dioxide at 37°C. Isometric tension was measured by a force transducer and recorded by a BI-OPAC data acquisition system (BI-OPAC MP150). Experiments began after 1 h of equilibration. The mean tension of spontaneous contractions was measured using a BI-OPAC digital polygraph system (AcqKnowledge); the same parameters were also determined after the addition of NmU, NmS, PGF2α, or oxytocin. At the start of each experiment, mean tension of spontaneous myometrial contractions was used as the reference response. Increases in mean tension (%) were expressed as percentages of (X/Y) − 100, where X is change in mean tension (g) induced by NmU, NmS, oxytocin, or PGF2α, and Y is the initial reference response (g).
Histological Analysis of Placental Villous Tissue
Villous tissue biopsies were fixed in 10% neutral buffered formalin and paraffin embedded. For immunohistochemistry, 5-μm-thick sections were obtained using a microtome and processed as previously described [23]. The following antibodies were used: NmU (1:50; Santa Cruz Biotechnology) and NmS (1:10; Cloud Corp.), with matched HRP-conjugated secondary antibodies (goat anti-rabbit-HRP; Bio-Rad). Staining was revealed using 3,3-diaminobenzidine (Amresco) and slides were counterstained with hematoxylin and mounted. Primary antibodies were omitted for negative controls. Images were obtained with a slide scanner (Axioscan; Zeiss) using Zen2 program.
Primary Cytotrophoblast Isolation and Culture
Primary cytotrophoblasts were isolated from term placentas from uncomplicated pregnancies obtained after cesarean delivery using a well-established method developed by Kliman et al. [24]. Briefly, villous tissue was dissected, minced, and rinsed in PBS prior to three enzymatic digestions in HBSS with trypsin and DNase. Cytotrophoblasts were obtained from these digestions after separation by centrifugation on a discontinuous Percoll gradient. Cytotrophoblasts were plated at 2 × 106 cells per milliliter in DMEM-F12 supplemented with 10% FBS and penicillin/streptomycin and washed with PBS to removed nonadherent cells after 12 h. Cells were treated with LPS (1 μg/ml; Sigma-Aldrich) for 24 or 48 h in Opti-MEM (Life Technologies). Cells were collected in lysis buffer (PBS with 1% Triton X-100 and protease inhibitor cocktail), they were centrifuged at 13 000 rpm for 10 min at 4°C, and supernatant was collected and kept at −20°C until it was used for analysis.
Immunocytochemistry
Cells were plated on coverslips precoated with poly-d-lysine and fixed in 4% paraformaldehyde. After blocking, cells were incubated overnight with a primary antibody for rabbit anti-α-actin and for 1 h at ambient temperature with a secondary antibody conjugated with Alexa Fluor 488 (Sigma). Nuclei were stained with 4′,6-diamidino-2-phenylindole (1:5000; Invitrogen). Images were captured using a 30× objective with an Eclipse E800 (Nikon) fluorescence microscope.
Statistical Analysis
Groups were compared by two-tailed Student t-test or one-way analysis of variance (ANOVA). Dunnett multiple-comparison method was employed when treatments were compared to a single control. Tukey multiple-comparison test was used for ex vivo contraction assays. A value of P < 0.05 was considered statistically significant. Data are presented as means ± SEM.
RESULTS
NmU-R2 Is Expressed in Murine Uterus and Induces Uterine Contractions and Labor upon Stimulation with NmU and NmS
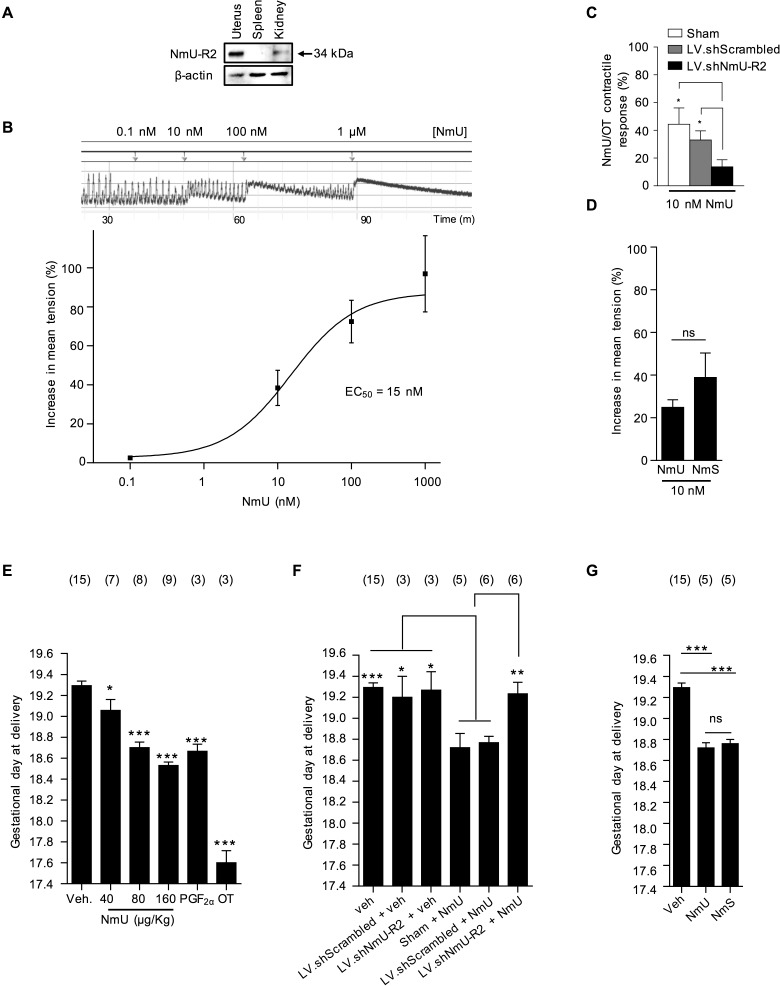
We confirmed the expression of NmU-R2 in murine pregnant uterus at term gestation (G19; Fig. 1A) and the uterocontractile effects of NmU (EC50 = 15 nM; Fig. 1B). Effects of NmU were NmU-R2 dependent, because uterine contraction was markedly diminished after Nmur2 knockdown (Fig. 1C; Supplemental Fig. S1, A–C) performed with an intrauterine injection of lentiviral-encoded shRNA-Nmur2. In contrast to Nmur2 knockdown, that of Nmur1 did not alter critical contraction-associated uterine smooth muscle NmU-triggered Ca++ mobilization (Supplemental Fig. S1, D and E), which is consistent with the documented NmU-R1-independent uterotonic effects of NmU [16]. Additionally, NmU-R2-specific ligand NmS [14] also increased uterine contractility, comparably to NmU (at ∼EC50 value; Fig. 1D). Hence, NmU exerts its effects on the uterus specifically via NmU-R2.
FIG. 1.
NmU and NmS induce uterine contractions and labor via NmU-R2. A) NmU-R2 immunoblot from term uterus. Spleen and kidney samples were used as negative and positive controls, respectively. B) Representative ex vivo contraction assay performed on a myometrium fragment from a pregnant mouse at term in response to increasing NmU concentrations (top) and dose-response curve (bottom). C) Pregnant mice were pretreated with LV.shNmU-R2 or LV.shScrambled at G15, and their uteri were collected at term for a contraction assay in response to NmU and OT. For each uterine strip, the contractile response to NmU was normalized to its response to OT (to control for interindividual variability). *P < 0.05. D) Ex vivo contraction assay on G19 uteri in response to 10−8 M NmU or NmS. E) Pregnant mice were injected intraperitoneally with increasing NmU doses twice a day from G17 to G18. Control animals were given an equivalent volume of saline. PGF2α and OT were used as positive controls at a dose of 160 μg/kg. *P < 0.05, ***P < 0.001 compared with vehicle only. F) Pregnant mice were pretreated with LV.shNmU-R2 or LV.shScrambled at G15 and then treated with 160 μg/kg NmU twice a day from G17 to G18. *P < 0.05, **P < 0.01, ***P < 0.001 compared with NmU or NmU + LV.shScrambled. G) Pregnant mice were injected intraperitoneally with 160 μg/kg NmS or NmU twice a day from G17 to G18. ***P < 0.001 compared with vehicle only. Data for A–D are representative of four to five mice per group. The number of mice used in E–G is displayed above each group, and mice treated with vehicle (control mice) were pooled together and repeatedly shown in each graph. Values are presented as mean ± SEM. Data were statistically analyzed using one-way ANOVA with comparison to control groups using Dunnett multiple-comparison test; ns, not significant.
NmU injected in vivo intraperitoneally twice daily on G17 and G18 to pregnant mice dose-dependently accelerated delivery, equivalent to that observed with established uterine contractile agonist PGF2α, but less effectively than oxytocin (Fig. 1E); shortened gestation induced by NmU was again NmU-R2 dependent, because it was abrogated in mice with uterine knockdown of Nmur2 (Fig. 1F); shRNA-Nmur2 did not significantly affect length of intact gestation. The NmU-R2-specific ligand NmS [14] exerted a modest shortening of gestation of 12 h, comparable to that by NmU (Fig. 1G).
Expression of NmS (but Not NmU) in Human and Murine Placenta and of NmU-R2 in Uterus Markedly Increases Near Term and During Labor, and Is Associated with Increased Uterocontractile Response
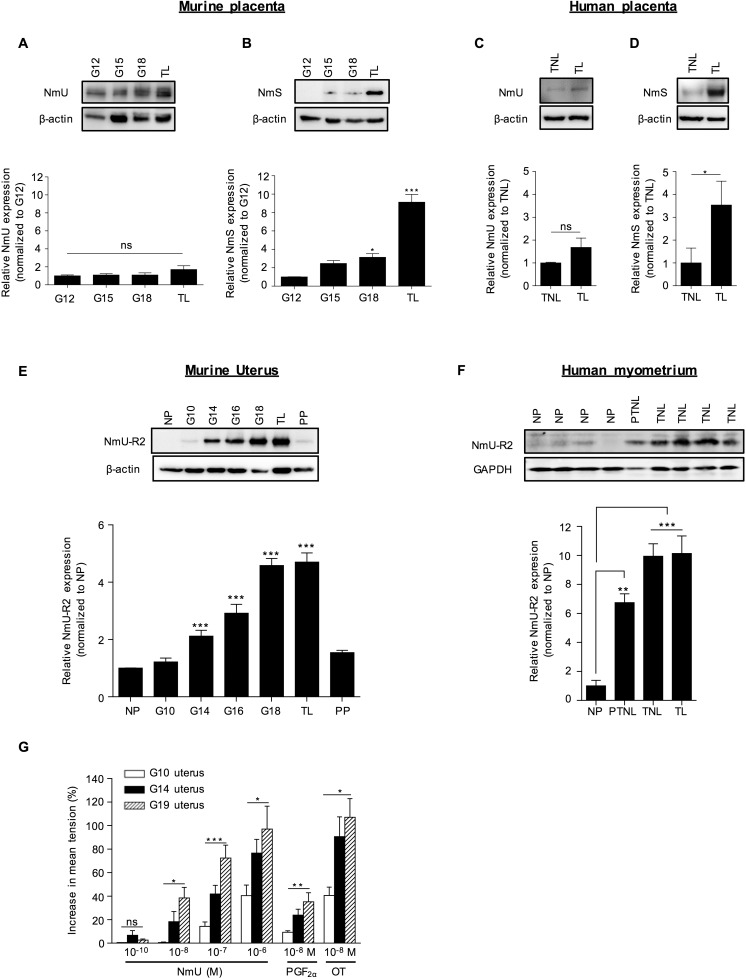
Given the labor-inducing action of NmU and NmS, we studied the endogenous expression of these NmU-R2 ligands in placenta, which is considered a key organ in the regulation of on-time labor [25]. We found placental protein and mRNA expression of NmU (Fig. 2A and Supplemental Fig. S2A) and NmS (Fig. 2B and Supplemental Fig. S2B); NmU expression was consistent throughout pregnancy, whereas NmS was exclusively and consistently expressed during spontaneous labor in mice. Study of human placental biopsies collected from women at term not in labor (TNL) or in established labor (TL) paralleled murine data, revealing expression of both NmU and NmS (Fig. 2, C and D), with a marked increase of NmS during labor.
FIG. 2.
The expression of NmS in placenta and of NmU-R2 in uterus increases near term and during labor in mice and humans, which is associated with increased NmU-induced myometrial contractility at term. A and B) NmU (A) and NmS (B) representative immunoblots of murine placentas collected at different gestational ages (G) and during spontaneous term labor (TL). Lower panels show densitometric analysis of protein bands normalized with β-actin and plotted as fold change versus the control group (G12). C and D) NmU (C) and NmS (D) immunoblots of human placenta biopsies from women at term not in labor (TNL) or in established labor (TL). Lower panels show densitometric analysis of protein bands normalized with β-actin and plotted as fold change versus the control group (TNL) of all patients screened (TNL, n = 6; TL, n = 6). E) NmU-R2 representative immunoblot of murine uteri collected at different gestational ages (G), during spontaneous term labor (TL) and 24 h postpartum (PP). The lower panel shows densitometric analysis of protein bands normalized with β-actin and plotted as fold change versus the control group (NP). F) Representative immunoblot of NmU-R2 from human myometrial tissue biopsies from four nonpregnant (NP) women (hysterectomy), one pregnant women at preterm without any clinical sign of labor (PTNL), and four pregnant women at term without any clinical sign of labor (TNL). The lower panel shows densitometric analysis of protein bands normalized with β-actin and plotted as fold change versus the control group (NP) of all patients screened (NP, n = 4; PTNL, n = 4; TNL, n = 9; TL, n = 5), as presented in F and Supplemental Figure S2G. G) Ex vivo contraction assay in response to increasing doses of NmU performed on myometrium fragments from pregnant mice at G10, G14, or G19. Uteri collected at G19 were only considered if the mice were still undelivered. PGF2α and OT were used as positive controls at a dose of 10−8 M. Data are representative of four to five mice per group and at least three independent experiments. Values are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with comparison to control groups using Dunnett multiple-comparison test; ns, not significant.
We also studied the expression of procontractile NmU-R2 in murine and human uterus. In murine uterus, NmU-R2 protein and mRNA expression rose near term, peaked during labor, and rapidly declined 24 h postpartum (Fig. 2E and Supplemental Fig. S2D). Study of human myometrial biopsies was again rigorously consistent with murine data, revealing a marked (>7-fold) and gradual increase of NmU-R2 protein expression in pregnant women at term and preterm (TL, n = 5; TNL, n = 9; preterm without any clinical sign of labor [PTNL], n = 4) compared with nonpregnant women (n = 4; Fig. 2F and Supplemental Fig. S2G). In contrast, NmU-R1 protein and gene expression in murine uterus did not increase during gestation or labor (Supplemental Figs. S1F and S2, C–F). Concordant with the gestational age-dependent rise in NmU-R2 expression, ex vivo uterine contractile response to NmU also significantly rose (dose dependently) with advancing gestation (Fig. 2G); NmU potency was comparable to that of PGF2α at any gestational age. Correspondingly, NmU, as seen with PGF2α, accelerated delivery when administered (intraperitoneally) in late but not early gestation (Table 3), confirming the gestational age-dependent effect of NmU.
TABLE 3.
NmU and PGF2α efficacy to induce labor is dependent on gestational age.
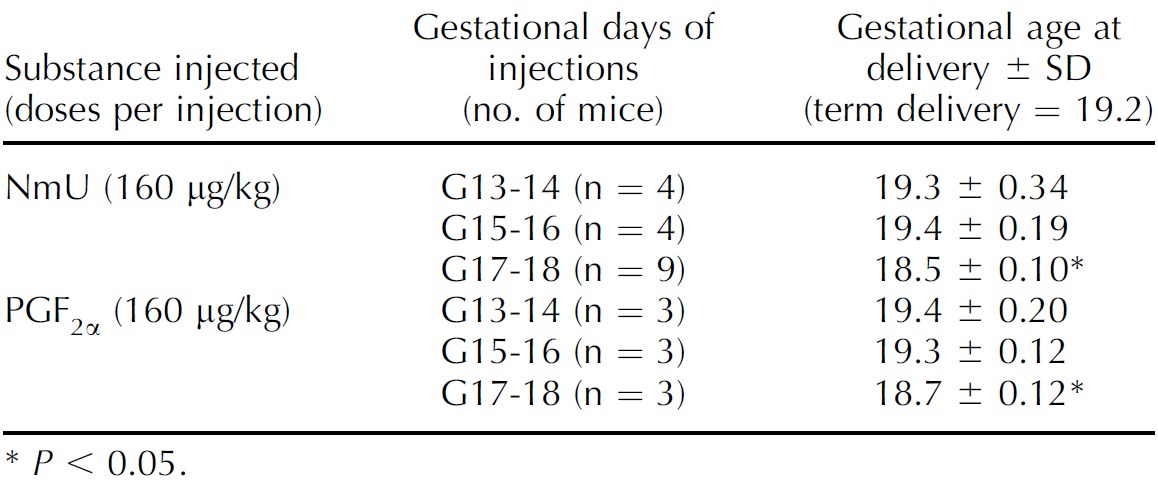
P < 0.05.
NmU-R2-Coupled Contraction-Associated Intracellular Signaling in Response to NmU Is Gestational Age Dependent
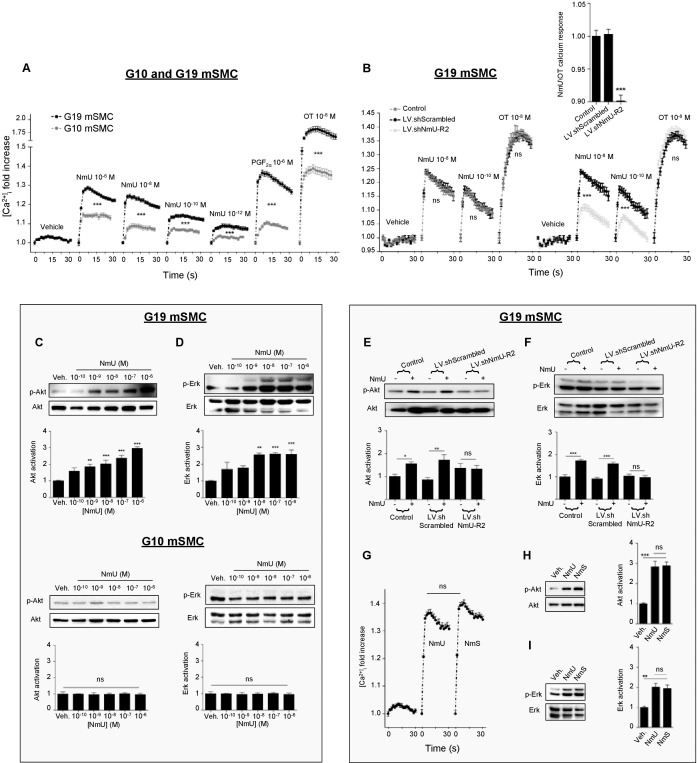
Primary murine mSMCs isolated at G10 (G10 mSMCs) and G19 (G19 mSMCs) were used to study NmU- and NmS-induced signaling responses. One first notes the purity of our cell isolate cultures (>95% of cells immunoreactive to α-actin), and significantly greater mRNA and protein expression of NmU-R2, but not NmU-R1, in cells isolated at G19 compared with G10 (Supplemental Fig. S3), as seen directly on uterine samples (Fig. 2E). NmU induced greater calcium transients on G19 mSMCs than G10 mSMCs (Fig. 3A); a similar profile was observed upon stimulation with known uterotonins PGF2α and oxytocin. As expected, NmU-induced calcium transients were NmU-R2 dependent (Fig. 3B and Supplemental Fig. S1E); the extent and duration of calcium transients are consistent with those observed for other G protein-coupled receptors [26, 27]. Exploration of NmU-R2 downstream mechanisms in G19 mSMCs revealed that NmU does not activate (by phosphorylation) p38, JNK, and c-jun (Supplemental Fig. S4A), but it does activate Akt and Erk (Fig. 3, C and D, and Supplemental Fig. S4, B and C); no effect was seen in G10 mSMCs. NmU-triggered Akt activation, but not that of Erk, was inhibited by calmodulin inhibition (W-7) and by phospholipase Cβ inhibition (U73122; Supplemental Fig. S4, D and E). Nmur2 knockdown abrogated NmU-induced Akt and Erk activation in G19 mSMCs (Fig. 3, E and F). A schematic diagram of NmU-R2 signaling pathway is presented in Supplemental Figure S4F. NmS, which acts specifically on NmU-R2 [14], also elicited calcium transients, as well as Akt and Erk activation, comparable to those seen with NmU (Fig. 3, G–I).
FIG. 3.
NmU-R2-associated signaling in mSMCs is potentiated by gestational age. A) Calcium assay performed on primary mSMCs from pregnant mice at either G10 or G19. PGF2α and OT were used as positive controls at a concentration of 10−6 M; n = 12–45 in each group. B) G19 myometrial cells were pretreated with LV.shNmU-R2 or LV.shScrambled for 72 h and then used in a calcium assay in response to 10−8 M NmU or OT. The calcium mobilization response to NmU is also presented when normalized with the response to OT (inset); n = 6–12 in each group. C and D) Akt (C) and Erk (D) phosphorylation immunoreactivity on primary mSMCs from pregnant mice at G10 or G19 stimulated with increasing concentrations of NmU for 10 min. Data are representative of four to five independent experiments. E and F) Akt (E) and Erk (F) immunoreactivity on G19 myometrial cells pretreated with LV.shNmU-R2 or LV.shSrambled for 72 h and then stimulated with 10−6 M NmU for 10 min. Data are representative of four to five independent experiments. G) Calcium assay of G19 myometrial cells treated with 10−6 M NmU or NmS; n = 6–45 in each group. H and I) Akt (H) and Erk (I) phosphorylation immunoblot of G19 myometrial cells treated with 10−6 M NmU or NmS for 10 min. Data are representative of four to five independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with comparison to control groups using Dunnett multiple-comparison test; ns, not significant.
IL1β Induces Uterine Expression of NmU-R2 and Potentiates Its Uterotonic Effects; Proinflammatory Infectious Stimuli Trigger NmS Expression in Human Decidua and Placenta
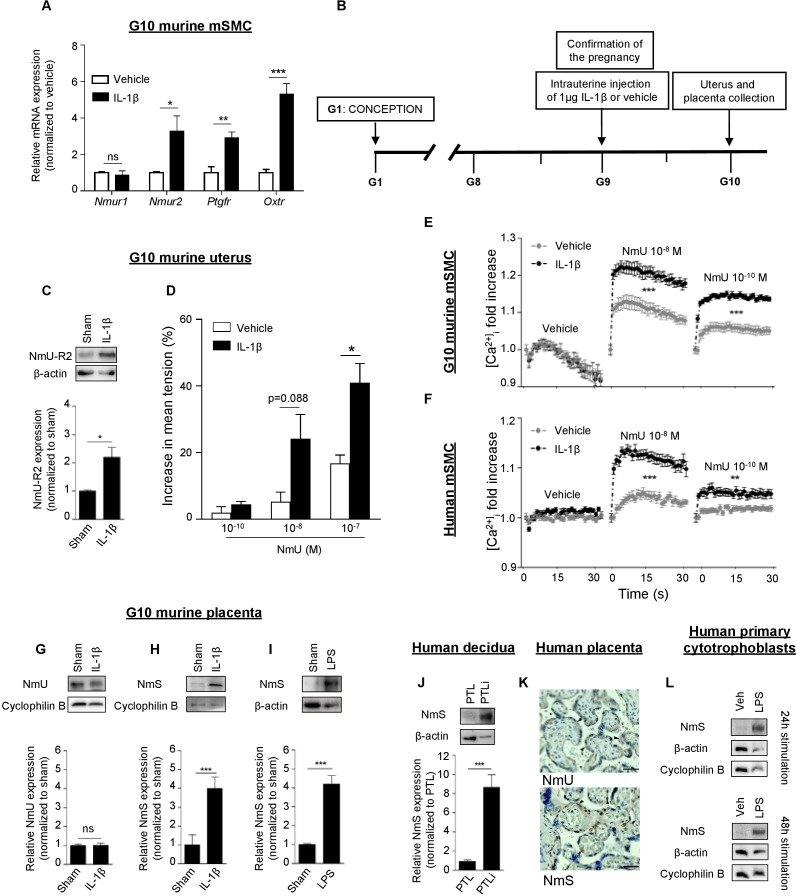
Chorioamnionitis is a major factor in triggering PTB [4–6]. We determined whether NmU-R2 is regulated by inflammatory factors. In line with the important role for IL1β in eliciting uterine inflammation and PTB [28–31], we stimulated G10 mSMCs with IL1β and measured the mRNA expression of Nmur1 and Nmur2, as well as Ptgfr and Oxtr; expression of the last three genes (but not Nmur1) was upregulated within 6 h by IL1β (Fig. 4A). Of relevance herein, progesterone (when anti-inflammatory PR-B is dominant [32]) had no effect on expression of these genes (data not shown). We then proceeded to explore whether inflammation (IL1β-induced) could reproduce these effects in vivo. Consistent with changes observed in mSMCs, IL1β (1 μg intrauterine, at G9 [Fig. 4B]), shown to amplify uteroplacental inflammation [30], induced protein and mRNA uterine expression of NmU-R2 (Fig. 4C and Supplemental Fig. S2I), but not NmU-R1, in mice (Supplemental Figs. S1G and S2H). Correspondingly, uteri isolated from pregnant mice (G10) treated with IL1β displayed increased uterocontractile response to NmU ex vivo (Fig. 4D). Concordantly, calcium transients triggered by NmU were significantly increased in murine mSMCs isolated at G10 and pretreated with IL1β (Fig. 4E); this effect was also observed in human mSMCs (Fig. 4F).
FIG. 4.
NmU-R2 in uterus and NmS in placenta are under the control of inflammation in mice and humans. A) Quantitative PCR performed on primary mSMCs collected from pregnant mice at G10 and stimulated with 5 ng/ml IL1β for 6 h. Results are relative to 18S and plotted as fold over the control group (vehicle). B) Schematic representation of the in vivo model used. C) Representative NmU-R2 immunoblot (top) and densitometric analysis (bottom) of uteri of pregnant mice collected 24 h after an intrauterine injection of saline (sham) or IL1β. D) Ex vivo contraction assay performed on uteri from pregnant mice exposed for 24 h to saline (sham) or IL1β. Data are representative of three to four mice per group. E and F) Pregnant mouse (G10) mSMCs or human mSMCs (hTERT-C3 cell line) were treated for 24 h with IL1β or vehicle, and were used in a calcium assay; n = 6–12 in each group. G and H) Immunoblots (top) and relative densitometric analysis of protein bands (bottom) showing NmU (G) and NmS (H) expression in placenta of pregnant mice 24 h after an intrauterine injection of either saline (sham) or IL1β. I) Immunoblot (top) and relative densitometric analysis of protein bands (bottom) showing NmS expression in placenta of pregnant mice 24 h after an intrauterine injection of either saline (sham) or LPS. J) NmS immunoblot from human decidual biopsies from women in preterm labor with (PTLi, n = 3) and without (PTL, n = 3) clinical evidence of infection. Bottom panel shows densitometric analysis of protein bands normalized with β-actin and plotted as fold over the control group (PTL). K) Immunohistochemistry analysis performed on term human placentas blotted against NmU and NmS. For the NmU panel, black arrows represent syncytiotrophoblasts; for the NmS panel, black arrows represent cytotrophoblasts and white arrows Hofbauer cells. Bar = 40 μm. L) Primary human cytotrophoblasts were stimulated with LPS for 24 h (top) or 48 h (bottom), and cell lysates were blotted against NmS. β-Actin and cyclophilin B were used as loading controls. Values are presented as mean ± SEM. Data are representative of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with comparison to control groups using Dunnett multiple-comparison test; ns, not significant.
Given the strong and consistent effect of IL1β in triggering NmU-R2 expression and actions in uterus, we determined whether IL1β triggered expression of NmU-R2 ligands in placenta. IL1β was found to increase placental expression of the NmU-R2-specific agonist NmS, but not NmU, in mice (Fig. 4, G and H). A similar induction of placental NmS was observed when mice were stimulated intrauterinely with known inflammasome-activating TLR4 ligand LPS (arising from Gram-negative bacteria) to mimic an infectious stimulus (Fig. 4I). Consistent with murine data, NmS expression was found to be markedly augmented (>8-fold) in decidual biopsies from women who delivered preterm with clinical evidence of infection (PTLi, n = 3) compared with those without (PTL, n = 3; Manchester, U.K., tissue bank; Fig. 4J). In human placenta, NmS was primarily expressed by cytotrophoblasts and Hofbauer (placental macrophage) cells (within placental villi), whereas NmU was specifically expressed by syncytiotrophoblasts (external layer of placental villi; Fig. 4K). Therefore, we stimulated primary human cytotrophoblasts isolated from term placenta with LPS for 24 and 48 h; LPS induced NmS expression from cytotrophoblasts (Fig. 4L), validating human and murine data (presented in Fig. 4, H–J).
NmU-R2 Plays a Key Role in Infection-Induced PTB
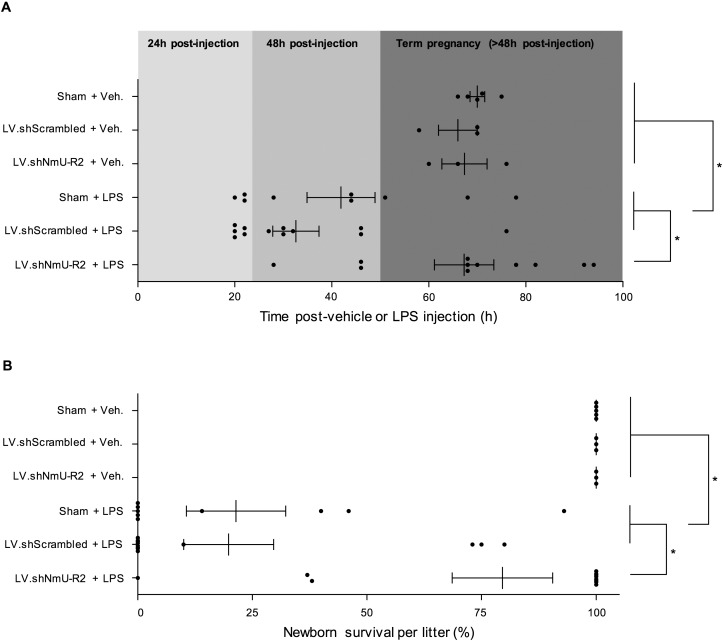
Previous results strongly suggest that NmS in placenta and NmU-R2 in uterus are regulated by inflammatory stimuli with or without infection. We proceeded to study the role of NmU-R2 in PTB associated with infection. For this purpose G16 mice were injected with LPS (to mimic infection), in animals previously treated or not treated intrauterinely with lentivirus encoded with shRNA against procontractile Nmur2 (or scrambled shRNA). Gestation was significantly prolonged in LPS-treated Nmur2 knocked down animals, by an average of ∼28 h (Fig. 5A); this effect was associated with improved neonatal survival rate (Fig. 5B).
FIG. 5.
NmU-R2 is important for infection-induced adverse gestation outcomes. A and B) Pregnant mice were pretreated with intrauterine injections of vehicle (sham), LV.shScrambled, or LV.shNmU-R2 at G13 (one injection in each uterine horn), and then treated with a single intraperitoneal injection of 10 μg of LPS or an equivalent volume of saline at G16. The timing of birth (A) and the survival of pups at birth per litter (B) were rigorously assessed. Values are presented as mean ± SEM. *P < 0.05 by one-way ANOVA with comparison to control groups using Dunnett multiple-comparison test.
DISCUSSION
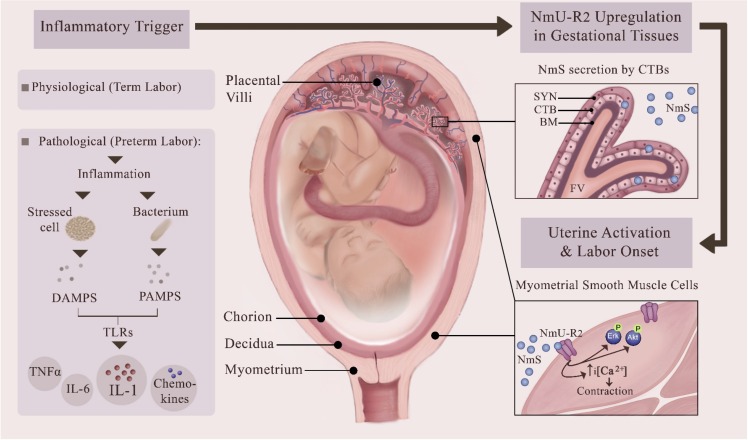
Inflammation, with or without infection, is considered to be implicated in more than 50% of PTBs, and its onset is often subclinical. Administration of bacteria or bacterial endotoxins in pregnancy triggers uterine activation pathways that can induce labor in rodents and nonhuman primates [30, 33–36]. Our findings indicate that NmU-R2 and its specific ligand NmS are regulated by inflammation in humans and animals, and play a critical role in infection-associated PTB. First, we found in murine gestational tissues that the expression of NmU-R2 and NmS was markedly increased upon intrauterine treatment with the major proinflammatory labor-inducing cytokine IL1β. Correspondingly, treatment with IL1β significantly increased the uterotonic effect and associated calcium transients coupled to NmU-R2 in murine and human mSMCs. Second, the inflammation-driven NmU-R2 upregulation observed in animals was also observed in human gestational tissues, because NmS was upregulated in women with clinical signs of infection; correspondingly, NmS was induced by LPS stimulation of primary human cytotrophoblasts isolated from term placenta. This suggests that the bacterial trigger needs to penetrate placental villi to induce NmS production from placenta and in turn promote labor. Interestingly, NmU-R2 is another labor-associated protein present in the central nervous system, as is the case for OXTR and IL-1RAcPb [37]. Third, knockdown of NmU-R2 in uterus significantly delayed preterm labor induced by Gram-negative bacterial product LPS. For these reasons, we suggest that infectious and noninfectious proinflammatory stimuli in pregnant gestational tissues, as well as advanced gestational age (in case of physiological term labor), trigger: 1) NmU-R2 upregulation in myometrium, thereby increasing the uterocontractile sensitivity to NmS; and 2) NmS production in placenta (specifically in cytotrophoblasts). This uterotonic system may contribute to the establishment of functional labor at term and preterm in the case of a pathological activation of an intrauterine inflammatory cascade (Fig. 6). Hence, NmU-R2 constitutes an interesting target for the prevention of inflammation-associated PTB.
FIG. 6.
Proposed role of NmU-R2 and its ligand NmS in physiological term labor and in pathological PTB. The schema provides a current view of inflammation-associated preterm birth whereby pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) activate pattern recognition receptors in uterus, predominantly Toll-like receptors (TLRs), to trigger an inflammatory cascade characterized by the local maturation and release of proinflammatory, prolabor cytokines, leading to uterine activation and PTB. Our study reveals that uterotonic NmS and its cognate receptor NmU-R2 are endogenously produced in human placenta and myometrium, respectively, and are upregulated by the PAMP LPS and by its downstream mediator IL1. We propose that this neuromedin system is implicated in sustaining or initiating uterine contractions in both physiological labor and pathological preterm labor associated with inflammation. This figure was made exclusively for this manuscript by the authors.
Preterm birth remains a challenge for clinicians because there are no available pharmacological agents sufficiently effective at prolonging preterm gestation by more than 48 h and improving newborn outcomes. The development of preventive therapies is limited because diagnostic tools with successful positive predictive values are also lacking, which hinders the identification of women at risk of preterm labor. Current therapies administered to women in labor (tocolytics) are largely ineffective and in many cases (e.g., indomethacin, nifedipine) are used off-label [38]. Only one tocolytic drug specifically designed to target uterine contraction has been approved in the last 30 yr, the oxytocin receptor antagonist atosiban. Although atosiban was demonstrated to be as efficacious as β-mimetics (which remains limited) and much better tolerated by women [39], its usage is limited to Europe, because it failed to gain U.S. Food and Drug Administration approval. Hydroxyprogesterone is effective for women with either short cervical length or prior history of preterm labor [40]. Yet there is still an unmet medical need for an effective tocolytic. Uterine activation proteins are interesting targets for the prevention of preterm birth because their induction directly precedes labor, and their functions are critical for successful labor.
Herein, we characterized a potential UAP, NmU-R2 (and its ligands NmU and NmS), in labor. NmU-R2 is upregulated in human and animal gestational tissue at term and during labor; NmS is concomitantly upregulated during parturition. The properties of NmU-R2 correspond to those of known UAPs: 1) its expression increases near labor and decreases in the immediate postpartum period; 2) it exhibits gestation age-dependent uterocontractile effects; 3) it is induced by proinflammatory uterotrophins, and its expression correspondingly increases during preterm labor with clinical evidence of infection; and 4) it contributes to the process of labor. In this study, NmU-R2 was found to be important for the onset of preterm labor associated with infection, but not necessary for labor at term, as seen with our NmU-R2 knocked-down model displaying normal parturition at term. This may be due to redundant mechanisms that are present in term uterus to ensure successful delivery, as is seen with the unaltered gestation length in germ line gene knockout mice for other critically important proteins, including IL1R1, TNFα, OT, COX-1, and others [19].
NmU-induced ex vivo uterine contractions have already been reported to be unaltered by NmU-R1 deficiency (using gene knockout mice), and thus are independent of NmU-R1 [16]. The present study is concordant and clarifies actions of NmU by showing that its contractile-associated effects (calcium transients) in uterus are specifically mediated by NmU-R2 (but not NmU-R1). In addition, uterine NmU-R2 knockdown considerably attenuated receptor-coupled signaling (calcium, Erk, and Akt), uterocontractile and prolabor effects induced by NmU, as well as preterm labor induced by inflammatory/infectious stimuli. In our murine experiments, NmU and NmS had similar efficacy to induce calcium, Erk, and Akt signaling in mSMCs, as well as uterine contraction and labor. Interestingly, only the NmU-R2-specific ligand NmS was endogenously induced by inflammation and upregulated during physiological labor, suggesting that NmU is not as important as NmS in initiating or sustaining labor contractions.
In summary, we hereby describe a procontractile, prolabor human and murine system wherein neuromedins U and (more importantly) S induce calcium (and other downstream) signals in mSMCs to promote potent uterine contractions and labor via NmU-R2 at term and particularly before term. NmU-R2, NmU, and NmS are expressed in human and murine gestational tissues, and NmU-R2 and NmS are upregulated (and potentiated) by gestation age, infection, and inflammation. Correspondingly, this system is important for the onset of inflammation-induced preterm labor in mice, which may plausibly apply to human labor because NmS is markedly upregulated in gestation with clinical evidence of infection. Overall, the present study expands our understanding of the physiological mechanisms underlying labor, and it uncovers new targets for potential therapeutic intervention to delay preterm delivery, and more opportunities to identify biomarkers to predict women at risk of preterm birth. Specifically, NmU-R2 antagonists may provide benefits to prolong gestation in threatened pregnancies.
ACKNOWLEDGMENT
We thank Dr. Sam Mesiano for providing myometrial biopsies samples from MacDonald Women's Hospital, Cleveland, and Dr. Rebecca Lee Jones for providing decidual samples from St. Mary's Hospital, Manchester. We also thank Dr. Derek Robertson for the technical help provided.
Footnotes
This study was funded by the Global Alliance for the Prevention of Prematurity and Stillbirth, an initiative of Seattle Children's, to S.C. and D.M.O.; the March of Dimes to S.C. and D.M.O.; the Canadian Institutes of Health Research (CIHR) to S.C. and D.M.O.; the “Réseau Québécois en Reproduction (RQR)” to S.G.; and the SickKids Foundation–CIHR Institute of Human Development Child and Youth Health to S.G. M.N.V. was supported by a Vanier Canada Graduate Scholarship (CIHR), by the Suzanne Veronneau-Troutman Funds in association with the Department of Ophthalmology of the Université de Montréal, by the Vision Research Network (RRSV), and by a scholarship from Fonds de Recherche du Québec–Santé. A.B. was supported by a scholarship from Centre Hospitalier Universitaire Sainte-Justine Foundation and RRSV. S.C. holds the Leopoldine Wolfe Chair in translational research in age-related macular degeneration and a Canada Research Chair (Vision Science). Presented in part at the 63rd Society for Reproductive Investigation meeting, March 16–19, 2016, Palais des Congrès, Montreal, Canada.
REFERENCES
- Cook JL, Shallow MC, Zaragoza DB, Anderson KI, Olson DM. Mouse placental prostaglandins are associated with uterine activation and the timing of birth. Biol Reprod. 2003;68:579–587. doi: 10.1095/biolreprod.102.008789. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. Preterm labour. In: Edmonds DK, editor. Dewhurst's Textbook of Obstetrics and Gynaecology, 8th ed. Oxford, UK: Wiley-Blackwell; 2012. pp. 338–355. In: (ed.) [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Stephen GL, Lui S, Hamilton SA, Tower CL, Harris LK, Stevens A, Jones RL. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol. 2015;73:36–55. doi: 10.1111/aji.12328. [DOI] [PubMed] [Google Scholar]
- Martinez VG, O'Driscoll L. Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin Chem. 2015;61:471–482. doi: 10.1373/clinchem.2014.231753. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Willars GB., Neuromedin U. and its receptors: structure, function, and physiological roles. Pharmacol Rev. 2004;56:231–248. doi: 10.1124/pr.56.2.3. [DOI] [PubMed] [Google Scholar]
- Kojima M, Haruno R, Nakazato M, Date Y, Murakami N, Hanada R, Matsuo H, Kangawa K. Purification and identification of neuromedin U as an endogenous ligand for an orphan receptor GPR66 (FM3) Biochem Biophys Res Commun. 2000;276:435–438. doi: 10.1006/bbrc.2000.3502. [DOI] [PubMed] [Google Scholar]
- Fukue Y, Sato T, Teranishi H, Hanada R, Takahashi T, Nakashima Y, Kojima M. Regulation of gonadotropin secretion and puberty onset by neuromedin U. FEBS Lett. 2006;580:3485–3488. doi: 10.1016/j.febslet.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Wise A, Dass NB, Willars GB. Paradoxical behavior of neuromedin U in isolated smooth muscle cells and intact tissue. J Pharmacol Exp Ther. 2008;325:154–164. doi: 10.1124/jpet.107.132803. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Payza K, Huang J, Liu R, Chen T, Coupal M, Laird JM, Cao CQ, Butterworth J, Lapointe S, Bayrakdarian M, Trivedi S, et al. Discovery and pharmacological characterization of a small-molecule antagonist at neuromedin U receptor NMUR2. J Pharmacol Exp Ther. 2009;330:268–275. doi: 10.1124/jpet.109.152967. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction. Mol Pharmacol. 2004;66:1544–1556. doi: 10.1124/mol.104.002337. [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, Kojima M, Kangawa K. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. EMBO J. 2005;24:325–335. doi: 10.1038/sj.emboj.7600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin U-8 and U-25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun. 1985;130:1078–1085. doi: 10.1016/0006-291x(85)91726-7. [DOI] [PubMed] [Google Scholar]
- Prendergast CE, Morton MF, Figueroa KW, Wu X, Shankley NP. Species-dependent smooth muscle contraction to Neuromedin U and determination of the receptor subtypes mediating contraction using NMU1 receptor knockout mice. Br J Pharmacol. 2006;147:886–896. doi: 10.1038/sj.bjp.0706677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WS, Xie QS, Wu XH, Liang QH., Neuromedin B. and its receptor induce labor onset and are associated with the RELA (NFKB P65)/IL6 pathway in pregnant mice. Biol Reprod. 2011;84:113–117. doi: 10.1095/biolreprod.110.085746. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fields MJ, Freidman S, Shemesh M, Ivell R. Oxytocin and the timing of parturition. Influence of oxytocin receptor gene expression, oxytocin secretion, and oxytocin-induced prostaglandin F2 alpha and E2 release. Adv Exp Med Biol. 1995;395:405–420. [PubMed] [Google Scholar]
- Kimura T, Ogita K, Kusui C, Ohashi K, Azuma C, Murata Y. What knockout mice can tell us about parturition. Rev Reprod. 1999;4:73–80. doi: 10.1530/ror.0.0040073. [DOI] [PubMed] [Google Scholar]
- Xu C, Long A, Fang X, Wood SL, Slater DM, Ni X, Olson DM. Effects of PGF2α on the expression of uterine activation proteins in pregnant human myometrial cells from upper and lower segment. J Clin Endocrinol Metab. 2013;98:2975–2983. doi: 10.1210/jc.2012-2829. [DOI] [PubMed] [Google Scholar]
- Shynlova OP, Oldenhof AD, Liu M, Langille L, Lye SJ. Regulation of c-fos expression by static stretch in rat myometrial smooth muscle cells. Am J Obstet Gynecol. 2002;186:1358–1365. doi: 10.1067/mob.2002.122415. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, Heazell AE, Derricott H, Allan SM, Sibley CP, Abrahams VM, Jones RL. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol. 2014;72:422–434. doi: 10.1111/aji.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Koike K, Kimura A, Masuhara K, Ikegami H, Ikebuchi Y, Kanzaki T, Touhara K, Sakaue M, Kobayashi Y, Akabane M, Miyake A, et al. Role of mitogen-activated protein kinase pathway in prostaglandin F2alpha-induced rat puerperal uterine contraction. Endocrinology. 1997;138:3103–3111. doi: 10.1210/endo.138.8.5305. [DOI] [PubMed] [Google Scholar]
- Stassen FL, Heckman G, Schmidt D, Papadopoulos MT, Nambi P, Sarau H, Aiyar N, Gellai M, Kinter L. Oxytocin induces a transient increase in cytosolic free [Ca2+] in renal tubular epithelial cells: evidence for oxytocin receptors on LLC-PK1 cells. Mol Pharmacol. 1988;33:218–224. [PubMed] [Google Scholar]
- Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Hirsch E. Effect of stimulation and antagonism of interleukin-1 signaling on preterm delivery in mice. J Soc Gynecol Investig. 2005;12:533–538. doi: 10.1016/j.jsgi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A, Duhamel F, Rivera JC, Zhu T, et al. Novel noncompetitive IL-1 receptor-biased ligand prevents infection- and inflammation-induced preterm birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Obari D, Quiniou C, Lubell WD, Olson DM, Girard S, Chemtob S. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev. 2016;28:37–51. doi: 10.1016/j.cytogfr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–E730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma C, Kuwayama C, Kaga N, Futamura Y, Katsuki Y, Shibutani Y. Trophoblastic apoptosis in mice with preterm delivery and its suppression by urinary trypsin inhibitor. Obstet Gynecol. 1997;90:117–124. doi: 10.1016/S0029-7844(97)00176-2. [DOI] [PubMed] [Google Scholar]
- Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods. 1998;39:147–154. doi: 10.1016/s1056-8719(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol. 2002;186:523–530. doi: 10.1067/mob.2002.120278. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Ishiguro TBH, Takeda J, Fang X, Olson DM. Interleukin (IL)-1 receptor I and IL-1 receptor accessory protein increase at delivery in rat uterus. Reprod Sci. 2014;21:238A. [Google Scholar]
- Olson DM, Christiaens I, Gracie S, Yamamoto Y, Mitchell BF. Emerging tocolytics: challenges in designing and testing drugs to delay preterm delivery and prolong pregnancy. Expert Opin Emerg Drugs. 2008;13:695–707. doi: 10.1517/14728210802568764. [DOI] [PubMed] [Google Scholar]
- Nisell H, Wolff K. Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. BJOG. 2001;108:133–142. [PubMed] [Google Scholar]
- Saccone G, Suhag A, Berghella V. 17-alpha-hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:16–22. doi: 10.1016/j.ajog.2015.01.054. [DOI] [PubMed] [Google Scholar]