Abstract
The discovery of the key roles of interleukin-17A (IL-17A) and IL-17A producing cells in inflammation, autoimmune diseases and host defense has led to the experimental targeting of the IL-17A pathway in animal models of diseases as well as in clinical trials in humans. These therapeutic agents include biological products that target IL-17A and IL-23, an upstream regulator of IL-17A production. IL-17A producing T helper cells (Th17 cells) are a distinct lineage from the Th1 and Th2 CD4+ lineages and have been suggested to represent a good drug target in certain inflammatory conditions. Targeting IL-17A has been proven to be a good approach as anti-IL-17A is FDA approved for the treatment of psoriasis in 2015. In host defense, IL-17A has been shown to be mostly beneficial against infection caused by extracellular bacteria and fungi. This review will overview the discovery of IL-17A, the receptors used by this cytokine and its role in mucosal immunity and inflammation.
Keywords: Interleukin-17A, Inflammation, neutrophil, cancer
Introduction
IL-17A is the most widely studied member of the IL-17 family, a group of proteins that have a highly conserved C-terminus containing a cysteine-knot fold structure (Weaver et al., 2007). IL-17A plays a critical role in host defense against various microbial pathogens as well as tissue inflammation. IL-17A and its closest relative IL-17F, signal through the same receptor complex (IL-17R) composed of the subunits IL-17RA and IL-17RC. Recently, IL-17A and IL-17A producing cells have become important targets for drug discovery for the treatment of various forms of autoimmune and inflammatory diseases. Anti-IL-17A is FDA approved for the treatment of psoriasis and this pathway has also been studied in asthma, rheumatoid arthritis, multiple sclerosis, transplant rejection, and inflammatory bowel disease.
Discovery and cellular sources of IL-17A
IL-17A, often time referred to as IL-17, was originally discovered at transcriptional level by Rouvier et al. in 1993 from a rodent T-cell hybridoma, derived from the fusion of a mouse cytotoxic T cell clone and a rat T cell lymphoma (Rouvier et al., 1993). Recent studies now suggest that what was thought to be a mouse sequence was actually derived from the rat lymphoma (Kennedy et al., 1996). Human and mouse IL-17A were cloned a few years later by Yao and Kennedy (Kennedy et al., 1996; Yao et al., 1995b). Yao demonstrated that the IL-17A transcript was expressed in CD4+ T-cells and the protein could induce IL-6 in fibroblasts. Early studies also suggested that IL-17A may be a major vehicle by which T cells communicate with the hematopoietic system (Fossiez et al., 1996; Schwarzenberger et al., 1998). Fossiez demonstrated that IL-17A could signal to bone marrow stromal cells in vitro to induce G-CSF that supported the differentiation of hematopoietic cells to the granulocyte lineage. Schwarzenberger demonstrated that IL-17A over expression in vivo also led to extramedullary granulopoiesis (Schwarzenberger et al., 1998) and this was due to in vivo induction of G-CSF and stem cell factor (Schwarzenberger et al., 2000). Subsequent studies showed that lymphocytes including CD4+, CD8+, gamma-delta T (γδ-T), invariant NKT and innate lymphoid cells (ILCs) are primary sources of IL-17A (Cua and Tato, 2010). Non-T cells, such as neutrophils, have also been reported to produce IL-17A under certain circumstances (Taylor et al., 2014). A critical advance in the field came in 2005 when several groups showed that Th17 cells could be derived from naïve CD4+ T-cells under the control of TGFβ, IL-6 and Th17 lineage commitment was independent of STAT4, STAT6 (Bettelli et al., 2006; Harrington et al., 2005; Park et al., 2005). Subsequent studies demonstrated a critical role of STAT3 (Mathur et al., 2007) and RORC (Ivanov et al., 2006) in Th17 lineage commitment in mice and humans. Accumulating data also suggest that IL-23 is essential for the maturation and maintenance of the Th17 lineage (Gaffen et al., 2014).
Discovery of IL-17A Receptors
A receptor for IL-17A was first isolated and cloned from mouse EL4 thymoma cells and the bioactivity of IL-17A was confirmed by stimulating the transcriptional factor NF-kappa B activity and interleukin-6 (IL-6) secretion in fibroblasts (Yao et al., 1995a). This receptor, IL-17RA, was the founding member of the IL-17 receptor family which includes IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. IL-17RA appears to be a common receptor chain shared with other IL-17R family members. For example IL-17RA pairs with IL-17RC to allow binding and signaling of IL-17A and IL-17F (Gaffen, 2009) and the IL-17RA/IL-17RC pair was discovered using flow cytometry binding assays as well as Biacore analysis (Kuestner et al., 2007)..
Role of IL-17A in autoimmune diseases
IL-17A producing CD4+ T helper cells, also called Th17 cells, have been studied extensively in the past decade and have been shown to be potent inducers of tissue inflammation and have been associated with the pathogenesis of many experimental autoimmune diseases and human inflammatory conditions. Substantial evidence suggests that IL-17A producing cells including Th17 cells are involved in human psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel diseases, and asthma (Korn et al., 2009). The aberrant IL17A expression in affected tissues and the reported cellular sources of IL-17A are summarized in Table 1 and each individual disease is discussed below. Although many studies have reported non-lymphoid cells such as mast cells and neutrophils may produce IL-17A, most of these studies drew these conclusions based on immunohistochemistry staining which does not discriminate between IL-17A producing cells and cells that have bounded IL-17A due the ubiquitous expression of IL-17RA. Regardless of the cellular sources of IL-17A, it is considered to act on the structural cells to initiate tissue inflammation. A cartoon version of IL-17A mediated inflammation in various diseases is illustrated in Figure 1.
Table 1.
IL-17A detection in human diseases.
| Disease | Affected tissue or organ | Detection sites of elevated IL-17A | Cellular sources of IL-17A | Reference |
|---|---|---|---|---|
| IBD | Intestine | Inflamed mucosa and serum | CD4+, CD8+ and ILCs. | (Fujino et al., 2003; Geremia et al., 2011; Holtta et al., 2008) |
| Psoriasis | Skin | Psoriatic skin lesions and serum | CD3+, neutrophils, mast cells | (de Oliveira et al., 2015; Johansen et al., 2009; Keijsers et al., 2014; Plee et al., 2015) |
| RA | Joint | Synovial fluid and serum | CD4+, NK, mast cells and B cells | (Church et al., 2010; Hueber et al., 2010a; Metawi et al., 2011; Schlegel et al., 2013) |
| MS | Brain and spinal cord | Blood and cerebrospinal fluid | CD4+ and CD8+ | (Balasa et al., 2013; Durelli et al., 2009; Tzartos et al., 2008) |
| Asthma | Lung | Bronchoalveolar lavage fluid and blood | CD4+, NK, γδ-T, ILC3, neutrophils and B cells | Reviewed in ref (Chesne et al., 2014; Nembrini et al., 2009) |
| COPD | Lung | Sputum and bronchial biopsy | CD3+ and mast cells | (Barczyk et al., 2003; Barnes, 2015; Roos et al., 2015) |
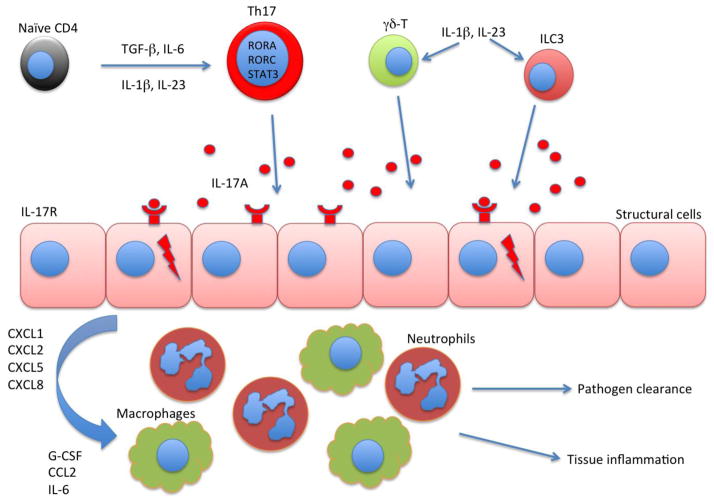
Figure 1. Schematic illustration of signaling pathway that involves IL-17A in inflammatory diseases.
Naïve CD4 T cells differentiate into IL-17 producing T cells under the cytokine environment including TGF-β, IL-6, IL-1β and IL-23. Th17 differentiation is controlled by transcription factors including RORA, RORC and STAT3. Type 3 innate lymphoid cell (ILC3) and γδ-T cell can also produce IL-17 in respond to IL-1β and IL-23 stimulation. IL-17A acts on structural cells such as epithelial cells, fibroblasts and keratinocytes in various tissue including skin, gut as well as lung. Structural cells that express IL-17 receptor produce inflammatory cytokines such as G-CSF and IL-6 as well as chemokines to attract neutrophils and macrophage to the inflamed tissues. These inflammatory cells can both clear the infection and initiate pathogenic inflammation.
Multiple sclerosis (MS) is a neurological disease caused by immune cells, which attack and destroy the myelin sheath that insulates neurons in the brain and spinal cord. This disease and its animal model experimental autoimmune encephalomyelitis (EAE) have historically been associated with the discovery of Th17 cells (Harrington et al., 2005; Park et al., 2005). However, elevated expression of IL-17A in multiple sclerosis (MS) lesions as well as peripheral blood has been documented before the identification of Th17 cells (Lock et al., 2002; Matusevicius et al., 1999). Human TH17 cells have been shown to efficiently transmigrate across the blood-brain barrier in multiple sclerosis lesions, promoting central nervous system inflammation (Kebir et al., 2007). Recent publications also suggest that GM-CSF instead of IL-17A produced by Th17 cells is a critical pathogenic factor in EAE (Codarri et al., 2011; El-Behi et al., 2011). Nonetheless, IL-17A may still be able to serve as a predictive or surrogate biomarker in MS based on the data from preclinical models.
Psoriasis is an auto-inflammatory skin disease characterized by circumscribed, crimson red, silver-scaled, plaque-like inflammatory lesions. Many types of immune cells can be found in these lesions such as infiltrating T cells (mainly CD4+ cells) and dendritic cells in the dermis as well as cytotoxic T cells and neutrophils in the epidermis (Lowes et al., 2007). These immune cells trigger rapid keratinocyte proliferation, abnormal keratinocyte differentiation, and dermal angiogenesis (Lowes et al., 2007). Initially, psoriasis was considered to be a Th1-mediated disease since elevated levels of IFN-γ, TNF-α, and IL-12 was found in the serum and lesions of psoriasis patients (Di Cesare et al., 2009). However, the finding of IL-17-producing cells as well as IL17A transcripts in the lesions of psoriatic patients suggested that Th17 cells may synergize with Th1 cells in driving the pathology in psoriasis (Cai et al., 2011; Harper et al., 2009). Furthermore, Th17 cells have also been found to be localized in the dermis in atopic dermatitis, with a higher prevalence present in acute compared to chronic lesions (Koga et al., 2008). Thus, IL-17 may be a target in inflammatory skin disorders beyond psoriasis. Secukinumab (anti-IL-17A) has been evaluated in psoriasis and the first report showing Secukinumab is effective when compared with placebo was published in 2010 (Hueber et al., 2010b). In 2015, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved anti-IL-17 for the treatment of psoriasis (Beringer et al., 2016).
Another autoimmune disease strongly associated with Th17 cells is rheumatoid arthritis (RA), a chronic disorder with symptoms include chronic joint inflammation, autoantibody production, which lead to the destruction of cartilage and bone (McInnes and Schett, 2011). Interestingly, the levels of IL-17A in the synovium correlate with tissue damage, whereas levels of IFN-γ correlate with protection (Kirkham et al., 2006). Direct clinical significance of IL-17A in RA comes from recent clinical trials which found that two anti-IL-17A antibodies, namely Secukinumab and Ixekizumab significantly benefit these patients (Genovese et al., 2014; Genovese et al., 2010). The first trial on Secukinumab in RA showed improved clinical response (Hueber et al., 2010b) but in another phase II trial, the same efficacy was not achieved (Genovese et al., 2013).
Th17 cells and IL-17 have also been linked to Crohn’s disease (CD) and ulcerative colitis (UC), the two main forms of inflammatory bowel diseases (IBD) in man. Th17 cells infiltrate massively to the inflamed tissue of IBD patients and both in vitro and in vivo studies have shown that Th17-related cytokines may initiate and amplify multiple pro-inflammatory pathways (Monteleone et al., 2012). Elevated IL-17A levels in IBD have been reported by several groups (Fujino et al., 2003; Rovedatti et al., 2009). Nonetheless, Th17 signature cytokines, such as IL-17A and IL-22, may target gut epithelial cells and promote the activation of regulatory pathways and confer protection in the gastrointestinal tract (Li et al., 2014; Sarra et al., 2010). To this end, recent clinical trials targeting IL-17A in IBD were negative and actually showed increased adverse events in the treatment arm (Hueber et al., 2012). This data raised the question regarding the role of IL-17A in IBD pathogenesis and suggested that the elevated IL-17A might be beneficial for IBD patients.
Systemic lupus erythematosus, commonly referred as SLE or lupus, is a complex immune disorder affects the skin, joints, kidneys, and brain. Although the exact cause of lupus is not fully known, it has been reported that IL-17 and Th17 cells are involved in disease pathogenesis (Garrett-Sinha et al., 2008). It has been reported that serum IL-17 levels are also elevated in SLE patients compared to controls (Vincent et al., 2013; Wong et al., 2008) and the Th17 pathway has been shown to drive autoimmune responses in pre-clinical mouse models of lupus (Hsu et al., 2008; Jacob et al., 2009). More importantly, IL-17 and IL-17 producing cells are also been detected in kidney tissue and skin biopsies from SLE patients (Crispin et al., 2008; Oh et al., 2011; Yang et al., 2009), suggesting a possible role for IL-17 in the pathophysiology of SLE and a potential therapeutic option of using anti-IL-17A in treating patients with SLE.
IL-17A in Lung Diseases
Asthma
Asthma is a major chronic disease characterized by airway inflammation and bronchial hyperresponsiveness, leading to recurrent chest wheezing, cough, and shortness of breath. Elevated levels of IL-17A have been found in the sputum and in bronchoalveolar lavage fluid of patients with asthma (Molet et al., 2001) and a positive correlation between IL-17A production and asthma severity has been established (Chesne et al., 2014). In murine models, treatment with dexamethasone inhibits the release of Th2-related cytokines but does not affect IL-17A production (McKinley et al., 2008). Furthermore, Th17 cell-mediated airway inflammation and airway hyperresponsiveness are steroid resistant, indicating a potential role for Th17 cells in steroid-resistant asthma (McKinley et al., 2008). Heterogeneous expression of IL-13 (Th2 signature cytokine) and IL-17 (Th17 signature cytokine) related genes have been observed in both patients and animal models, suggesting combination therapy targeting both pathways could be effective in treating asthma (Choy et al., 2015). However, a recent trial using anti-IL-17RA did not show efficacy in subjects with asthma (Busse et al., 2013). Further studies in stratifying and phenotyping patients are needed to determine the contributions of IL-17A in asthma.
COPD
Chronic obstructive pulmonary disease (COPD) is a pulmonary disease marked by progressive emphysematic changes in the lung. Recent studies have suggested the involvement of immunological mechanisms in COPD (Alcorn et al., 2010). An increase in Th17 cells was observed in patients with COPD compared with current smokers without COPD and healthy subjects, and inverse correlations were found between Th17 cells with lung function (Vargas-Rojas et al., 2011). Increased production of IL-17A in the lungs were also found by different research groups (Cazzola and Matera, 2012). Gene expression profiling of bronchial brushings obtained from COPD patients also linked lung function to several Th17 signature genes such as SAA1, SAA2, SLC26A4 and LCN2 (Steiling et al., 2013). Animal studies have shown that cigarette smoke promotes pathogenic Th17 differentiation and induces emphysema (Chen et al., 2011b), while blocking IL-17A using neutralizing antibody significantly decreased neutrophil recruitment and the pathological score of airway inflammation in tobacco-smoke-exposed mice (Cazzola and Matera, 2012; Shen et al., 2011).
Role of IL-17A in host defense
The primary function of Th17 cells appears to be control of the gut microbiota (Ivanov et al., 2009; Kumar et al., 2016) as well as the clearance of extracellular bacteria and fungi. IL-17A and IL-17 receptor signaling has been shown to be play a protective role in host defenses against many bacterial and fungal pathogens including Klebsiella pneumoniae, Mycoplasma pneumonia, Candida albicans, Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis (Chen and Kolls, 2013). However, IL-17A seems to be detrimental in viral infection such as influenza through promoting neutrophilic inflammation (Crowe et al., 2009).
The requirements of IL-17A and IL-17 receptor signaling in host defense were well documented and appreciated before the identification of Th17 cells as an independent T helper cell lineage (Ye et al., 2001). In experimental pneumonia models, IL-17A or IL-17RA knock mice have increased susceptibility to various Gram-negative bacteria, such as Klebsiella pneumoniae (Ye et al., 2001) and Mycoplasma pneumonia (Wu et al., 2007). Upon K. pneumoniae infection, IL-17RA knockout mice showed reduced G-CSF production and neutrophil recruitment in the lungs and, as a consequence, the IL-17RA knockout mice had higher bacterial burdens in the lung as well as increased systemic dissemination to the spleen when compare to their littermate controls (Ye et al., 2001). Early IL-17A production, principally from γδ T-cells, in response to K. pneumonia requires TLR4 signaling and IL-23 production (Happel et al., 2003). Mice lacking IL-23p19, a subunit that is IL-23 specific, have decreased levels of IL-17A and exhibit similar defects as the IL-17RA knockout mice with reduced production of proinflammatroy cytokines and chemokines such as G-CSF and CXCL1, 2, and 5 (Happel et al., 2005). In IL-23p19 knockout mice, mucosal administration of recombinant IL-17A rescued the chemokine production and neutrophil infiltration to the lungs and resulted in decreased bacterial burden as well as systemic dissemination (Happel et al., 2005). Similar results have also been observed in M. pneumoniae infection (Wu et al., 2007). In contrast, data suggest that IL-23 and IL-17A are not required for protection against primary infection by the intracellular bacteria Mycobacterium tuberculosis. Both the IL-17RA knock out mice and the IL-23p19 knock out mice cleared primary infection with M. tuberculosis (Khader et al., 2005; Sun and Metzger, 2008). However, M. tuberculosis infection induces Il23a expression in the lungs and overexpression of IL-23p19 in M. tuberculosis vaccination significantly boosted antigen-specific Th1 responses (Khader et al., 2007; Wozniak et al., 2006a; Wozniak et al., 2006b). Interestingly, IL-17A is required for protection against primary infection with a different intracellular bacteria, Francisella tularensis. It has been shown in the mouse model that IL-17A is required for the production of IL-12p70 after infection and the Th1 responses promoted by IL-17p70 is responsible for the ultimate clearance of the infection (Lin et al., 2009).
Mouse model studies using the IL-17RA knock out mice and the IL-17A knock out mice with the murine adapted influenza strain (PR8) (Crowe et al., 2009) as well as the 2009 pandemic H1N1 stain (Li et al., 2012) both support that IL-17A plays a detrimental role in mediating the acute lung injury, suggesting that therapeutic anti-IL-17A treatment may be useful in an influenza pandemic.
While the essential role of the IL-17A in protection against primary infection caused by a variety of extracellular bacteria and fungi has been documented for many years, the role of adaptive immune responses mediated by antigen specific Th17 has been investigated more recently. The development of antigen specific memory Th17 cells could benefit the host by mounting a more robust pathogen specific recall response upon re-countering the same pathogen. Thus, Th17 cells may have an advantage as a source of IL-17A as innate sources of IL-17A such as γδ-T and ILC3 cells do not mount typical immunological memory responses. In addition, antigen specific Th17 cells were also shown to recognize conserved protein antigens among different K. pneumoniae strains and provide broad-spectrum serotype-independent protection (Chen et al., 2011a). This concept has also been extended to fungal infection where an IL-17 producing clone with a TCR specific for calnexin from Blastomyces dermatitidis confers protection with evolutionary related fungal species including Histoplasma spp (Wuthrich et al., 2015). In another pneumonia mouse model, it has been shown that antigen specific CD4 T cells limit nasopharyngeal colonization of S. pneumoniae (Trzciński et al., 2008). More importantly, CD4 T cell-derived IL-17A, but not IFNγ or IL-4, was required for the clearance of Pneumococcal colonization (Lu et al., 2008). Furthermore, immunization with pneumococcal whole cell antigen and several derivatives provided IL-17-mediated, but not antibody dependent, protection against S. pneumoniae challenge (Malley et al., 2006; Moffitt et al., 2012). These studies demonstrated an essential role of antigen specific Th17 in host defense in the lungs. Antigen specific Th17 responses are also observed in other mucosal site such as the gastrointestinal tract. It has been shown that segmented filamentous bacterium (SFB) is sufficient to induce the Th17 development in the small intestine of mice (Ivanov et al., 2009) and these Th17 cells have been shown to be SFB antigen specific (Yang et al., 2014). These cells appear to constrain SFB growth in the intestine as loss of IL-17R specifically in the gut epithelium leads to SFB expansion (Kumar et al., 2016).
Role of IL-17A in Cancer
The IL-17A response, while constituting a protective arm defending the body against various infections, also functions as a double-edged sword constituting a risk factor that mediates the development of autoimmune diseases. The two sides of IL-17A can be even observed in one disease such as cancer. Recently, a pathogenic role of IL-17A in cancer is suggested (Chang et al., 2014; Coffelt et al., 2015; Wu et al., 2014). In contrast, anti-tumor effect of IL-17 producing cells was also observed in animal models (Martin-Orozco et al., 2009). With the prevalence of cancer increasing year to year, it is of importance to find new pathogenic pathways as well as new therapeutics to combat this disease.
In tumorigenesis, IL-17A has been shown to recruit myeloid derived suppressor cells (MDSCs) to dampen anti-tumor immunity (Chang et al., 2014; He et al., 2010). IL-17A can also enhance tumor growth in vivo through the induction of IL-6, which in turn activates oncogenic transcription factor signal transducer and activator of transcription 3 (STAT3) and upregulates pro-survival and pro-angiogenic genes in tumors (Wang et al., 2009). The exact role of IL-17A in angiogenesis has yet to be determined and current data suggest that IL-17A can promote or suppress tumor development (Houghton, 2013). IL-17A seemed to facilitate development of colorectal carcinoma by fostering angiogenesis via promote VEGF production from cancer cells (Liu et al., 2011) and it has been show that IL-17A also mediates tumor resistance to anti-VEGF therapy through the recruitment of MDSCs (Maniati and Hagemann, 2013). In a murine lung cancer model, Th17 cells have been shown to recruit myeloid cells and promote tumor proliferation (Chang et al., 2014) and in a murine model of pancreatic cancer, IL-17 also accelerates pancreatic intraepithelial neoplasia initiation and progression (McAllister et al., 2014). However IL-17A KO mice were more susceptible to developing metastatic lung melanoma (Martin-Orozco et al., 2009), suggesting that IL-17A can possibly promote the production of the potent antitumor cytokine IFN-γ, produced by cytotoxic T cells. Indeed, data from ovarian cancer suggest that Th17 cells are positively correlated with NK cell–mediated immunity and anti-tumor CD8 responses (Kryczek et al., 2009). A novel immune evasion mechanism by the tumors through the inhibition of Th17 cell development is proposed (Kryczek et al., 2009).
Targeting IL-17A in diseases
IL-17A producing T cell subsets are attractive targets for pharmaceutical intervention. In contrast, the generation of pathogen-specific T cell responses may be desired for advances in vaccine development. Other than the monoclonal antibodies that have been discussed above, highly specific and potent inhibitors targeting Th17 specific transcription factor RORγt have been identified and found to be highly effective (Huh and Littman, 2012). These small molecules will also lead us to the better understanding of how IL-17A functions both in normal development and disease settings. Vitamin D, a potent immunomodulator, has also been shown to suppress Th17 cell differentiation and function by several research groups (Hayes et al., 2015). Since IL-17A signals through the receptor complex composed of the subunits IL-17RA and IL-17RC, both can serve as targets for inhibiting IL-17A signaling (Gaffen, 2009). IL-17RA is expressed in nearly every cell type of the body, including epithelial cells, endothelial cells, fibroblasts and myeloid cells. Although IL-17RC is also expressed on epithelial cells and fibroblasts, its expression on myeloid cells is lower (Ge and You, 2008). Based on this more restricted expression of IL-17RC, it is thought that fibroblasts, epithelial cells and endothelial cells are a major target of IL-17A and IL-17F and targeting IL-17RC could be beneficial to the patients with a better specificity and selectivity.
Concluding remarks
Recent studies on IL-17A and Th17 subset provide new evidence of the contribution of T helper cells (other than Th1 and Th2) to chronic inflammation and tissue destruction. An increasing numbers of diseases have been linked to IL-17A and Th17 cells and their specific contribution to disease pathogenesis is being revealed. Both pre-clinical research and clinical trials are underway to test these concepts. Knowledge of the molecular pathways responsible for the regulation of the Th17 lineage and the production of IL-17A is expanding rapidly. This information will undeniably be essential for the development of novel therapeutic strategies for the treatments of an array of inflammatory diseases in humans.
Highlights.
Overview of the discovery, cloning and identification of the cellular sources of IL-17A
Summary of the roles of IL-17A in mucosal immunity and autoimmune diseases
Summary of IL-17A targeted drug development
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE.
The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/IL17A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- Balasa R, Bajko Z, Hutanu A. Serum levels of IL-17A in patients with relapsing-remitting multiple sclerosis treated with interferon-beta. Mult Scler. 2013;19:885–890. doi: 10.1177/1352458512468497. [DOI] [PubMed] [Google Scholar]
- Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136:531–545. doi: 10.1016/j.jaci.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Beringer A, Noack M, Miossec P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol Med. 2016;22:230–241. doi: 10.1016/j.molmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Matera MG. IL-17 in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2012;6:135–138. doi: 10.1586/ers.12.7. [DOI] [PubMed] [Google Scholar]
- Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111:5664–5669. doi: 10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol. 2013;31:605–633. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011a;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011b;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- Church LD, Filer AD, Hidalgo E, Howlett KA, Thomas AM, Rapecki S, Scheel-Toellner D, Buckley CD, Raza K. Rheumatoid synovial fluid interleukin-17-producing CD4 T cells have abundant tumor necrosis factor-alpha co-expression, but little interleukin-22 and interleukin-23R expression. Arthritis Res Ther. 2010;12:R184. doi: 10.1186/ar3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015 doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- de Oliveira PS, Cardoso PR, Lima EV, Pereira MC, Duarte AL, Pitta Ida R, Rego MJ, Pitta MG. IL-17A, IL-22, IL-6, and IL-21 Serum Levels in Plaque-Type Psoriasis in Brazilian Patients. Mediators Inflamm. 2015;2015:819149. doi: 10.1155/2015/819149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, Novelli F. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- Ge D, You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1:19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion JA, Lee SH, Codding CE, Kellner H, Ikawa T, et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol. 2014;41:414–421. doi: 10.3899/jrheum.130637. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, Aelion JA, Lee SH, Codding CE, Kellner H, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M, Blauvelt A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hayes CE, Hubler SL, Moore JR, Barta LE, Praska CE, Nashold FE. Vitamin D Actions on CD4(+) T Cells in Autoimmune Disease. Front Immunol. 2015;6:100. doi: 10.3389/fimmu.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtta V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, Kolho KL, Farkkila M, Savilahti E, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–1184. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010a;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, et al. Psoriasis Study G. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010b;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Littman DR. Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–2237. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao HX, Putterman C, Koss MN, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol. 2009;182:2532–2541. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijsers RR, Joosten I, van Erp PE, Koenen HJ, van de Kerkhof PC. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp Dermatol. 2014;23:799–803. doi: 10.1111/exd.12487. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Rossi DL, Zurawski SM, Vega F, Jr, Kastelein RA, Wagner JL, Hannum CH, Zlotnik A. Mouse IL-17: a cytokine preferentially expressed by alpha beta TCR + CD4-CD8-T cells. J Interferon Cytokine Res. 1996;16:611–617. doi: 10.1089/jir.1996.16.611. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Zou Z, Yan Y, Wang W, Chen Z, et al. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Gong C, Zhao MH, Feng BS. Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol. 2014;20:18177–18188. doi: 10.3748/wjg.v20.i48.18177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, et al. Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, Anderson PW. Antibody-Independent, Interleukin-17A-Mediated, Cross-Serotype Immunity to Pneumococci in Mice Immunized Intranasally with the Cell Wall Polysaccharide. Infection and Immunity. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniati E, Hagemann T. IL-17 mediates resistance to anti-VEGF therapy. Nat Med. 2013;19:1092–1094. doi: 10.1038/nm.3333. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201–1207. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- Moffitt KL, Malley R, Lu YJ. Identification of Protective Pneumococcal TH17 Antigens from the Soluble Fraction of a Killed Whole Cell Vaccine. PLoS ONE. 2012;7:e43445. doi: 10.1371/journal.pone.0043445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012;12:592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995–986. [DOI] [PubMed] [Google Scholar]
- Oh SH, Roh HJ, Kwon JE, Lee SH, Kim JY, Choi HJ, Lim BJ. Expression of interleukin-17 is correlated with interferon-alpha expression in cutaneous lesions of lupus erythematosus. Clin Exp Dermatol. 2011;36:512–520. doi: 10.1111/j.1365-2230.2010.03996.x. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plee J, Le Jan S, Giustiniani J, Barbe C, Joly P, Bedane C, Vabres P, Truchetet F, Aubin F, Antonicelli F, et al. Integrating longitudinal serum IL-17 and IL-23 follow-up, along with autoantibodies variation, contributes to predict bullous pemphigoid outcome. Sci Rep. 2015;5:18001. doi: 10.1038/srep18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos AB, Sanden C, Mori M, Bjermer L, Stampfli MR, Erjefalt JS. IL-17A Is Elevated in End-Stage Chronic Obstructive Pulmonary Disease and Contributes to Cigarette Smoke-induced Lymphoid Neogenesis. Am J Respir Crit Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- Rovedatti L, Kudo T, Biancheri P, Sarra M, Knowles CH, Rampton DS, Corazza GR, Monteleone G, Di Sabatino A, Macdonald TT. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- Schlegel PM, Steiert I, Kotter I, Muller CA. B cells contribute to heterogeneity of IL-17 producing cells in rheumatoid arthritis and healthy controls. PLoS One. 2013;8:e82580. doi: 10.1371/journal.pone.0082580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol. 2011;23:212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- Steiling K, van den Berge M, Hijazi K, Florido R, Campbell J, Liu G, Xiao J, Zhang X, Duclos G, Drizik E, et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med. 2013;187:933–942. doi: 10.1164/rccm.201208-1449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzciński K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against Nasopharyngeal Colonization by Streptococcus pneumoniae Is Mediated by Antigen-Specific CD4+ T Cells. Infection and Immunity. 2008;76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Rojas MI, Ramirez-Venegas A, Limon-Camacho L, Ochoa L, Hernandez-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med. 2011;105:1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Wozniak TM, Ryan AA, Britton WJ. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006a;177:8684–8692. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- Wozniak TM, Ryan AA, Triccas JA, Britton WJ. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect Immun. 2006b;74:557–565. doi: 10.1128/IAI.74.1.557-565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Brandhorst TT, Sullivan TD, Filutowicz H, Sterkel A, Stewart D, Li M, Lerksuthirat T, LeBert V, Shen ZT, et al. Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes. Cell Host Microbe. 2015;17:452–465. doi: 10.1016/j.chom.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995a;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995b;155:5483–5486. [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]