Abstract
The three papers in this symposium are based on presentations to an RSM meeting on the Diabetic Eye, held on 9 April 2003. The matter is particularly topical because the National Service Framework for Diabetes calls for a high-quality retinal screening programme. After a review of the various ophthalmic conditions likely to be encountered in diabetic patients (A Negi, S A Vernon) we proceed to the most important, diabetic retinopathy, with a discussion of screening methods (D M Squirrell, J F Talbot) and an account of laser treatments (J G F Dowler). Colour versions of the clinical photographs are available online [www.jrsm.org]. Publication was coordinated by Professor Susan Lightman, of Moorfields Eye Hospital, London, UK.
Diabetes is one of the world's greatest health challenges and its prevalence appears to be increasing. In the UK some 1.4 million people are known to have diabetes and the number with undiagnosed diabetes is probably similar. Of the known cases, around 200 000 are type 1 and 1.2 million type 2. 1 The increasing prevalence of obesity in the young is expected to cause an epidemic of early-onset type 2 disease in the western world, with a rise in prevalence from the 1995 figure of 51 million to 72 million in 2025.
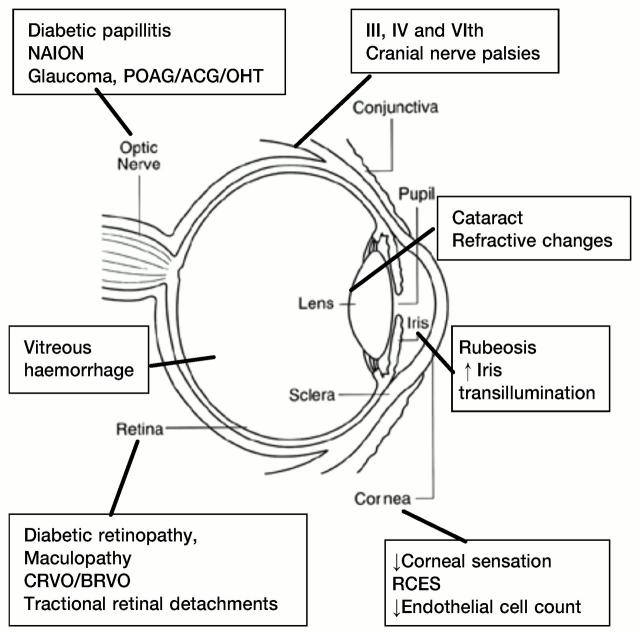
Type 1 diabetes affects young lean people (usually diagnosed at <30 years); they are insulin deficient and always need insulin. Type 2 diabetes tends to affect older obese persons (>30 years but see above); the abnormality is partial insulin deficiency, insulin resistance or both. In both types, macrovascular disease leads to coronary artery disease, stroke and peripheral vascular disease while microvascular involvement causes nephropathy and retinopathy. Here we review the effects of diabetes on the eye, with an emphasis on the most important and characteristic—namely, diabetic retinopathy. Cataract apart, retinopathy is responsible for most of the sight-threatening complications of diabetes. However, ophthalmic practitioners, general physicians, general practitioners and optometrists should be aware of the various other diabetesassociated disorders so as to recognize them promptly and refer appropriately for treatment. The principal ophthalmic manifestations are summarized in Figure 1.
Figure 1.
Common eye abnormalities in diabetes. RCES=recurrent corneal erosion syndrome, BRVO=branch retinal vein occlusion, CRVO=central retinal vein occlusion, NAION=non-arteritic ischaemic optic neuropathy, OHT=ocular hypertension, POAG=primary open-angle glaucoma, ACG=angle-closure glaucoma
Despite over 20 years of laser therapy, diabetic retinopathy remains the leading cause of blindness in people of working age. 2 8% of those registered legally blind (vision < 3/60 in the better eye) have diabetes. A much larger number suffer ocular morbidity from retinopathy and are visually impaired or handicapped. Diabetics are twentynine times more likely to be blind than non-diabetics of a similar age. Retinopathy is the commonest complication of diabetes and up to 10% of diabetics at any one time will have sight-threatening retinopathy requiring specialist ophthalmological management. 3
DIABETIC RETINOPATHY
Risk factors
In the Wisconsin Epidemiology Study of Diabetic Retinopathy, the younger-onset patients showed a higher frequency of proliferative diabetic retinopathy (PDR) in males than in females but in the older-onset patients there were no sex differences in prevalence, incidence, or rates of progression to PDR. 4 A high prevalence is reported in Asians and Afro-Caribbeans in the UK, 5 with diagnosis often delayed. In the Newcastle study, 6 about 18% of south-Asians aged 25-74 were found to have diabetes and an additional 19% had impaired glucose tolerance.
The prevalence and severity of diabetic retinopathy increase with age and duration of diabetes. The condition is infrequent before the age of 13 years irrespective of the duration of diabetes. After diagnosis of diabetes, retinopathy is more frequent in the older-onset group. Indeed, 20% of type 2 diabetics have retinopathy at presentation, whereas those with type 1 diabetes can expect to be free of clinically significant retinopathy for the first 5 years. After 10-15 years, 25-50% of type 1 patients show some signs of retinopathy. This prevalence increases to 75-95% after 15 years and approaches 100% after 30 years. Proliferative disease is rare in the first decade from diagnosis but increases to 14-17% by 15 years and 50% after 25 years.
Of patients with type 2 diabetes, 23% have nonproliferative disease after 11-13 years, 41% after 14-16 years and 60% after 16 years. PDR can be found in 3% of patients 11 or more years after the diagnosis. Type 2 patients tend to develop maculopathy first, but many go on to develop proliferative retinopathy later. 7
Up to 10% of all patients with diabetes develop macular oedema during their lifetime and in nearly half of these the central fovea is affected. The likelihood depends upon the severity of retinopathy and the duration and type of diabetes. Only 3% of patients with mild non-proliferative disease have macular oedema, but the prevalence reaches 40% in those with moderate to severe disease and 70% in those with PDR.
Macular oedema is rare in patients with type 1 diabetes of less than 8 years' duration and the prevalence after 20 years is 25-30%. In type 2 patients receiving insulin the prevalence is 10% after 10 years and 30-35% after 20 years; in those not on insulin the prevalence is almost half the above.
The Diabetes Control and Complications Trial8 compared intensive glycaemic control with conventional insulin treatment in type 1 diabetes. Patients on the intensive regimen showed a lower incidence of retinopathy, less progression to preproliferative and proliferative retinopathy, less macular oedema and less need for panretinal photocoagulation. The UK Prospective Diabetes Study (UKPDS) showed similar advantages for intensive control in type 2 diabetes. 9 Data from various epidemiological studies suggest a positive relation between prevalence of retinopathy and hypertension. Increased blood pressure, through an effect on blood flow, has been hypothesized to damage the retinal capillary endothelial cells, resulting in development and progression of retinopathy. The UKPDS showed that beta-blockers and angiotensin converting enzyme inhibitors were equally effective in reducing the risk of progression of retinopathy in newly diagnosed persons with type 2 diabetes. Data from most studies suggest an association between the prevalence of diabetic nephropathy, as manifest by microalbuminuria or gross proteinuria, and retinopathy. 10 Although smoking causes tissue hypoxia by increasing blood carbon monoxide levels and can promote platelet aggregation, most epidemiological data show no relation between cigarette smoking and diabetic retinopathy. Pregnancy is a significant predictor of progression of diabetic retinopathy, and women with type 1 diabetes who get severe retinopathy in pregnancy are at excess risk of giving birth to a child with congenital abnormalities. 11
Physical activity, socioeconomic status, body mass index and consumption of alcohol are some of the other risk factors with a bearing on the incidence and progression of diabetic retinopathy.
Clinical features (see Figures 2,3,4,5)
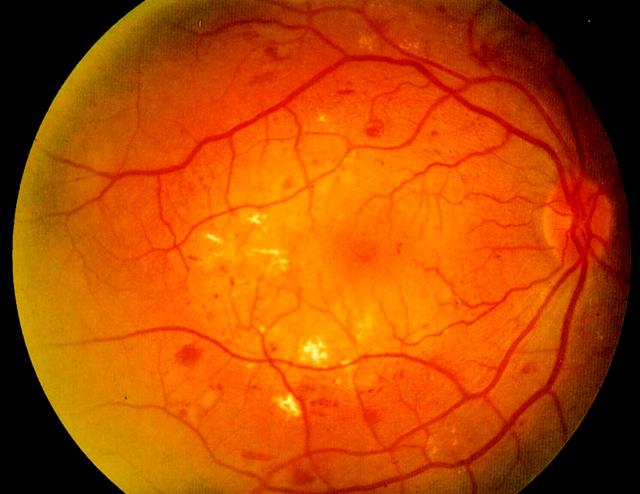
Figure 2.
Sight-threatening maculopathy with normal acuity. Note the microaneurysms nasal to the fovea, the dot and blot haemorrhages, and the hard exudates in relation to the microvascular abnormalities. Colour version available on [www.jrsm.org]
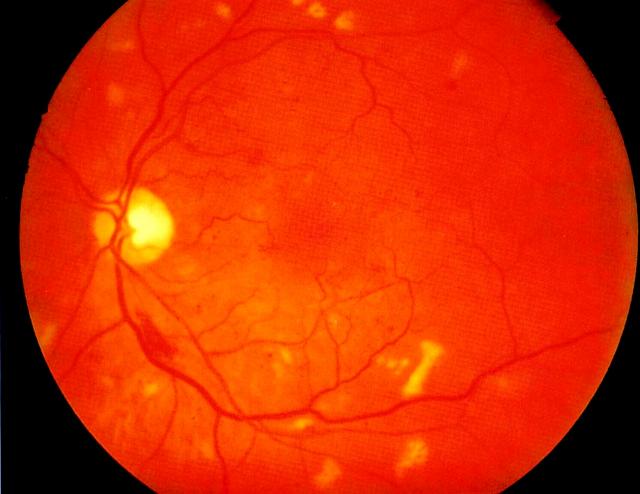
Figure 3.
Preproliferative retinopathy with multiple cotton-wool spots and a flame-shaped haemorrhage below the disc. Normal acuity. Colour version available on [www.jrsm.org]
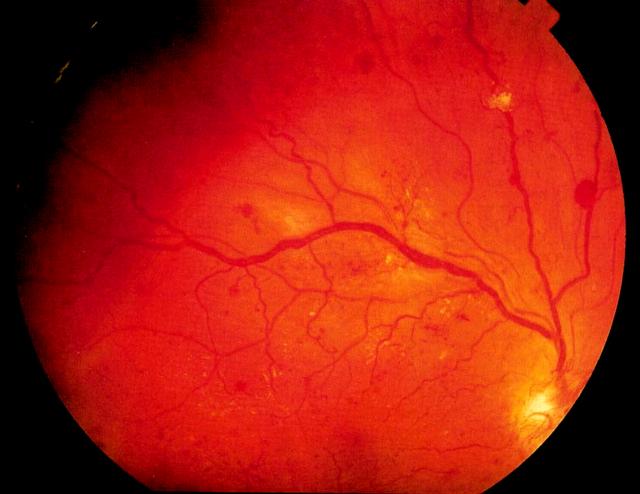
Figure 4.
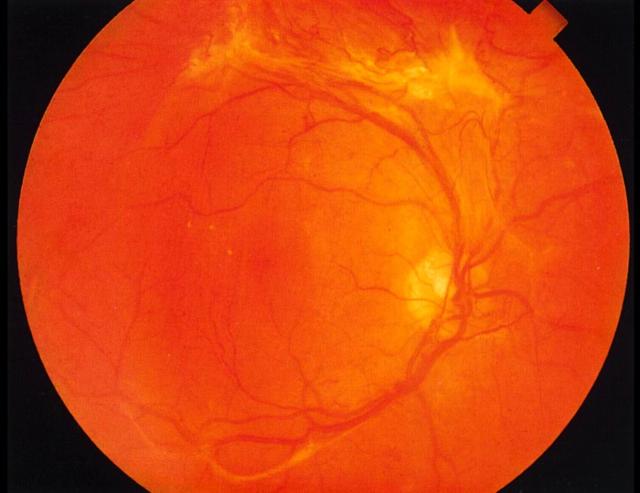
Proliferative retinopathy. A gliotic neovascular tuft is seen arising from the vein running directly upwards from the disc. There is severe beading of the superotemporal vein (this sign is often followed by rapid progression to proliferation). Disc new vessels are also present. Colour version available on [www.jrsm.org]
Figure 5.
Proliferative retinopathy with disc vessels, gliosis and traction retinal detachment superiorly. The acuity is still 6/6 in this patient, who presented with a few floaters. Colour version available on [www.jrsm.org]
The clinical signs of diabetic retinopathy are divided into background, preproliferative and proliferative stages (Table 1).
Table 1.
Classification of diabetic retinopathy
| Stage | Clinical features |
|---|---|
| Non-proliferative | |
| Background | Microaneurysms (dots) |
| Intraretinal haemorrhages (blots) | |
| Retinal vasodilation | |
| Hard exudates | |
| Nerve fibre layer infarcts (cotton-wool spots) | |
| Preproliferative | Venous beading and looping |
| Intraretinal microvascular abnormality, ‘white lines’ | |
| Proliferative | Retinal signs: neovascularization of optic disc, retina, and/or iris |
| Vitreous signs: vitreous cells, contraction, and opacification of posterior hyaloid face, partial posterior vitreous detachment with epiretinal membranes and tractional retinal detachments | |
| Maculopathy | |
| Ischaemic | Clinically extensive blot haemorrhages in the macula, and on fluorescein angiography evidence of enlarged foveal avascular zone and destruction of its outline |
| Oedematous* | Focal: retinal thickening at or within 500 μm of the centre of the fovea |
| Diffuse: hard exudates within 500 μm of the centre of the fovea with thickening of the adjacent retina; retinal thickening more than 1 disc area any part of which is within 1 disc diameter from the centre of the fovea |
Clinically significant macular oedema
Background diabetic retinopathy (mild non-proliferative diabetic retinopathy)
Early circulatory changes Retinal vasodilation, one of the earliest findings in diabetic retinopathy, possibly results from the loss of capillary pericytes and thickening of the capillary basement membrane. 12
Microaneurysms Retinal microaneurysms are usually the first sign of diabetic retinopathy and are located within the inner nuclear layer in capillaries linking the superficial and deep capillary network. They range in diameter from 12 to 100 μm but only those greater than 30 μm in diameter can be seen ophthalmoscopically.
Intraretinal haemorrhages Ruptured microaneurysms, capillaries and venules are all sources of intraretinal haemorrhages, which are mostly located within the outer plexiform and inner nuclear layers. Deep haemorrhages are dots and blots while superficial ones in the nerve fibre layer are flame-shaped. A predominance of flame-shaped haemorrhages points to the presence of concomitant venous obstruction or arterial hypertension.
Hard exudates These are extracellular collections of lipid within the outer plexiform layer, derived from leakage of serum from the abnormal vessels. They are yellowish and vary in size. They may be confluent or arranged in a circinate pattern around a cluster of microvascular abnormalities. Scattered hard exudates often come and go, but continued focal leakage will result in plaques that can cause permanent functional impairment if located on the fovea.
Cotton-wool spots (soft exudates) Cotton-wool spots result from focal abnormalities of axoplasmic transport in the nerve fibre layer from localized capillary ischaemia and appear as superficial, white, fluffy edged patches. Large numbers of them may signify the presence of arterial hypertension.
Preproliferative diabetic retinopathy (moderate and severe non-proliferative diabetic retinopathy)
This is the intermediate stage between background and proliferative retinopathy. Signs of increasing ischaemia develop, including arterial thinning and occlusion (seen as white lines in the retina) or more commonly, venous beading and looping, and intraretinal microvascular abnormalities.
Proliferative diabetic retinopathy
Neovascularization originating from the disc, retina or iris is the hallmark of proliferative retinopathy. New vessels can grow across the surface of the retina or on the posterior vitreous face, when they may be associated with fibrous tissue seen as gliotic bundles. Neovascular fronds can result in preretinal or vitreous haemorrhages. Preretinal haemorrhage is seen as localised boat-shaped haemorrhage between the posterior hyaloid face and the internal limiting membrane of the retina. In the presence of a detached vitreous (as occurs in many elderly people) subsequent new vessel growth is limited to focal raspberry-like lesions without fibrosis. Small haemorrhages into the vitreous cause ‘floaters’, whereas more extensive vitreous haemorrhage can cause sudden and painless loss of vision. Advanced proliferative disease is characterized by fibrovascular proliferation and contraction that can lead to continued vitreous haemorrhage and tractional retinal detachment. Rubeosis iridis (see below) and secondary glaucoma complete the picture of advanced disease.
Although sometimes unilateral, proliferative diabetic retinopathy nearly always becomes bilateral in due course. 13
OTHER VITREO-RETINAL ABNORMALITIES
Central and branch retinal vein occlusions are over-represented in diabetes and must be distinguished clinically from the various stages of retinopathy. In both, the deterioration of vision is more sudden. Among the predisposing causes to be excluded are hypertension, hyperlipidaemia and hyperviscosity syndromes. These occlusions can lead to retinal ischaemia and retinal and optic disc neovascularization. Lipaemia retinalis is a rare condition associated with severe hypertriglyceridaemia and tends to clear with reduction of serum lipids.
NON-RETINAL OCULAR ABNORMALITIES
Lids and conjunctiva
Recurrent styes and blepharoconjunctivitis are sometimes the first indications of diabetes and should prompt tests to exclude it. Xanthelasma, a fatty deposit in the subcutaneous tissue of the lids, is commonly seen in diabetics, especially with associated hyperlipidaemia.
Cornea
Corneal sensitivity is commonly impaired in diabetes, and is inversely related to the severity of retinopathy. 14 This sensory deficit may predispose to bacterial corneal ulcers, neurotropic ulcers and difficulties with contact lenses; indeed, diabetic patients who wear contact lenses must take extra care with lens hygiene and be warned to seek advice early if trouble develops. Intrinsic abnormalities of the epithelial basement membrane complexes, with impaired barrier function, lead to superficial punctate keratitis and poor healing after trauma and the formation of persistent epithelial defects. 15 Epithelial defects and recurrent corneal erosions can develop after photocoagulation and vitrectomy. 16 The treatment in most cases is topical lubricants; in some cases bandage contact lenses are required.
Glaucoma
Primary open-angle glaucoma
Diabetic patients have higher intraocular pressures than the normal population, especially those treated with insulin, 17 but evidence of an association between primary open-angle glaucoma and diabetes is conflicting. Case-control studies show relative risks of 1.6 to 4.7 in diabetes. 18,19 The Baltimore Eye Survey found little evidence of an association between glaucoma and diabetes, 20 whereas the Blue Mountains study was more positive. Two important considerations regarding intraocular pressure in diabetes are as follows: first, the patients are at excess risk of visual field loss and this happens at relatively low pressures;21 second, use of topical beta-blockers (e.g. timolol maleate) to lower the pressure can mask hypoglycaemic symptoms and reduce glucose tolerance.
Narrow-angle glaucoma
Patients with narrow-angle glaucoma are more likely to have abnormal glucose tolerance than either those with primary open-angle glaucoma or healthy controls, and there is an excess prevalence of type 2 diabetes.22 This seems to be a consequence of the abnormally large lens in diabetes. Also, in hyperglycaemia the lens can swell rapidly, precipitating angle-closure glaucoma. In diabetic patients glycerol should not be used as hyperosmotic agent during an acute attack since it is metabolized to glucose.
Iris neovascularization (rubeosis iridis) (Figure 6)
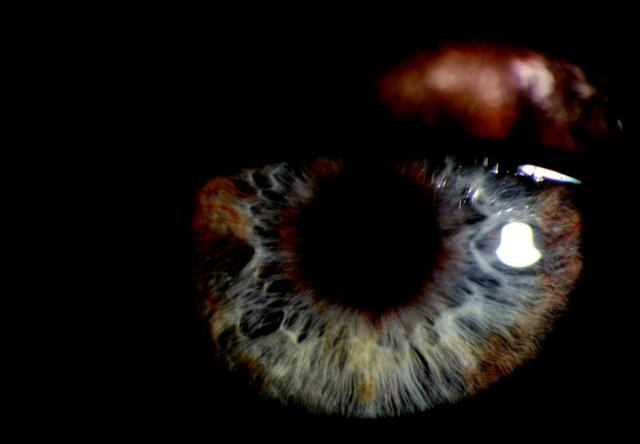
Figure 6.
Iris rubeosis. Note new vessels around the pupil margin and in the iris periphery. Cornea is clear but intraocular pressure was raised. Colour version available on [www.jrsm.org]
Proliferative diabetic retinopathy remains a leading cause of neovascular glaucoma, second only to central retinal vein occlusion. Profound retinal ischaemia seems to stimulate production of vascular endothelial growth factor, which diffuses into the anterior segment of the eye and causes neovascularization of the iris. Early neovascularization appears as small tufts of blood vessels along the pupillary margin or in the anterior chamber angle. It may cause glaucoma even without secondary closure, through exudation of plasma proteins into the aqueous.
The treatment of established neovascular glaucoma is difficult. Usually, extensive panretinal photocoagulation is required to induce neovascular regression. This may be accomplished by conventional laser, vitrectomy with endo-laser, or retinal treatment through the sclera by diode laser/cryotherapy. Filtering glaucoma surgery with antimetabolite (5-fluorouracil or mitomycin-C) enhancement or the insertion of drainage tubes into the eye can sometimes control the intraocular pressure. Diode laser cyclophotocoagulation, to reduce aqueous production, is gaining popularity.23
Pupil abnormalities
Some diabetic patients have small pupils in dim illumination which dilate poorly to mydriatic agents. The cause is an autonomic neuropathy partially denervating both the sphincter and the dilator muscles.
Lens
Refractive error
Fluctuating myopia can be a presenting sign in diabetes. Collection of the sugar alcohol sorbitol in the lens, due to increased aldose reductase activity, causes the lens to swell and change its refractive power. In newly diagnosed diabetes and when therapy is changed, patients should be advised not to get new spectacles until the blood glucose has stabilized.
Cataract formation
Data from the Framingham and other eye studies indicate a three to fourfold increased prevalence of cataract in patients with diabetes under 65, and up to a twofold excess prevalence in patients above 65.24,25 The risk is increased with the duration of diabetes and in those with poor metabolic control. A special type of cataract known as snowflake cataract is seen in young type 1 patients and tends to progress rapidly. Cataracts may be reversible in young diabetics with improvement in metabolic control.
The most frequently seen type of cataract in diabetics is the age-related or ‘senile’ variety, which tends to occur earlier and progresses more rapidly than in nondiabetics. Common early symptoms are frequent changes of spectacles, glare and dazzle, and monocular diplopia or polyopia (double or multiple images in one eye).
Cataract surgery in diabetics
There has been a recent shift in emphasis towards earlier cataract extraction in diabetics. Pre-existing macular oedema is the major risk factor for poor visual outcome after cataract surgery,26 but the surgery may cause a rapid acceleration of retinopathy and induce rubeosis.27 Cataract surgery is advisable before lens opacity precludes detailed fundus examination, and pre-existing maculopathy should be adequately treated with focal laser beforehand.
Phacomulsification results in less postoperative inflammation and astigmatism, more rapid visual rehabilitation and, with modern foldable lenses, a lower incidence of capsulotomy than with the outdated extracapsular surgery, and is now the preferred technique in most types of cataract. However, no difference in retinopathy progression between the two techniques has been reported.28
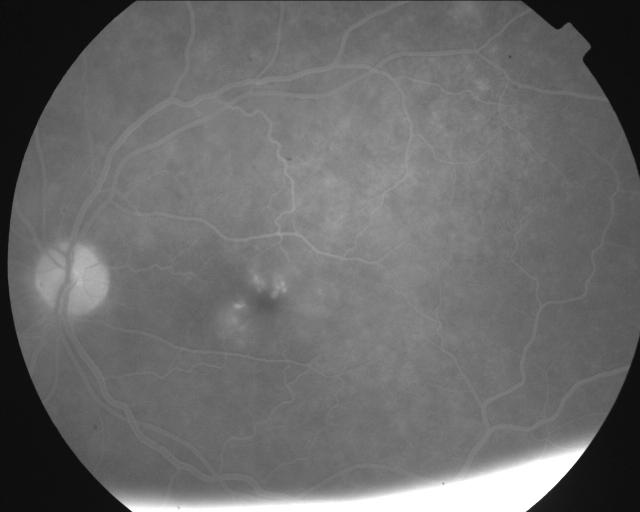
Cystoid macular oedema is a sight-threatening complication occurring after cataract surgery and is related to postoperative inflammation. Diabetics with or without evidence of diabetic retinopathy demonstrate a compromised blood aqueous barrier leading to an increased risk of postoperative inflammation. Other factors influencing the amount of postoperative inflammation and incidence of clinical and angiographic cystoid macular oedema (Figure 7) are duration of surgery, wound size and posterior capsular rupture or vitreous loss.
Figure 7.
Oral fluorescein digitally enhanced angiographic image of cystoid macular oedema
Careful postoperative follow-up is essential after cataract surgery not only to check for development of cystoid macular oedema but also to make sure there has been no acceleration of diabetic retinopathy. Regarding the operation itself, the continuous curvilinear capsulorrhexis technique performed in most phacoemulsification cases provides many advantages over previous capsulotomy techniques. One disadvantage is anterior capsular contraction29 and opacification that can hamper adequate retinal evaluation and retinal laser treatment.
Optic nerve abnormalities
Acute disc oedema
Acute disc oedema (diabetic papillopathy) affects patients in their second to fourth decades. It is often bilateral and usually causes no symptoms.30 On routine ophthalmoscopic examination it is characterized by diffuse oedema or severe disc swelling with soft exudates, cystoid macular oedema and occasionally macular star formation. There is no correlation with the severity of diabetic retinopathy but individuals require close observation since rapid progression to proliferative retinopathy may ensue. Distinction of this condition from papilloedema is important.
Optic atrophy (Wolfram's syndrome)
Wolfram's syndrome is a complication of diabetes mellitus associated with progressive optic atrophy and multiple other neurological and systemic abnormalities. Patients show neurosensory hearing loss and some also have diabetes insipidus, anosmia and gonadal dysfunction.31 It is autosomal recessive or sporadic in inheritance.
Non-arteritic ischaemic optic neuropathy
This condition causes acute visual loss in older patients with diabetes and must be distinguished from the arteritic variety, in which the optic disc appearance is similar with pale swelling (sometimes sectoral). Pointers to the arteritic type are a history of typical symptoms of inflammatory vasculopathy and the finding of tender thickened temporal arteries.
Optic atrophy
The causes of optic atrophy in diabetic patients include previous diabetic papillopathy, previous non-arteritic ischaemic optic neuropathy, multiple nerve fibre layer infarcts and extensive pan-retinal photocoagulation.32
Visual field abnormality
Common reasons for a visual field abnormality in diabetes are cerebrovascular accident, coexisting glaucoma and pan-retinal photocoagulation. All patients undergoing pan-retinal photocoagulation must be warned about the potential for significant visual field loss. Small-burn laser therapy with increased burn numbers minimizes the effect on the binocular driving visual field test demanded by the Driver and Vehicle Licensing Authority.33 Macular laser treatment, delivered in a grid fashion, does not significantly affect the DVLA field test.34
Other cranial nerve abnormalities
In 25-30% patients above the age of 45 years with acute extraocular muscle palsy, diabetes is the underlying cause. Isolated third, fourth and sixth nerve palsies are due to small vessel occlusion. Third nerve palsies may be painful in up to 20% cases and the pupil is spared in 80%. If the pupil is involved (20%), the effect is usually subtle with an anisocoria of <1 mm rather than the fully dilated unreactive pupil found in compressive lesions.35 Most patients recover after weeks or months but the condition may recur. Simultaneous involvement of two or more nerves is rare and so is the presence of other focal neurological signs, progressive deterioration or lack of complete recovery in three months. The presence of any of the above,or a palsy at age <45 years should be investigated to exclude a compressive lesion.
References
- 1.Chief Medical Officer. Annual Report of the Chief Medical Officer of the Department of Health for the Year 1997. London: Stationery Office, 1998
- 2.Evans J. Causes of Blindness and Partial Sight in England and Wales 1990-91. London: HMSO
- 3.McLeod BK, Thompson JR, Rosenthal AR. The prevalence of retinopathy in the insulin-requiring diabetic patients of an English country town. Eye 1988;2: 424-30 [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102: 527-32 [DOI] [PubMed] [Google Scholar]
- 5.Hawthorne K, Mello M, Tomlinson S. Cultural and religious influences in diabetes care in Great Britain. Diabet Med 1993;10: 8-12 [DOI] [PubMed] [Google Scholar]
- 6.Unwin N, Alberti KGMM, Bhopal R, Harland J, Watson W, White M. Comparison of the current WHO and new ADA criteria for the diagnosis of diabetes mellitus in three ethnic groups in the UK. Diabet Med 1998;15: 554-7 [DOI] [PubMed] [Google Scholar]
- 7.Tong L, Vernon SA, Kiel W, Sung V, Orr GM. Association of macular involvement with proliferative retinopathy in type 2 diabetes. Diabet Med 2001;18: 388-94 [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329: 977-86 [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risks of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352: 837-53 [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: V. Proteinuria and retinopathy in a population of diabetic persons diagnosed prior to 30 years of age. In: Friedman EA, L'Eserance Jr FA, eds. Diabetic Renal-syndrome, vol. 3. Orlando: Grune & Stratton, 1986
- 11.Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol 1982;93: 745-56 [DOI] [PubMed] [Google Scholar]
- 12.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns: IV. Diabetic retinopathy. Arch Ophthalmol 1961;66: 366 78 [DOI] [PubMed] [Google Scholar]
- 13.Valone JA, McNeel JW, Franks EP. Unilateral proliferative diabetic retinopathy. II. Clinical course. Arch Ophthalmol 1981;99: 1362-6 [DOI] [PubMed] [Google Scholar]
- 14.Rogell GD. Corneal hypesthesia and retinopathy in diabetes mellitus. Ophthalmology 1980;87: 229-33 [DOI] [PubMed] [Google Scholar]
- 15.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol 1977;95: 2193-6 [DOI] [PubMed] [Google Scholar]
- 16.Arentsen J, Tasman W. Using a bandage contact lens to prevent recurrent corneal erosion during photocoagulation in patients with diabetes mellitus. Am J Ophthalmol 1981;92: 714-16 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell P, Smith W, Chey T, IIealey PR. Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology 1997;104: 712-18 [DOI] [PubMed] [Google Scholar]
- 18.Morgan RW, Drance SM. Chronic open angle glaucoma and ocular hypertension: an epidemiological study. Br J Ophthalmol 1975;59: 211-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson MR, Hertzmark E, Walker AM, et al. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol 1987;105: 1066-71 [DOI] [PubMed] [Google Scholar]
- 20.Tielsch JM, Katz J, Quigley HA, et al. Diabetes, intraocular pressure and primary open angle glaucoma in the Baltimore Eye Survey. Ophthalmology 1995;102: 48-53 [DOI] [PubMed] [Google Scholar]
- 21.Becker B. Diabetes mellitus and primary open angle glaucoma. Am J Ophthalmol 1971;71: 1-13 [DOI] [PubMed] [Google Scholar]
- 22.Mapstone R, Clark CV. Prevalence of type 2 diabetes in glaucoma. BMJ 1985;291: 93-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer AF, Vernon SA. ‘Cyclodiode’—results of a standard protocol. Br J Ophthalmol 1999;83: 311-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein BE, Klein R, Moss SE. Prevalence of cataract in a population-based study of persons with diabetes mellitus. Ophthalmology 1985;92: 1191-6 [DOI] [PubMed] [Google Scholar]
- 25.Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol 1981;91: 381-95 [DOI] [PubMed] [Google Scholar]
- 26.Chew EY, Benson WE, Remley NA, et al. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol 1999;117: 1600-6 [DOI] [PubMed] [Google Scholar]
- 27.Sadiq SA, Chatterjee A, Vernon SA. Progression of diabetic retinopathy and rubeotic glaucoma following cataract surgery. Eye 1995;9: 728-32 [DOI] [PubMed] [Google Scholar]
- 28.Dowler JG, Hykin PG, Hamilton AM. Phacoemulsificatio'n versus extracapsular cataract surgery in diabetes. Ophthalmology 2000;107: 457-62 [DOI] [PubMed] [Google Scholar]
- 29.Kato S, Oshika T, Numaga J, et al. Anterior capsular contraction after cataract surgery in eyes of diabetic patients. Br J Ophthalmol 2001;85: 21-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson WE, Brown GC, Tasman W. Diabetes and Ocular Complications. Philadelphia: WB Saunders, 1988: 116-17
- 31.Lessell S, Rosman NP. Juvenile diabetes mellitus and optic atrophy. Arch Neurol 1977;34: 759-66 [DOI] [PubMed] [Google Scholar]
- 32.Sadun AA. Neuro-ophthalmic manifestations of diabetes. Ophthalmology 1999;106: 1047-8 [DOI] [PubMed] [Google Scholar]
- 33.Hulbert MF, Vernon SA. Passing the DVLC field regulations following bilateral pan-retinal photocoagulation in diabetics. Eye 1992;6: 456-60 [DOI] [PubMed] [Google Scholar]
- 34.Tong L, Vernon SA. Passing the DVLA driving field regulations following macular photocoagulation. Eye 2000;14: 35-8 [DOI] [PubMed] [Google Scholar]
- 35.Jacobson DM. Pupil involvement in patients with diabetes-associated oculomotor nerve palsy. Arch Ophthalmol 1998;116: 723-7 [DOI] [PubMed] [Google Scholar]