ABSTRACT
Objective:
This systematic review sought to identify the association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and function in people with chronic obstructive pulmonary disease (COPD).
Data sources:
We searched electronic databases including PubMed, CINAHL, MEDLINE, EMBASE, The Cochrane Library, ProQuest Dissertations and Theses, Scopus, Google Scholar, Trove, and WHO International Clinical Trials Registry Platform and reference lists of retrieved articles published prior to August 2014.
Inclusion criteria:
We considered observational studies that evaluated dietary intake of omega-3 (eicosapentaenoic acid, docosahexaenoic acid or α-linolenic acid) and/or omega-6 fatty acids (γ-linoleic acid or arachidonic acid), and experimental studies that evaluated omega-3 fatty acid supplementation (containing predominantly one or more omega-3 fatty acids) on airway and systemic inflammatory markers and/or functional capacity outcomes in people with COPD-related diagnoses.
Data synthesis:
Since statistical pooling was not possible, the findings were presented in narrative form including tables and figures to aid in data presentation when appropriate.
Results:
One 8-week randomized controlled trial conducted in 80 COPD patients in the Netherlands showed polyunsaturated fatty acid supplementation significantly improved exercise capacity compared with the control condition [between-group difference in mean peak workload was 9.7 W (2.5–17.0; P = 0.009); and mean duration was 4.3 min (0.6–7.9; P = 0.023)]. One cross-sectional study conducted in 250 COPD patients in Spain found associations of specific dietary omega-3 fatty acids with inflammation were inconsistent.
Conclusions:
Limited evidence provides weak support for the use of omega-3 fatty acid supplementation for reducing chronic inflammation and some support for improving functional capacity in COPD patients. There is no consistent evidence showing that low dietary intake of specific omega-3 fatty acids worsens inflammation and/or function. More evidence is required before routinely incorporating this therapy within COPD management plans.
Keywords: chronic obstructive pulmonary disease, diet, fatty acids, omega-3, omega-6, supplement
Introduction
Chronic obstructive pulmonary disease (COPD) is currently ranked the fifth leading cause of global disability (health loss).1 The Burden of Obstructive Lung Disease (BOLD) study estimates that the prevalence of COPD, defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as stage 2 or higher, was 7.5% among Australian people aged 40 years or older in 2006–2010.2 The prevalence of COPD was highest among people aged 75 years or older.2 A recent report by Access Economics estimates that COPD cost the Australian economy about $98 billion in 2008.3 Since Australia's population is ageing, the projected rise in the health burden of COPD will undoubtedly cause significant stress on the national healthcare system. Effective treatments to slow the progression of COPD and/or prevent exacerbations will likely result in significant health and economic benefits.
Chronic obstructive pulmonary disease is characterized by persistent airflow limitation that is usually progressive and associated with chronic inflammation in the airways and lungs from exposure to noxious particles or gases.4 Prolonged exposure to noxious particles and gases is likely to bring about a chronic (innate and adaptive) inflammatory response in COPD patients which increases production and accumulation of mucus, infiltration of the airway walls by inflammatory cells, thickening of the airway wall and narrowing of the lumen.5 The progression of COPD (i.e. an increase in the severity of airflow limitation by the GOLD grading system) is positively associated with the degree of the inflammatory response,6 and the narrowing and disappearance of the small conducting airways.7 In addition, prospective cohort studies have shown that airway and systemic inflammation, independent of smoking, increases the risk of worsening lung function and exacerbations, which, in turn, are associated with reduced survival.8 Another cohort study observed decreases in airway and systemic inflammation in COPD patients hospitalized for acute exacerbation at the time of discharge (i.e. after treatment and recovery).9 Although there is considerable uncertainty about the origin and role of inflammation in COPD, this body of evidence suggests that new therapies for reducing the chronic inflammatory response might improve COPD prognosis.
Low dietary intake of omega-3 fatty acids may be a novel reversible risk factor for chronic inflammation, and subsequent functional decline, in COPD patients, through a variety of mechanisms, including cell surface and intracellular receptors, that control inflammatory cell signalling and gene expression.10 The major food sources of omega-3 fatty acids – docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) – are fish, fish oils and animal tissues (brain), whereas some plant oils contain α-linolenic acid (ALA).11 Omega-6 fatty acids are predominately derived from plant oils, although small amounts of animal tissues contain γ-linoleic acid (GLA) and arachidonic acid.11 Omega-3 and omega-6 fatty acids have competitive (inhibiting) interactions that are important in modulating inflammation.12 Epidemiological evidence (cross-sectional and prospective cohort studies) consistently shows an inverse association between dietary intake of omega-3 fatty acids and systemic inflammation across different populations including people with COPD.13–16 Moreover, the most recent systematic review and meta-analysis of relevant randomized controlled trials (RCTs) show that short-term marine-derived omega-3 supplementation significantly reduces systemic inflammatory biomarkers including C-reactive protein (CRP), interleukin 6 (IL-6) and tumour necrosis factor α (TNF-α) in different population groups.17 The effectiveness of omega-3 supplementation for lowering inflammatory markers was most evident in patients with cardiovascular risk factors, which are highly prevalent in COPD patients.18 In contrast, two cross-sectional studies on omega-6 fatty acids reported opposing associations between dietary intake of arachidonic acid and systemic inflammation.13,16
Although the systematic review by Li et al.17 is an excellent synthesis of evidence on effectiveness, none of the studies reviewed focussed on COPD patients. Furthermore, the equivocal results reported in one RCT raise questions about whether a reduction in chronic inflammation with polyunsaturated fatty acid (PUFA) supplementation is actually beneficial in patients with COPD.19 For instance, a cross-sectional study found no evidence of benefit and several adverse associations between dietary intake of individual omega-3 and omega-6 fatty acids and airflow limitation in a regionally representative sample of Dutch adults.20 To our knowledge, there is no completed or in-progress systematic review of evidence on this topic. We therefore sought to systematically assess relevant studies on the association of dietary intake and supplementation of specific PUFAs with inflammatory markers and functional capacity outcomes in COPD to inform guidelines and clinical practice.
Objectives
This review sought to identify the association of dietary intake and supplementation of specific PUFAs with chronic inflammation and functional capacity outcomes in people with COPD. More specifically, the objectives were the following:
To identify the association of low dietary intake of omega-3 fatty acids (DHA, EPA or ALA) and/or omega-6 (GLA or arachidonic acid) fatty acids with chronic inflammation and functional capacity outcomes in people with COPD compared with controls (high dietary intake reference group).
To identify the effectiveness of omega-3 fatty acid supplementation (containing predominantly one or more omega-3 fatty acids) in reducing chronic inflammation and improving functional capacity outcomes compared with placebo/no intervention in people with COPD.
Protocol
The Joanna Briggs Institute (JBI) protocol21 has been registered with PROSPERO, the International Prospective Register of Systematic Reviews hosted by the Centre for Reviews and Dissemination (Registration Number is CRD42015015847).
Inclusion criteria
Types of participants
This review considered studies that included people with chronic lung disease within the spectrum of COPD-related diagnoses (i.e. emphysema or chronic bronchitis).
Types of intervention(s)/phenomena of interest
This review considered observational studies that evaluate dietary intake of omega-3 (EPA, DHA or ALA) and/or omega-6 fatty acids (GLA or AA), and experimental studies that evaluate omega-3 fatty acid supplementation (containing predominantly one or more omega-3 fatty acids).
Types of studies
This review considered both experimental and non-experimental (observational) study designs including RCTs, non-RCTs, quasi-experimental, before and after studies, prospective and retrospective cohort studies, case-control studies and cross-sectional studies for inclusion.
Types of outcomes
This review considered studies that included the following outcome measures: airway and systemic inflammatory markers and functional capacity outcomes. Inflammatory markers included, but were not limited to, CRP, TNF-α and interleukins. Functional capacity outcomes included, but were not limited to, exercise-based physical performance measures (e.g. muscle strength and endurance), and health status or health-related quality of life (HRQoL) measures.
Search strategy
The search strategy aimed to find both published and unpublished studies. A three-step search strategy was utilized in this review. An initial limited search of CINAHL was undertaken followed by an analysis of the text words contained in the title and abstract, and of the index terms used to describe the article. A second search was conducted independently by two reviewers using all identified keywords and index terms across all included databases. Thirdly, the reference list of all identified reports and articles was searched for additional studies. Studies published in English prior to August 2014 were considered for inclusion in this review. When possible, efforts were made to contact authors for information about unpublished trials. Details and examples of full electronic database search strategies appear in the appendix (Appendix I).
Assessment of methodological quality
Papers selected for retrieval were assessed by two independent reviewers for methodological quality using the standardized critical appraisal instruments from the JBI Meta Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI).22 Any disagreements that arose between the reviewers were resolved through discussion.
Data collection
Data were extracted from papers included in the review independently by two reviewers using the standardized data extraction tools from JBI-MAStARI.22 The data extracted included specific details about the interventions, populations, study methods and outcomes.
Data synthesis
Since statistical pooling was not possible because of the diverse types of studies reviewed, the findings were presented in narrative form, including tables to aid in data presentation, when appropriate.
Results
The search strategy identified 187 citations after duplicates were removed (Fig. 1). Nine potentially eligible citations were identified after the first screening of titles and/or abstracts. After further screening, seven citations were excluded resulting in two eligible studies for review (Appendix II).
Figure 1.
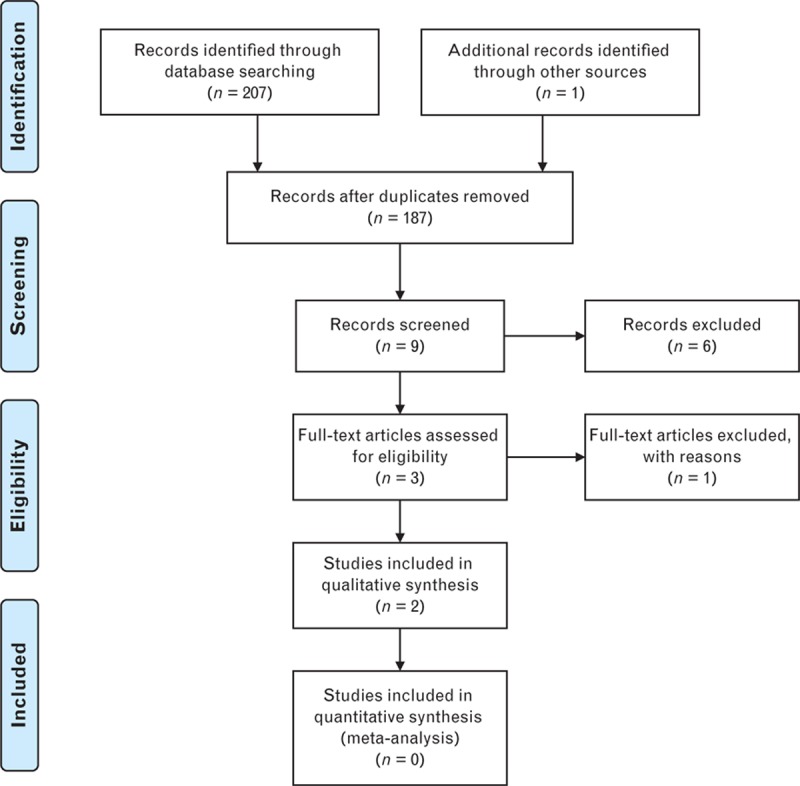
Flowchart summarizing identification of studies included for review.
Description of studies
Study characteristics of two studies included for review appear in Table 1. The RCT was conducted in 80 clinically stable COPD patients admitted to an inpatient pulmonary rehabilitation centre between 2000 and 2002 in the Netherlands (Asthma Center Hornerheide, Horn, the Netherlands).19 The RCT was conducted in predominately male patients (about 70%), with a mean age of 63 years and without malignancies, gastrointestinal or kidney abnormalities, metabolic or endocrine diseases, and traditional ‘inflammatory diseases’. The RCT investigated the effect of nine 1-g capsules daily, containing a total dose of 3.4 g PUFA (400 mg stearidonic acid; 760 mg GLA; 1200 mg ALA; 700 mg EPA; 340 mg DHA) on changes in exercise capacity, lung function and systemic inflammation outcomes at 8 weeks. All capsules were enriched with 3.5 mg/g vitamin E to ‘stabilize the oil and to serve as an antioxidant’. The placebo-controlled comparison group was administered nine 1-g capsules daily, containing 80% palm oil and 20% sunflower oil, and the same caloric (9 kcal/capsule) and vitamin E content as the PUFA capsules administered in the experimental group. The PUFA supplementation significantly improved exercise capacity compared with the control condition [between-group difference in mean peak workload was 9.7 W (2.5–17.0; P = 0.009); and mean duration was 4.3 min (0.6–7.9; P = 0.023)]. Between-group differences in other outcomes were not statistically significant.
Table 1. Study characteristics of the two studies reviewed.
| Study details | Study design/duration | Participant details | Experimental/exposure condition (group 1) | Control/non-exposure condition (group 2) | Outcomes (measures, units) | Study results | Author conclusions and reviewer's comments |
| Broekhuizen et al. (2005), the Netherlands | Randomized control trial (RCT), double-blind, placebo-controlled/8 weeks | Sample size: 80 | 9 times 1-g capsules daily, dose of 3.4 g PUFA (containing: 400 mg stearidonic acid; 760 mg GLA; 1200 mg ALA; 700 mg EPA; 340 mg DHA). All capsules were enriched with 3.5 mg/g vitamin E to ‘stabilize the oil and to serve as an antioxidant’; and PR including supervised endurance and strength training exercise. | 9 times 1-g capsules daily, containing 80% palm oil and 20% sunflower oil, and same caloric (9 kcal/capsule) and vitamin E content as the PUFA capsules; and PR including supervised endurance and strength training exercise. | Change in exercise capacity (peak workload in Watts and duration in minutes, bicycle ergometer). | Statistical methods: Linear regression with baseline value and intervention group predictors, associated P value | Author's conclusion: PUFA has beneficial effects on exercise capacity in patients with COPD. |
| Inclusion criteria: Clinically stable COPD (GOLD stage 2–4), admission to inpatient PR centre from 2000 to 2002. | N = 38/80. | N = 42/80. | Change in muscle strength (isokinetic quadriceps strength in Nm). | Between-group difference (95% CI): 9.7 W (2.5–17.0; P = 0.009); 4.3 min duration (0.6–7.9; P = 0.023), both favoured experimental group. | Reviewer's comments: This trial was well conducted, but lacks details about methods used for randomization and blinding. Limitations include analysis of study completers, use of placebo containing potentially pro-inflammatory supplements, and results may not be relevant to other populations (females, COPD patients with common comorbidities and other countries). | ||
| Exclusion criteria: Malignancies, gastrointestinal or kidney abnormalities, metabolic or endocrine diseases, and inflammatory diseases. | 24 patients ‘depleted’ or with recent ‘weight loss’ in group 1 also received 3.4 g daily liquid nutritional supplements, Respifor 375 ml (containing 3.4 g PUFA of 2.85 g LA; and 0.6 g ALA). | 24 patients ‘depleted’ or with recent ‘weight loss’ in group 2 also received 3.4 g daily liquid nutritional supplements, Respifor 375 ml. | Change in lung function (FEV1, FVC, spirometry). | Other outcomes not statistically significant. | |||
| Mean ± SD age, % sex at trial entry: group 1 64±10 years, male 71% (36/51); group 2 62±8 years, male 69% (35/51) | Change in systemic inflammation (plasma IL-6, TNF-α, hs-CRP). | ||||||
| Setting: Inpatient PR centre (Asthma Center Hornerheide, Horn, the Netherlands). | |||||||
| de Batlle et al. (2012), Spain | Observational study (cross-sectional) | Sample size: 250 | Above median for daily dietary intake of omega-3 fatty acids in past 2 years (DHA >0.42 g/day; EPA >0.21 g/day; ALA >1.22 g/day) and omega-6 fatty acids (LA >11.21 g/day; AA >0.18 g/day), and ratios (ALA/LA; EPA/AA; DHA/AA). | Below median for daily dietary intake of omega-3 and omega-6 fatty acids. | Above median (high) for systemic inflammatory markers: high TNF-α, high IL-6, high IL-8, high hs-CRP. | Statistical methods: Multivariate logistic regression to determine odds ratios for associations between PUFA and outcomes adjusted for covariates (BMI, smoking, caloric intake, other PUFAs or ratios), associated P value. | Author's conclusion: There is an association between dietary intake of omega-3 (negative) and omega-6 (positive) fatty acids and serum inflammatory markers in COPD patients. |
| Inclusion criteria: Confirmed COPD (post-bronchodilator FEV1/FVC ≤0.70, GOLD criterion), first hospital admission and clinically stable ≥3 months post-discharge between Jan 2004 and March 2006, available PUFA and outcome data. | N = 125/250. | N = 125/250. | Reviewer's comments: Although reasonable quality, the cross-sectional study design limits findings to associations only. Associations with specific omega-3 fatty acids were inconsistent, and DHA/AA was opposite from expected. Further limitations include recall bias, and results may not be relevant to other populations (female patients, recent or frequent exacerbation, and other countries). | ||||
| Exclusion criteria: None stated. | Odds ratios (95% CI): ALA 0.46 (0.21–0.99; P = 0.49); DHA/AA 3.02 (1.03–8.91; P = 0.045); AA 1.96 (1.05–3.64; P = 0.034); AA 1.95 (1.03–3.66; P = 0.039). | ||||||
| Mean ± SD age: 68 ± 8 years. | Other associations not statistically significant. | ||||||
| Sex: Male 94% (234/250) | |||||||
| Setting: Nine university hospitals, Spain. |
Notes: Sample sizes analyzed in the study by Broekhuizen et al. were smaller in the treatment (N = 38 or less) and control groups (N = 42 or less).
COPD, chronic obstructive pulmonary disease; LA, linoleic acid; PR, pulmonary rehabilitation; FEV1/FVC, forced expiratory volume in the first second to forced vital capacity ratio; hs-CRP, high-sensitivity C-reactive protein; PUFA, polyunsaturated fatty acid.
The cross-sectional study was conducted in 250 out of 342 COPD patients recruited during their first hospital admission at one of the nine university hospitals in Spain between January 2004 and March 2006. Patients had confirmed diagnosis of COPD, and were clinically stable for at least 3 months after discharge, and had dietary PUFA data and outcome data available.13 They were predominately male patients (94%), with a mean age of 68 years. The study investigated the associations of exposure to high daily dietary intake of specific PUFAs and of omega-3 to omega-6 PUFA ratios with measures of systematic inflammation, using levels of inflammatory markers [TNF-α, IL-6, IL-8 and high-sensitivity C-reactive protein (hs-CRP)]; the median values discriminating between high and low level systemic inflammation. Daily dietary PUFA intake was estimated from analysis of dietary habits over the past 2 years. The comparison non-exposure condition was low PUFA intake (below the median). The study reported the following associations: high ALA was associated with decreased odds of high TNF-α [odds ratio (OR) 0.46, 95% confidence interval (CI) 0.21–0.99, P = 0.49]; high DHA/arachidonic acid was associated with increased odds of high TNF-α (OR 3.02, 95% CI 1.03 to 8.91, P = 0.045), which is in the opposite direction to what is expected; high arachidonic acid was associated with increased odds of high IL-6 (OR 1.96, 95% CI 1.05–3.64, P = 0.034) and hs-CRP (OR 1.95, 95% CI 1.03–3.66, P = 0.039). Other associations were not statistically significant.
Methodological quality
The RCT was well-conducted and had 8 of the 10 quality items (Appendix III), but lacked details about methods used for randomization and blinding. The cross-sectional study was well conducted and had five of the six applicable quality items (Appendix III), but did not use a random sampling procedure.
Discussion
On the basis of limited evidence of effectiveness from one 8-week RCT [National Health and Medical Research Council (NHMRC) Level II]23 conducted in the Netherlands19 and aetiological evidence from one cross-sectional study (NHMRC Level IV) conducted in Spain,13 there is only weak support for the use of omega-3 fatty acid supplementation to reduce chronic inflammation and some support to improve functional capacity in COPD patients (NHMRC grade C). Specifically, there is no evidence showing that low dietary intake of specific omega-3 fatty acids worsens inflammation and/or function, nor evidence about the effectiveness of specific types and combinations of omega-3 fatty acids, appropriate dosing and duration of treatment required for improving inflammation and/or function. Thus, despite their excellent safety profile, wide availability, low cost and feasibility for implementation, recommendations for omega-3 fatty acid supplementation in patients with COPD should be applied with caution in country-specific healthcare settings.
The size of the effect (i.e. benefit) of mixed omega-3 fatty acids on exercise capacity observed in the RCT was large (increase from baseline was approximately 33%) and would likely be clinically relevant to COPD patients24 and their clinicians.25 However, the improvements in exercise capacity found in the RCT could not be attributed to a decrease in systemic inflammation, since no changes were observed in plasma IL-6, TNF-α or hs-CRP. This suggests that omega-3 fatty acids may improve exercise capacity through other physiological mechanisms including improvements in endothelial function and skeletal muscle metabolism. For example, a systematic review of RCTs conducted mostly in adults showed that supplementation of omega-3 fatty acids significantly improves endothelial function (assessed by flow-mediated dilation and endothelium-independent vasodilation) at a daily dose ranging from 0.45 to 4.5 g in the short term (over a median of 56 days).26 Previous research shows that endothelial function is a strong predictor of exercise capacity.27–29 In addition, supplementation of omega-3 fatty acids alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle, which may improve exercise capacity through improvements in metabolic efficiency during exercise.30
In contrast, the lack of effectiveness for reducing chronic inflammation is at odds with the positive findings in a previous systematic review, which showed that marine-derived omega-3 fatty acid supplementation significantly reduced CRP, IL-6 and TNF-α levels in other (non-COPD) population groups.17 It is possible that the daily dose of omega-3 fatty acids in the RCT19 reviewed was inadequate, since subgroup analysis in the systematic review revealed that significant reductions in hs-CRP and TNF-α were largest in the group of studies with dosing equal to or above the median daily dose of total omega-3 fatty acids and EPA.17
Alternatively, the anti-inflammatory effect/benefit of omega-3 fatty acid supplementation may have been exaggerated and attributable to the type of placebo used in the previous studies, resulting in bias. For instance, subgroup analysis in the previous systematic review revealed that the size of the effect of omega-3 fatty acids on hs-CRP was larger and statistically significant [weighted mean difference was −0.22 (−0.33 to −0.11) vs. 0.03 (−0.13 to 0.20)] only when linoleic acid (LA) (an omega-6 fatty acid) was the major fatty acid used for placebo rather than oleic acid (an omega-9 fatty acid).17 High (above median) daily dietary intake of LA was associated with increased crude odds of high hs-CRP (OR 1.67, 95% CI 1.02–2.76) in the cross-sectional study reviewed herein.13 Conversely, the RCT evidence reviewed herein suggests that LA supplementation (8 g of safflower oil daily for 16 weeks) effectively reduces serum CRP levels. Given the conflicting effectiveness evidence on inflammation, the degree of bias, if any, attributable to the use of LA as placebo is unclear.
Several limitations require further consideration. Limitations in the RCT reviewed herein include analysis of study completers, use of a placebo containing potentially pro-inflammatory supplements (palm oil)31,32 and that results may not be relevant to other COPD populations (patients with common comorbidities, and in other countries). Limitations of the cross-sectional study include recall bias and uncertainty about the applicability of the results to other COPD populations (female patients, patients with recent or frequent exacerbations and other countries). Finally, reviewer-level limitations include incomplete retrieval of information for several of the citations excluded, and the existence of other relevant studies not identified by the search strategy, potentially resulting in bias. However, this level of bias was likely minimal since the results and conclusions reported in the three citations excluded because complete information could not be retrieved, were conflicting.
Conclusion
Collectively, the results of this review provide weak support for the routine use of omega-3 fatty acid supplementation for reducing chronic inflammation, and some support for improving functional capacity in COPD patients. Furthermore, there was no consistent evidence showing that low dietary intake of specific omega-3 fatty acids worsens inflammation and/or function. The generation of new research evidence should focus on the effectiveness of specific types and combinations of omega-3 fatty acids, the appropriate doses and duration of treatment required to improve inflammation and/or function and on the risks or benefits of low dietary intake of specific PUFAs, with a view to informing healthcare policy makers, COPD patients and their clinicians.
Acknowledgements
The authors are grateful to Melissa Burley, Nursing and Midwifery School Librarian, and her team for their work on developing and conducting the electronic database searches.
Authors’ contributions: Authorship order is according to percentage contribution. E.A. is guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. E.A. conceived and designed the review, identified studies for inclusion, extracted and interpreted data and drafted the article. B.C. extracted and interpreted data, and revised the article. Both authors approved the final completed article.
Conflicts of interest
There are no conflicts of interest.
Appendix I: Search strategy
The databases searched were: PubMed, CINAHL, MEDLINE, EMBASE, The Cochrane Library, ProQuest Dissertations & Theses, Scopus, Google scholar, Trove, and WHO International Clinical Trials Registry Platform (ICTRP).
Search keywords were mapped to database headings where possible and included:
Omega-3 fatty acids
Omega-6 fatty acids
Long-chain polyunsaturated fatty acids (LCPUFA)
Polyunsaturated fatty acids (PUFA)
Essential fatty acids (EFA)
free fatty acids
DHA
docosahexaenoic acid (DHA)
eicosapentaenoic acid (EPA)
a-linolenic acid (ALA)
linoleic acid (LA)
arachidonic acid (AA)
fatty acids
fish
fish oil
egg oil
squid oil
krill oil
flaxseed oil
diet
nutrition
nutritional intervention
dietary modification
dietary intervention
dietary supplement
nutritional supplement
chronic inflammation
chronic lung disease
bronchitis
obstructive pulmonary
obstructive lung
airflow obstruction
Lung diseases, obstructive
chronic obstructive airway disease
COAD
COPD
C.O.P.D.
chronic obstructive pulmonary disease
Pulmonary Disease, Chronic Obstructive
emphysema
pulmonary emphysema.
Examples of full electronic database search strategies:
CINAHL:
’Omega-6 fatty acids’ OR ‘Omega-3 fatty acids’ OR ‘nutritional supplement’ OR (MH ‘Dietary Supplements’) OR ‘dietary intervention’ OR ‘dietary modification’ OR ‘nutritional intervention’ (MH ‘Nutrition’) OR (MH ‘Diet’) OR (MH ‘Linseed Oil’) OR ‘flaxseed oil’ OR ‘krill oil’ OR ‘squid oil’ OR ‘egg oil’ OR (MH ‘Fish’) OR ‘Fish’ OR (MH ‘Fish Oils’) OR (MH ‘Fatty Acids, Omega-6’) OR (MH ‘Fatty Acids, Omega-3’) OR (MH ‘Fatty Acids’) OR (MH ‘Arachidonic Acids’) OR ‘arachidonic acid’ OR (MH ‘Linoleic Acids’) OR ‘linoleic acid’ OR (MH ‘alpha-Linolenic Acid’) OR ‘a-linolenic acid’ (MH ‘Eicosapentaenoic Acid’) OR (MH ‘Docosahexaenoic Acids’) OR ‘docosahexaenoic acid’ OR LCPUFA OR PUFA OR EFA OR DHA OR ‘free fatty acids’ (MH ‘Fatty Acids, Essential’) OR ‘Essential fatty acids’ OR ‘Polyunsaturated fatty acids’ OR (MH ‘Fatty Acids, Unsaturated’) OR ‘Long-chain polyunsaturated fatty acids’
AND
bronchitis OR (MH ‘Bronchitis’) OR ‘obstructive pulmonary’ OR ‘obstructive lung’ OR ‘airflow obstruction∗’ OR (MH ‘Lung Diseases, Obstructive’) OR ‘chronic obstructive airway disease’ OR COAD OR COPD OR C.O.P.D. OR ‘chronic obstructive pulmonary disease’ OR (MH ‘Pulmonary Disease, Chronic Obstructive’) OR emphysema∗ OR ‘pulmonary emphysema’ OR (MH ‘Emphysema’)
(NB MH = CINAHL heading search)
MEDLINE via Ovid:
Omega-3 fatty acids.mp. or Fatty Acids, Omega-3/ OR Omega-6 fatty acids.mp. or Fatty Acids, Omega-6/ OR Fatty Acids, Unsaturated/ or Long-chain polyunsaturated fatty acids.mp. OR Polyunsaturated fatty acids.mp. OR Fatty Acids, Essential/ OR free fatty acids.mp. or Fatty Acids, Nonesterified/ OR Docosahexaenoic Acids/ OR Eicosapentaenoic Acid/ OR alpha-Linolenic Acid/ OR Linoleic Acid/ OR Arachidonic Acid/ OR Fatty Acids/ OR Fishes/ OR Fish Oils/ OR egg oil.mp. OR squid oil.mp. OR krill oil.mp. OR flaxseed oil.mp. or Linseed Oil/ OR Diet/ OR nutrition.mp. OR nutritional intervention.mp. OR Nutritional Status/ OR dietary modification.mp. or Food Habits/ OR dietary intervention.mp. OR Dietary Supplements/ OR nutritional supplement.mp.
AND
COPD.mp. or Pulmonary Disease, Chronic Obstructive/ OR chronic lung disease.mp. OR Bronchitis/ OR ‘obstructive pulmonary’.mp. OR ‘obstructive lung’.mp. OR Airway Obstruction/ or Lung Diseases, Obstructive/ or airflow obstruction∗.mp. OR ‘chronic obstructive OR airway disease’.mp. OR COAD.mp. OR Emphysema/ OR Pulmonary Emphysema/ OR Dyspnea/ OR Bronchitis, Chronic/ OR ‘chronic obstructive pulmonary disease’.mp.
(NB mp = keyword search, all other terms mapped to MESH headings)
PubMed:
- (COPD[MeSH Terms]) AND Omega-3 fatty acids[MeSH Terms] OR Omega-6 fatty acids[MeSH Terms]
- (COPD) AND Omega-3 fatty acids
- (COPD AND Omega-3 fatty acids OR Omega-6 fatty acids[Title/Abstract])
- ((chronic inflammation OR chronic lung disease[Title/Abstract])) AND (AND Omega-3 fatty acids OR n-3 OR w-3 OR Omega-6 fatty acids OR n-6 OR w-6[Title/Abstract])
Embase:
- (((COPD or ‘chronic obstructive pulmonary disease’) and Omega-3 fatty acids) or Omega-6 fatty acids).mp.
- (((COPD or ‘chronic obstructive pulmonary disease’) and Omega-3 fatty acids) or Omega-6 fatty acids).m_titl.
- (COPD or ‘chronic obstructive pulmonary disease’).ab,ti. AND (’Omega-6 fatty acids’ or n-6).mp. or w-6.ab,ti.
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘Long-chain polyunsaturated fatty acids’.ab,ti.
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘Polyunsaturated fatty acids’.mp. or PUFA.ab,ti.
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘Essential fatty acids’.mp. or EFA.ab,ti.
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND DHA.mp. or ‘docosahexaenoic acid’.ab,ti.
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘eicosapentaenoic acid’.mp. or EPA
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘a-linolenic acid’.mp. or ALA
- (COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND ‘linoleic acid’.mp. or LA.ab,ti.
- COPD.mp. or ‘chronic obstructive pulmonary disease’.ab,ti.) AND (Fish or ‘fish oil’ or ‘egg oil’ or ‘squid oil’ or ‘krill oil’).mp. or ‘flaxseed oil’.ab,ti.
Appendix II: Excluded citations/records with reasons.
Barr RG. The Chronic Obstructive Pulmonary Disease Fish Oil Pilot Trial. 2008.
Reason: Citation for a registered trial.
Barr RG, Campos H, Ahmed F, Austin JH, Biancardi A, Henschke C et al. Higher N-3 PUFA levels predict a lower rate of respiratory exacerbations and exacerbation-related hospitalizations: A prospective cohort study. American journal of respiratory and critical care medicine. 2011;183 (1 MeetingAbstracts).
Reason: Incomplete retrieval of information for assessment of methodological quality, data extraction, and data synthesis
Bottle L, Engel B, Hart K, Klopper T. The efficacy of dietetic intervention in patients with chronic obstructive pulmonary disease. Selected abstracts from the British Dietetic Association Conference 2008. Journal of Human Nutrition & Dietetics. 2008;21(4):381-2.
Reason: Incomplete retrieval of information for assessment of methodological quality, data extraction, and data synthesis
Fulton AS, Hill AM, Williams MT, Howe PR, Frith PA, Wood LG et al. Feasibility of omega-3 fatty acid supplementation as an adjunct therapy for people with chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. Trials. 2013;14:107. doi:10.1186/1745-6215-14-107.
Reason: Citation for a study protocol.
Metin S, Kiziltan G, Ucar N, Saka M. Nutritional support with omega-3 rich diets improve inflammation, respiratory functions and exercise capacity in stable COPD. Annals of Nutrition and Metabolism. 2013;63:1169. doi:http://dx.doi.org/10.1159/000354245.
Reason: Incomplete retrieval of information for assessment of methodological quality, data extraction, and data synthesis
Novgorodtseva TP, Denisenko YK, Zhukova NV, Antonyuk MV, Knyshova VV, Gvozdenko TA. Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. BioMed research international. 2013;12:117.
Reason: Did not meet inclusion criteria (no COPD control group).
Thomashow M, A., Yip N, H., Parikh M, Burkart K, M., Lo Cascio C, M., Shimbo D et al. Randomization To Omega-3 Polyunsaturated Fatty Acid Supplementation And Endothelial Function In Chronic Obstructive Pulmonary Disease: The COD-Fish Pilot Randomized Controlled Trial. American Thoracic Society International Conference: American Thoracic Society; 2014. p. A6016-A.
Reason: Incomplete retrieval of information for assessment of methodological quality, data extraction, and data synthesis
Appendix III: Methodological quality items assessed using JBI-MAStARI instruments.
Randomized Control Trial / Cross-sectional Study

References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toelle BG, Xuan W, Bird TE, et al. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust 2013; 198:144–148. [DOI] [PubMed] [Google Scholar]
- 3.Access Economics Pty Limited. Economic impact of COPD and cost effective solutions. 2008. [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Revised 2011). 2011. [Google Scholar]
- 5.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004; 364:709–721. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 7.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005; 128:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C, Zhu H, Shen N, et al. Bacterial infection, airway and systemic inflammation and clinical outcomes before and after treatment of AECOPD: a longitudinal and cross-sectional study. J Chronic Obstruct Pulmon Dis 2015; 12:19–30. [DOI] [PubMed] [Google Scholar]
- 10.Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr 2006; 83 (6 Suppl):1520S–1525S. [DOI] [PubMed] [Google Scholar]
- 11.Mann J, Truswell AS. Essentials of human nutrition. 3rd ed.Oxford: Oxford University Press; 2007. [Google Scholar]
- 12.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev 2001; 23:268–287. [DOI] [PubMed] [Google Scholar]
- 13.de Batlle J, Sauleda J, Balcells E, et al. Association between 3 and 6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem 2012; 23:817–821. [DOI] [PubMed] [Google Scholar]
- 14.Poudel-Tandukar K, Nanri A, Matsushita Y, et al. Dietary intakes of α-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese men. Nutr Res 2009; 29:363–70. [DOI] [PubMed] [Google Scholar]
- 15.Niu K, Hozawa A, Kuriyama S, et al. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr 2006; 84:223–229. [DOI] [PubMed] [Google Scholar]
- 16.Julia C, Touvier M, Meunier N, et al. Intakes of PUFAs were inversely associated with plasma C-reactive protein 12 years later in a middle-aged population with vitamin E as an effect modifier. J Nutr 2013; 143:1760–1766. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Huang T, Zheng J, et al. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS One 2014; 9:e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest 2012; 142:1126–1133. [DOI] [PubMed] [Google Scholar]
- 19.Broekhuizen R, Wouters EFM, Creutzberg EC, et al. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005; 60:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeever TM, Lewis SA, Cassano PA, et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax 2008; 63:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atlantis E, Cochrane B. The association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and functional capacity outcomes in chronic obstructive pulmonary disease: a systematic review protocol. JBI Database Syst Rev Implement Rep 2014; 12:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2014 edition. The Joanna Briggs Institute; 2014. [Google Scholar]
- 23.National Health and Medical Research Council (NHMRC). NHMRC additional levels of evidence and grades for recommendations for developers of guidelines STAGE 2 CONSULTATION: early 2008 till end June 2009. [Google Scholar]
- 24.Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91:221–225. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189:250–255. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Liang X, Wang L, et al. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis 2012; 221:536–543. [DOI] [PubMed] [Google Scholar]
- 27.Heffernan KS, Karas RH, Patvardhan EA, Kuvin JT. Endothelium-dependent vasodilation is associated with exercise capacity in smokers and non-smokers. Vasc Med 2010; 15:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuvin JT, Patel AR, Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol 2001; 38:1843–1849. [DOI] [PubMed] [Google Scholar]
- 29.Patel AR, Kuvin JT, Sliney KA, et al. Peripheral vascular endothelial function correlates with exercise capacity in women. Clin Cardiol 2005; 28:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst EA, Paglialunga S, Gerling C, et al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol 2014; 592 (Pt 6):1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W, Wu JH, Wang Q, et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J Clin Nutr 2015; 101:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu L, Mukamal KJ, Naqvi AZ. Erythrocyte saturated fatty acids and systemic inflammation in adults. Nutrition 2014; 30:1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]


