Abstract
Objective:
To determine the predictive value of clinical assessment and dopamine transporter (DAT) uptake for the early development of neurodegenerative synucleinopathy diseases from idiopathic REM sleep behavior disorder (iRBD) over 5 years in a Chinese population.
Methods:
Forty-three patients with iRBD were administered clinical assessment tests, and 35 were examined by DAT-SPECT imaging during 2011. Cox proportional hazard and Kaplan-Meier analyses were used to evaluate the predictive value of the markers in a follow-up study over 5 years.
Results:
Eighteen patients (41.9%) developed neurodegenerative synucleinopathy diseases after a median of 4.1 years of prospective follow-up (median interval of 10.5 years from the estimated onset of iRBD symptoms). Patients with higher scores on the Nonmotor Symptom Questionnaire (hazard ratio [HR] 3.11, 95% confidence interval [CI] 1.15–8.40, p = 0.026) and Scale for Outcomes in Parkinson Disease–Autonomic questionnaire (HR 4.46, 95% CI 1.64–12.10, p = 0.003) were more likely to develop neurodegenerative synucleinopathy diseases. Furthermore, the population with decreased 99mTc-TRODAT-1 binding in the left striatum (HR 2.7, 95% CI 1.02–7.14, p = 0.046) and putamen (HR 3.23, 95% CI 1.16–8.33, p = 0.024) had a relatively higher risk of developing neurodegenerative synucleinopathy diseases.
Conclusions:
Our findings elucidate the predictive value of autonomic dysfunction and DAT uptake in identifying patients with iRBD at a high risk of progressing into neurodegenerative synucleinopathy diseases and could form a basis for future disease-prevention trials.
Neurodegenerative synucleinopathy diseases such as Parkinson disease (PD), multiple systems atrophy (MSA), and dementia with Lewy bodies (DLB) have a prodromal period of several years before manifestation of parkinsonism or dementia symptom, during which neuropathologic changes likely occur.1 Diagnostic markers for this period are therefore critical. Accumulating symptoms of constipation, olfactory dysfunction, depression, and cardiac autonomic disorder often appeared in the premotor phase of PD.2–4 However, these features are also common in the general population and are not sufficient for a certain diagnosis.5,6 REM sleep behavior disorder (RBD) is a distinct feature of the prodromal phase of parkinsonism diseases; a majority of patients with an idiopathic form of RBD (iRBD) will develop synucleinopathy diseases over several years.1,7–9 However, the median interval is quite long, ranging between 12 and 14 years,1,7,10 and factors leading to an increased short-term risk for developing neurodegenerative synucleinopathy are not well known. iRBD has also been associated with nonmotor symptoms of parkinsonism diseases and decreased dopamine transporter (DAT) uptake.11–13 However, there has been rare study to date of the role of clinical assessment index with DAT-SPECT imaging tests in patients with iRBD during the conversion. The aim of our study was to evaluate the predictive value of these markers for conversion to neurodegenerative synucleinopathy diseases in patients with iRBD over an observation period of 5 years in a Chinese population.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and all participants or their guardians provided written informed consent.
Participants.
All study participants were recruited from the Department of Neurology, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, in 2011. Fifty patients with iRBD were enrolled in our study; 7 were lost during the 5-year follow up. The diagnosis of definite iRBD met the following International Classification of Sleep Disorder-II criteria: history of problematic sleep behaviors that were disturbing to self or sleep partner or disruptive of sleep continuity, excessive tonic or phasic chin electromyographic activity during REM sleep, and elaborate motor activities associated with dream content during REM sleep by video recordings (not associated with periodic leg movement syndrome or respiratory events).14 Duration of iRBD symptoms was recorded by patient self-report. Moreover, overnight video-polysomnograph examination was done for every patient to confirm the diagnosis of iRBD with a Compumedics E-Series EEG/PSG Recording System (Compumedics Ltd, Melbourne, Australia).
Baseline neurologic examinations were performed for every participant to exclude defined neurodegenerative diseases. Diagnosis of parkinsonism or cognitive impairment was made according to current clinical diagnostic criteria by at least 2 movement disorders specialists.15 Because some patients diagnosed earlier with PD would slowly progress to other neurodegenerative diseases later on, we recognized these patients as having neurodegenerative synucleinopathy diseases. Follow-up occurred annually until the development of parkinsonism or cognitive impairment symptoms.
Clinical assessment.
A series of validated and comprehensive questionnaires covering autonomic symptoms, depression, olfactory testing, and cognitive function identically were administered at baseline, including the Nonmotor Symptom Questionnaire (NMSQ), REM sleep behavior disorder screening questionnaire, Scale for Outcomes in Parkinson Disease–Autonomic (SCOPA-AUT), Hamilton Depression Rating Scale-17 (HAMD-17), Mini-Mental State Examination (MMSE), and Montreal Cognitive Assessment (MOCA). NMSQ was used to evaluate nonmotor symptoms and SCOPA-AUT to assess autonomic dysfunction.16 An olfaction test (Sniffin Sticks 16-item test [SS-16]) was also performed to assess the olfactory function through 16-item odor identification.17,18 HAMD-17 was administered to measure depressive symptoms,19 and MMSE and MOCA were performed to evaluate global cognitive functioning resulting from PD.20
99mTc-TRODAT-1 SPECT imaging.
DAT-SPECT was performed as we reported previously.21 Thirty-five patients participated in the SPECT imaging study, and they did not take any medications affecting experimental observation within the 48 hours before the IV administration of 2 mL solution, which contains 20 mCi 99mTc-TRODAT-1 prepared from a preformulated lyophilized kit obtained from Jiangsu Nuclear Medicine Institute (WuXi, China). Two hours later, the binding to DAT was comprehensively assessed with SPECT imaging by a double-headed gamma camera (SymbiaT16, Siemens, Munich, Germany). The energy window was 140 ± 14 keV, with each head containing a matrix of 128 × 128 in circular orbit with step-and-shoot movements of 64 steps. The diameter and degree of rotation were 30 cm and 360°; acquisition time was 30 seconds; zoom was 1.45; and slice thickness was 3 mm. Brain MRI (1.5T, Siemens) with 3-mm cuts was also performed for each patient at the level of the basal ganglia to establish a reference to determine regions of interest for the uptake of 99mTc-TRODAT-1. As we reported before, the regions of interest (776 ± 33 mm2) were placed over the left and right striata to split them into the caudate nucleus and putamen, respectively, with the reference background placed on same summed image of the cerebellum.21 The tracer uptake on both side of the putamen, caudate nucleus, and striatum was calculated as follows with an average value: BPND = ST-CB/CB, where BPND is binding potential of the nondisplaceable radioligand, ST is striatum (caudate and putamen), and CB is cerebellum.
Statistical analysis.
Categorical variables were compared with the χ2 or Fisher exact test to assess the difference of baseline parameters between the disease-free and disease-developed group. The primary endpoint, predicted progression-free survival, was defined as the time from the establishment of iRBD to disease progression of neurodegenerative synucleinopathy diseases. We estimated the rates of progression-free survival by using the Kaplan-Meier method to analyze disease risk. To test prodromal markers, Cox proportional hazards were performed, and for stratification, predictive markers were classified by relative points as cutoff values: 7 for RBD, 12 for NMSQ, 8 for SS-16, 8 for HAMD-17, 11 for SCOPA-AUT, 25 for MOCA, and 27 for MMSE. Constipation index was classified binary, and DAT tracer uptake values were classified with the use of median cutoff values in our study. Each variable was assessed with Cox regression, with hazard ratios (HRs) adjusted for age and sex. Two-tailed values of p < 0.05 were considered statistically significant differences. All statistical analyses were performed with SPSS statistical software (version 22.0, SPSS Inc, Chicago, IL).
RESULTS
Demographic data.
Demographic and lifestyle factors of the patients are shown in table 1. Average age was 65 years, and 34 patients (79%) were male. There were no differences in age, sex, smoking history, or constipation between the nonconverters and converters. Over follow-up until April 2016, 18 of 43 patients (41.9%) developed defined neurodegenerative synucleinopathy diseases (DLB = 2, MSA = 3, PD/mild cognitive impairment = 4, and PD = 9) after a median of 4.1 years of prospective follow-up (median interval of 10.5 years from the estimated onset of iRBD symptoms) (figure 1). Median iRBD duration was 6.9 years in neurodegenerative synucleinopathy converters and 5.9 years in nonconverters; there was no statistical difference between the 2 groups.
Table 1.
Demographics of the 2 patient groups
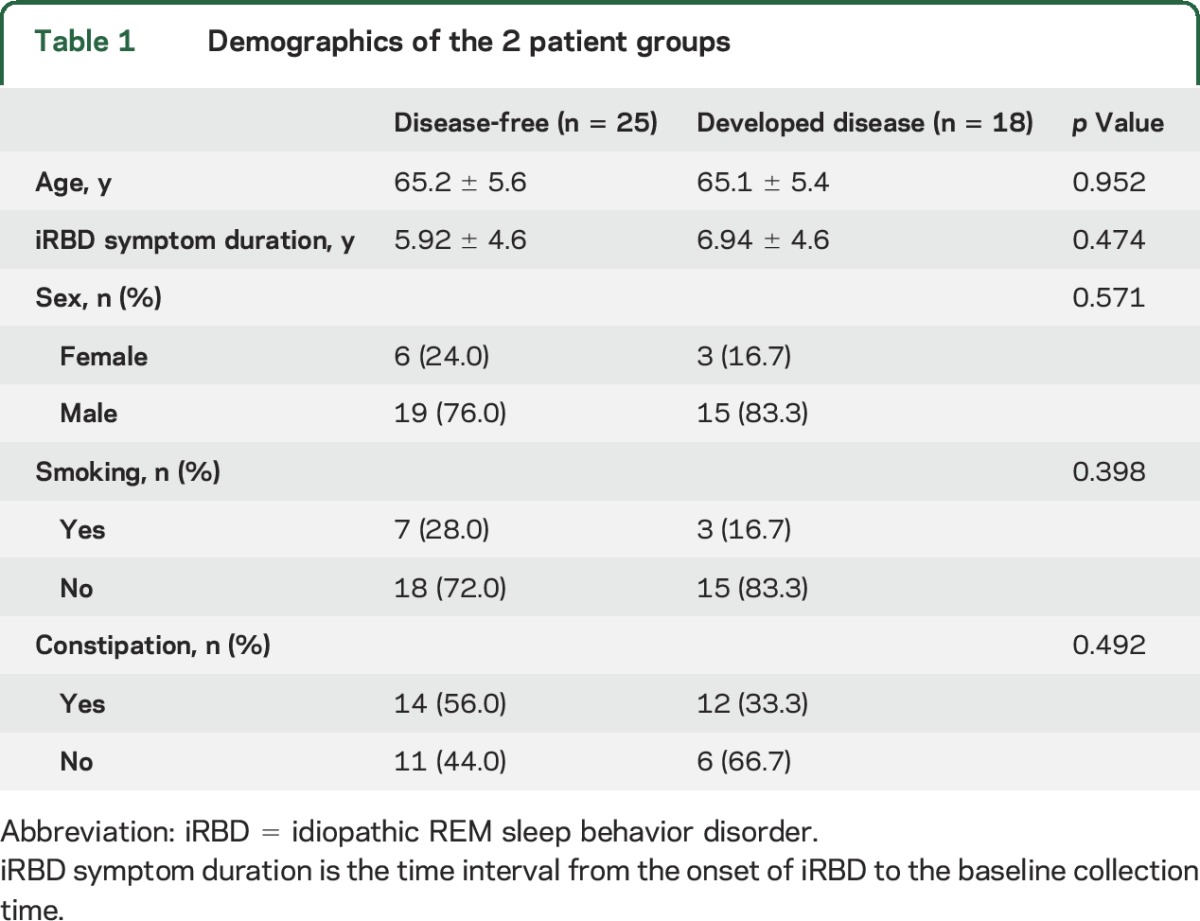
Figure 1. Development of defined neurodegenerative synucleinopathy diseases in patients with iRBD.
Kaplan-Meier plot of disease-free survival of patients with idiopathic REM sleep behavior disorder (iRBD). Conversion time was measured from iRBD diagnosis time. Ticks indicate censoring events.
Potential predictors of disease progression from iRBD to neurodegenerative synucleinopathy diseases.
All participants were evaluated with the olfaction test (SS-16), RBD, NMSQ, SCOPA-AUT, HAMD-17, MMSE, and MOCA questionnaire, and 35 patients underwent DAT-SPECT. All subsequent analyses were adjusted for age and sex (table 2). Patients with higher scores on the NMSQ (HR = 3.11 95% confidence interval [CI] 1.15–8.40, p = 0.026) and SCOPA-AUT questionnaire (HR = 4.46, 95% CI 1.64–12.10, p = 0.003) were more likely to develop neurodegenerative synucleinopathy diseases. For the olfaction, depression, MMSE, and MOCA questionnaire, although the converter group had slightly higher scores, no statistical difference was observed. Furthermore, DAT tracer uptake in the 2 groups showed that the population with decreased 99mTc-TRODAT-1 binding in the left striatum (HR 2.7, 95% CI 1.02–7.14, p = 0.046; table 3) and putamen (HR 3.23, 95% CI 1.16–8.33, p = 0.024; table 3), especially the left putamen (HR 3.7, 95% CI 1.27–11.11, p = 0.017; table 3), had a higher risk of developing neurodegenerative synucleinopathy diseases.
Table 2.
Evaluation of the converters and nonconverters
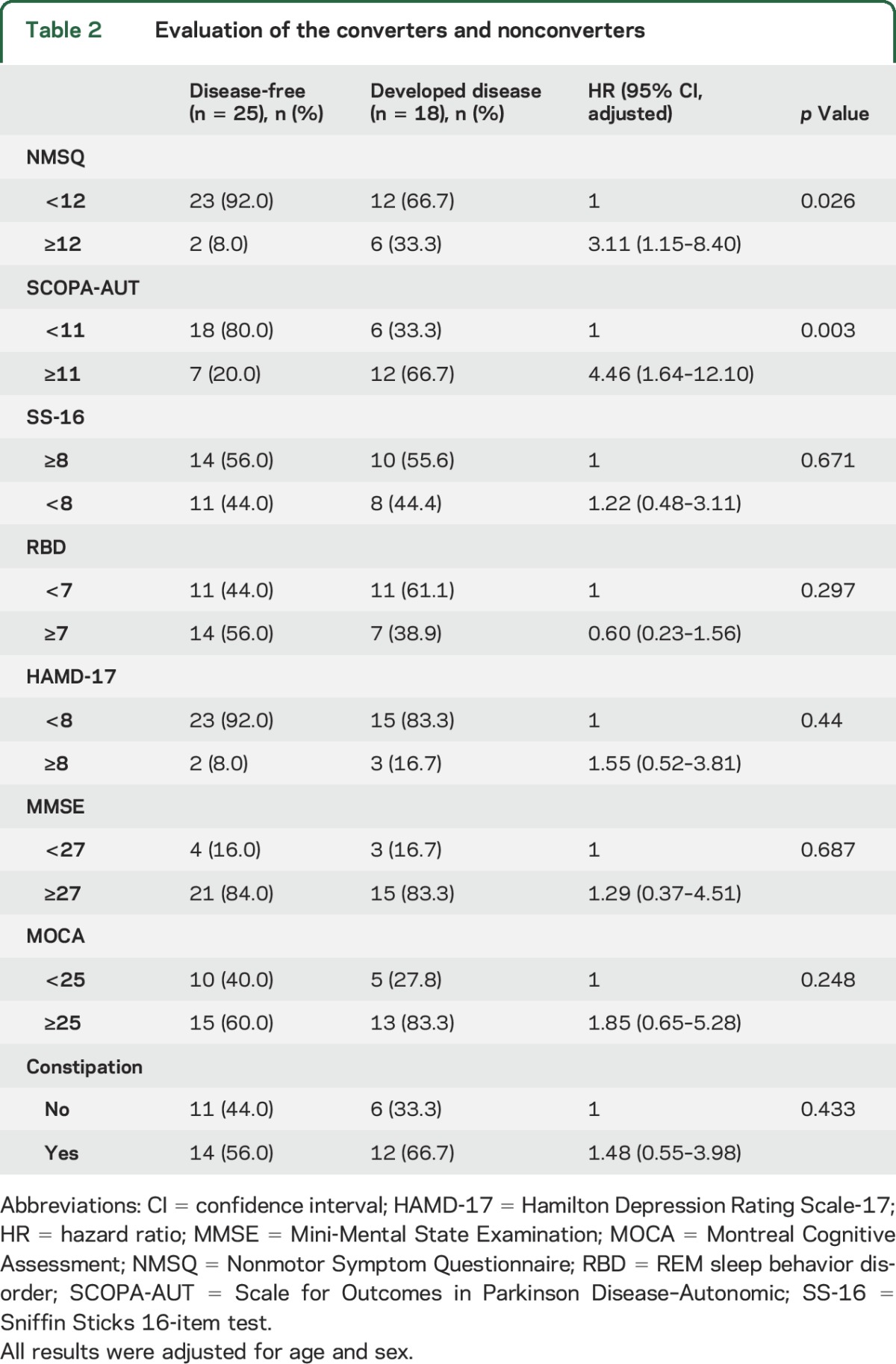
Table 3.
Evaluation of DAT intake between the converters and nonconverters
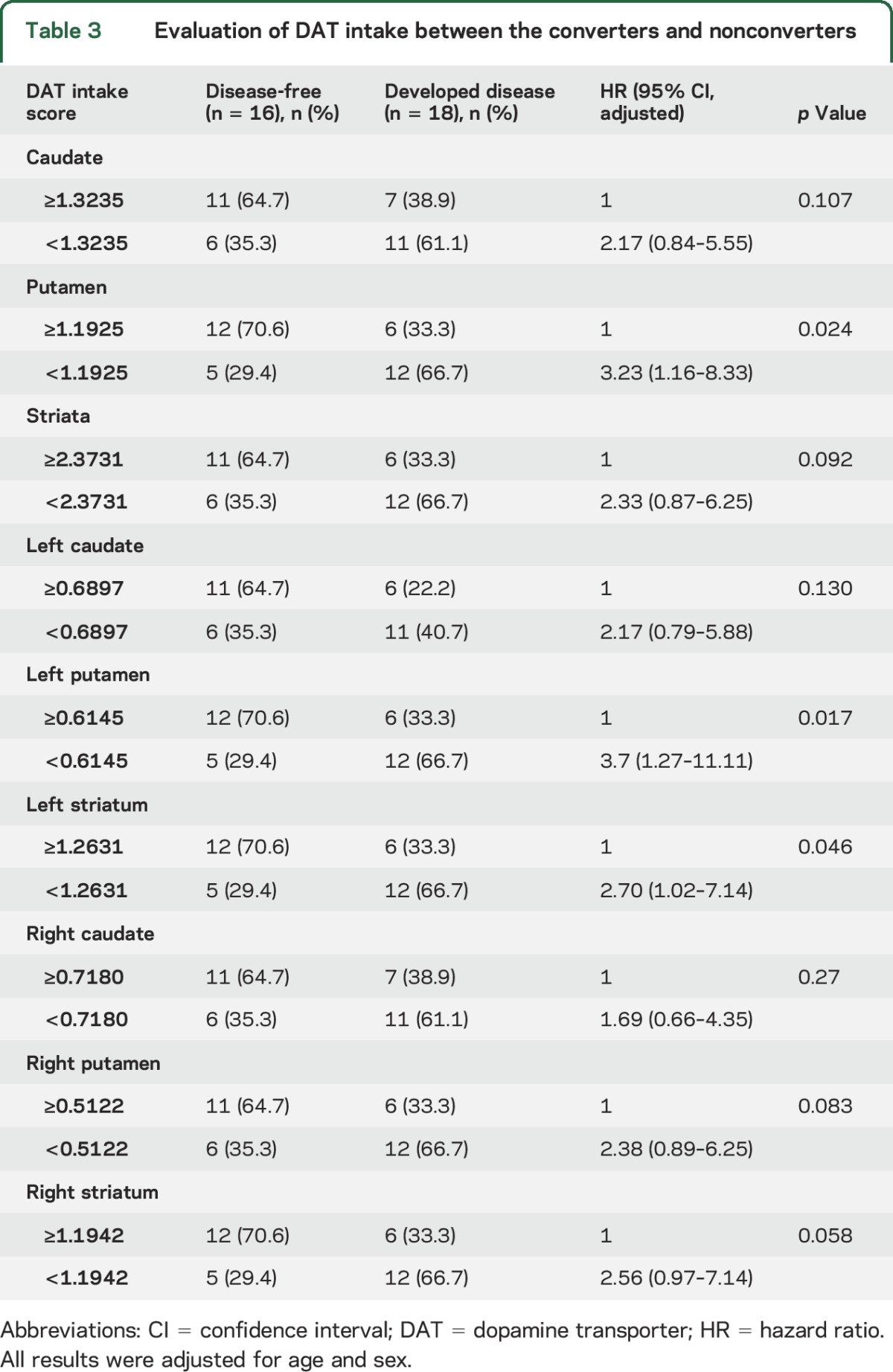
Furthermore, Kaplan-Meier analysis indicated median progression-free survival times of 4.4 years in the group with scores <12 in NMSQ and 3.1 years in the remaining population (p = 0.0064; figure 2). Patients with scores <11 on the SCOPA-AUT questionnaire appeared to have longer progression-free survival than patients with scores >11 (p = 0.0072). Moreover, patients with higher DAT tracer uptake in the putamen (≥1.1925) exhibited longer progression-free survival than the remaining patients (3.7 vs 4.3 years, p = 0.039).
Figure 2. Predictive markers of neurodegenerative synucleinopathy diseases in iRBD.
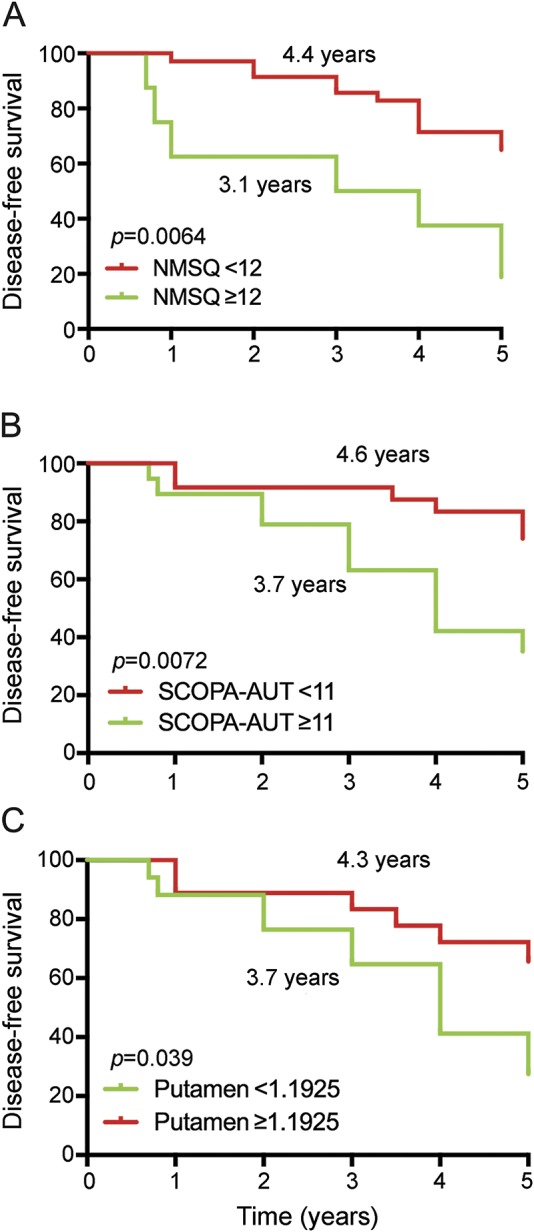
Kaplan-Meier plot of disease-free survival of patients with idiopathic REM sleep behavior disorder (iRBD), stratified according to presence of baseline questionnaires: (A) Nonmotor Symptom Questionnaire (NMSQ), (B) Scale for Outcomes in Parkinson Disease–Autonomic (SCOPA-AUT), and (C) dopamine transporter (DAT) tracer uptake of putamen. Conversion time was measured from iRBD diagnosis time. Solid line indicates patients with less or equal relevant scores; dashed line, higher scores.
Subgroup analysis of potential predictors.
According to the baseline characteristics, 34 patients (79%) were male, 26 (60%) had constipation, and 19 (44%) experienced dysosmia. We therefore investigated the potential predictors of disease progression in specific subgroups. First, we found that male patients with higher SCOPA-AUT scores (HR 4.67, 95% CI 1.52–14.28, p = 0.007; table e-1 at Neurology.org) were more likely to develop neurodegenerative synucleinopathy diseases. Simultaneously, male patients with lower 99mTc-TRODAT-1 binding values in the left putamen had a higher risk of developing neurodegenerative synucleinopathy diseases (HR 3.7, 95% CI 1.11–12.5, p = 0.033).
Moreover, we found that patients without olfactory disorders who presented higher NMSQ (HR 5.51, 95% CI 1.16–26.16, p = 0.032) and SCOPA-AUT (HR 6.99, 95% CI 1.42–14.37, p = 0.017; table e-2) scores were more likely to develop neurodegenerative synucleinopathy diseases than the remaining patients. In those with dysosmia, no significant difference was found between the 2 groups.
In patients with constipation, those who obtained higher SCOPA-AUT scores were more likely to develop neurodegenerative synucleinopathy diseases than the others (HR 5.35, 95% CI 1.85–15.44, p = 0.025; table e-3). Furthermore, the patients with constipation with lower 99mTc-TRODAT-1 binding values in both left putamen and left striatum showed higher risks of developing neurodegenerative synucleinopathy diseases (HR 4.17, 95% CI 1.27–12.5, p = 0.018).
Subsets analysis of NMSQ and SCOPA-AUT.
Because the NMSQ is a composite score and SCOPA-AUT contains several specific domains, we further performed a secondary, exploratory analysis to screen for potential factors. For the 30 items of NMSQ, we performed the Cox proportional hazards analysis and found that patients with iRBD with urgency (HR 2.62, 95% CI 1.03–6.66, p = 0.043), nocturia (HR 3.97, 95% CI 1.48–10.63, p = 0.006), daytime sleepiness (HR 2.73, 95% CI 1.06–7.05, p = 0.038), and sweating (HR 4.78, 95% CI 1.76–12.98, p = 0.002) were more likely to develop neurodegenerative synucleinopathy diseases (table e-4). For domains of the SCOPA-AUT, we found that the converters had higher scores in the gastrointestinal (p = 0.024), urinary (p = 0.006), and cardiovascular (p = 0.037) subsets than the nonconverters (table e-5).
DISCUSSION
In our study, the median interval from the estimated onset of iRBD to the manifestation of defined neurodegenerative synucleinopathy diseases was 10.5 years, and the median interval from the diagnosis time was 4.1 years. Patients with iRBD with more nonmotor symptoms (especially autonomic disturbances) were more likely to develop neurodegenerative synucleinopathy diseases. Furthermore, patients with higher NMSQ and SCOPA-AUT scores exhibited shorter progression-free survival time, while patients with lower DAT uptake of the left striatum and putamen showed longer median progression-free survival time.
In some cases, iRBD could be the initial manifestation and a prodromal symptom of PD according to the Braak staging system,1,22,23 similar to mild cognitive impairment for Alzheimer disease. However, the long median time for conversion limits the feasibility of designing future preventive trials; thus, basic markers for those at risk of early conversion are required. A study in Spain last year reported that patients with iRBD frequently exhibit nonmotor symptoms that occur in prodromal PD, particularly hyposmia and constipation.24 In our study, the overall nonmotor symptoms scores were consistent with it. Patients with iRBD with higher NMSQ scores are more likely to develop neurodegenerative synucleinopathy diseases, and for single items of NMSQ, urgency, nocturia, daytime sleepiness, and sweating acted as specific items. This finding is in agreement with a previous study that showed that patients with PD had significantly higher scores for complaints of many nonmotor symptoms such as urinary urgency, sweating,25 and daytime sleepiness, which have been shown to be predictors of more rapid conversion to parkinsonism/dementia.26 Thus, the predictive value of nonmotor symptoms and their specific items in the conversion progress was highlighted.
Among the typical nonmotor symptoms occurring in patients with parkinsonism, autonomic dysfunction is frequently observed and often precedes the onset of motor symptoms.27 Moreover, patients with iRBD often present with impaired autonomic functions and higher SCOPA-AUT scores.28 In this study, the severity of autonomic disturbances was evaluated by the SCOPA-AUT questionnaire (including the urinary, gastrointestinal, cardiovascular, and thermoregulation systems), and the results showed that patients with higher scores more frequently developed neurodegenerative synucleinopathy diseases. During further analysis of these 4 domains of SCOPA-AUT (sexual disorder being excluded because most information about it could not be collected as a result of Chinese cultures), we found that the converters had higher scores for urinary, gastrointestinal, and cardiovascular systems. This is in agreement with several previous reports that indicated that patients with iRBD had more severe autonomic disturbances, including constipation, erectile dysfunction, impaired orthostatic blood pressure regulation, and reduced tracer uptake in cardiac iodine-123-metaiodobenzylguanidine scintigraphy.29–31 Thus, the results showed the predictive value of autonomic symptoms in the conversion of iRBD to neurodegenerative synucleinopathy diseases and indicated that patients with iRBD with higher SCOPA-AUT scores would develop neurodegenerative synucleinopathy faster. Furthermore, patients with iRBD who had constipation were more likely to develop neurodegenerative synucleinopathy diseases; however, this finding was not statistically significant, which may be due to the small sample size.
Previous studies have shown the predictive value of olfactory function during the progression from iRBD to Lewy body diseases.32 In our study, patients with olfactory disorders were more likely to develop Lewy body diseases (even excluding patients with MSA; table e-6); however, this finding was not significant, likely because of racial variation and limited population. Because the SS-16 test was designed primarily for the routine clinical assessment of olfactory performance in Americans,17 it may not perfectly fit in other countries and cultural biases, and smell familiarity could influence the test results.33 For the Chinese population, several odors among the items such as cinnamon and cloves are not appropriate. Moreover, a study in China has shown the importance of using SS-12 instead of SS-16 in screening people at high risk of PD.34 A more feasible test of olfaction to screen its predictive value in a large cohort is needed in the future.
DAT imaging is a useful method for the diagnosis of parkinsonism. Patients in the early stages of PD usually show decreased striatal DAT uptake, which indicates substantia nigra dopaminergic dysfunction, particularly in the putamen rather than in the caudate nucleus.35 In a 2.5-year follow-up study in 2010, a combination of transcranial sonography and DAT-SPECT was used to identify 5 patients with iRBD who developed classic features of PD and DLB with a specificity of 55%.12 In 2011, a prospective study showed that 3 patients in whom iRBD progressed to PD had the lowest [123I]-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane uptake at baseline and a significant reduction, particularly in the putamen, in a 3-year follow-up prospective study.36 In the present study, we postulated that neuroimaging changes could occur in a proportion of patients with iRBD who might be at risk of developing neurodegenerative synucleinopathy diseases and found that 18 patients with iRBD with decreased DAT uptake in the putamen (especially the left side) rather than the caudate converted to neurodegenerative synucleinopathy diseases, which was consistent with the previous results of postmortem studies in parkinsonism.37 However, we supposed that it was the coincidence of which side was more affected. Furthermore, Kaplan-Meier analysis showed that patients with lower DAT uptake in the putamen exhibited a shorter progression-free survival time compared to the population with higher DAT uptake, indicating that serial DAT-SPECT could be used to monitor the progression of nigrostriatal damage in patients with iRBD. Aside from a study in 2010, our work is the second published study to prove the value of DAT imaging in predicting the risk of PD diseases in iRBD.
Our study had a few potential limitations. First, although a 5-year follow-up was performed, the time of the final event was difficult to define, and the primary outcome was based on clinical diagnosis without pathologic confirmation. Moreover, as for the conversion time, we agreed that the conversion measured from iRBD symptoms onset would be “softer” than the time of diagnosis. Thus, we also calculated the conversion rate starting with the diagnosis of iRBD by polysomnograph as listed in table e-7, showing that the NMSQ and SCOPA-AUT were still significant. However, the iRBD diagnosis was also based on clinical history, especially the score on the iRBD questionnaires, and our latest study showed good coherence between iRBD questionnaires and polysomnograph-based diagnosis of RBD.38 Simultaneously, a statistic bias may have occurred if the polysomnograph diagnosis time was used because the duration time of iRBD was actually longer than the polysomnograph diagnosis time, and particular attention had been paid to the clinical history regarding onset time. Finally, for the subgroup analysis, higher SCOPA-AUT scores and decreased DAT uptake in the left putamen showed predictive value in male patients and those with constipation. We did not perform the analysis in the female subgroup because of the limited sample size.
It is very likely that SCOPA-AUT, a simple and useful tool for evaluating autonomic symptoms, together with DAT uptake, could be used to identify patients with iRBD who are at a high risk of disease conversion into neurodegenerative synucleinopathy diseases, which could also provide a cue for the criteria of prodromal PD. This study, which used Cox proportional hazards and Kaplan-Meier analyses to evaluate the value of clinical assessment with imaging examination to predict iRBD developing into neurodegenerative synucleinopathy diseases, could form a basis for disease-prevention trials and the development of neuroprotective therapies in the future.
Supplementary Material
GLOSSARY
- CI
confidence interval
- DAT
dopamine transporter
- DLB
dementia with Lewy bodies
- HAMD-17
Hamilton Depression Rating Scale-17
- HR
hazard ratio
- iRBD
idiopathic REM sleep behavior disorder
- MMSE
Mini-Mental State Examination
- MOCA
Montreal Cognitive Assessment
- MSA
multiple systems atrophy
- NMSQ
Nonmotor Symptom Questionnaire
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- SCOPA-AUT
Scale for Outcomes in Parkinson Disease–Autonomic
- SS-16
Sniffin Sticks 16-item test
Footnotes
Editorial, page 1486
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jun Liu and Shengdi Chen conceived and supervised the project. Jun Liu and Shengdi Chen contributed to patient recruitment. Yuanyuan Li, Jun Liu, and Wenyan Kang drafted the manuscript. Yuanyuan Li, Wenyan Kang, Qiong Yang, Linyuan Zhang, and Fangyi Dong contributed to sample collection. Yuanyuan Li, Lina Zhang, and Fangyi Dong performed data management and statistical analyses. All authors read and approved the final version of the manuscript.
STUDY FUNDING
This work was supported by grants from the National Natural Science Foundation of China (81471287, 81071024, 81171202, 81371407, 30872729, and 30870879), the National Key Research and Development Program (2016YFC1306505), the Shanghai Shuguang Program (11SG20), the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20152201), and the Fifth National Undergraduate Student Innovating Program (2011015). This study was sponsored by the Department of Neurology and Institute of Neurology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology 2009;72:S12–S20. [DOI] [PubMed] [Google Scholar]
- 3.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 2008;63:167–173. [DOI] [PubMed] [Google Scholar]
- 4.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–462. [DOI] [PubMed] [Google Scholar]
- 5.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–1591, quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 6.Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev 2011;31:1117–1125. [DOI] [PubMed] [Google Scholar]
- 7.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 8.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 9.Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postuma RB, Iranzo A, Hogl B, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 2015;77:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of “idiopathic” REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 2005;128:126–137. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected]. Lancet Neurol 2010;9:1070–1077. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011;69:811–818. [DOI] [PubMed] [Google Scholar]
- 14.ICD-10 (International Classification of Diseases): a new way with speed bumps? Discussed on the example of depression, anxiety and sleep disorders [in German]. Psychiatrische Praxis 1996;23:1–8. [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology 2007;69:333–341. [DOI] [PubMed] [Google Scholar]
- 17.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin' sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997;22:39–52. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Chen S, Kang WY, et al. Application of odor identification test in Parkinson's disease in China: a matched case-control study. J Neurol Sci 2012;316:47–50. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 1990;27:428–437. [DOI] [PubMed] [Google Scholar]
- 21.Kang WY, Yang Q, Jiang XF, et al. Salivary DJ-1 could be an indicator of Parkinson's disease progression. Front Aging Neurosci 2014;6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord 2012;27:677–689. [DOI] [PubMed] [Google Scholar]
- 23.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre-Mardones C, Iranzo A, Vilas D, et al. Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol 2015;262:1568–1578. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord 2006;21:916–923. [DOI] [PubMed] [Google Scholar]
- 26.Arnulf I, Neutel D, Herlin B, et al. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep 2015;38:1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology 2009;73:1752–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein G, Burghaus L, Vaillant M, Pieri V, Fink GR, Diederich N. Dysautonomia in narcolepsy: evidence by questionnaire assessment. J Clin Neurol 2014;10:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frauscher B, Nomura T, Duerr S, et al. Investigation of autonomic function in idiopathic REM sleep behavior disorder. J Neurol 2012;259:1056–1061. [DOI] [PubMed] [Google Scholar]
- 30.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006;66:845–851. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 2006;67:2236–2238. [DOI] [PubMed] [Google Scholar]
- 32.Mahlknecht P, Iranzo A, Hogl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015;84:654–658. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Violante M, Gonzalez-Latapi P, Camacho-Ordonez A, Martinez-Ramirez D, Morales-Briceno H, Cervantes-Arriaga A. Comparing the accuracy of different smell identification tests in Parkinson's disease: relevance of cultural aspects. Clin Neurol Neurosurg 2014;123:9–14. [DOI] [PubMed] [Google Scholar]
- 34.Huang SF, Chen K, Wu JJ, et al. Odor identification test in idiopathic REM-behavior disorder and Parkinson's disease in China. PLoS One 2016;11:e0160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson's disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 2000;15:692–698. [DOI] [PubMed] [Google Scholar]
- 36.Iranzo A, Valldeoriola F, Lomena F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2011;10:797–805. [DOI] [PubMed] [Google Scholar]
- 37.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114(pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 38.Ma JF, Hou MM, Tang HD, et al. REM sleep behavior disorder was associated with Parkinson's disease: a community-based study. BMC Neurol 2016;16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


