Abstract
Objective:
To explore the association of intracranial atherosclerotic disease (ICAD) with mild cognitive impairment (MCI) and dementia.
Methods:
From 2011 to 2013, 1,744 participants completed high-resolution vessel wall MRI from the population-based Atherosclerosis Risk in Communities Study by a sampling strategy that allowed weighting back to the cohort. We defined ICAD by plaque features (presence, territory, stenosis, number). Trained clinicians used an algorithm incorporating information from interviews and neuropsychological and neurologic examinations to adjudicate for MCI and dementia. We determined the relative prevalence ratio (RPR) of MCI or dementia after adjusting for risk factors at midlife using multinomial logistic regression.
Results:
A total of 601 (34.5%) participants had MCI (mean age ± SD, 76.6 ± 5.2 years), 83 (4.8%) had dementia (79.1 ± 5.3 years), and 857 (49.1%) were current or former smokers. Anterior cerebral artery (ACA) plaque (adjusted RPR 3.81, 95% confidence interval [CI] 1.57–9.23), >2 territories with plaque (adjusted RPR 2.12, 95% CI 1.00–4.49), and presence of stenosis >50% (adjusted RPR 1.92, 95% CI 1.01–3.65) were associated with increased prevalence of dementia in separate models. Posterior cerebral artery plaque was associated with MCI but did not reach statistical significance for dementia (adjusted RPR MCI 1.43, 95% CI 1.04–1.98; adjusted RPR dementia 1.58, 95% CI 0.79–2.85). There were no associations with middle cerebral artery atherosclerotic lesions or cognitive impairment. Many participants had plaque in >1 territory (n = 291, 46%) and participants with ACA plaques (n = 69) had the greatest number of plaques in other territories (mean 6.0, SD 4.4).
Conclusions:
This study demonstrates associations between ICAD and clinical MCI and dementia.
Dementia and cognitive decline affect millions of US adults with an economic burden over and above that of heart disease and cancer combined.1 As a consequence, there is growing interest in identifying modifiable risk factors that can delay or prevent the development of dementia. Vascular risk factors such as diabetes and hypertension are significantly associated with the risk of incident dementia.2 Intracranial atherosclerotic disease (ICAD) is also associated with vascular risk factors.2,3 Although other vascular contributors to dementia such as arteriolosclerosis and stroke are well-established,4–6 few studies have evaluated the association of ICAD and dementia.4–6 At autopsy the brains of patients with dementia, some of whom have Alzheimer disease (AD), often contain evidence of all types of cerebrovascular disease at higher rates than is observed in patients without dementia.7,8 Other pathologic studies have observed that ICAD in the circle of Willis is associated with dementia.9,10 Studies using CT angiography in highly selected samples suggest an association of ICAD with dementia,11,12 although CT angiography may miss ICAD without stenosis. This suggests that data on the association of ICAD without stenosis and dementia in representative samples of community-dwelling adults are lacking.
High-resolution black blood MRI (BBMRI) has recently enabled identification and characterization of ICAD in large population studies.13 This technique has emerged as highly reliable and able to detect the thickness and burden of ICAD.14 In this study, we used 3D BBMRI in a community-based cohort of adults to examine associations of ICAD with MCI and dementia.
METHODS
The Atherosclerosis Risk in Communities (ARIC) Study began with recruitment of 15,792 middle-aged (45–64 years) adults in 1987–1989 at 4 US sites (Washington County, Maryland; Forsyth County, North Carolina; Minneapolis, Minnesota; and Jackson, Mississippi) using probability sampling to ensure representative samples of white and African American participants.15 There were 5 main study visits: visits 1–4 (1987–1989, 1990–1992, 1993–1995, 1996–1999) and the ARIC Neurocognitive Study (ARIC-NCS) visit in 2011–2013 when study participants were 66–90 years of age.
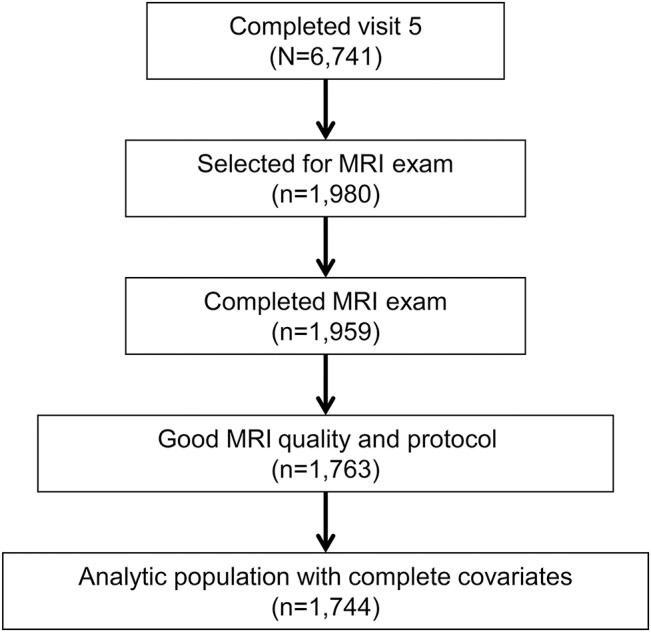
ARIC-NCS was designed to investigate risk factors and vascular markers of MCI and dementia. All surviving ARIC participants were invited for an in-person ARIC-NCS visit and 6,741 participants attended. Participants who were unable or unwilling to undergo an in-person assessment were offered a telephone cognitive assessment. For those unable or unwilling to undergo a telephone cognitive assessment and if cognitive impairment was suspected based on hospital discharge codes or annual telephone interviews, a family member was interviewed about the participant's functional and cognitive status.16 For ARIC-NCS, 1,980 participants underwent brain MRI, of which 1,959 participants completed the scan.17 Participants who attended the ARIC-NCS visit and participated in MRI scans were selected on the basis of a prespecified sampling strategy that included having had a prior ARIC research brain MRI from 2004 to 2006 (n = 438), having evidence of cognitive decline (based on longitudinal data from cognitive testing at prior ARIC visits 2 and 4 [n = 672]), or being sampled from a set of remaining individuals (n = 868). Of these participants, 1,744 completed cognitive testing and MRI examinations with good image quality and protocol adherence and were without missing covariates (figure). Data from these participants were weighted using the sampling strategy to be representative of all participants attending visit 5.
Figure. Schematic describing the Atherosclerosis Risk in Communities Neurocognitive Study and analytic population.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards approved the study at each site and participants provided written informed consent.
MRI.
MRI scans were performed at all 4 field centers on 3.0T scanners and vascular sequences were acquired at the end of the standardized brain MRI protocol by trained MRI technologists. The vascular protocol has been described previously17 and consisted of a 3D time-of-flight magnetic resonance angiography (MRA) (acquired resolution 0.50 × 0.50 mm2; slice thickness 0.55 mm) and 3D high isotropic resolution vessel wall MRI (acquired resolution 0.5 mm3)13,18 oriented to cover the major intracranial vessels.
Seven certified readers blinded to participant characteristics analyzed all MRI. MRI examinations were graded for image quality and protocol adherence. Atherosclerotic plaque was defined as eccentric wall thickening, with or without luminal stenosis on MRA.17,19 We recorded the number of detectable plaques and the degree of narrowing for the most stenotic plaque in each of 11 predefined vascular territories (right and left internal carotid artery [ICA], right and left anterior cerebral artery [ACA], right and left middle cerebral artery [MCA], right and left posterior cerebral artery [PCA], right and left vertebral artery, and basilar artery). We recorded the number of detectable plaques using the definition of atherosclerotic plaque as eccentric wall thickening with or without luminal stenosis seen at MRA.17 Using criteria established in the Warfarin-Aspirin Symptomatic Intracranial Disease Trial, the ordinal degree of narrowing (i.e., no detectable stenosis, <50% stenosis, 51%–70% stenosis, 71%–99% stenosis, and occlusion) was recorded for the most stenotic plaque.20 A subset of participants (n = 102) underwent repeat MRA examinations for determination of intrarater and interrater reliability. Percentage agreement for plaque identification and stenosis was high (plaque identification: 87.0% interreader estimate, 89.2% intrareader estimate; ordinal stenosis: 93.8% interreader estimate, 94.4% intrareader estimate).17
Assessment of MCI and dementia.
ARIC-NCS neuropsychological examinations have been described in detail.16 Briefly, participants were tested in the domains of memory, sustained attention and processing speed, and language (table e-1 at Neurology.org). All participants who underwent an MRI scan and those identified as at risk for dementia as well as a random sample of those who likely did not have dementia completed a more detailed in-person interview including a neurologic examination, the Clinical Dementia Rating scale, the Functional Activities Questionnaire, and the Neuropsychiatric Inventory. In addition to the complete neuropsychological evaluation at the ARIC-NCS visit, a brief cognitive examination was performed at visits 2 and 4 (table e-1). The visit 2 and 4 testing was used to identify those with cognitive decline who were selected for the MRI at visit 5.
Ascertainment of MCI and dementia occurred by expert review using a predetermined algorithm and incorporated data from the ARIC-NCS visit and from the longitudinal cognitive evaluations at visits 2 and 4. The algorithm was based on the National Institute on Aging–Alzheimer's Association workgroup formulations of MCI and dementia21,22 and DSM-5.23 MCI and dementia diagnoses were established by review of all information by a physician (neurologist or geriatrician) and a neuropsychologist. If the 2 disagreed, a third clinician reviewed the case to arrive at a final diagnosis.16 Etiologic diagnoses for dementia were assigned for individuals seen in person during the more detailed interview, who were given a diagnosis of MCI or dementia. Reviewers designated at least one of the following, but were allowed to choose more than one: AD-related, cerebrovascular disease–related, Lewy body disease–related, or other.16
Other variables.
ARIC visits included a clinic visit with standardized evaluation of vascular risk factors. Vascular risk factors were ascertained at visit 1 to represent midlife contributions to vascular disease later in life. Education, smoking history, and alcohol use were assessed using standard questionnaires. Prevalent stroke was defined as a self-report of stroke with symptoms confirmed by clinical personnel. Height and weight were measured and body mass index calculated in kilogram per square meter. Systolic and diastolic blood pressure were measured in the right arm with the patient seated, and were recorded as the mean of the last 2 of 3 measurements.
Serum glucose and a lipid profile were measured in fasting blood samples. Low-density lipoprotein (LDL) was calculated using the Friedewald formula.24 Diabetes mellitus was defined as fasting glucose ≥126 mg/dL, a self-reported history of diabetes, or self-reported treatment for diabetes. Blinded APOE genotyping was determined from blood samples at visit 1 and was done using the TaqMan assay (Applied Biosystems, Foster City, CA).
Statistical analysis.
We conducted a cross-sectional analysis evaluating the association of ICAD with prevalent cognitive status (classified as normal, MCI, or dementia) at the ARIC-NCS visit. We compared participant characteristics by cognitive status category using one-way analysis of variance for continuous variables and χ2 tests for categorical variables. Participants were selected for the MRI scan using the probabilistic sampling plan described above. In order to represent the full visit 5 cohort, sampling weights were derived as the product of the inverse sampling fractions and the inverse probability of completing the examination.25 We used multinomial logistic regression to estimate the relative prevalence ratios (RPRs) and 95% confidence intervals (CIs) of ICAD presence with cognitive outcomes, using normal cognition as the reference group for MCI or dementia. The RPR for the dementia outcome can be interpreted as the ratio of 2 prevalence ratios: the ratio of the prevalence of dementia comparing participants with and without ICAD and the ratio of the prevalence of being in the normal category comparing participants with and without ICAD. A similar interpretation applies to MCI. If MCI or dementia prevalence is higher among those with ICAD, the RPR will be greater than 1.0. In addition to overall presence of ICAD, we evaluated plaque location (ACA, MCA, PCA, vertebral, basilar, ICA) and presence of stenosis (>50%, >70%). Next we stratified number of plaques and number of territories with plaque into 3 categories (0, 1–2, and ≥2). The categorical variable number of plaques and number of territories were used as the predictors in multinomial logistic regression models to identify the RPR with cognitive outcomes (normal reference; MCI or dementia).
For multivariable analyses, we used several models with progressive degrees of adjustment. Models were first adjusted for sociodemographic factors and then for vascular risk factors, including systolic blood pressure and LDL. We tested for differences in the association between ICAD and cognitive status by race and sex, but none of the tests for interaction was statistically significant (not shown). Sensitivity analyses were performed excluding all participants with prevalent stroke (n = 60) and in participants with minimal arteriolosclerosis (without lacunar stroke and in the lowest 2 quintiles of white matter hyperintensity volume). Table e-2 shows the RPR of ICAD presence with MCI and dementia excluding those with a history of prior stroke. The sensitivity analyses for those with minimal arteriolosclerosis are further described in the e-Methods and table e-3. A 2-sided p value of <0.05 was considered significant for all analyses.
RESULTS
Participant characteristics.
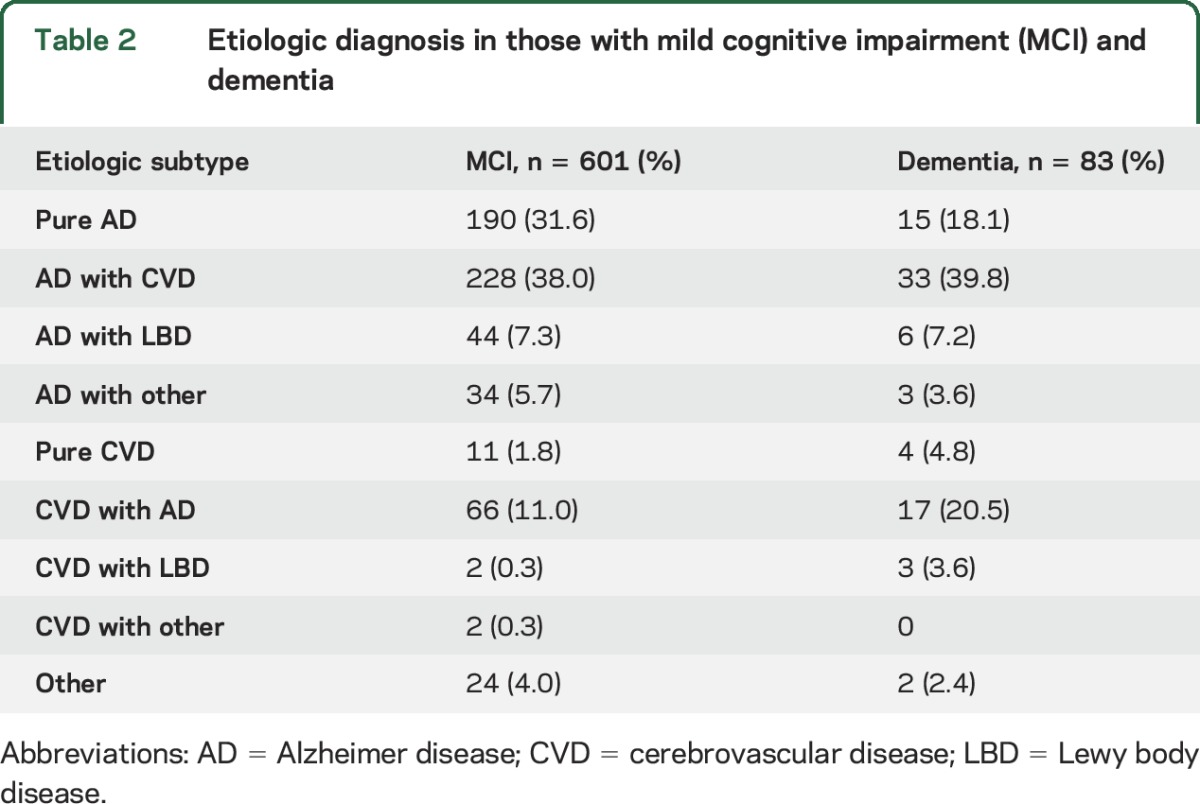
The mean age of participants undergoing brain MRI was 76.3 ± 5.3 years, and participants with MCI and dementia were, on average, older compared to those with normal cognition (table 1). Although women comprised 58.6% (n = 1,022) of participants undergoing brain MRI, relatively fewer women had MCI and dementia (table 1). Of 506 black participants (29.0% of participants), 131 had MCI and 27 had dementia (table 1). Participants with MCI and dementia were more likely to have had a prior stroke, and had higher burden of midlife diabetes and higher blood pressure and LDL (table 1). Among 1,744 participants, 601 and 83 participants were classified as MCI and dementia, respectively (weighted prevalence of 22% and 3%, respectively). Compared with cognitively normal participants, those with dementia were slightly older, less educated, and more likely to have a history of diabetes and stroke (table 1). The overall prevalence of current or former smoking was 49.1%, and this did not vary by cognitive status (table 1). Table 2 shows the etiologic diagnosis in participants with MCI and dementia. The most common subtypes of MCI and dementia were pure AD, AD with cerebrovascular disease, and cerebrovascular disease with AD. The weighted prevalence of ICAD in at least one territory was 34.5% (n = 636). Almost half of participants with ICAD had plaque in more than one territory (n = 291, 46%). Participants with ACA plaques had the greatest number of plaques in other territories (mean 6.2 other, SD 4.5) and the greatest number of other territories affected (table e-4).
Table 1.
Participant characteristics
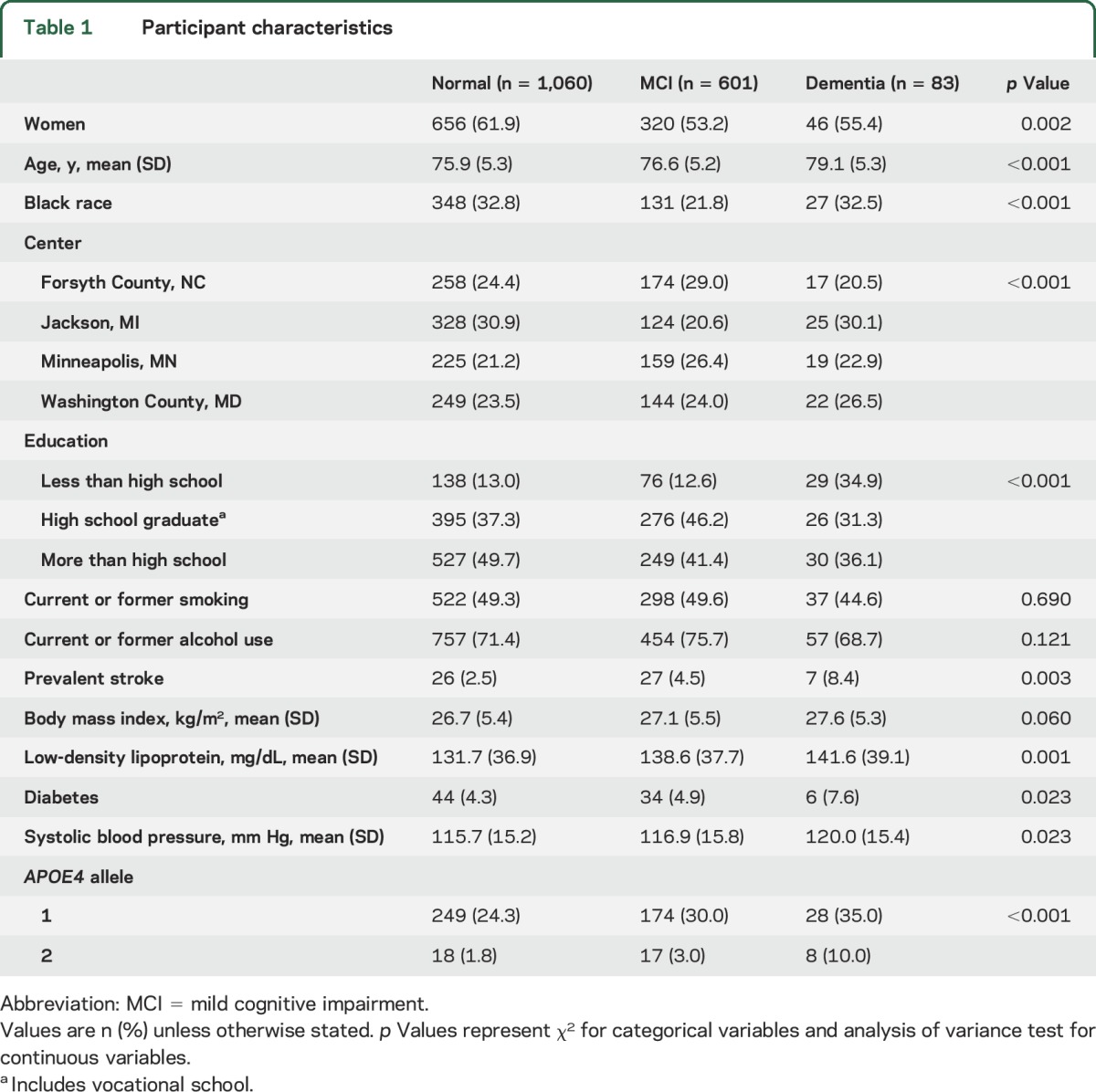
Table 2.
Etiologic diagnosis in those with mild cognitive impairment (MCI) and dementia
ICAD characteristics and MCI and dementia.
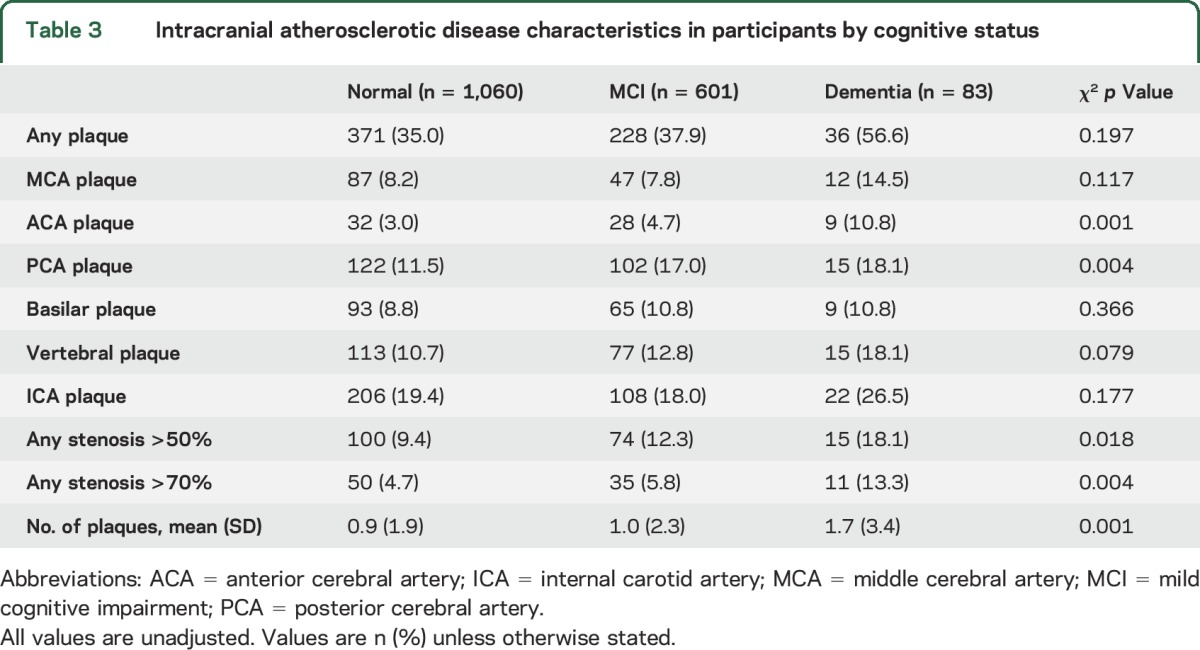
The prevalence of ICAD in participants with normal cognition, MCI, and dementia was 35.0, 37.9, and 56.6%, respectively (table 3). Having any plaque, an ACA plaque, a plaque in the posterior circulation (PCA, vertebral, basilar), or a stenosis >50% were significantly associated with the prevalence of MCI (table 3), while having any plaque, plaque in any individual territory except the basilar artery, or any stenosis >50% or >70% were significantly associated with the prevalence of dementia (table 3). The mean number of plaques in participants with normal cognition, MCI, and dementia was 0.9, 1.0, and 1.7, respectively (table 3).
Table 3.
Intracranial atherosclerotic disease characteristics in participants by cognitive status
In multivariable analysis, the presence of a PCA plaque was associated with an increased prevalence of MCI (table 4). Other territories, number of plaques, and number of territories with plaque were not associated with a significantly higher RPR of MCI (table 5). For dementia, the presence of ACA plaque was strongly associated with the prevalence of dementia even after adjustment for vascular risk factors (RPR 3.81, 95% CI 1.57–9.23, table 4), as were the presence of stenosis >50% or >70% in any territory and the presence of plaque in >2 territories (tables 4 and 5). Other territories with plaque and number of plaques were not significantly associated with increased risk of dementia (table 5).
Table 4.
Intracranial atherosclerotic disease and risk of mild cognitive impairment (MCI) and dementia
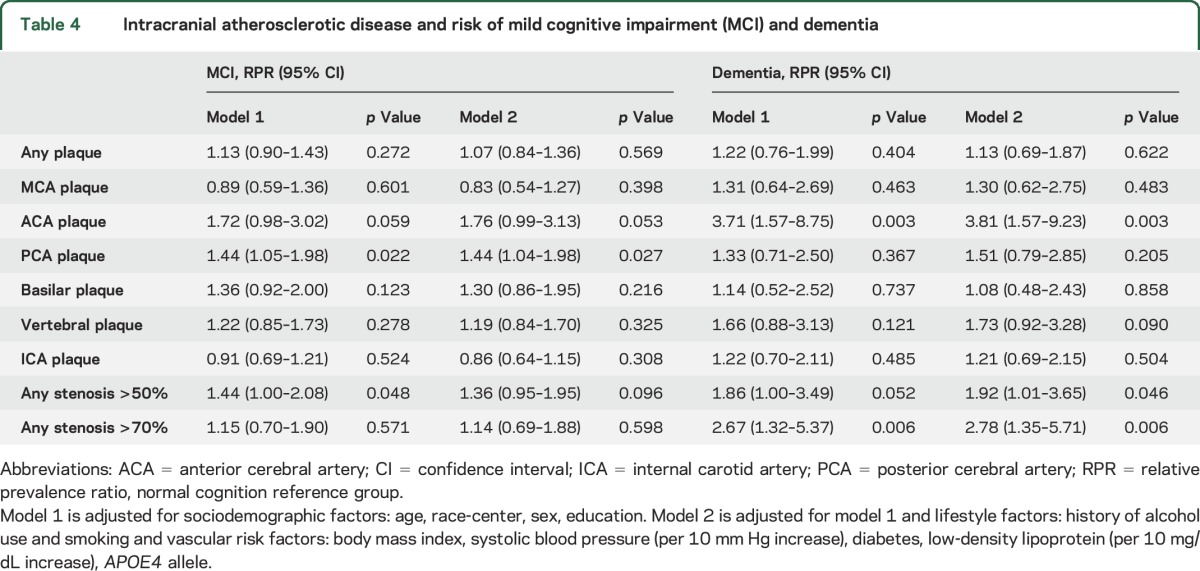
Table 5.
Number of plaques and territories affected and risk of cognitive impairment and dementia
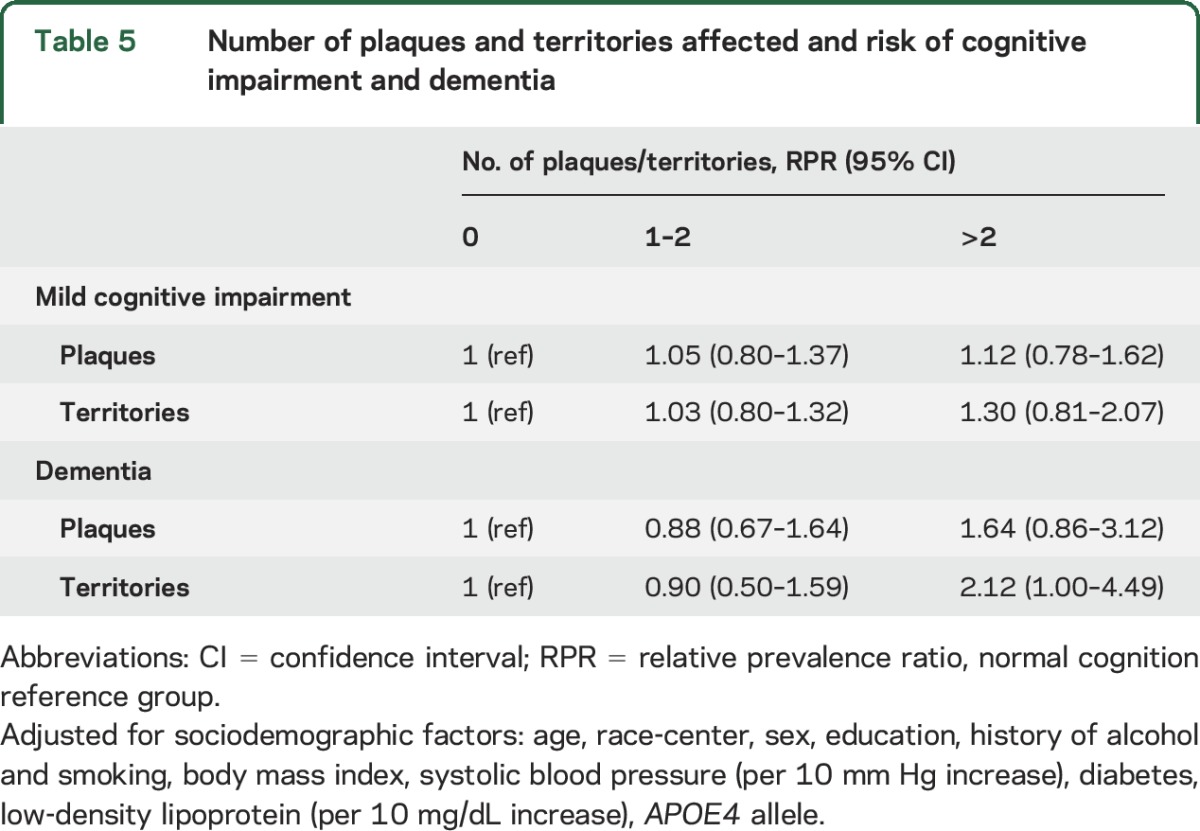
Sensitivity analyses did not change the magnitude or interpretation of the results (tables e-2 and e-3). Only 5 of 640 participants (<1%) with low burden of arteriolosclerosis/small vessel disease had dementia, limiting interpretation of this subgroup.
DISCUSSION
Our major finding was that ICAD, specifically, >2 vascular territories with plaque ACA plaque presence and stenosis >50%, was associated with dementia, even when controlling for traditional vascular risk factors. These findings support the growing body of research suggesting that atherosclerosis is an important contributor to late-life cognitive impairment.2,26,27 It is not clear from our studies what the mechanisms are by which large vessel disease could cause neuronal dysfunction or whether this is only an association. It could simply be that atherosclerosis in large vessels like the ACA is driven by the same mechanisms that drive arteriolosclerosis or microvascular disease, both of which have also been linked to late-life cognitive impairment. Although there are suggestions that there are associations between atherosclerosis and excess β-amyloidosis, definitive evidence for a synergistic interaction between cerebrovascular disease and AD pathophysiology has been elusive. As this study is cross-sectional, it is only hypothesis-generating regarding this point.
Current theories of dementia pathogenesis suggest there is a continuum between vascular disease and dementia such as AD.28 There is increasing recognition that microvascular disease and β-amyloid and tau deposition often coexist. Many believe that identification and treatment of vascular risk factors is equally important to prevent cognitive decline.27 This study highlights that it may be important to investigate the associations with ICAD and dementia because many people have ICAD alone. In addition, ICAD prevalence is higher in ethnic groups such as black and Asian as compared to persons of European extraction.29 We are unable to comment on racial differences in the associations between ICAD and dementia for white and black participants in this study because the number of black participants with dementia was too small. Finally, arteriolosclerosis explains only a small part of age-related cognitive decline, highlighting the need to identify other contributors to cognitive impairment.5
A small number of studies have examined the link between ICAD and dementia.11,12,30 Our results support cadaveric studies that have shown that the number of atherosclerotic plaques and degree of stenosis is associated with AD.9,10,31,32 These studies have not elucidated which locations may be more associated with dementia to confirm our findings.
Associations with ACA plaque and dementia were not fully accounted for by the number of plaques in other territories. One possible explanation is that ACA plaques could develop later, making them a better indicator of disease severity, as opposed to, for example, ICA plaques, which were more frequent and were not significantly associated with dementia. Another important location may be the PCA, which was associated with MCI. The ACA, as well as the PCA, supply 2 anatomic areas important to cognitive functioning and memory processing: the cholinergic nucleus basalis of Meynert supplied by the ACA and the hippocampus supplied by the PCA.9,33 This does not, however, explain why the MCA, which supplies areas of the brain important for language and interpretation of spatial information, did not reach statistical significance in association in our population.
Limitations of this study include its cross-sectional design, which does not account for reverse causality (the possibility that dementia/MCI develop before ICAD) or for the possibility that associations simply represent independent age-related phenomena that happen to develop simultaneously. In addition, as participants were enrolled in midlife, participants with extreme measures of disease were less likely to survive or enroll in visit 5, 21 years later. This selection bias towards the less sick participants would be expected to attenuate the results to the null. There were few participants with plaques in only one territory, limiting our ability to evaluate the implications of involvement of each territory alone. The number of participants with dementia was not sufficient to adequately power subgroup analyses by race or other potential interactions. A strength is the availability of midlife risk factors and reliable measurement of confounders. The study design allowed for state-of-the-art classification of ICAD and a rigorous dementia adjudication protocol.
This cross-sectional analysis provides the basis for future work and follow-up in this cohort. Future directions from this study are to longitudinally study participants with ICAD to evaluate incident dementia, allowing for a prospective evaluation of risk. If this work confirms our observed associations with plaque characteristics and dementia, ICAD may be a biomarker to identify those at risk of future cognitive decline. Further, evidence of a prospective relationship between ICAD and conversion to dementia would emphasize the importance of prevention and management of ICAD, with the goal of preventing cases of dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of the ARIC Study for their contributions and the dementia adjudication panel.
GLOSSARY
- ACA
anterior cerebral artery
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- ARIC-NCS
Atherosclerosis Risk in Communities Neurocognitive Study
- BBMRI
black blood MRI
- CI
confidence interval
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- ICA
internal carotid artery
- ICAD
intracranial atherosclerotic disease
- LDL
low-density lipoprotein
- MCA
middle cerebral artery
- MRA
magnetic resonance angiography
- PCA
posterior cerebral artery
- RPR
relative prevalence ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jennifer L. Dearborn: primary authorship of manuscript, statistical analysis, interpretation of data, approval of final manuscript. Yiyi Zhang: statistical input, manuscript revision, approval of final manuscript. Ye Qiao: data interpretation, manuscript revision, approval of final manuscript. Muhammad Fareed K. Suri: study design, approval of final manuscript, obtained funding. Li Liu: statistical input, data interpretation, approval of final manuscript. Rebecca F. Gottesman: manuscript revision, approval of final manuscript. Dr. Gottesman is an Associate Editor for Neurology®. Andreea Rawlings: manuscript revision, approval of final manuscript. Thomas H. Mosley: manuscript revision, approval of final manuscript. Alvaro Alonso: manuscript revision, approval of final manuscript. David S. Knopman: manuscript revision, approval of final manuscript. Eliseo Guallar: data interpretation, statistical input, manuscript revision, approval of final manuscript. Bruce A. Wasserman: study concept, interpretation of data, manuscript revision, approval of final manuscript, obtained funding.
STUDY FUNDING
This study was supported by NIH RO1HL105930, RO1HL105626, and K99HL106232. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825.
DISCLOSURE
J. Dearborn and Y. Zhang report no disclosures relevant to the manuscript. Y. Qiao has a patent application pending (13/922,111) for the 3D high-resolution vessel wall MRI technique used in this study. M. Suri, L. Liu, R. Gottesman, A. Rawlings, T. Mosley, and A. Alonso report no disclosures relevant to the manuscript. D. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals, and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH. E. Guallar reports no disclosures relevant to the manuscript. B. Wasserman has a patent application pending (13/922,111) for the 3D high-resolution vessel wall MRI technique used in this study. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation: a systematic review. BMC Neurol 2006;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–2399. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 5.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 2000;356:628–634. [DOI] [PubMed] [Google Scholar]
- 6.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 7.Neuropathology Group of the Medical Research Council Cognitive F, Ageing S. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001;357:169–175. [DOI] [PubMed] [Google Scholar]
- 8.Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke 2010;41:S144–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roher AE, Tyas SL, Maarouf CL, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimer's Demen 2011;7:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 2007;113:13–21. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Wang Y, Li J, Deng J, Zhou H. Intracranial artery stenosis and progression from mild cognitive impairment to Alzheimer disease. Neurology 2014;82:842–849. [DOI] [PubMed] [Google Scholar]
- 12.Bos D, Vernooij MW, de Bruijn RFAG, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement 2015;11:639–647. [DOI] [PubMed] [Google Scholar]
- 13.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011;34:22–30. [DOI] [PubMed] [Google Scholar]
- 14.Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke 2016;47:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study. Design and objectives: the ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016;280:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astor BC, Sharrett AR, Coresh J, Chambless LE, Wasserman BA. Remodeling of carotid arteries detected with MR imaging: atherosclerosis risk in communities carotid MRI study. Radiology 2010;256:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association ; 2013. [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities–Neurocognitive Study. Stroke 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci 2007;257:80–87. [DOI] [PubMed] [Google Scholar]
- 27.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. J Am Med Assoc 1997;277:813–817. [PubMed] [Google Scholar]
- 28.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging studies. Neurology 2016;86:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke 1986;17:648–655. [DOI] [PubMed] [Google Scholar]
- 30.De Silva DA, Ancalan M, Doshi K, Chang HM, Wong MC, Chen C. Intracranial large artery disease in Alzheimer's disease and vascular dementia among ethnic Asians. Eur J Neurol 2009;16:643–645. [DOI] [PubMed] [Google Scholar]
- 31.Kalback W, Esh C, Castano EM, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer's disease. Neurol Res 2004;26:525–539. [DOI] [PubMed] [Google Scholar]
- 32.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US national Alzheimer's coordinating center. Neurology 2005;64:494–500. [DOI] [PubMed] [Google Scholar]
- 33.Liu A, Chang RC, Pearce RB, Gentleman S. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer's and Parkinson's disease. Acta Neuropathol 2015;129:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


