In the human lower respiratory tract, influenza A (INFA) can activate an inflammatory response that interferes with the innate immune response to bacterial pathogens, increasing the probability of subsequent infection by opportunistic organisms such as methicillin-susceptible (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA).1,2 Some hypervirulent strains of community-acquired and hospital-acquired MSSA and MRSA can express Panton-Valentine Leukocidin (PVL), which is a 2-component pore-forming cytotoxin that primarily destroys neutrophils in both healthy and immunocompromised patients.3 As a result of white blood cell destruction by PVL-positive MSSA/MRSA pathogens during the inflammatory response of an INFA-positive patient, bacterial infection can quickly intensify into severe necrotizing pneumonia.1 PVL-positive MSSA/MRSA infections are associated with a 40% to 75% patient mortality rate.4,5 Here, we present high-resolution bronchoscopic images and describe the diagnosis and treatment of a patient with INFA-H1N1-09 and PVL-positive MRSA pneumonia.
CASE REPORT
A 64-year-old man with a history of COPD and diabetes was admitted with progressive shortness of breath, cough, and generalized weakness despite outpatient antibiotics following influenza-like illness. He was septic, hyperglycemic, hypoxemic, and serum creatinine was mildly elevated (1.37 mg/dL). A chest x-ray showed a questionable early infiltrate and linear densities in the right base of the lung. Empiric vancomycin, cefepime, and levofloxacin were started; however, respiratory failure rapidly progressed requiring intubation. The patient developed atrial fibrillation with rapid ventricular response with normal proBNP level. The CT angiogram demonstrated multifocal airspace disease in both lower lobes, a poorly defined, fairly dense infiltrate in the left upper lobe, but no pulmonary embolism. The CT findings were most consistent with infiltrates indicative of pneumonia. A subacute lower extremity DVT was found on ultrasound. On the second day, renal failure progressed, renal replacement therapy was initiated, and the patient required vasopressors for septic shock. A bronchoscopy was performed revealing diffuse intense hemorrhagic tracheobronchitis with profound mucosal sloughing almost obstructing the airway (Fig. A). The findings were so extreme that biopsies were obtained and an urgent pathologic evaluation for malignancy and viral and fungal infection was requested. In the meantime, empiric liposomal amphotericin B and acyclovir were added.
Histologic evaluation of biopsies revealed acute inflammatory exudates, but was negative for malignancy, fungal organisms, viral pathogens, and PCP smears. Bronchial wash specimens were tested through culture and Target Enriched Multiplex Polymerase Chain Reaction Viral Respiratory Panel (TEM-PCR VRP; Diatherix Laboratories, LLC; Huntsville, AL).6 On day 3, the TEM-PCR VRP was positive for INFA-H1N1-09, therefore, oseltamivir was added. On day 4, the bronchial wash specimen was additionally tested with the TEM-PCR Staphylococcus Differentiation Panel and identified the presence of MRSA and the PVL cytotoxin. On the fifth day, the bronchial wash culture grew MRSA.
Later on day 5, vancomycin was switched to linezolid due to worsening leukocytosis and the presence of the PVL cytotoxin. A second bronchoscopy that day demonstrated decreased, but still significant, necrotizing inflammation (Figs. 1A, B). Eventually, the patient’s renal function recovered and septic shock and atrial fibrillation had resolved. On day 10, a third bronchoscopy demonstrated drastic improvement of previously seen endobronchial findings (Figs. 1C, D). On day 13, despite development of delirium, the patient was successfully extubated and on day 20, the patient was eventually discharged to the rehab after completion of a 2-week course of linezolid.
FIGURE 1.
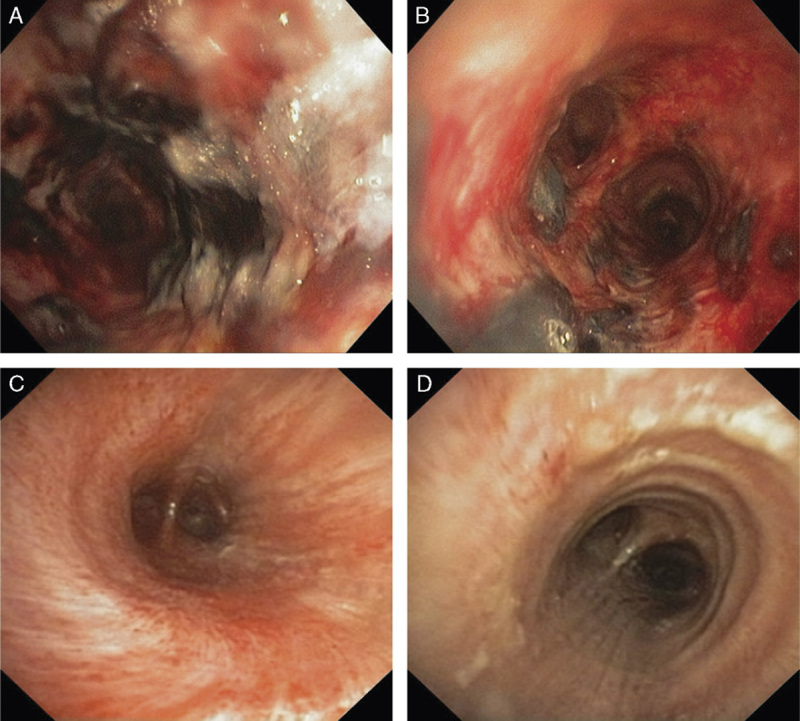
Bronchoscopy images. On day 5, after therapy was altered to cover all pathogens, areas of mucosal hemorrhage remained in the trachea (A) and mainstem bronchus (B), but there was partial improvement seen from day 2. On day 10, a third bronchoscopy showed near-full resolution of the trachea (C) and mainstem bronchus (D).
FIGURE A.
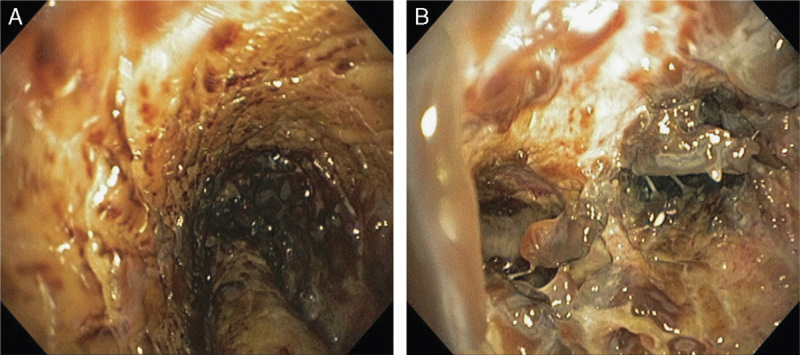
Bronchoscopy images of pneumonia. On day 2, severe necrotizing hemorrhagic tracheobronchitis (A) and sloughing of mucosa obstructing the right mainstem bronchus (B) are seen.
DISCUSSION
In this case, a variety of possible causes of necrotizing tracheobronchitis seen through bronchoscopy were investigated, including fungal, viral, and/or bacterial infection(s) and malignancy. Initial fungal and histologic tests for malignancy were negative, but subsequent diagnostics were positive for INFA-H1N1-09 and PVL-positive MRSA.
The suggested treatment differs between PVL-negative MSSA/MRSA and PVL-positive MSSA/MRSA infections. Therefore, identifying which pathogen(s), antibiotic resistance gene(s), and/or cytotoxin(s) are present impacts the optimal choice of medication and influences the probability of full patient recovery. For patients infected with PVL-negative MSSA pneumonia, the suggested treatment involves a β-lactam antimicrobial effective against gram-positive bacteria.7,8 However, if the MSSA is PVL positive, the use of a β-lactam antibiotic may actually increase toxin production, leading to further patient deterioration.9 Guidelines suggest that PVL-negative MRSA be treated with vancomycin because the pathogen is resistant to β-lactam antimicrobials.7,8 However, vancomycin is not recommended for the treatment of PVL-positive MRSA because vancomycin has suboptimal tissue penetration characteristics and cannot reach the concentration necessary in the lungs to fully eliminate the PVL-producing MRSA.10 Low tissue concentration of vancomycin may have been a contributing factor to the continued mucosal hemorrhaging in our patient after 5 days of empiric vancomycin treatment.
PVL-positive MRSA infection can only be successfully neutralized with medications that not only destroy the bacterial pathogen, but also eliminate their ability to produce the PVL toxin and deactivate any toxins that were released into the bloodstream.9 Whereas vancomycin and β-lactam antimicrobials mechanistically inhibit proper cell wall synthesis and can actually spread active PVL toxins to neighboring healthy cells during bacterial eradication, linezolid and clindamycin are both bacterial protein synthesis inhibitors that prohibit the formation of ribosomes in gram-positive bacteria.9 As a result, translation of the PVL toxin cannot occur.
In addition, linezolid has excellent respiratory pharmacokinetics, the medication can penetrate bronchial fluids and tissue more efficiently, and the medication’s activity is not affected if the patient has weakened renal functionality.10 These characteristics make linezolid one of the most effective antibiotics for combating PVL-positive MSSA/MRSA infection and, as seen in Figures 1A–D, the implementation of this medication resulted in full patient recovery.
CONCLUSIONS
This case demonstrates the successful diagnosis and treatment of a patient with rapidly progressing necrotizing pneumonia and multiorgan failure caused by PVL-positive MRSA complicating INFA-H1N1-09 infection. Impressive bronchoscopic dynamics of extensive hemorrhagic tracheobronchitis were seen. These images can be valuable in the clinic and aid in the correct diagnosis of patients with complex respiratory conditions of similar presentation.
ACKNOWLEDGMENTS
The authors thank Karen Wolters for testing the specimens for this patient through TEM-PCR. In addition, the authors thank Vicki Caneer and Michelle Hammond for proofreading manuscript drafts, and Brint Roden and Pete Garrett for formatting the bronchoscopy images.
Footnotes
Supported by Fort Hamilton Hospital and Diatherix Laboratories, LLC.
Presented as a poster (Abstract: 2615) at the ERS International Congress 2014 meeting in Munich, Germany.
After this study, M.G. volunteered to be on the Medical Advisory Council for Diatherix. M.D.H. is a full-time employee of Diatherix Laboratories, LLC. C.Q. was a paid consultant for Diatherix Laboratories, LLC at the time of this study, but C.Q. have since retired their roles. M.I.G. declares no conflict of interest or other disclosures.
REFERENCES
- 1.Niemann S, Ehrhardt C, Medina E, et al. Combined action of influenza virus and Staphylococcus aureus Panton-Valentine Leukocidin provokes severe lung epithelium damage. J Infect Dis. 2012;206:1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MH, Arrecubieta C, Martin FJ, et al. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loffler B, Hussain M, Grundmeier M. Staphylococcus aureus Panton-Valentine Leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. [DOI] [PubMed] [Google Scholar]
- 5.Kreienbuehl L, Charbonney E, Eggimann P. Community-acquired necrotizing pneumonia due to methicillin-sensitive Staphylococcus aureus secreting Panton-Valentine leukocidin: a review of case reports. Ann Intensive Care. 2011;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassoun MD, Huff MD, Weisman D, et al. Seasonal variation of respiratory pathogen colonization in asymptomatic health care professionals: a single-center, cross-sectional, 2-season observational study. Am J Infect Control. 2015;43:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health Protection Agency. Guidance on the Diagnosis and Management of PVL-associated Staphylococcus aureus Infections (PVL-SA) in England, 2nd ed London: Health Protection Agency; 2008. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322857/Guidance_on_the_diagnosis_and_management_of_PVL_associated_SA_infections_in_England_2_Ed.pdf. Accessed October 19, 2015. [Google Scholar]
- 8.American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 9.Stevens DL, Ma Y, Salmi DB, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–211. [DOI] [PubMed] [Google Scholar]
- 10.Stein GE, Wells EM. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia complicated skin and soft-tissue infection caused by methicillin-resistant Staphylococcus aureus: vancomycin and linezolid. Curr Med Res Opin. 2010;26:571–588. [DOI] [PubMed] [Google Scholar]


