Abstract
Background:
Electromagnetic navigation bronchoscopy (ENB) may aid in the diagnosis of solitary pulmonary lesions with a lower complication rate than conventional diagnostic modalities. A curved-tip catheter is now available for use with ENB; however, the diagnostic yield of this device has not been previously reported.
Methods:
A single-center, single-operator retrospective chart review was performed on patients who underwent ENB for the diagnosis of pulmonary lesions. A curved-tip catheter was used in all procedures; angle options were chosen depending on lesion location. After navigation to the target lesion, fine-needle aspiration, brushings, biopsies, and bronchoalveolar lavage were performed in all patients. Correct localization was confirmed with fluoroscopy.
Results:
Thirty-one consecutive patients underwent ENB between February and October 2014. The mean lesion size was 1.8 cm (range, 0.4 to 4.0 cm) and 35% were in the right upper lobe. The probe tip was navigated to the target lesion in all cases. A diagnosis was obtained in 30/31 patients (96.8%). Twenty-two (71%) had a definitive histologic diagnosis of malignancy. One case was nondiagnostic requiring a repeat fine-needle aspiration. The remaining 8 nonmalignant cases were followed radiologically and demonstrated no progression of lesion size through at least 1 year. Fiducials were placed in 48% of cases. There were 2 pneumothoraces (6.5%), one of which required chest tube placement (3.2%).
Conclusions:
This study suggests that the curved-tip catheter is a useful modality for diagnosing peripheral pulmonary lesions with ENB. The diagnostic yield of ENB using this catheter was superior to that reported in other studies utilizing straight catheters.
Key Words: bronchoscopy, electromagnetic navigation bronchoscopy, image-guided biopsy, lung cancer, lung neoplasms, solitary pulmonary nodule, superDimension navigation system
Lung cancer is the leading cause of cancer death among both men and women in the United States, with an estimated 158,040 deaths in 2015. Although most cancers have shown a steady increase in survival rates over the past 3 decades, the prognosis for lung cancer remains poor. The 5-year survival rate for lung cancer is only 18%, largely due to late-stage diagnosis, for which the survival rate is only 4%.1 Thus, early diagnosis is critical.
For patients with solitary pulmonary nodules suspicious for lung cancer, the diagnostic treatment plan is typically determined based on the preprocedure probability of malignancy. In cases where the probability of malignancy is high, thoracoscopic excision is usually recommended.2
However, a significant percentage of patients are medically inoperable due to cardiac and pulmonary comorbidities or prior contralateral surgery.2–4 Furthermore, many peripheral lung lesions are beyond the reach of conventional bronchoscopy, representing an additional challenge for the management of the inoperable patient. The risk of bleeding and pneumothorax after transthoracic needle biopsy is also considerably higher in patients with pulmonary comorbidities. Anatomic limitations preclude transthoracic biopsy for many lesions as well. Predictors of pneumothorax after transthoracic needle biopsy include chronic obstructive pulmonary disease (COPD), age ≥60 years, tobacco use, small diameter lesions, and distance from pleura.5 COPD has also been associated with increased bleeding risk.5 In a meta-analysis of 20 studies, pneumothorax rates of 15% to 25% (4% to 6% requiring a chest tube) were reported after transthoracic needle lung biopsy.5
Electromagnetic navigation bronchoscopy (ENB) has been recommended for the diagnosis of peripheral lung lesions difficult to reach with conventional bronchoscopy.6 In inoperable patients and those with comorbidities that would increase the risk of complications after transthoracic biopsy, ENB offers an alternative where none existed previously, with low pneumothorax rates of approximately 3% (1.6% requiring a chest tube).7 ENB has also been successfully used for fiducial marker placement.8–10
Prior studies of ENB have demonstrated rates of diagnostic yield ranging from 59.9% to 94%, with a pooled sensitivity rate of 82% in 1 recent meta-analysis (mean reported diameter range 20.0 to 70.0 mm).11 Diagnostic yield rates are higher in more recent studies,12–15 possibly related to software updates and advances in catheter design and associated tools. In particular, a recently available curved-tip directional catheter designed for use with the ENB system may allow better angulation capability and maneuverability compared with straight catheters. To date, there are no publications specifically evaluating the performance of this curved-tip catheter in the evaluation of peripheral lung lesions.
The objective of this single-center, retrospective chart review was to examine diagnostic yield of ENB using the curved-tip catheter to aid in the biopsy of pulmonary lesions.
METHODS
This study was conducted in accordance with the amended Declaration of Helsinki and the Health Care Portability and Accountability Act of 1996. The protocol was deemed exempt from review by the Midwest Health System Institutional Review Board (Lee Summit, MO, November 25, 2014) in accordance with US Department of Health and Human Services Regulation 45 CFR 46.101(b)(4) for the collection of existing anonymous patient data.
Materials
The device under study was the Edge catheter [manufactured by Covidien (now Medtronic), Mansfield, MA] which was used with the superDimension navigation system version 7 (Medtronic). Like the traditional straight superDimension navigation catheters (Medtronic), the Edge catheter includes a radiopaque material on the distal 2 mm for x-ray visibility. In contrast to straight catheter, the Edge catheter uses a preformed curved tip, in 45, 90, or 180 degrees configurations for angulation and steerability (Fig. 1).
FIGURE 1.
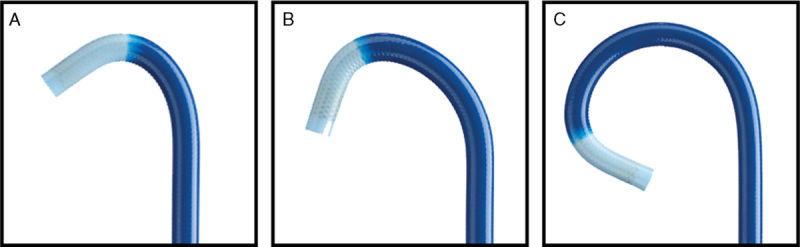
Curved-tip catheter in 45 degrees (A), 90 degrees (B), and 180 degrees (C) configurations. All rights reserved. Used with the permission of Medtronic. Copyright 2016 Medtronic.
Procedures
Data from 31 consecutive patients undergoing ENB for the diagnosis of peripheral lung lesions between February 2014 and October 2014 were retrospectively reviewed. Patients were deemed inoperable due to severe COPD, the presence of bilateral disease, or other contraindications. Procedures were performed by a single pulmonologist (M.C.) who had undergone prior training in ENB and were conducted with the patient under general anesthesia in a similar manner to prior reports.12,13 After planning the target location and advancing the bronchoscope, the curved-tip extended working channel catheter was inserted and advanced to the target lesion. C-arm fluoroscopy was used to confirm correct localization in all cases. Endobronchial ultrasound was not used. Once the catheter reached the target, the locatable guide was removed and endoscopic tools were inserted. Fine-needle aspiration (FNA), brushings, transbronchial biopsy, and bronchoalveolar lavage were performed in all patients (although not necessarily in that order). Rapid on-site evaluation was conducted by a cytotechnologist in the operating suite for immediate feedback on atypical cells. Fiducial markers were placed through the extended working channel when warranted. Retrospective data collected include the location and width of the lesions, complications, and final diagnosis. Follow-up imaging data were available through 1-year follow-up for all patients with an initial nonmalignant diagnosis.
Analyses
Data were summarized by descriptive statistics (for continuous variables) or frequencies and percentages (for categorical variables). Diagnostic yield was calculated as the percentage of patients who received an ENB-aided diagnosis (malignant or nonmalignant) and was confirmed with radiologic follow-up through at least 1 year.
RESULTS
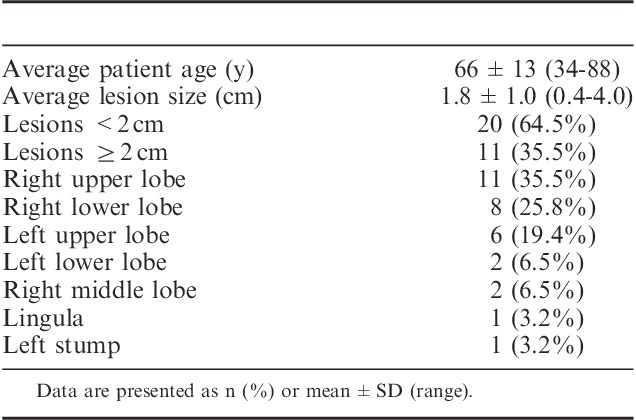
Thirty-one patients underwent ENB between February 2014 and October 2014. The mean lesion size was 1.8 cm (range, 0.4 to 4.0 cm) and 35.5% were in the right upper lobe. More than half (64.5%) of patients had lesions <2 cm in diameter (Table 1). On the basis of computed tomography imaging, distance from the pleura was <3 cm in 26 patients and >3 cm in 5 patients [mean 1.4±1.2 cm (range, 0.1 to 4.0 cm)].
TABLE 1.
Patient Demographics and Lesion Characteristics (N=31 Patients)
The probe tip was navigated to the target lesion in all cases (100% navigation success). No cases were terminated due to an inability to reach the lesion. A single target lesion was evaluated in each case. Fiducial markers were placed in 48% of cases.
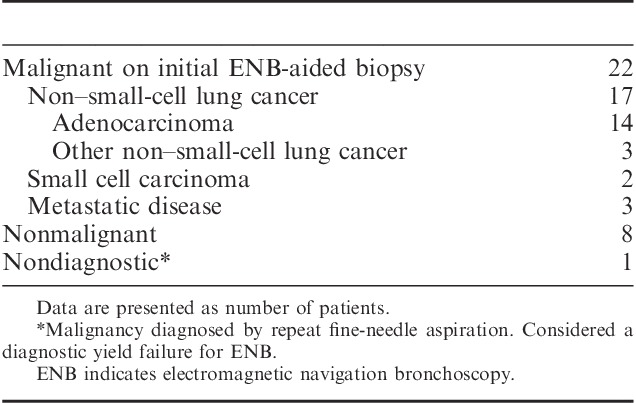
A diagnosis was obtained in 30/31 patients for a diagnostic yield of 96.8%. One case (a patient with a 2.7 cm right upper lobe lesion) was nondiagnostic by ENB requiring a repeat FNA, which led to a final malignant diagnosis. Twenty-two patients (71%) had a definitive histologic diagnosis of malignancy aided by ENB (Table 2), including 14 cases of adenocarcinoma, 3 cases of other non–small-cell lung cancer, 2 cases of small-cell lung cancer, and 3 cases of metastatic disease (breast, colon, and renal). The remaining 8 nonmalignant cases (1 infection, 2 granuloma, 5 lymphocytes) were followed radiologically and demonstrated no progression of lesion size through 1 year.
TABLE 2.
ENB-aided Diagnosis (N=31 Patients)
There were 2 pneumothoraces (6.5%), one of which required chest tube placement (3.2%) in a patient with emphysema. There was no major bleeding observed.
DISCUSSION
Published studies of ENB use in the diagnosis of peripheral lung lesions have demonstrated promising results; however, the diagnostic yield of the curved-tip catheter has not been previously reported. The results of this study suggest that the catheter is useful to aid in the diagnosis of peripheral lung lesions, providing a diagnostic yield rate (96.8%) higher than that seen in prior publications, ranging from 59.9% to 94%.11
Subjective experience of the operator in this study was that the curved-tip catheter allowed maneuverable access to peripheral lesions. Other reasons for the high yield rate observed in this study could also be the use of general anesthesia and intubation of the patient, availability of rapid on-site evaluation by a cytotechnologist for immediate feedback on atypical cells, location confirmation by fluoroscopy, and the employment of multiple biopsy methods, all of which have been recommended in the literature to improve yield.7,15–20 Loo et al14 have reported that concurrent ENB-guided bronchial brushing, transbronchial biopsy, and FNA with rapid on-site evaluation resulted in a diagnostic yield of 95%. Choice of catheter may also impact yield. Catheter selection was made at the discretion of the operator based on the curve of the route identified during the planning phase of each navigational bronchoscopy. A precurved catheter was selected from the 180, 90, and 45 degree options for navigating to the lesion, followed by the deployment of aspirating needle, biopsy forceps, and biopsy brush through the extended working channel. This retrospective study did not capture which catheter was used for each case. Future prospective studies should specifically delineate the success of each individual tool.
Another factor in the variation in diagnosis yield across studies is the definition of diagnostic yield. In the current study all 22 cases initially diagnosed as malignant went on to further tumor treatment (radiosurgery, or a combination of chemotherapy and radiation) with confirmation of malignancy (no false positives). The 8 patients with an initial nonmalignant diagnosis by ENB have demonstrated no progression of lesion size over at least 1 year of radiographic follow-up. At the current time, these cases are therefore considered true negatives. Thus, only 1 patient was nondiagnostic by ENB for a 1-year diagnostic yield rate of 30/31 (96.8%). Most studies consider granuloma or lymphocytes an accurate nonmalignant diagnosis provided there is confirmation of no disease progression over time,12,16–18,20–23 whereas others consider these nondiagnostic absent of a specific benign diagnosis such as bacterial infection, tuberculosis, hamartoma, etc.24 In a worst case sensitivity analysis in which the 2 cases diagnosed as granuloma and the 5 cases diagnoses as lymphocytes in the current study were ultimately determined to be malignant, the diagnostic yield could be as low as 23/31 (74%). However, that scenario is not consistent with the clinical presentation and subsequent follow-up.
Safety outcomes in the current series were comparable to results of prior studies. Two patients experienced a pneumothorax. A chest tube was inserted in one patient and the other was observed overnight and sent home with no further treatment. In our experience, most pneumothoraces are predictable and occur in patients with emphysema or lesions close to the pleura. Thus, close observation and care must be taken with such patients.
Limitations
This was a retrospective, single-arm, single-center, single-operator study with no direct comparison with the straight catheter or prospective comparison between catheters types or biopsy tools. Follow-up beyond 1 year will also be important in future studies to confirm initially negative diagnoses.
CONCLUSIONS
This study suggests that the curved-tip catheter is a useful modality for diagnosing peripheral pulmonary lesions with ENB. The diagnostic yield of ENB using this catheter was superior to that reported in other studies utilizing straight catheters. ENB provides an alternative approach to the diagnosis of peripheral lung lesions in inoperable and high-risk patients.
Footnotes
The authors independently collected and analyzed the data, which was presented as a poster at the 2015 American Thoracic Society Annual Meeting. Kristin L. Hood, PhD of Medtronic provided medical writing support for this manuscript based on content and conclusions determined by the authors. No funding or compensation was provided for the collection of data or the preparation of the manuscript. Open Access fees were paid by Medtronic.
Disclosure: M.C. has a publication support agreement with Medtronic for the completion of this work. There is no conflict of interest or other disclosures for the remaining authors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–313S. [DOI] [PubMed] [Google Scholar]
- 3.Mentzer SJ, Swanson SJ. Treatment of patients with lung cancer and severe emphysema. Chest. 1999;116:477S–479S. [DOI] [PubMed] [Google Scholar]
- 4.Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–190S. [DOI] [PubMed] [Google Scholar]
- 5.Wiener RS, Wiener DC, Gould MK. Risks of transthoracic needle biopsy: how high? Clin Pulm Med. 2013;20:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e142S–165S. [DOI] [PubMed] [Google Scholar]
- 7.Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87:165–176. [DOI] [PubMed] [Google Scholar]
- 8.Mungal V, Sarsour RM, Siddiqui AM, et al. The utility of bronchoscopy for the placement of fiducial markers for stereotactic body radiotherapy. Clin Pulm Med. 2015;22:294–297. [Google Scholar]
- 9.Bolton WD, Richey J, Ben-Or S, et al. Electromagnetic navigational bronchoscopy: a safe and effective method for fiducial marker placement in lung cancer patients. Am Surg. 2015;81:659–662. [PubMed] [Google Scholar]
- 10.Nabavizadeh N, Zhang J, Elliott DA, et al. Electromagnetic navigational bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy: analysis of safety, feasibility, and interfraction stability. J Bronchology Interv Pulmonol. 2014;21:123–130. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Chen S, Dong X, et al. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis. 2015;7:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowling MR, Kohan MW, Walker P, et al. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol. 2015;22:5–13. [DOI] [PubMed] [Google Scholar]
- 13.Odronic SI, Gildea TR, Chute DJ. Electromagnetic navigation bronchoscopy-guided fine needle aspiration for the diagnosis of lung lesions. Diagn Cytopathol. 2014;42:1045–1050. [DOI] [PubMed] [Google Scholar]
- 14.Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol. 2014;122:191–199. [DOI] [PubMed] [Google Scholar]
- 15.Mohanasundaram U, Ho LA, Kuschner WG, et al. The diagnostic yield of navigational bronchoscopy performed with propofol deep sedation. ISRN Endoscopy. 2013;2013:1–5. [Google Scholar]
- 16.Wilson DS, Bartlett RJ. Improved diagnostic yield of bronchoscopy in a community practice: combination of electromagnetic navigation system and rapid on-site evaluation. J Bronchology Interv Pulmonol. 2007;14:227–232. [Google Scholar]
- 17.Balbo PE, Bodini BD, Patrucco F, et al. Electromagnetic navigation bronchoscopy and rapid on site evaluation added to fluoroscopy-guided assisted bronchoscopy and rapid on site evaluation: improved yield in pulmonary nodules. Minerva Chir. 2013;68:579–585. [PubMed] [Google Scholar]
- 18.Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center’s early success. Ann Thorac Surg. 2012;93:944–949; discussion 949-950. [DOI] [PubMed] [Google Scholar]
- 19.Karnak D, Ciledag A, Ceyhan K, et al. Rapid on-site evaluation and low registration error enhance the success of electromagnetic navigation bronchoscopy. Ann Thorac Med. 2013;8:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchology Interv Pulmonol. 2012;19:91–97. [DOI] [PubMed] [Google Scholar]
- 21.Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007;131:1800–1805. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol. 2011;18:133–137. [DOI] [PubMed] [Google Scholar]
- 23.Jensen KW, Hsia DW, Seijo LM, et al. Multicenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodules. J Bronchology Interv Pulmonol. 2012;19:195–199. [DOI] [PubMed] [Google Scholar]
- 24.Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


