Abstract
Perturbations in neuregulin-1 (NRG1)/ErbB4 function have been associated with schizophrenia. Affected patients exhibit altered levels of these proteins and display hypofunction of glutamatergic synapses as well as altered neuronal circuitry. However, the role of NRG1/ErbB4 in regulating synapse maturation and neuronal process formation has not been extensively examined. Here we demonstrate that ErbB4 is expressed in inhibitory interneurons at both excitatory and inhibitory postsynaptic sites. Overexpression of ErbB4 postsynaptically enhances size but not number of presynaptic inputs. Conversely, knockdown of ErbB4 using shRNA decreases the size of presynaptic inputs, demonstrating a specific role for endogenous ErbB4 in synapse maturation. Using ErbB4 mutant constructs, we demonstrate that ErbB4-mediated synapse maturation requires its extracellular domain, whereas its tyrosine kinase activity is dispensable for this process. We also demonstrate that depletion of ErbB4 decreases the number of primary neurites and that stimulation of ErbB4 using a soluble form of NRG1 results in exuberant dendritic arborization through activation of the tyrosine kinase domain of ErbB4 and the phosphoinositide 3-kinase pathway. These findings demonstrate that NRG1/ErbB4 signaling differentially regulates synapse maturation and dendritic morphology via two distinct mechanisms involving trans-synaptic signaling and tyrosine kinase activity, respectively.
Although central nervous system synapses utilize a variety of brain-specific molecules to mediate contact formation and maturation, some of the proteins implicated in this process are also major players in neuromuscular junction development. Among these shared molecules are NRG13 and its receptor, ErbB4, which are expressed in both the developing and adult brain. Neuregulins comprise a family of four related genes (nrg1–4), each producing a large number of isoforms via differential promoter usage and alternative splicing (1, 2). NRGs contain EGF-like repeats, which enable them to bind to and activate EGF family receptors (ErbB2–4). Previous studies showed that NRG1 is initially synthesized as a transmembrane protein, which then undergoes proteolytic processing, whereby the extracellular EGF-containing fragment is released into the extracellular environment. The remaining intracellular fragment has been shown to translocate into the nucleus, where it regulates neuronal survival and transcription of PSD-95 (3, 4). Proteolytic processing of NRG1 is also regulated by neuronal activity and by interaction with ErbB receptors (3, 5). NRG1 is widely expressed throughout development and adulthood, with the highest expression in nervous tissue (6) and is essential for survival. In the central nervous system NRG1 is also required for differentiation, migration, and development of neurons and glia as well as for axonal myelination and pathfinding, dendritic development, and neurotransmitter receptor maintenance. During development, NRG1-ErbB signaling mediates radial glia maintenance and elongation, whereas glial-derived NRG1 directs the migration of cortical and cerebellar neurons (7, 8). Moreover, NRG1-ErbB4 signaling is required to direct axons of thalamocortical projections to their targets (9). In the brain and spinal cord, NRG1 regulates oligodendrocyte differentiation, and in spinal cord explants from NRG1−/− mice, oligodendrocytes fail to develop (10–12).
NRG1 signaling is mediated through its receptors; that is, the ErbB family of proteins. There are four members in the ErbB family, named one through four. However, ErbB1 specifically binds EGF and does not respond to NRG1. ErbB2 contains the functional kinase domain but is unable to bind NRG1 (13). ErbB3 on the other hand binds NRG1 but is unable to propagate the signal to the cells because of lack of kinase activity (14). ErbB4 is a single-pass, 160-kDa transmembrane protein with extra- and intracellular regions of approximately equal size (15, 16). A kinase domain is located within the intracellular region of ErbB4, which also contains a PDZ binding motif at the C terminus, enabling ErbB4 to interact with PDZ-domain containing proteins such as PSD-95 (17, 18).
Several isoforms of ErbB4 occur due to alternative splicing of the gene: juxtamembrane-a and -b (JM-a and JM-b) isoforms differ in their sensitivity to proteolytic cleavage by TACE (tumor necrosis factor-α-converting enzyme) metalloprotease, with only the JM-a isoform being sensitive to this cleavage (19). The CYT-1 isoform differs from CYT-2 in that it contains a PI3K binding region (15). The presence of multiple ErbB4 isoforms and their differential distribution may contribute to the diversity of ErbB4 signaling. Although the function of the soluble extracellular fragment is unknown, it raises the possibility of retrograde signaling (15). The intracellular fragment that remains after cleavage has been shown to act as a constitutive kinase (20) and propagate signaling from the cell surface. ErbB4 interacts with PSD-95, SAP102, and b2-syntrophin, proteins enriched at the postsynaptic density (17, 18, 21). Interestingly, the interaction between ErbB4 and PSD-95 is regulated by neuronal activity (22), and the increased interaction between these proteins is detected in brain lysates from schizophrenia patients (23). Moreover, previous findings showed that ErbB receptor-mediated NRG1 signaling modulates N-methyl-d-aspartate receptor (NMDAR) function (24), indicating that NMDARs constitute an immediate target of NRG1/ErbB4 signaling. At the synapse, ErbB4 also interacts with and regulates activation of two non-receptor protein kinases, Fyn and pyk2. These molecules have been implicated in regulation of N-methyl-d-aspartate receptor phosphorylation and long term potentiation induction (25).
Here we examine the role of ErbB4 in synapse maturation and neuronal morphology in primary hippocampal cultures by overexpressing wild-type and mutant forms of ErbB4, knocking down endogenous protein, and/or treating cells with NRG1. We show that, although ErbB4 is not sufficient to induce presynaptic differentiation in neuron-COS7 cell co-cultures, ectopic expression of ErbB4 in dissociated hippocampal neurons enhances the size of presynaptic terminals contacting ErbB4-expressing cells. Furthermore, knockdown of endogenous ErbB4 results in a decrease in the size of presynaptic protein clusters. Interestingly, the number of presynaptic terminals is unchanged in cells with altered ErbB4 expression, highlighting a trans-synaptic role for ErbB4 in synapse maturation but not initial formation. Finally, we show that stimulation of ErbB4 signaling by NRG1 alters process outgrowth via activation of the PI3K signaling pathway.
EXPERIMENTAL PROCEDURES
cDNA Cloning and Mutagenesis—The original full-length ErbB4 (JM-a, CYT-2 isoform) cDNA and ErbB4 shRNA constructs were gifts from Dr. Lin Mei. HA-tagged transmembrane isoform of neuregulin-1 (HA-NRG1) was a gift from Dr. Jianxin Bao (Medical College of Georgia, Augusta, Georgia). The HA tag (YPYDVPDYA) was inserted after amino acid 997. HA-tagged wild type NL1 amplified from mouse cerebellum was a gift from Dr. Peter Scheiffele (Columbia University). GFP transfections were carried out using pEGFP-C1 plasmid (Clontech).
The generation of ErbB4-HA, ErbB4-HA ΔPDZb, and ErbB4-HA ΔNT, was carried out by PCR subcloning in two steps. First, a construct that contains the ErbB4 signal sequence followed by the HA tag (ss-HA) was generated by PCR using oligonucleotides containing AflII and XbaI restriction sites followed by subcloning the resulting fragment into the pCDNA3.1(+) vector (Invitrogen). Generation of the deletion mutants was carried out by PCR using oligonucleotides with the following restriction sites: ErbB4-HA ΔNT-NotI/XbaI (forward, ggactcGCGGCCGCCATTCCACTTTACCACAACATG; reverse, GGGCCCTCTAGATTACACCACAGTATTCC) and subcloning amplified fragments into the ss-HA construct. Finally, ErbB4-HA ΔPDZb was made by amplification of the intracellular region of ErbB4 using oligonucleotides containing KpnI and XbaI restriction sites (forward, GTATTTGGGTACCTGAAGGAG; reverse, GGGCCCTCTAGATTACCGGTGTCTGTAAGGTGG) and subcloning the resulting fragment into the ErbB4-HA ΔCT construct, restoring the full sequence of ErbB4 lacking only the last four amino acids. A single point mutation in the ErbB4 kinase domain (K751R) was introduced using the QuikChange site-directed mutagenesis kit (Stratagene). Briefly, the entire plasmid was amplified by PCR using oligonucleotides containing one base substitution (AAG → AGG) (GTGAAGATTCCTGTGGCTATTAGGATTCTTAATGAGACAACTGG), the template methylated strand was destroyed enzymatically with Dpn1, and purified construct was transformed into bacteria and verified by direct sequencing. All constructs were verified by direct sequencing.
To suppress the expression of endogenous ErbB4, the pFUGW vectors expressing short hairpin RNAs specifically directed against rat ErbB4 (26) were transfected into hippocampal neurons using Lipofectamine 2000 (Invitrogen) 3 or 5 days before. The target sequences of two short hairpin RNAs for erbB4 are 5′-CCAGACTACCTGCAGGAATAC-3′ (hp2) and 5′-GCCCGCAATGTGTTGGTGAAA-3′ (hp3). A vector expressing GFP was used as a control.
Cell Culture and Mixed Culture Assay—Dissociated primary neuronal cultures were prepared from hippocampi of embryonic day 18/19 Wistar rats. Cells were dissociated by papain digestion followed by brief mechanical trituration and plated on poly-d (or l)-lysine (Sigma)-treated coverslips at a density of 105 per 8-mm glass coverslip in minimal essential medium (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), glucose (Sigma), sodium pyruvate, GlutaMAX, and penicillin/streptomycin (Invitrogen). After 2 h the medium was replaced with NeuroBasal medium (Invitrogen) supplemented with B-27, GlutaMAX, and penicillin/streptomycin (Invitrogen) as previously described (27). Every 3–4 days, half of the volume of maintenance medium was taken out and replaced with fresh solution. Cultures were transfected by lipid-mediated gene transfer using Lipofectamine 2000 reagent following the manufacturer’s protocol (Invitrogen) or by the calcium phosphate technique (Clontech) as previously described (28).
COS7 cells were grown in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), sodium pyruvate, and penicillin/streptomycin (Invitrogen). For protein expression cells were transfected with Lipofectamine 2000 reagent (Invitrogen) and collected/fixed 24–36 h later.
Neuron-COS7 cell co-cultures were performed as previously described (29). Briefly, neurons were grown on glass coverslips suspended above a glial feeder layer in minimal essential medium with N2 supplements. COS7 cells were transfected with Lipofectamine 2000 (Invitrogen) and trypsinized 24 h later. Cells were washed twice with fetal bovine serum-supplemented Dulbecco’s modified Eagle’s medium and plated onto neurons that had been pre-grown for 9–10 days. COS7 cells were allowed to adhere and grow on neurons for 24 h before fixation.
HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), sodium pyruvate, and penicillin/streptomycin (Invitrogen). For protein expression, cells were transfected with Lipofectamine 2000 reagent (Invitrogen) for 12 h, grown for an additional 6 h, collected, and resuspended in Dulbecco’s modified Eagle’s medium. To examine transcellular interaction between proteins, cells transfected with the appropriate constructs were co-cultured on 10-mm glass coverslips (Fisher) and allowed to grow for 24 h before fixation with 4% paraformaldehyde supplemented with 4% sucrose (Sigma).
Immunocytochemistry and Reagents—Coverslips were fixed in −20 °C methanol for staining for synaptic proteins or in 4% paraformaldehyde with 4% sucrose (Sigma) and permeabilized with 0.3% Triton-X-100 in phosphate-buffered saline. The following primary antibody solutions were used: HA (mouse, 1:1000, Berkeley Antibody Co., Inc., and rat, 1:1000, Roche Applied Science), GFP (chicken, 1:1000, Abcam), synaptophysin (mouse, 1:1000, Sigma, and rabbit, 1:500, Pharmingen), VGLUT1 (rabbit, 1:1000, Synaptic Systems and guinea pig, 1:2000, Chemicon), VGAT (rabbit, 1:1000, Synaptic Systems and Chemicon), PSD-95 (mouse 1:500, Affinity BioReagents, and rabbit, custom made by Affinity BioReagents), gephyrin (mouse, 1:1000, Synaptic Systems), ErbB4 (mouse, 1:200, Neo-Markers, and rabbit, 1:200, Santa Cruz), MAP2 (mouse, 1:500, BD Pharmingen), and p-ErbB4 (mouse, Cell Signaling). Secondary antibodies were generated in goat and conjugated with Alexa 488 (1:1000), Alexa 568 (1:1000, Molecular Probes), or aminomethyl coumarin acetate (1:100, Jackson Immuno-Research). All antibody reactions were performed in blocking solution containing 2% normal goat serum for 1 h at room temperature or overnight at 4 °C. Preparation and purification of the protein coding for the extracellular domain of ErbB4 fused to Fc (ecto-ErbB4) was previously described (30). Human recombinant NRG1 containing the EGF motif was purchased from R&D Systems (Minneapolis, MN), resuspended in phosphate-buffered saline with 0.1% bovine serum albumin at a concentration of 10 mg/ml, separated into aliquots, and stored at −20 °C. PI3K inhibitor (LY 294002) and MAPK inhibitor (PD 98059) were purchased from Calbiochem and resuspended in dimethyl sulfoxide at a concentration of 10 mm and 5 mg/ml, respectively, separated into aliquots, and stored at −20 °C.
For tissue section immunohistochemistry, adult (postnatal day 120) female rat brains were rapidly extracted, embedded in OCT medium, and flash-frozen in isopentane cooled in liquid nitrogen. Sections were cut on a cryostat at thicknesses of 8 mm to allow detection of synaptic puncta. Sections were kept frozen until fixation in −20 °C methanol for 10 min. After thorough washing in phosphate-buffered saline, sections were incubated in blocking solution (2.5% bovine serum albumin, 0.1% Triton-X-100, 0.02% sodium azide) for 45 min followed by primary antibody diluted in blocking solution overnight at 4 °C. After washing, sections were incubated with Alexa-conjugated secondary antibodies (fluorescent immunohistochemistry; Molecular Probes) and mounted with Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL).
Western Blotting—To assess phosphorylation levels of ErbB4 deletion mutants, constructs were transfected into COS7 cells for 24 h, and proteins were analyzed by Western blot. COS7 cells were washed with ice-cold phosphate-buffered saline and resuspended in 500 μl of lysis buffer containing 20 mm HEPES, pH 7.0, 0.5% deoxycholic acid, 0.1% Nonidet P-40, 150 mm NaCl, 2 mm EDTA, 10 mm NaF, 2 mm sodium orthovanadate, 0.25 mm phenylmethylsulfonyl fluoride, and 1 protease inhibitor tablet/10 ml (Roche Applied Science). After extracting for 20 min at 4 °C, insoluble material was removed by centrifugation at 13,000 × g for 15 min at 4 °C. Samples were boiled for 3 min upon the addition of 4× SDS-PAGE sample buffer containing 10% β-mercaptoethanol and analyzed by SDS-PAGE.
Nitrocellulose membranes were blocked in 1% bovine serum albumin or 5% milk and incubated with primary antibody solutions overnight at 4 °C. Western blot signals were detected with the Odyssey machine (Li-Cor) using infrared-conjugated antibodies as previously described (31) or by ECL (Amersham Biosciences).
Imaging and Analysis—Images were acquired on a Zeiss Axiovert M200 motorized microscope with a 63 × 1.4 NA ACROMAT oil immersion lens and a monochrome 14-bit Zeiss Axiocam HR charge-coupled camera with 1300 × 1030 pixels. The exposure time was adjusted per individual experiment to achieve maximal brightness without saturation; for intensity measurement experiments, all pictures were taken at equal exposure for all experimental conditions. To correct for out-of focus areas within the field of view, focal plane (z) stacks were collected, and maximum intensity projections were compiled. Images were scaled to 16 bits and analyzed in Northern Eclipse (Empix Imaging Inc., Mississauga, ON, Canada) using custom written software routines as previously described (32).
In brief, images were processed at a constant threshold level (of 32,000 pixel values), and dendrites visualized by immunofluorescence signal were outlined. Only clusters with average pixel values three times greater than background (diffuse dendritic shaft pixel values) were selected for analysis. The number of dendritic clusters per unit length was measured as a function of dendritic length and normalized to controls.
For intensity analysis, the average background intensity was subtracted from the average intensity of individual puncta and multiplied by the puncta area to obtain integrated intensity. For colocalization analysis, background-subtracted immunofluorescence clusters for all imaging channels (red, green, and blue) were correlated for overlapping signal. Colocalization was scored if clusters in two channels were overlapping by at least 1 pixel for a postsynaptic and presynaptic protein. Two-tailed Student’s t test was performed to calculate the statistical significance of results between experimental groups.
To quantify the extent of protein accumulation at the area of cell-cell contact in HEK cell experiments, a line was drawn through the area of contact, and pixel intensity was measured using ImageJ software. Average intensity was then normalized to the level of protein expression in the cell in question, which was determined by taking the average intensity along a line of equal length drawn through the cell body.
To analyze the ability of ErbB4 expressed in heterologous cells to induce clustering of VGLUT1 and VGAT, a co-culture system was used. COS7 cells transfected with ErbB4 or NL1 were co-cultured with hippocampal neurons. Immunostaining for VGLUT1/PSD-95 and VGAT/gephyrin combinations was used to assess the induction of excitatory and inhibitory presynaptic terminals, respectively. Random ErbB4- or NL1-immunopositive COS cells showing contact with neuronal processes by phase contrast were classified as to whether they exhibited any associated clusters of VGLUT1 lacking PSD95 or VGAT lacking gephyrin (presumed induced clusters in the contacting axons).
RESULTS
Localization of Endogenous ErbB4 at Excitatory and Inhibitory Synapses—Previous studies have demonstrated that ErbB4 expression is restricted to inhibitory GABAergic interneurons of the developing and adult cortex and hippocampus (33–35). Consistent with this, ErbB4 expression in dissociated hippocampal neurons at DIV 14 demonstrated a punctate pattern of immunostaining in neurons positive for the inhibitory neuronal markers, VGAT and GAD65 (the GABA synthesizing enzyme), with no signal detectable in GAD65-negative cells (Fig. 1A and data not shown). Other studies have demonstrated that ErbB4 clusters at synaptic sites and colocalizes with a number of markers for excitatory synapses, including the NR1 subunit of N-methyl-d-aspartate receptors and PSD-95 (17). As inhibitory neurons receive both excitatory and inhibitory inputs, we assessed the distribution of ErbB4 at excitatory and inhibitory synapses. Hippocampal neurons were double-labeled with ErbB4 and either VGLUT1 or VGAT, markers for excitatory or inhibitory presynaptic terminals, respectively (Fig. 1A). Consistent with previous findings, a significant proportion of ErbB4 clusters (64 ± 3%) was associated with VGLUT1-positive puncta (Fig. 1B). However, a smaller proportion of ErbB4 clusters (32 ± 6%) were apposed to VGAT puncta (Fig. 1B), indicating that ErbB4 is present not only at excitatory but also at a subset of inhibitory postsynaptic sites. Together these results indicate that ErbB4 is expressed in inhibitory GABAergic hippocampal interneurons and is localized largely at the postsynaptic compartment of excitatory synapses, with a smaller proportion found at inhibitory contacts.
FIGURE 1.
Localization of ErbB4 at excitatory and at a subset of inhibitory synapses. A, hippocampal neurons were fixed at DIV 14 and immunostained for endogenous ErbB4 (green) and either VGLUT1 (top panel) or VGAT (bottom panel) (red), presynaptic markers for excitatory and inhibitory synapses, respectively. ErbB4 clusters were apposed to the majority of VGLUT1 and a subset of VGAT presynaptic puncta (arrows). Scale bars, 10 μm; insets, 5 μm). B, quantification of the localization of ErbB4 clusters at sites apposed to excitatory and inhibitory synaptic markers. 64 ± 3% of VGLUT1 clusters were apposed to ErbB4, whereas 32 ± 6% of VGAT puncta colocalized with ErbB4 (n = 7 cells). C, a subset of ErbB4 puncta is colocalized with the inhibitory GABAergic postsynaptic marker, gephyrin. Adult rat brain sections were stained for ErbB4 and gephyrin. The inset shows colocalization of ErbB4 and gephyrin in the CA1 region of the hippocampus. Scale bar, 20 μm.
To confirm that the localization of ErbB4 at inhibitory contacts is not an artifact of the culture system, we immunostained adult rat brain sections for ErbB4 and the inhibitory postsynaptic marker, gephyrin. In the cortex and hippocampus, expression of ErbB4 was limited to GABAergic interneurons (data not shown). A proportion of ErbB4 clusters was colocalized with gephyrin, consistent with the localization of a subset of ErbB4 at inhibitory synapses (Fig. 1C).
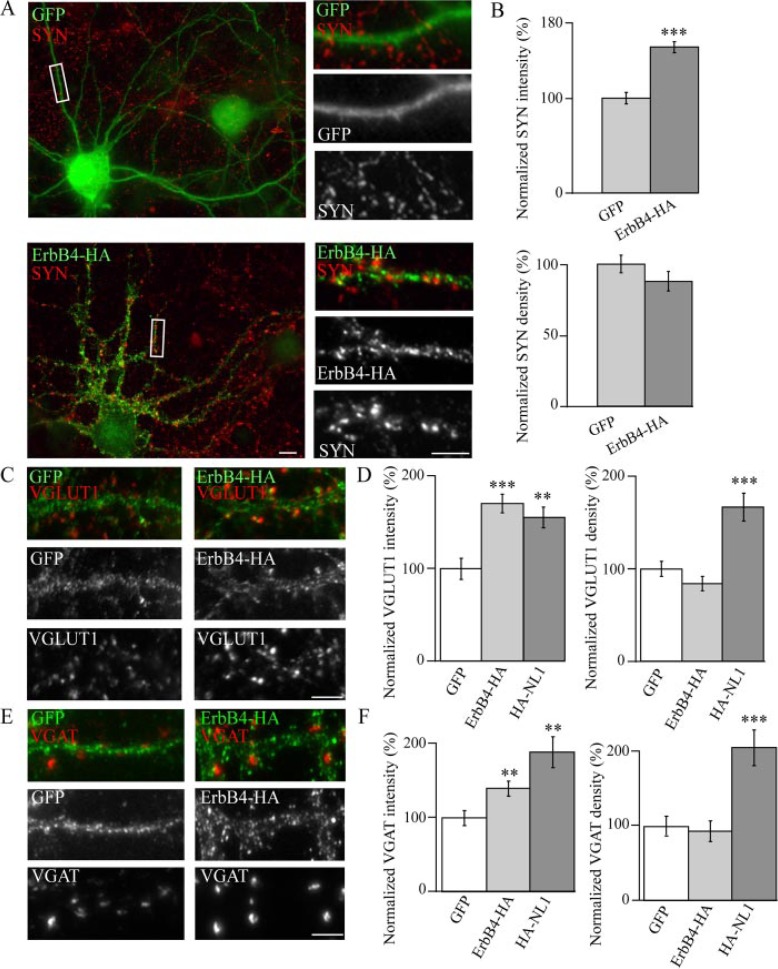
ErbB4 Overexpression Enhances the Maturation of Presynaptic Terminals—To determine the role of ErbB4 at the synapse at early stages of neuronal development, we generated an HA-tagged version of full-length ErbB4, placing the tag immediately after the tyrosine kinase domain to avoid interference with PDZ-dependent interactions (see Fig. A) (17). This construct was then expressed in dissociated embryonic hippocampal neurons at DIV 5–6 followed by fixation at DIV 10–11, a period at which synaptogenesis occurs. Levels of ectopic ErbB4 expression in excitatory neurons were comparable with endogenous ErbB4 levels observed in inhibitory neurons (supplemental Fig. 1). Synapses were visualized by immunostaining cultures with the presynaptic terminal marker, synaptophysin (SYN) (Fig. 2A). Analysis revealed an increase in the integrated intensity (herein referred to as size) of SYN-positive puncta in untransfected neurons forming synapses with ErbB4-HA-expressing cells compared with cells expressing GFP (Fig. 2B). Despite a 155% increase in the intensity of SYN-positive puncta associated with ErbB4-expressing cells, no significant difference was seen in synapse density (Fig. 2B). The enhancement of size, but not number, of synapses suggests that ErbB4 modulates certain aspects of synapse maturation rather than the initial induction of synapses.
FIGURE 2.
ErbB4 induces excitatory and inhibitory presynaptic maturation. A, hippocampal neurons were transfected with GFP (top panel) or ErbB4 tagged with HA in the C-terminal region (ErbB4-HA), and immunostained for the presynaptic marker, SYN. B, analysis reveals that ErbB4 expression enhances the intensity, but not the density, of SYN-positive puncta when compared with cells expressing GFP (n = 27 cells). C and E, neurons were transfected with either GFP, ErbB4-HA, or HA-tagged neuroligin 1 (HA-NL1) and examined for changes in VGLUT1 and VGAT puncta intensity and density. D and F, summary of changes in VGLUT1 (D) and VGAT (F) cluster size (intensity) and number (density) in cells overexpressing ErbB4-HA (n = 16 and 17 cells, respectively) or HA-NL1 (n = 8 and 7 cells, respectively), compared with GFP (n = 16 and 12 cells)0expressing cells. NL1 significantly enhances the number of VGAT and VGLUT1 puncta per unit dendritic length compared with GFP-expressing cells. In contrast, ErbB4 expression increased puncta intensity but had no effect on cluster number when compared with GFP controls. ***, p < 0.001; **, p < 0.01. Scale bars, 5 μm.
To confirm that the HA tag did not interfere with the normal function of ErbB4, another construct with the HA-tag inserted immediately after the signal sequence (HA-ErbB4) was generated. Both constructs had similar effects on presynaptic maturation, confirming that the observed effects are not due to altered protein folding or function caused by insertion of the HA epitope (see Fig. 4, B and C).
FIGURE 4.
Protein domains required for ErbB4-mediated effects on presynaptic maturation. A, Schematic diagram summarizing the mutant forms of ErbB4 used. B, hippocampal neurons were transfected with either GFP, wild type (ErbB4-HA), or with mutant forms of ErbB4 and immunostained for SYN. C, summary of changes in SYN cluster intensity. Both full-length ErbB4 (ErbB4-HA and HA-ErbB4) and ErbB4-HA KD (n = 17 cells) significantly enhanced SYN cluster intensity compared with GFP (n = 20 cells). However, deletion of the N-terminal (n = 18 cells) and PDZ binding motif (n = 9 cells) abolished the effects of ErbB4 on SYN puncta intensity. ***, p < 0.001 (scale bar, 5 μm).
ErbB4 Enhances the Maturation of Both Excitatory and Inhibitory Synapses—The level of excitation in the brain is kept in check through inhibitory control exerted by GABAergic neurons (36). Thus, a proper ratio of excitatory and inhibitory synapses is critical for neuronal excitability and brain function. In this manner, neurons strike a “balance” that allows them to become activated and convey discrete synaptic signals while preventing excessive excitation. This balance can be disturbed by even small changes in the level of inhibition, resulting in epileptiform activity. Factors that regulate both excitatory and inhibitory contact formation and/or maintenance may, therefore, represent key elements for fine-tuning neuronal excitability. Because ErbB4 is detected at excitatory and inhibitory synapses, we analyzed whether ErbB4 expression modulates the maturation of a heterogeneous population of synaptic contacts. Similar to the effects on SYN, there was an overall increase in the intensity of VGLUT1- and VGAT-positive puncta associated with ErbB4-expressing cells relative to GFP controls (Fig. 2, C–F). Again, consistent with the data relating to SYN, no significant changes were detected in the density of VGLUT1 and VGAT clusters (Fig. 2, D and F). These results are in contrast to the enhanced VGLUT1 and VGAT cluster density and intensity in cells transfected with NL1, a potent inducer of synapse formation and maturation (Fig. 2, D and F) (32).
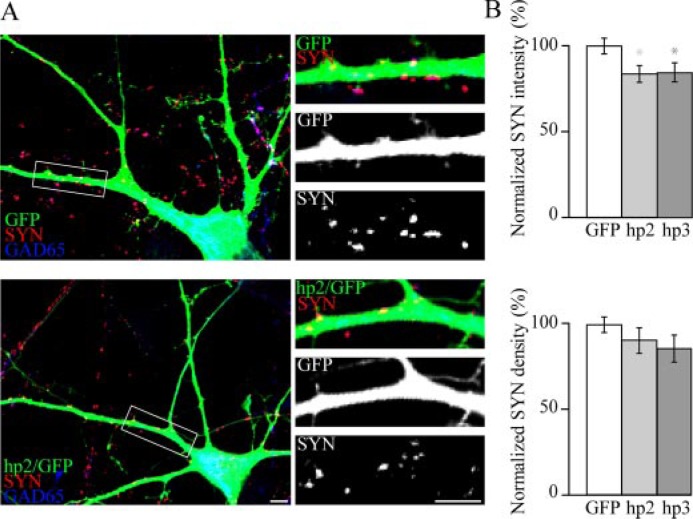
Presynaptic Terminal Intensity Is Decreased in Neurons Expressing ErbB4 shRNA—To assess a role for endogenous ErbB4 in synapse maturation, we employed a knockdown approach. The number and intensity of presynaptic terminals synapsing onto neurons expressing shRNAs specifically directed against ErbB4 was measured. The efficacy and specificity of these shRNA in reducing ErbB4 expression has been previously demonstrated (45). Because ErbB4 is specifically expressed in inhibitory GABAergic neurons, only ErbB4 shRNA-transfected neurons immunopositive for GAD65 were analyzed. Complementary to the effects seen after ErbB4 overexpression, reduction in endogenous ErbB4 expression resulted in an ∼16% decrease in the intensity of SYN clusters associated with knockdown cells as compared with GFP-expressing control cells (Fig. 3). Consistent with overexpression studies, the density of presynaptic terminals associated with knockdown cells remained unchanged (Fig. 3B).
FIGURE 3.
shRNA-mediated knockdown of ErbB4 in GABAergic neurons reduces presynaptic maturation in contacting axonal terminals. A, hippocampal neurons were transfected with either GFP alone or a vector expressing both GFP and shRNA directed against ErbB4 (hp2 or hp3) and immunostained for SYN as well as GAD65 to identify inhibitory neurons. B, quantification of changes in SYN cluster intensity and density. Although SYN cluster density remains unchanged upon ErbB4 knockdown (hp2, n = 40 cells; hp3, n = 23 cells), cluster size is significantly reduced as compared with cells transfected with GFP only (n = 46 cells). *, p < 0.05 (scale bars, 5 μm).
The Extracellular Domain of ErbB4, but Not Its Tyrosine Kinase Domain, Is Required for Presynaptic Maturation—To determine how ErbB4 enhances the maturation of presynaptic sites, a number of deletion mutant forms of ErbB4 were generated (Fig. 4A). The extracellular N-terminal domain of ErbB4 consists of two cysteine-rich repeats flanking a putative ligand binding site, whereas the intracellular C-terminal region contains the tyrosine kinase domain and the PDZ binding domain. To address the importance of the kinase domain in presynaptic maturation, an ErbB4 mutant form with a single point mutation at the ATP binding site (K751R) was generated, resulting in a tyrosine kinase dead ErbB4 (ErbB4-HA KD) (Fig. 4A and supplemental Fig. 4C). Expression of this mutant form enhanced SYN intensity to a similar extent as wild type ErbB4, indicating that kinase domain-mediated signaling is not required for the effects of ErbB4 on presynaptic maturation (Fig. 4, B and C).
The ErbB4 PDZ binding motif can directly interact with the excitatory postsynaptic protein, PSD-95 (17, 18). Furthermore, we have previously shown that overexpression of PSD-95 can promote the maturation of synapses both pre- and postsynaptically (37). Thus, it is plausible that ErbB4 indirectly enhances the size of presynaptic clusters by recruiting PSD-95 to postsynaptic sites. To further explore this idea, we transfected cells with the ErbB4 deletion mutant lacking the PDZ-binding motif (PDZb) (ErbB4-HA ΔPDZb) (Fig. 4A). Expression of this mutant failed to enhance presynaptic maturation (Fig. 4, B and C). However, upon further analysis it was determined that the ErbB4-HA ΔPDZb was not localized to synapses, indicating that the PDZ binding domain is essential for proper targeting of ErbB4 to synapses (supplemental Fig. 2A).
One way in which the synaptic localization of ErbB4 may be affected by its PDZ binding domain is if PDZ interactions regulate the trafficking of ErbB4 to the cell surface. To investigate this possibility, we analyzed surface expression of ErbB4-HA ΔPDZb by immunostaining under non-permeabilizing conditions. Subsequently, neurons were permeabilized, and staining of surface versus intracellular pools of ErbB4 was analyzed. MAP2 staining was used to monitor the extent of permeabilization (data not shown). ErbB4-HA was mainly present at the cell surface. In contrast, ErbB4-HA ΔPDZb was not observed at the cell surface but instead accumulated in the perinuclear region and formed intracellular aggregates present throughout the dendrites (supplemental Fig. 2B). These trafficking defects of ErbB4-HA ΔPDZb precluded the ability to determine whether ErbB4-mediated recruitment of PDZ proteins is required for its effects on synapse maturation.
Because many adhesion molecules mediate synapse maturation via trans-synaptic interaction with their ligands, we investigated whether the extracellular domain of ErbB4 is required for presynaptic maturation using a deletion mutant lacking the N-terminal domain (Fig. 4A). This mutant form was generated in the context of a K751R kinase dead mutant (ErbB4-HA ΔNTKD), as it has been previously shown that deletion of the N-terminal region results in a constitutively active tyrosine kinase domain (38) (supplemental Fig. 4C). SYN-positive clusters in neurons synapsing onto ErbB4-HA ΔNTKD-expressing cells were similar in intensity and number of cells expressing GFP alone (Fig. 4, B and C). Taken together our analysis reveals that the extracellular domain of ErbB4, but not its kinase activity, is essential for presynaptic maturation.
To confirm our observation that ErbB4 enhances presynaptic maturation via its extracellular domain, cells were treated with a soluble fusion protein coding for the extracellular domain of ErbB4, which is predicted to inhibit all extracellular ErbB4 interactions (ecto-ErbB4) (30). Treatment of cells transfected with GFP alone did not influence the intensity of SYN clusters (Fig. 5B). This is expected, because excitatory neurons, which constitute ∼90% of transfected neurons in our culture system, do not typically express ErbB4. In contrast, treatment with the ecto-ErbB4 peptide abolished the effects of ErbB4 overexpression on presynaptic maturation, suggesting that trans-synaptic signaling is required to mediate the effects of ErbB4 on synapse maturation (Fig. 5).
FIGURE 5.
The ectodomain of ErbB4 is required for presynaptic maturation induced by ErbB4. A, hippocampal neurons were transfected with full-length ErbB4 in the absence (n = 13 cells) or presence (n = 23 cells) of an Fc fusion protein containing the ectodomain of ErbB4 (ecto-ErbB4). Three days post-transfection, neurons were fixed and immunostained for SYN. Treatment with ecto-ErbB4 abolishes ErbB4-induced effects on presynaptic cluster size. B, quantification of results seen in A. ErbB4-HA overexpression significantly enhances the intensity of SYN puncta in the absence of the ecto-ErbB4 peptide when compared with GFP-expressing cells. ErbB4-HA expressing neurons treated with ecto-ErbB4 showed a reduction in SYN cluster intensity with respect to untreated controls. ***, p < 0.001 (scale bar, 5 μm).
Although the function of soluble NRG1 has been heavily focused on in previous studies, the full-length transmembrane form has been shown to exist in the presynaptic compartment (39, 40). It is, therefore, possible that ErbB4 may enhance presynaptic development via trans-synaptic interactions between NRG1 and ErbB4. To test whether transcellular associations can occur, we examined whether membrane-associated NRG1 regulates accumulation of ErbB4 at contact sites. HEK293 cells were transfected with either ErbB4 or HA-NRG1. One day post-transfection, these two populations of cells were co-cultured, and the subcellular distribution of ErbB4 and NRG1 was determined (supplemental Fig. 3A). Interestingly, ErbB4 showed enhanced accumulation at sites of contact between ErbB4- and NRG1-transfected cells but not at sites of contact between ErbB4- and GFP-transfected cells (supplemental Fig. 3A). These results reveal that intercellular NRG1/ErbB4 interactions enhance the aggregation of ErbB4 at sites of contact. It is, therefore, plausible that postsynaptic ErbB4 enhances the maturation of presynaptic terminals directly by its trans-synaptic interaction with membrane-bound NRG1. However, due to the existence of many isoforms of NRG1, the distribution of NRG1 at developing excitatory and inhibitory presynaptic terminals has yet to be determined. Alternatively, the presynaptic effects of ErbB4 may be indirectly mediated through interaction with other postsynaptic or extracellular partners.
Other trans-synaptic adhesion systems are sufficient to induce the formation of presynaptic terminals (41). To explore whether this applies to ErbB4 as well, we employed a mixed co-culture system using primary neurons and heterologous cells (29, 42). COS7 cells expressing ErbB4 or NL1 were co-cultured with dissociated hippocampal neurons (DIV 9–10) for 24 h, and induction of presynaptic protein clusters in contacting axons were subsequently detected (supplemental Fig. 3, B and C). To distinguish between clusters induced by transfected protein and endogenous clusters that coincidentally colocalize with COS7 cell contact interfaces, cultures were immunostained for pre- and postsynaptic markers, and only presynaptic clusters lacking apposing postsynaptic sites were analyzed. Immunostaining for VGLUT1/PSD-95 and VGAT/gephyrin combinations was used to assay the induction of the excitatory and inhibitory presynaptic terminals, respectively. Consistent with previous findings (41), NL1 induced VGLUT1 and VGAT clustering in contacting axons (supplemental Fig. 3, B and C). In contrast, COS7 cells expressing ErbB4 failed to induce clustering of either VGLUT1 or VGAT in four independent experiments (supplemental Fig. 3, B and C). These results are consistent with the finding that ErbB4 enhances the intensity but not the density of presynaptic terminals. Thus, we show that ErbB4 is not sufficient to induce clustering of VGLUT1 and VGAT and that the enhancement of presynaptic terminal size observed upon overexpression in neurons more likely requires recruitment of neuron-specific factors that participate in synapse maturation. Together these finding demonstrate a role for ErbB4 in synapse maturation but not in initiation of synaptic contacts.
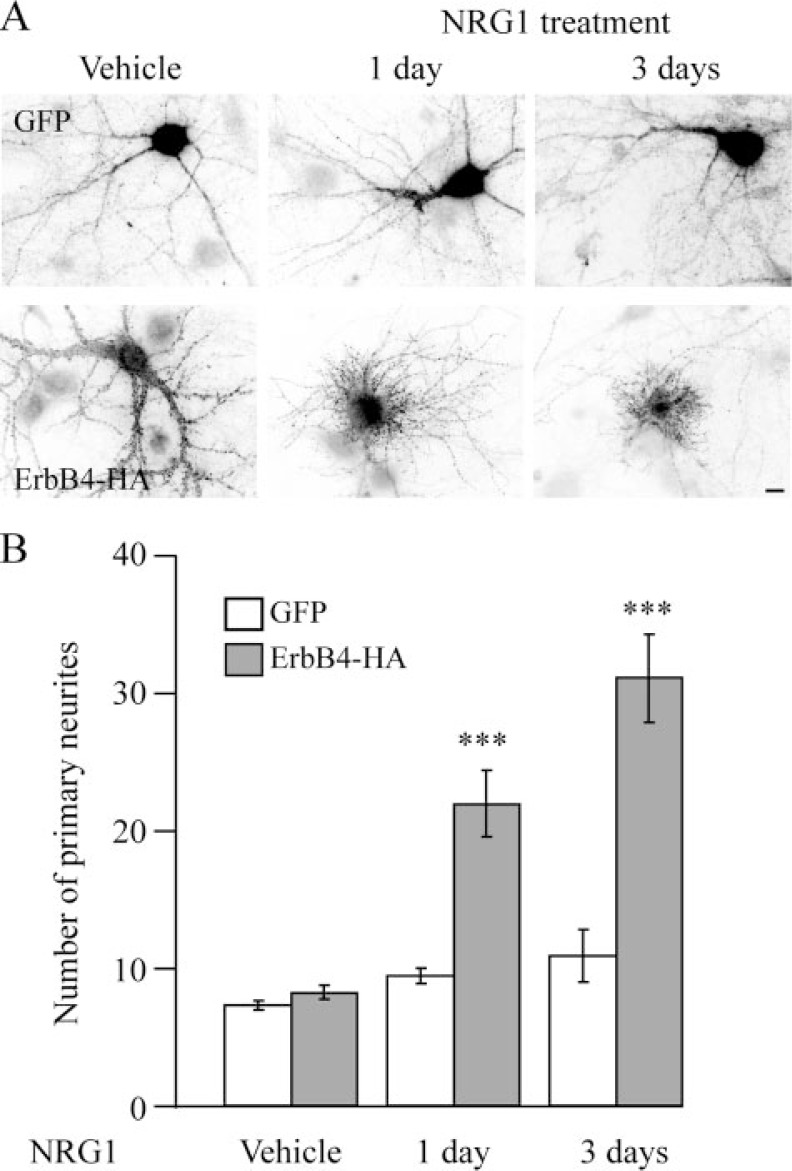
Treatment with a Soluble Form of NRG1 Alters Primary Neurite Formation in Neurons Ectopically Expressing ErbB4—Previous studies have demonstrated that NRG1 is subject to cleavage and that the soluble form activates ErbB4-mediated signal transduction pathways (1). To elucidate the role of soluble NRG1 on ErbB4 function, dissociated hippocampal neurons were transfected with full-length ErbB4-HA and then treated for 1 day with 10 nm soluble NRG1-β1 containing the extracellular EGF-like domain. Surprisingly, this treatment resulted in a dramatic increase in the number of primary neurites (defined here as processes originating from the cell body) and a concomitant loss of mature elongated dendrites (Fig. 6A). These alterations were observed only in cells overexpressing ErbB4. ErbB4-HA-expressing cells that were untreated as well as GFP-expressing cells treated with NRG1 had a similar number of primary neurites compared with untreated, GFP-expressing cells (Fig. 6). These results are reminiscent of previous findings reporting that ErbB4 activation in pheochromocytoma 12 (PC12) cells promotes neurite formation (43) and induces neurite outgrowth in cerebellar granule cells (44), suggesting the involvement of ErbB4 signaling in neurite formation. Evidence presented here, however, also suggests that NRG1-ErbB4 signaling can inhibit mature dendritic arborization in developing hippocampal neurons. Aberrant dendritic outgrowth and the formation of immature neurites in ErbB4-expressing cells treated with NRG1 precluded the assessment of synapse maturation. To determine the time course for induction of changes in neuronal morphology upon NRG1 treatment, neurons were transfected with GFP or full-length ErbB4-HA, treated with 10 nm NRG1 for 1 or 3 days, and analyzed for primary neurite number (Fig. 6). Treatment of cells with soluble NRG1 for 30 min and analysis 1 day later also resulted in a significant alteration in neurite number (data not shown). The time-dependent alteration in neurite number and morphology as well as its induction after only 30 min of exposure to NRG1 indicate a robust and long-lasting effect.
FIGURE 6.
Time course of changes in neurite outgrowth upon treatment with soluble NRG1. A, hippocampal neurons transfected with either GFP or ErbB4-HA were treated with vehicle or a soluble form of NRG1 (10 nm) for 1 and 3 days. Cells were then fixed and immunostained for HA to visualize transfected cells. In cells overexpressing ErbB4-HA, the number of primary neurites gradually increases with prolonged NRG1 treatment, with a corresponding loss of mature, elongated dendrites. GFP-expressing cells were unaffected by this treatment. B, quantification of results seen in A. Statistical comparisons were made between NRG1-treated cells and GFP controls within a particular time group (n = 8 cells). ***, p < 0.001 (scale bar, 5 μm).
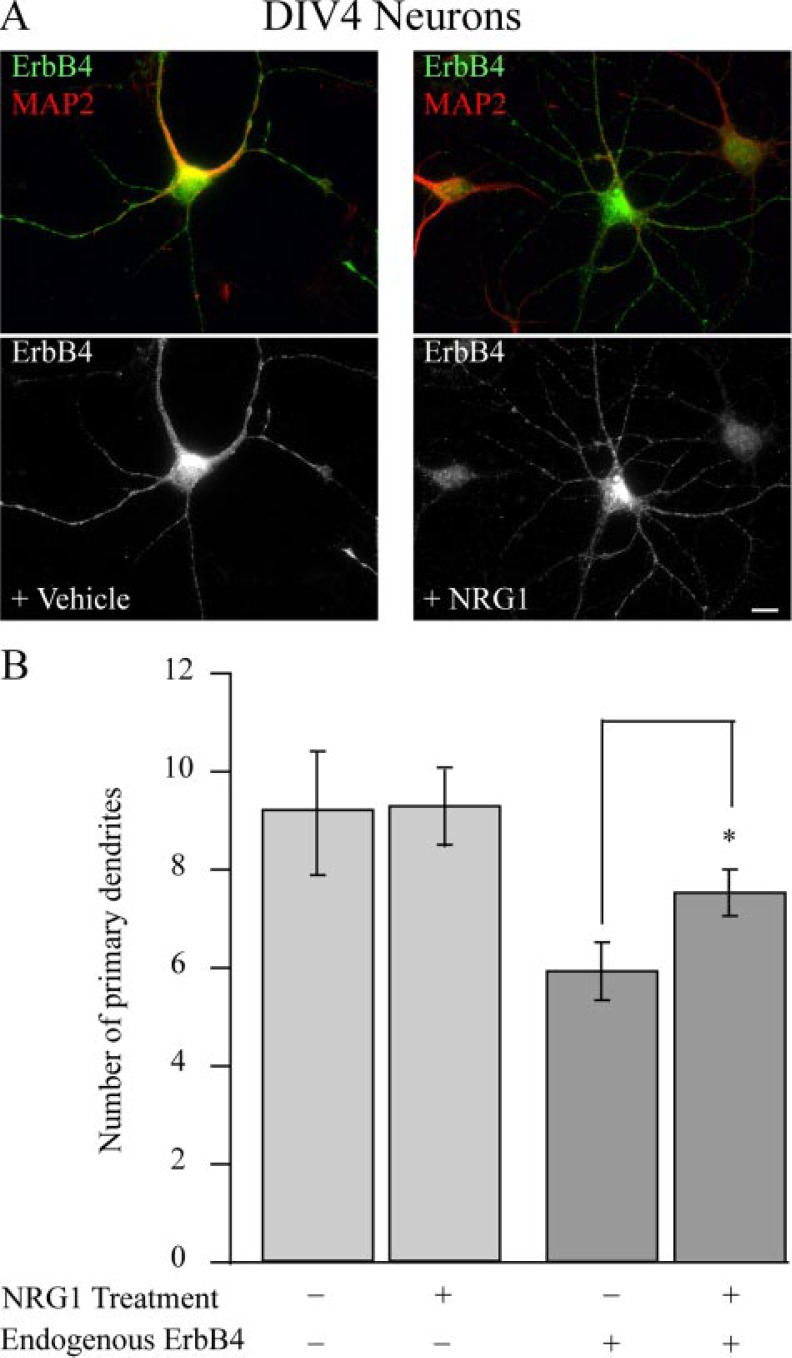
Endogenous ErbB4 Regulates Neurite Outgrowth—To establish whether the effects observed in cells ectopically expressing ErbB4 and treated with NRG1 were not an artifact of overexpression, the effect of NRG1 treatment was studied on inhibitory interneurons that express endogenous ErbB4 at DIV 4, a period that correlates with rapid dendritic growth and branching. Typically, excitatory neurons (ErbB4-negative cells) have on average more primary neurites than interneurons positive for ErbB4 (Fig. 7B). Thus, we contrasted changes in primary neurites upon NRG1 treatment in ErbB4-positive and -negative cells. Consistent with the effects observed in neurons transfected with ErbB4, a significant increase in primary neurite formation in ErbB4-expressing inhibitory neurons was observed after NRG1 treatment compared with ErbB4-negative controls (Fig. 7). In this instance there was no apparent inhibition of dendrite maturation. These results reveal that application of soluble NRG1 activates postsynaptic signaling pathways that modulate dendritic arborization. These robust effects of soluble NRG1 on the cytoskeleton are in holding with the previous findings that this protein can act as a guidance cue (8).
FIGURE 7.
Soluble NRG1 enhances dendritic arborization in ErbB4 expressing inhibitory neurons. A, hippocampal neurons were treated with either vehicle (left panels) or with 10 nm soluble NRG1 (right panels) for 2 days, fixed at DIV 4, and immunostained for ErbB4 (green) and MAP2 (red). Treatment of ErbB4-positive, but not negative, neurons with soluble NRG1 increases the number of primary neurites. B, quantification of results seen in A. n = 8 cells. *, p < 0.05 (scale bar, 10 μm).
To further support a role for endogenous ErbB4 in the extension of primary neurites, ErbB4 levels were depleted in inhibitory neurons using shRNAs. Inhibitory neurons have a simple morphology compared with excitatory cells, typically extending ∼5 primary neurites. In ErbB4 knockdown cells, however, primary neurite number was decreased to 3–4 (Fig. 8). This modest yet highly significant reduction in primary neurite number is in accord with the similarly modest yet significant increase in neurite number after NRG1 treatment of inhibitory neurons (Fig. 7B).
FIGURE 8.
Decreased number of primary neurites in inhibitory GABAergic neurons expressing ErbB4 shRNA. A, hippocampal neurons were transfected with either GFP alone or a vector expressing both GFP and ErbB4 shRNA (hp2 or hp3) and immunostained for GAD65 to identify inhibitory neurons. B, quantification of the number of primary neurites. Cells transfected with ErbB4 shRNA (hp2, n = 52 cells; hp3, n = 30) showed a statistically significant reduction in the number of primary neurites as compared with GFP control cells (n = 51 cells). **, p < 0.01; ***, p < 0.001 (scale bars, 10 μm).
ErbB4 Kinase Activity Alters Neurite Outgrowth—To characterize which ErbB4 domains are involved in the regulation of neurite outgrowth, the deletion mutant forms of ErbB4 described above were used. We first examined changes in neurites in the absence of NRG1 treatment upon expression of the deletion mutant forms of ErbB4. Expression of each of the mutants did not alter neurite outgrowth. The only exception was in the case of cells transfected with ErbB4-HA ΔNT, which showed a 2-fold increase in the number of primary neurites accompanied again by a loss of mature elongated dendrites (supplemental Fig. 4, A and B). To examine whether changes in neurite outgrowth are due to altered tyrosine kinase activity, we tested basal tyrosine phosphorylation in heterologous cells. Both full-length ErbB4-HA and ErbB4-HA ΔPDZb demonstrated similar phosphorylation levels (supplemental Fig. 4C), indicating that deletion of the PDZ binding motif does not compromise kinase activity of ErbB4. As expected, ErbB4-HA KD showed no detectable tyrosine phosphorylation (supplemental Fig. 4C). In contrast, ErbB4-HA ΔNT was strongly phosphorylated compared with full-length, even in the absence of NRG1 treatment (supplemental Fig. 4C). The robust phosphorylation of ErbB4-HA ΔNT is consistent with a previous report showing that deletion of the extracellular domain of ErbB4 results in a constitutively active kinase (20). To confirm the importance of the tyrosine kinase domain in neurite arborization, cultured hippocampal neurons were transfected with full-length ErbB4-HA or ErbB4-HA KD, and the effects of 4-day treatment with 10 nm soluble NRG1 were contrasted to vehicle-treated controls. Expression of ErbB4-HA KD failed to alter primary neurite outgrowth after NRG1 treatment (Fig. 9, A and B). These results further support the notion that activation of downstream signaling pathways mediated by ErbB4 kinase activity are involved in the morphological changes observed upon treatment with soluble NRG1.
FIGURE 9.
ErbB4 kinase activity is required for effects of ErbB4 on neurite outgrowth. A, hippocampal neurons were transfected at DIV 5 with full-length ErbB4-HA or the kinase dead mutant form (ErbB4-HA KD) in the presence of vehicle (left panels) or 10 nm soluble NRG1 (right panels) for 4 days. Cells were then fixed and immunostained for HA to visualize transfected neurons. Neurons expressing full-length ErbB4 displayed an enhanced number of primary neurites and loss of mature elongated dendrites after NRG1 treatment. In contrast, ErbB4-HA KD-expressing neurons were not affected by NRG1 treatment. B, quantification of changes in the number of primary neurites in neurons expressing either GFP, full-length ErbB4-HA, or ErbB4-HA KD with or without NRG1 treatment (GFP: n =10 and 11 cells, respectively; ErbB4-HA: n = 24 and 20 cells, respectively; ErbB4-HA KD: n = 24 and 29 cells, respectively). C, ErbB4-HA-transfected neurons were treated with NRG1 either alone (n = 10 cells) or in the presence of the PI3K inhibitor, LY 294002 (LY, 20 mm; n = 11 cells) or the MAPK inhibitor, PD 98059 (PD, 20 nm; n = 15 cells) and then assessed for changes in neurite number 1 day post-treatment. ErbB4-transfected cells which were not treated with NRG1 or drug were used as controls (n = 11 cells). Inhibition of PI3K, but not MAPK, abolishes the NRG1-mediated increase in the number of primary neurites. ***, p < 0.001 (scale bar, 5 μm).
Two major signaling pathways activated by ErbB4 tyrosine kinase signaling could potentially influence dendrite morphology by altering both microtubule and actin dynamics. NRG1-triggered tyrosine phosphorylation of ErbB4 causes activation of the Ras/MAPK signaling pathway, and the resulting phosphorylation of MAP2 decreases its affinity for microtubules, thus decreasing microtubule stability. ErbB4 also activates the PI3K signaling pathway that regulates actin dynamics, and thus, PI3K recruitment to the membrane may also influence neurite outgrowth (42). To determine which of these pathways is involved, we next analyzed changes in process outgrowth upon manipulation of both MAPK and PI3K signaling pathways by using kinase-specific inhibitors. We found that treatment with the PI3K inhibitor, LY-294002, but not the MAPK inhibitor, PD 98059, abolished the effects of NRG1 on neurite outgrowth (Fig. 9C). These results reveal that a PI3K-dependent signaling pathway is required for NRG1-ErbB4 mediated neurite reorganization. Our findings establish that distinct mechanisms are involved in mediating ErbB4 effects on synapse maturation and neurite outgrowth (Fig. 10).
FIGURE 10.
Model for the role of ErbB4 in synapse maturation and neurite morphology. A, postsynaptic ErbB4 expression modulates the maturation of presynaptic compartments through a mechanism that is dependent upon the extracellular domain of ErbB4. Two possible ways by which ErbB4 may regulate presynaptic maturation are illustrated 1) by binding directly to NRG1 type I/II in the presynaptic compartment and 2) through direct interaction with protein X, possibly through PDZ-dependent interactions, which recruits postsynaptic protein Y, which in turn directly binds unknown presynaptic protein Z. B, binding of soluble NRG1 (sNRG1) to ErbB4 activates the ErbB4 receptor tyrosine kinase (TK), activating the PI3K pathway and inducing cytoskeleton reorganization and dendrite arborization.
DISCUSSION
Establishing functional contacts between neurons is essential for successful information transfer in the brain. The current study reveals that postsynaptic ErbB4 bridges extracellular signals with intracellular signaling complexes to control synapse maturation and dendritic arborization, processes fundamental for building fully functional neuronal networks. ErbB4-mediated synapse maturation requires the extracellular domain of ErbB4, which potentially enhances presynaptic protein clustering via intercellular NRG1/ErbB4 interactions. In contrast, ErbB4 tyrosine kinase activity modulates primary neurite formation and dendritic arborization via a PI3 kinase-dependent pathway. Together these results reveal that NRG1 and ErbB4 influence neuronal process development and synaptic contact maturation through distinct mechanisms.
ErbB4 Modulates Synapse Maturation but Not Formation—Numerous studies have pointed to a restricted pattern of ErbB4 expression that is exclusive to inhibitory GABAergic interneurons (17, 35). Although the expression of ErbB4 in inhibitory neurons is undisputed, the subcellular localization of this protein remained less clear. Indeed, some reports suggested that ErbB4 is limited to excitatory postsynaptic densities (17, 18), whereas more recent work has demonstrated the presence of ErbB4 at inhibitory contact sites in dissociated cultures (28). In the present study we confirm that ErbB4 is localized at both excitatory and inhibitory contact sites in dissociated hippocampal neurons and also demonstrate excitatory and inhibitory synapse distribution in adult brain tissue.
Importantly, the results herein support a model whereby ErbB4 modulates transmission at both excitatory glutamatergic and inhibitory GABAergic contacts. Indeed, our analysis shows that ectopic ErbB4 expression increases the size of both excitatory and inhibitory presynaptic terminals. Conversely, reducing ErbB4 expression in GABAergic interneurons decreases presynaptic terminal size. However, the number of synaptic contacts is unaltered in either case. This implies a role for ErbB4 in synapse maturation rather than initial formation, consistent with the finding that expression of ErbB4 in heterologous cells is insufficient to drive new synapse formation in co-culture assays.
It has recently been shown that NRG1-mediated ErbB4 signaling modulates the function of GABAergic terminals by enhancing GABA release (30). The data presented here also support a role for ErbB4 in modulating the maturation of inhibitory presynaptic terminals. However, in this study we reveal that these effects are mediated by postsynaptic expression of ErbB4. Further studies assessing subcellular localization of endogenous ErbB4 using electron microscopy are required to further characterize the proportion of ErbB4 localized at pre- and postsynaptic sites, and thus, clarify the issue of retrograde versus anterograde signaling through the NRG1/ErbB4 pathway.
Enhanced clustering of presynaptic proteins is not unique to ErbB4, as overexpression of other molecules such as NL1 and PSD-95 have been shown to exert similar effects (32, 37). Because many proteins are able to redundantly perform similar biological functions, it is likely that these proteins work in concert to ensure the proper recruitment of presynaptic proteins involved in synaptic maturation and function.
A Putative Trans-synaptic Role for NRG1/ErbB4 at the Synapse—The enhancement in size of presynaptic protein clusters observed upon ErbB4 expression could be mediated via the direct trans-synaptic action of ErbB4 through presynaptic transmembrane isoforms of NRG1. Three major NRG1 isoforms (types I–III) are expressed in adult brain and are differentially distributed throughout cerebral cortex and brainstem (30, 39). All three isoforms exist as transmembrane proteins in addition to secreted ectodomain products of proteolytic processing of NRG1 and, thus, could potentially interact trans-synaptically with postsynaptic ErbB4 (1). In contrast to NRG1 types I and II, type III isoform appears to be important for signaling in glial cells (40). Therefore, NRG1 type I/II are more likely candidates in mediating trans-synaptic NRG1/ErbB4 interaction in neurons. Moreover, NRG1 type I/II are found throughout the hippocampus (30) and contain immunoglobulin domains important for protein-protein interactions (1) and are, thus, positioned appropriately to participate in trans-synaptic interaction with ErbB4.
In support of this notion, we show that treatment of neurons with a soluble form of the ectodomain of ErbB4 (ecto-ErbB4) abolishes the effects of ErbB4 on presynaptic development. Moreover, the extracellular domain of ErbB4 is required to induce presynaptic maturation, which further supports the importance of trans-synaptic interactions in this process. Thus, these data, in combination with the findings that expression of a kinase dead form of ErbB4 retains the ability to induce maturation of presynaptic terminals suggest a prevailing role for trans-synaptic protein-protein interactions rather than postsynaptic downstream signaling involving ErbB4 kinase activity in controlling presynaptic maturation.
One may also envision a paradigm whereby ErbB4, through PDZ-dependent interactions, would promote clustering of other postsynaptic molecules. This could in turn lead to recruitment of postsynaptic cell adhesion molecules to contact sites, where they would act trans-synaptically to promote presynaptic maturation (Fig 10). This is a plausible mechanism that could act in concert with the requirement for the trans-synaptic NRG1/ErbB4 interactions established above. Indeed, we have shown that NRG1 is able to cluster ErbB4 intercellularly. Thus, it is possible that NRG1-induced ErbB4 clustering results in the recruitment of PDZ proteins. PSD-95 is a leading candidate at glutamatergic synapses, as it has been shown to directly interact with ErbB4 via its PDZ-binding motif (17, 18). Moreover, studies on PSD-95 have demonstrated that overexpression of PSD-95 in neurons promotes maturation of existing synapses, both pre- and postsynaptically. However, PSD-95 overexpression does not increase the number of synapses (37). Thus, these results are similar to those observed in the case of cells overexpressing ErbB4 in the present study. Although intriguing, we have not been able to thoroughly test whether PSD-95 participates in ErbB4-mediated presynaptic maturation, as PSD-95 has been shown to be involved in a number of synaptic processes and altering levels of PSD-95 alone is sufficient to alter presynaptic maturation (32). Further work must be conducted to assess the role of PSD-95 in ErbB4-mediated presynaptic development.
At GABAergic synapses, ErbB4 may promote clustering of scaffolding molecules such as glutamate receptor-interacting protein, syntrophin, S-SCAM, or gephyrin. In addition, ErbB4 could also enhance the recruitment/retention of GABAergic synapse-specific adhesion molecules such as L1-CAM or neuroligin 2 at these sites, which could act trans-synaptically to influence presynaptic contact maturation (45). In the future it will be important to determine whether clustering of ErbB4 drives clustering of other postsynaptic proteins at excitatory and inhibitory contacts.
Physiological Role for ErbB4 at Synapses—Our data showing altered presynaptic maturation upon manipulating the levels of expression of ErbB4 in neurons is supportive of a role for this protein in regulating synaptic efficacy. Another recent study showed that ErbB4 overexpression enhances activity-dependent α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA) receptor-mediated currents, suggesting an ErbB4-mediated increase in the number of AMPA receptors at synaptic sites (26). ErbB4 overexpression in CA1 pyramidal neurons also influences spine morphology, increasing the size of these protrusions, whereas it has no effect on the number of spines per unit length (26). Although the physiological relevance of the ability of ErbB4 to enhance spine maturation is unclear because it is endogenously expressed in aspiny GABAergic interneurons, this study parallels our findings that altering ErbB4 levels both ectopically and endogenously affects maturation of excitatory glutamatergic synapses. Although expression of a mutant form of ErbB4 lacking the PDZ binding motif blocks ErbB4-mediated activity-dependent enhancement of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor currents (26), the contribution of PDZ-dependent interactions in ErbB4-mediated synaptic maturation remains unclear, as our data show that this mutant does not traffic normally to the cell surface.
Two recent reports have supported a synaptic role for ErbB4 in vivo using independent conditional ErbB4 knock-out mouse models. Golub et al. (46) demonstrate reduced motor activity and altered cognition in mice exhibiting ErbB4 ablation in neurons and glia. Furthermore, a recent study by Pitcher et al. (47) showed that mutant mice lacking ErbB4 (in which the lethal cardiac defect was rescued by α-myosin heavy chain promoter-driven expression in the heart) displayed enhanced long term potentiation at Schaffer collateral CA1 synapses.
ErbB4 Stimulation with Soluble NRG1 Regulates Neurite Outgrowth—Given the proposed role of ErbB4 in synapse maturation and stability, it is interesting to discover that prolonged treatment with soluble NRG1 enhances neurite formation in cells that express ErbB4 and that a corresponding reduction occurs upon knockdown of ErbB4. A role for NRG1/ErbB4 signaling in neurite outgrowth has previously been suggested based on the findings that NRG1 application promotes neurite outgrowth in PC12 cells and that ErbB4 signaling enhances primary neurite sprouting in cerebellar granule cells (43, 44). In contrast to the ErbB4-mediated mechanism involved in presynaptic terminal maturation, which is dependent on extracellular interactions, two pieces of evidence indicate that altered neurite formation relies on ErbB4 kinase activity. First, expression of constitutively active ErbB4 lacking the extracellular domain resulted in altered primary neurite outgrowth in the absence of NRG1 stimulation. Second, expression of ErbB4 with an inactive kinase domain fails to alter primary neurite formation induced by NRG1 treatment. Thus, ErbB4/NRG1 signaling at the synapse is mediated by two distinct mechanisms; although the extracellular domain of ErbB4 regulates synapse maturation, activation of the ErbB4 kinase domain by soluble NRG1 alters neurite outgrowth.
Two major signaling pathways activated by ErbB4 kinase activity could potentially influence dendrite morphology by altering both microtubule and actin dynamics. One such pathway involves activation of the MAPK signaling pathway, which induces phosphorylation of MAP2. This process decreases MAP2 binding affinity for microtubules, suppressing microtubule polymerization and increasing microtubule instability, ultimately promoting changes in dendritic extension. ErbB4 also activates the PI3K signaling pathway, which induces membrane recruitment of Cdc42, Rac, and Rho, guanine-nucleotide exchange factors for the Rho family of GTPases (42, 48). Although activation of Cdc42 and Rac1 induces dendritic branching, RhoA activity decreases actin dynamics and causes retraction of dendrites (49, 50). Overall, the concerted action of these GTPases regulates the rate of branch addition and retraction in an activity-dependent manner. Although the ErbB4 isoform used in our experiments specifically lacks the PI3K binding site, ErbB4 is still capable of activating the PI3K kinase pathway via Ras, a mechanism that is of particular importance in central nervous system neurons (51). Our analysis reveals that activation of the PI3K rather than the MAPK pathway is required for ErbB4-mediated neurite extension (Fig. 9C).
Both NRG1 and the ErbB4 receptor have recently been associated with schizophrenia (52, 53). In particular, altered NRG1 expression, rather than loss of function, has been associated with this disease (54). Consistent with this finding, a number of groups have observed altered expression patterns for various NRG1 isoforms (54–56). How altered NRG1/ErbB4 signaling could result in symptoms associated with schizophrenia disorders remains elusive. Based on our findings, altered expression or prolonged stimulation of ErbB4 may have adverse effects on synapse maturation, synaptic balance, and dendritic arborization. These findings may, therefore, provide the molecular basis for synaptic changes and altered behavior associated with schizophrenia.
In conclusion, this work reveals novel roles for NRG1/ErbB4 signaling in synapse maturation and process outgrowth. These effects are differentially modulated by extracellular interactions that affect presynaptic development as well as activation of postsynaptic downstream PI3K signaling via ErbB4 tyrosine kinase function, which results in altered neuronal morphology. These results also suggest that the precise duration of ErbB4 stimulation by NRG1 may finely modulate different cellular processes that influence synapse maturation and dendritic arborization.
Acknowledgments
We thank Dr. Jianxin Bao for the NRG1-HA construct and Ester Yu, Xiling Zhou, Souraya Mansour, Dr. Rujun Kang, Kimberly Gerrow, and Marie-France Lise for technical assistance.
Footnotes
The abbreviations used are: NRG, neuregulin; PDZ, PDS-95/Dlg/ZO-1; PI3K, phosphoinositide 3-kinase; EGF, epidermal growth factor; PSD-95, postsynaptic density protein 95; HA, hemagglutinin; NL1, neuroligin-1; GFP, green fluorescent protein; VGLUT1, vesicular glutamate transporter-1; VGAT, vesicular GABA transporter; GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; SYN, synaptophysin; MAP2, microtubule-associated protein 2; MAPK, mitogen-activated protein kinase; DIV, days in vitro; KD, kinase dead; shRNA, short hairpin RNA.
This work was supported by grants from Canadian Institutes of Health Research and Michael Smith Foundation for Health Research. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
This work is dedicated to the memory of Dr. Alaa El-Husseini, a teacher, a mentor, and a friend.
REFERENCES
- 1.Buonanno A, Fischbach GD. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 2.Mei L, Xiong WC. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao J, Wolpowitz D, Role LW, Talmage DA. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim T-W, Mei L, Dai P, Ohlemiller KK, Ambron RT. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- 6.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 7.Anton E, Marchionni M, Lee K, Rakic P. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 8.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calaora V, Rogister B, Bismuth K, Murray K, Brandt H, Leprince P, Marchionni M, Dubois-Dalcq M. J Neurosci. 2001;21:4740–4751. doi: 10.1523/JNEUROSCI.21-13-04740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 12.Vartanian T, Fischbach G, Miller R. Proc Natl Acad Sci U S A. 1999;96:731–735. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. Proc Natl Acad Sci U S A. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., III Proc Natl Acad Sci U S A. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter G. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 16.Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Proc Natl Acad Sci U S A. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 18.Garcia RAG, Vasudevan K, Buonanno A. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rio C, Buxbaum JD, Peschon JJ, Corfas G. J Biol Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 20.Linggi B, Cheng QC, Rao AR, Carpenter G. Oncogene. 2006;25:160–163. doi: 10.1038/sj.onc.1209003. [DOI] [PubMed] [Google Scholar]
- 21.Huang YZ, Wang Q, Won S, Luo ZG, Xiong WC, Mei L. Int J Dev Neurosci. 2002;20:173–185. doi: 10.1016/s0736-5748(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 22.Xie F, Padival M, Siegel RE. J Neurochem. 2007;100:62–72. doi: 10.1111/j.1471-4159.2006.04182.x. [DOI] [PubMed] [Google Scholar]
- 23.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 24.Gu Z, Jiang Q, Fu AKY, Ip NY, Yan Z. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Woo RS, Mei L, Malinow R. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer GJ, Torricelli JR, Evege EK, Price PJ. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 28.Jiang M, Deng L, Chen G. Gene Ther. 2004;11:1303–1311. doi: 10.1038/sj.gt.3302305. [DOI] [PubMed] [Google Scholar]
- 29.Graf ER, Kang Y, Hauner AM, Craig AM. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Swayze RD, Lise MF, Levinson JN, Phillips A, El-Husseini A. Neuropharmacology. 2004;47:764–778. doi: 10.1016/j.neuropharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Fox IJ, Kornblum HI. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- 35.Yau HJ, Wang HF, Lai C, Liu FC. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 36.Levinson JN, El-Husseini A. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 37.El-Husseini AE-D, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 38.Linggi B, Carpenter G. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Falls DL. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 41.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers EE, Theibert AB. Int J Dev Neurosci. 2002;20:187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 43.Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. J Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- 44.Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. J Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerrow K, El-Husseini A. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- 46.Golub MS, Germann SL, Lloyd KC. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichardt LF. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. J Neurosci. 1999;19:8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Van Aelst L, Cline HT. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan DR, Miller FD. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 52.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison PJ, Law AJ. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 56.Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, Medeiros HM, Carvalho C, Macedo A, Dourado A, Valente J, Ferreira CP, Patterson NJ, Azevedo MH, Daly MJ, Pato CN, Pato MT, Sklar P. Mol Psychiatry. 2005;10:366–374. 328. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]