Abstract
Background and Aims
The beneficial effect of one graft on another has been reported in combined transplantation but the associated mechanisms and biological influence of each graft have not yet been established.
Methods
In multiple analyses, we explored the PBMC phenotype and signature of 45 immune-related messenger RNAs and 754 microRNAs from a total of 235 patients, including combined liver–kidney transplant recipients (CLK), patients with a liver (L-STA) or kidney (K-STA) graft only under classical immunosuppression and patients with tolerated liver (L-TOL) or kidney grafts (K-TOL).
Results
CLK show an intermediary phenotype with a higher percentage of peripheral CD19+CD24+CD38Low memory B cells and Helios+ Treg cells, two features associated with tolerance profiles, compared to L-STA and K-STA (P < 0.05, P < 0.01). Very few miRNA were significantly differentially expressed in CLK vs. K-STA and even fewer when compared to L-STA (35 and 8, P < 0.05). Finally, CLK are predicted to share common miRNA targets with K-TOL and even more with L-TOL (344 and 411, P = 0.005). Altogether CLK display an intermediary phenotype and gene profile, which is closer to that of liver transplant patients, with possible similarities with the profiles of tolerant patients.
Conclusion
These data suggest that CLK patients show the immunological influence of both allografts with liver having a greater influence.
Keywords: combined transplantation, gene expression, human, kidney transplantation, liver transplantation, microRNA, phenotype
Since the first in 1983 (1), the number of combined liver and kidney transplants has increased each year. Despite a higher postoperative mortality rate, the incidence of acute cellular rejection is lower and the rate of renal allograft survival is higher compared to kidney transplant patients (2–7), suggesting a beneficial impact of the liver on the kidney graft. Early clinical reports suggest that combined transplant patients (CLK) have less hyperacute rejection (8,9) and that patients with a positive cross-match convert to a negative one and exhibit fewer Panel Reactive Antibodies (PRA) (8). The UNOS database reports show lower renal failure and liver allograft rejection in these patients (10). However, the existence of a protective effect of the liver on kidney graft is not accepted unanimously. Saidman et al., for example, report that both patient and kidney allograft survival were lower in cross-match positive patients than in negative ones in CLK transplant recipients (11). Similarly, Katznelson et al. report no difference in 3-year survival rate compared to patients with a single renal transplant, and identical functional renal graft survival in patients with more than two HLA mismatches or high pretransplant levels of PRA (12).
Thus, the protective mechanism in combined grafts and the real effect of one graft on the other, usually pointing towards a beneficial effect of the liver on the renal graft, remain contentious yet and their mechanisms are not fully understood. Various hypotheses have been postulated: the production and secretion into the systemic circulation of soluble HLA class I antigens neutralizing pre-existing allo-antibodies and CTL (13); a microchimerism (14); a Th2 immune deviation induced by hematopoietic precursors present in the liver graft (15); the involvement of regulatory T cells (16); and, more recently, higher expression of HLA-G (17). None of these hypotheses fully explain the biological mechanisms of this protective effect. The objectives of this study were to assess the peripheral blood profile (phenotype and gene expression, messenger RNAs (mRNAs) and microRNAs (miRNAs)) of combined liver–kidney transplant patients (CLK) and to compare them with patients with a stable graft function following a single kidney (K-STA) or liver (L-STA) transplant. Finally, to assess if there may be a potential positive effect of combined grafts, we compared their patterns with those of transplant recipients with highly stable graft function and who operationally tolerate a liver or a kidney transplant (L-TOL and K-TOL) (18–20).
Patients and methods
Patients
A total of 235 patients were included in this study: 80 from Barcelona (Spain), 19 from Rennes (France), 60 from Nantes (France), 76 from San Francisco (USA). Local ethics committees approved all aspects of this study and all patients gave their informed consent. Criteria for each group were: (i) Long-term immunosuppressive drug-free kidney transplant recipients (K-TOL; n = 9): stable kidney graft function (blood creatinemia <150 µmol/L and proteinuria <1 g/24 h) in the absence of IS for at least 1 year. Immunosuppressive treatment was stopped because of non-compliance (n = 7), post-transplant lymphoproliferative disorder (PTLD) (n = 1) or calcineurin inhibitor toxicity (n = 1); (ii) Kidney transplant recipients with stable graft function under standard IS (K-STA; n = 51): proteinuria <1 g/24 h and stable creatinemia (variations <25%) for at least 3 years. (iii) Liver recipients maintaining stable graft function in the absence of immunosuppressive therapy (L-TOL; n = 9): intentional weaning from IS under medical supervision at least 1 year prior to this study. (iv) Liver recipients with stable graft function (L-STA; n = 110): >3 years after transplantation and under standard IS; (v) Combined liver and kidney recipients (CLK; n = 56): at least 1.5 year after a simultaneous liver and kidney graft from a single non-living donor and under standard IS. Written informed consents were obtained in accordance with the Declaration of Helsinki, and this study was approved by the local ethics committee: Groupement Nantais d’Ethique dans le Domaine de la Santé.
Phenotyping, mRNA and miRNA assays were performed on peripheral blood as described in the Data S1.
Results
Demographic parameters
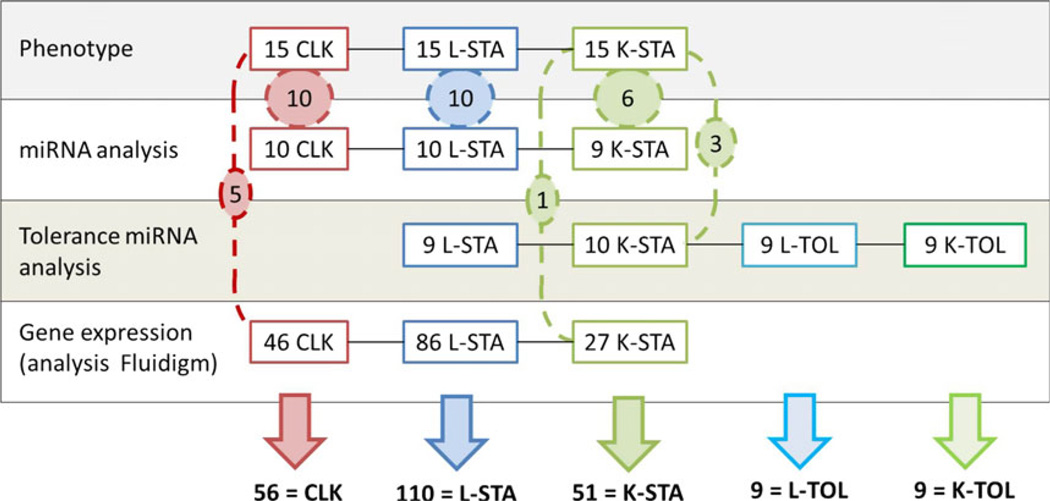
A total of 235 patients were enrolled and included in five groups (Table S1). Blood samples from these patients were used in four analytic experiments, as described in Fig. 1. Reasons for transplantation varied depending on the graft. Alcoholic liver disease was the leading cause in CLK (27%, n = 15) and L-TOL 33% (n = 3), and the second in L-STA (23%, n = 24). Polycystic disease was the leading cause in K-STA (27%, n = 14) and the third in CLK (24%, n = 13). The second cause in K-STA was IgA nephropathy (18%, n = 9). Viral hepatitis (B or C) accounted for 24% of CLK (n = 14), 50% of L-STA (n = 52) and 44% L-TOL (n = 4). 24% of CLK patients (n = 14) showed a combination of primitive hepatic and renal pathologies. The 51 K-STA were significantly younger than others (P < 0.001). Mean sex ratio was 0.63 (M/F) except for L-TOL, who were all male. The mean time between graft and analysis was significantly shorter for stable than for tolerant patients (P < 0.001), but post transplantation time was not found to be significantly associated with any of the parameters analysed in uni/multivariate analyses. Induction therapy was given to 72% of K-STA [n = 36; antilymphocyte serum (n = 17), anti-IL2 receptor antibody (n = 15) and other drugs (n = 4)], 7% of the CLK [n = 4; antilymphocyte serum (n = 1) anti-IL2 receptor antibody (n = 3)] and none of the L-STA (P < 0.001). CLK patients had higher creatininemia and proteinuria means compared to the other groups (respectively, 155 ± 17 µmol/L, P < 0.001 and 0.51 ± 0.13 g/L, P < 0.05) and a normal hepatic function comparable to the others. CLK patients (18 out of 19) from Rennes (FR) were followed up for an average of 1 year 4 (±7) months. At end of the follow-up, 14 (77%) of these patients displayed no biological evidence of rejection for either kidney (mean creatinemia 103 ± 10 µmol/L and Cockroft score 77 ± 11 mL/min) or liver graft (mean AST 20.6 ± 2.3 and ALT 22.6 ± 5.9). The four remaining patients displayed: histological evidence of humoral rejection in liver and kidney (1), histological cirrhosis of unknown aetiology (1) and renal insufficiency leading to dialysis (2).
Fig. 1.
Study design. Four analytic experiments were performed on samples from 235 patients. Number of samples per experiment is displayed and common samples between experiments are shown in dashed circles.
CLK phenotype
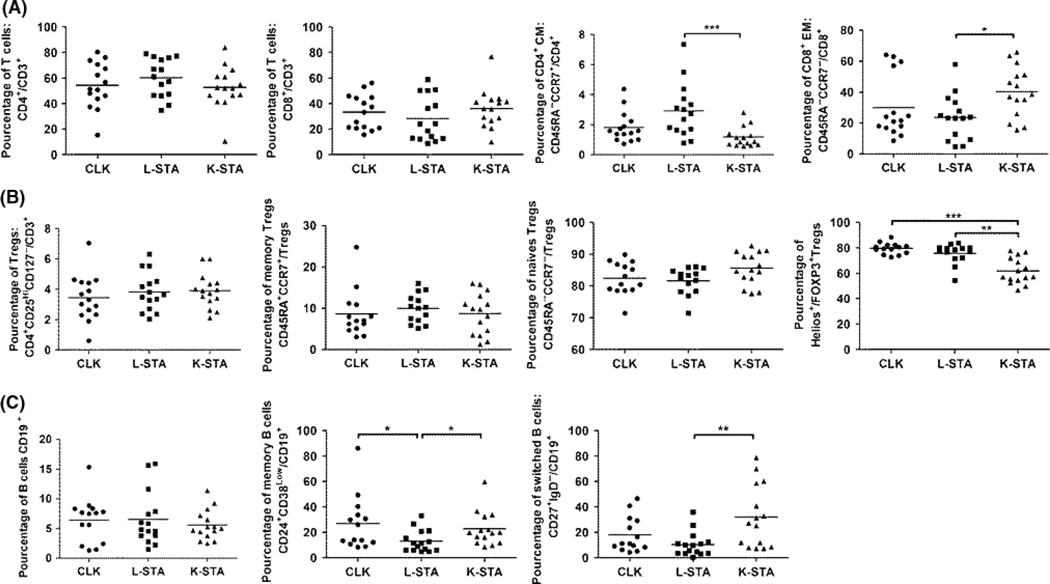
A detailed phenotype analysis using flow cytometry was performed on blood mononuclear cells from 15 patients in each group which were representative of the whole population (15 CLK, 15 L-STA and 15 K-STA) (Figs. S1 and S2). No difference was found in percentages of CD3+CD4+, CD3+CD8+ and CD8+CD28− T cells between the three groups of patients (Fig. 2A; Fig. S2). Neither was any difference found for CD8+EM and CD4+CM T cells between CLK and L-STA and K-STA, with L-STA displaying less CD8+EM (P < 0.05) and more CD4+CM (P < 0.001) than K-STA (Fig. 1A). Regarding the Treg compartment, CD3+CD4+CD25HighCD127Low Treg cell percentages, FOXP3 expression and proportions of naïve (CD45RA+CCR7+) and memory (CD45RA− CCR7−) Tregs, no difference was observed between the three groups. The percentage of FOXP3+Helios+Treg was significantly higher in CLK and L-STA than in K-STA (P < 0.01 and P < 0.001) (Fig. 2B). Regarding other cell subsets, no significant difference was observed between the three groups for Tγδ, NKT, CD3−CD56+NK (Fig. 2) and CD19+ B cells. CLK and K-STA showed a significantly higher rate of CD19+CD24+CD38Low memory B cells compared to L-STA (P < 0.05) (Fig. 2C). These results correlated with the CD19+CD27+ memory staining (R = 0.97 (IC95% [0.95–0.98]), P < 0.001) (data not shown). Finally, CLK displayed an intermediate profile for switched CD19+CD27+IgD− memory B cells compared to the two other groups (Fig. 2C). All together, these data show that CLK exhibit an intermediary blood cell phenotype closer to L-STA for the T cells and closer to K-STA for the memory B cell compartment.
Fig. 2.
CLK phenotype. A) Frequencies of T lymphocyte subsets within CLK, L-STA and K-STA. B) Frequencies of Treg subsets. C) Frequencies of B lymphocyte subsets. Cell frequencies are shown as median.
mRNA tolerance profile in CLK
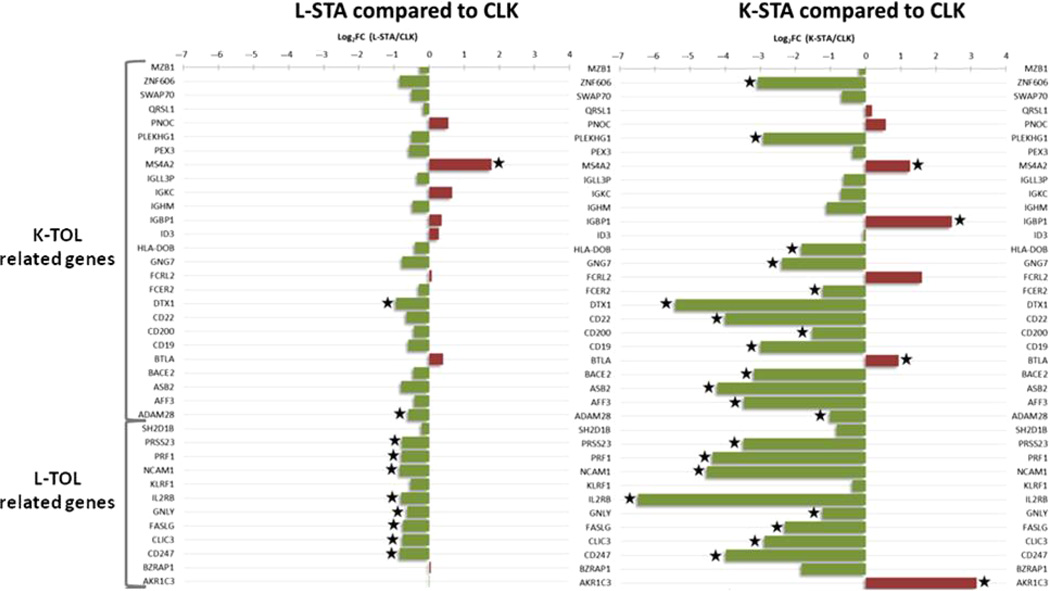
The expression of 26 renal and 12 liver-related mRNAs, previously described as associated with a tolerance profile (21–23) (for more details see supporting information), was quantified in PBMC from 46 CLK, 86 L-STA and 27 K-STA patients using Fluidigm real-time PCR (Fig. 3). Twenty-five and eleven (P < 0.05) mRNAs were found differentially expressed in CLK compared with K-STA and L-STA, respectively; the smaller number of changes between CLK and L-STA suggests closer gene expression in these two groups of patients (P < 0.05). Among all of them, 16 mRNAs mainly related to B cells previously associated with K-TOL (24) and eight mRNAs related to NK cells previously associated with L-TOL (23) were significantly up-regulated in CLK. Altogether, these results show that CLK mRNA expression patterns are closer to the profile of liver transplant patients and display some similarities with the profiles of liver and kidney tolerant recipients.
Fig. 3.
mRNA expression profile in CLK. Relative expression of 26 kidney operational tolerance-related transcripts and 12 liver tolerant-related transcripts, in L-STA (left panel) and K-STA (right panel) compared to CLK (Log2 of fold-changes). Green bars indicate an under-expression and red bars an over-expression compared to CLK. Significant differential expressions (P < 0.05) are highlighted with a star.
miRNA profile in CLK
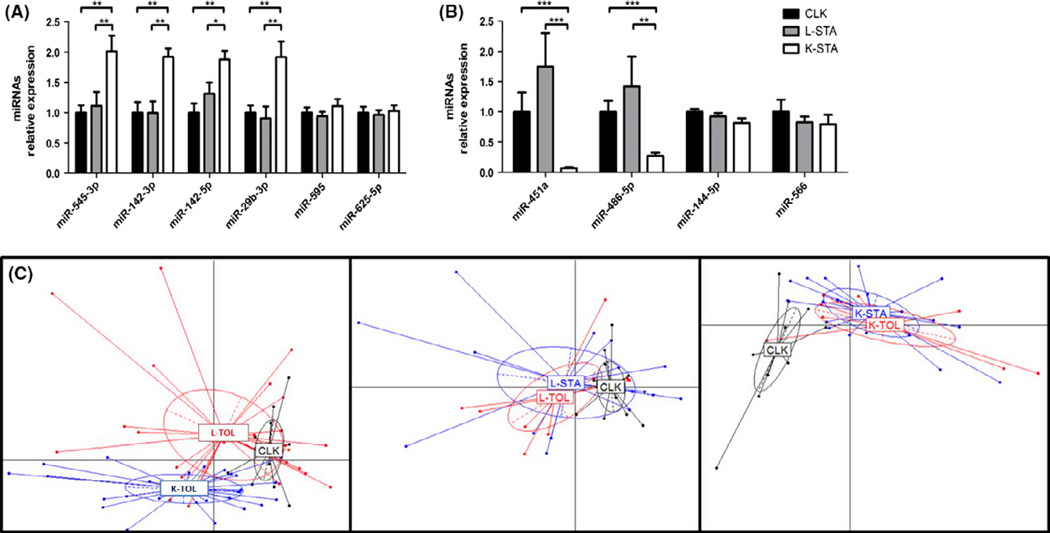
We measured the expression of 754 miRNAs in 10 CLK, 9 K-STA and 10 L-STA by qPCR using TaqMan Low Density Array (TLDA). A total of 542 miRNAs were expressed (Cq<35) in at least half of the samples of each group. Among them, a total of 35 and eight miRNAs were differentially expressed between CLK, and K-STA and vs. L-STA, respectively (P < 0.05) (Table S3). The 10 miRNAs exhibiting sufficient expression (Cq<30) and a two or more fold change (FC) were selected and validated by individual assays. Differential expression was confirmed for 6 of them, all referring to the CLK vs. K-STA comparison: miR-142-3p, miR142-5p, miR-29b-3p, miR-545-3p, miR-451a and miR-486-5p (P < 0.05; Table S3 and Fig. 4A and B). miRNA expression levels were previously measured in PBMC from liver and kidney tolerant patients in an independent set of 37 patients (25) (9 K-TOL, 10 K-STA, 9 L-TOL and 9 L-STA). A total of 448 miRNAs were expressed (Cq<35) in at least half of the samples of each group. Among them, only 13 miRNA were differentially expressed in K-TOL compared to K-STA. Similarly, only 12 miRNAs were different between L-TOL and L-STA (Table S4A and B) (P < 0.05), suggesting very few differences between stable and tolerant patterns in these two allografts. We then compared the miRNA expression patterns of CLK with those of L-TOL and K-TOL. The two TLDA datasets were combined (10 CLK, 19 L-STA, 19 K-STA, 9 L-TOL and 9 L-TOL, Fig. 1 and Fig. S3). Principal component analyses (PCA) showed a CLK superposition with L-TOL and distance from K-TOL (Fig. 4C), confirming that CLK patients are characterized by a miRNA profile closer to L-TOL.
Fig. 4.
miRNA expression pattern in CLK and deduced PCA. miRNAs up regulated in K-STA vs. CLK (A) and down-regulated in K-STA vs. CLK (B) in internal validation using qPCR. PCA based on the expression of the miRNAs associated with CLK (C). Left panel, CLK vs. tolerant patients (L-TOL and K-TOL) (likelihood 84%). Centre, CLK vs. liver recipients (L-TOL and L-STA) (likelihood 85%). Right panel CLK vs. kidney recipients (K-TOL and K-STA) (likelihood 78%).
miRNA target profile in CLK and TOL
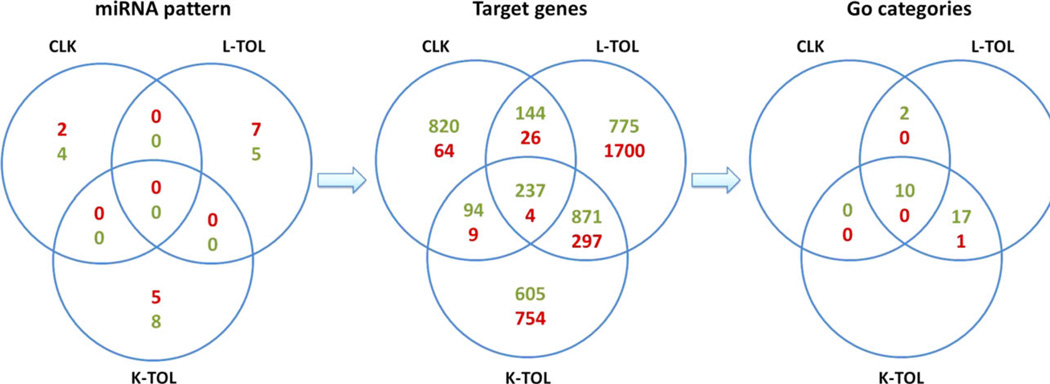
Very few common transcripts of potential targets of miRNAs are differentially expressed in L-TOL and in K-TOL (detailed in supporting information and Fig. 5), in agreement with our previous results (21,26). As the CLK miRNA expression profile displayed some similarities with that of tolerant recipients and distinct miRNAs can target common transcripts, we investigated the transcripts predicted as potential targets of the 6, 13 and 12 differentially expressed miRNAs identified in blood from CLK, K-TOL and L-TOL (Fig. 5). We restricted our analysis to predict target scores higher than 60, as recommended by the miRDB database (27). Among the 1,295 genes predicted to be up-regulated in CLK, 381 and 331 were common with L-TOL and K-TOL respectively (P = 0.031). For the 103 genes predicted to be down-regulated in CLK, 30 were in common with L-TOL and 13 with K-TOL (P = 0.0057). These data suggest that, as for mRNA, miRNA targets from CLK exhibit similarities with both L-TOL and K-TOL (411 and 344 common predicted targets) with significantly more similarities with L-TOL (P = 0.005).
Fig. 5.
Identification of differential miRNAs and targeted genes associated with CLK, L-TOL and K-TOL profiles. Summary of the common genes and genes ontologies predicted as potential targets from the 6, 13 and 12 differentially expressed miRNAs identified in blood from CLK, K-TOL and L-TOL according to the miRDB database. Down- and up-regulated miRNAs or target genes are highlighted in green and red respectively.
Discussion
The purpose of this study was to explore the unknown phenotype and gene expression profiles of patients with combined liver–kidney transplant compared to those with a liver or kidney transplant alone. As clinical studies report a potential protective effect of combined liver–kidney graft (4,5), we also compared their profile with that of patients displaying hepatic or renal tolerance (18,23). This analysis aims to decipher the influence of one graft on the other one and to further explore a possible “beneficial” influence of each graft in the combined transplantation.
We report that CLK exhibit an intermediary blood cell phenotype with some of the phenotypical characteristics of patients with operational tolerance (kidney and liver). CLK show more memory CD19+CD24+CD38Low B cells, a phenotype that has been shown to be associated with tolerance in kidney transplantation in various studies, including our own (20,26,28,29). Previous studies on combined liver–kidney transplant patients have shown an increased frequency of CD3+CD4Low, CD3+CD8Low and FOXP3-negative T-cell populations with immunosuppressive properties associated with graft acceptance (30,31). These cells have been shown to be over-represented in patients with better graft acceptance and higher concentrations of HLA-G in their serum (31). We did not observe significant difference in CD3+CD4Low FOXP3-negative T-cell frequency between the different groups of patients (data not shown), but for technical reasons we have been unable to study the frequency of CD3+CD8low population. Thus, the implication of HLA-G in our study is unlikely even though we cannot exclude it (31). But interestingly, we report on a higher frequency of CD4+CD25HighCD127LowFOXP3+Helios+ in both CLK and L-STA. FOXP3+Helios+ Treg has been demonstrated to exhibit greater TGF-β mRNA expression (32), lower effector cytokine production (IFNγ, IL17A and IL2) (33) (34) and to have demethylated Foxp3 TSDR regions (34,35). This Treg population is probably more stable, and less likely to convert to effector T cells during the course of an inflammatory response in vivo, therefore less prone to inducing graft rejection that FOXP3+Helios−. Further study including cytokine production assays (e.g. TGF-β, IL17A and IFNγ) are necessary to analyse whether this increase in FOXP3+Helios+ Treg frequency is effectively accompanied by modification of cytokine profiles. The higher blood frequency of FOXP3+Helios+ in such transplanted populations and particularly in CLK may thus reflect some immune-regulatory phenomenon. Thus, CLK patients exhibit a higher rate of CD19+CD24+CD38Low memory B cells than L-STA and CD3+CD4+CD25HighCD127LowFOXP3+Helios+ Treg than K-STA. These data suggest that CLK patients exhibit some features associated with the potential regulatory processes which probably arise from the concomitance of the transplantation of both organs. To what extent these regulatory profiles can be attributed to a specific “regulatory” role of each organ on the other remains to be established but at least this clearly suggests an influence of both organs. Ideally, the identification of CLK tolerant patients and their analysis would help deciphering such regulatory mechanisms. Direct phenotypic comparison of CLK and tolerant patients would reinforce our findings, but further investigation of operational tolerance mechanisms, which are far from being understood themselves, are required.
To further explore this point, we analysed the gene expression profiles (mRNA and miRNA) in CLK and compared them with the profiles of liver and kidney transplant patients with stable graft function. Our results clearly suggest that CLK patients display a profile closer to L-STA than to K-STA patients, based on the smaller amplitude of variations measured for the differentially expressed genes. These observations, based on mRNA expression, supported by miRNA PCA analyses and reinforced by their predicted target expression, suggest that liver graft influences the combined graft more. Interestingly, we found that within the studied set of 38 genes associated with tolerance (21–23), 8 of the differentially expressed genes are linked to NK cells, previously described as associated with L-TOL (23) and 16 are related to B cells, previously described as associated with K-TOL (21). Although our study does not prove that expression of these mRNAs varies with the same magnitude in CLK and tolerant patients, this result is interesting with regard to a number of clinical studies that report a potential protective effect of liver on kidney graft (10,36). The hypothesis of a shared genetic profile with tolerant patients is reinforced by the analysis of miRNA targets, which shows similarities between CLK, L-TOL and K-TOL. In our study, CLK patients had good hepatic and renal functions at inclusion, i.e. on average 72.7 months post transplantation. Since no biopsy has been performed and an absence of subclinical rejection cannot be excluded, a longitudinal analysis would be necessary to determine whether function remains stable over time. Nevertheless, no impaired outcome was observed for the 18 CLK patients followed up in Rennes. A limitation of this study is the heterogeneity of IS, which remains a major issue when comparing patients with different transplant origins coming from different centres. Beside the absence of IS in L-TOL and K-TOL, K-STA received induction therapy more frequently than CLK and L-STA. We tested IS as a confounding factor in a multivariate analysis based on absence or presence of induction therapy or based on the drug’s nature. We found no significant influence of induction therapy on the phenotypic and miRNA profiles of the patients. Only maintenance immunosuppressive treatment with MPA was significantly associated with variation in memory B cells (global or switched) (P < 0.001) and with the expression of miR-486-5p (P = 0.014). This suggests a slight influence of IS on both phenotypic and gene expression profiles of our patients. Finally, the fact that we also observed some similarities between CLK and tolerant patients who received no IS, reinforces the idea of a limited influence of IS. Similarly, we tested whether the underlying diseases leading to transplantation may influence on the immunological profile in a uni/multivariate analysis. Only the expression of miR-144-5p was significantly increased in patients with viral hepatitis (P = 0.039, multivariate analysis) but with a meaningless expressional fold change of 0.76, which suggested initial disease is not a confounding factor in our analyses. Another limitation of this study is the use of uncorrected statistical tests for multitesting and miRNA target predictions (37) which could both cause the return of a substantial number of false positives. However, this does not seem to be a major issue considering the few common miRNA targeted genes found between L-TOL and K-TOL, evidencing their highly differential profile. Thus, even if similar proportions of false positives were expected for the common predicted targets between CLK and L-TOL, and K-TOL, this issue, together with the limited number of patients used in each comparison, suggests that further validation is needed to ascertain definite conclusions.
In summary, although this study remains exploratory and descriptive, phenotype and gene expression of combined transplanted recipients suggest an effect from both grafted organs and throws doubt on the generally held view of a potential beneficial phenomenon being driven by the hepatic graft. Since CLK gene profile share similarities with that of tolerant patients, combined transplantation may favour a tolerating process, which could participate in the positive outcomes commonly observed. To what extent such similarities with tolerant patients contribute to long-term graft maintenance in CLK remains to be established. In any case, this would not preclude these patients from being weaned of their IS, taking into account the double risk of such a decision in combined transplantation. Although tolerance is increasingly regarded as an ideal solution, IS weaning still remains a very sensitive point in transplantation, and the identification of “low-risk” patients among cohorts of transplanted recipients, as performed for heart allograft rejection diagnosis (38,39), is not yet commonly accepted for other transplanted organs. Our study may serve as a basis to aid to such future studies.
Supplementary Material
Key points.
PBMC phenotype and gene expression of CLK patients suggest an effect from both grafted organs.
However, a greater influence of the liver allograft on PBMC phenotype and gene profile of CLK is suggested compared to that of the renal allograft.
CLK PBMC phenotype and gene profile share potential similarities with the profiles of tolerant recipients.
Maintenance of liver and of kidney tolerance is likely driven by different mechanisms as suggested by their distinct transcriptomic and immunological profiles.
Acknowledgments
We thank all the patients who participated in this study and the physicians who helped us recruit patients: JF. Subra, F. Villemain, C. Legendre, E. Thervet, FJ. Bemelman, G. Roussey, G. Orlando, A. Garnier, H. Jambon, H. Le Monies De Sagazan, L. Braun, C. Noël, E. Pillebout, MC. Moal, C. Cantarell, A. Hoitsma, M. Ranbant, A. Testa.
Financial support: R.D. was supported by the “Fondation Centaure” (RTRS) which supports a French research network in transplantation and by a grant from the “Fondation pour la Recherche Médicale” (FRM). E.D. was supported by a grant from the French National Academia of Medicine. INSERM U1064 was supported by the “Agence de la Biomédecine” and by the Association Française pour l’Etude du Foie, AFEF. PAV was supported by the ASTS/Novartis Fellowship in Transplantation Award. This work was carried out in the context of the IHU-Cesti project, which received financial support from the French government, managed by the National Research Agency (“Investment Into The Future” program ANR-10-IBHU-005), and from Nantes Metropole and the Pays de la Loire Region. The Institute of Liver Studies (KCL) is supported by the Medical Research Council (MRC) Centre for Transplantation, King’s College London, UK – MRC grant no. MR/J006742/1 and by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Abbreviations
- CM
central memory
- CLK
combined liver–kidney graft
- EM
effector memory
- IS
immunosuppression
- K-STA
kidney stable graft
- K-TOL
kidney operational tolerant
- L-STA
liver stable graft
- L-TOL
liver operational tolerant
- MELD score
model for end-stage liver disease score
- PBMC
peripheral blood mononuclear cells
- TEMRA
terminally differentiated effector
- Treg
regulatory T cells
- UNOS
united network for organ sharing
Footnotes
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1111/liv.12917/suppinfo
Conflict of interest: The authors do not have any disclosures to report.
References
- 1.Margreiter R, Kramar R, Huber C, et al. Combined liver and kidney transplantation. Lancet. 1984;1:1077–1078. doi: 10.1016/s0140-6736(84)91486-7. [DOI] [PubMed] [Google Scholar]
- 2.Chava SP, Singh B, Stangou A, et al. Simultaneous combined liver and kidney transplantation: a single center experience. Clin Transplant. 2010;24:E62–E68. doi: 10.1111/j.1399-0012.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Créput C, Durrbach A, Samuel D, et al. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3:348–356. doi: 10.1034/j.1600-6143.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 4.Faenza A, Fuga G, Nardo B, et al. Combined liver-kidney transplantation: the experience of the University of Bologna and the case of preoperative positive cross-match. Transplant Proc. 2006;38:1118–1121. doi: 10.1016/j.transproceed.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Lang M, Neumann U, Kahl A, et al. Long-term outcome of 27 patients after combined liver-kidney transplantation. Transplant Proc. 2001;33:1440–1441. doi: 10.1016/s0041-1345(00)02545-8. [DOI] [PubMed] [Google Scholar]
- 6.Mehrabi A, Fonouni H, Ayoub E, et al. A single center experience of combined liver kidney transplantation. Clin Transplant. 2009;23(Suppl. 21):102–114. doi: 10.1111/j.1399-0012.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 8.Fung J, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:88–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Mjörnstedt L, Friman S, Bäckman L, Rydberg L, Olausson M. Combined liver and kidney transplantation against a positive cross match in a patient with multispecific HLA-antibodies. Transplant Proc. 1997;29:3164–3165. doi: 10.1016/s0041-1345(97)00827-0. [DOI] [PubMed] [Google Scholar]
- 10.Fong T-L, Bunnapradist S, Jordan SC, Selby RR, Cho YW. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76:348–353. doi: 10.1097/01.TP.0000071204.03720.BB. [DOI] [PubMed] [Google Scholar]
- 11.Saidman SL, Duquesnoy RJ, Demetris AJ, et al. Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies. Transpl Immunol. 1994;2:61–67. doi: 10.1016/0966-3274(94)90080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katznelson S, Cecka JM. The liver neither protects the kidney from rejection nor improves kidney graft survival after combined liver and kidney transplantation from the same donor. Transplantation. 1996;61:1403–1405. doi: 10.1097/00007890-199605150-00021. [DOI] [PubMed] [Google Scholar]
- 13.Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;50:678–682. doi: 10.1097/00007890-199010000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang YJ, Lu L, Fung JJ, Qian S. Liver-derived dendritic cells induce donor-specific hyporesponsiveness: use of sponge implant as a cell transplant model. Cell Transplant. 2001;10:343–350. doi: 10.3727/000000001783986729. [DOI] [PubMed] [Google Scholar]
- 16.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Créput C, Durrbach A, Menier C, et al. Human leukocyte antigen-G (HLA-G) expression in biliary epithelial cells is associated with allograft acceptance in liver-kidney transplantation. J Hepatol. 2003;39:587–594. doi: 10.1016/s0168-8278(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 18.Brouard S, Pallier A, Renaudin K, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 19.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774–1780. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 20.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano JJ, Pallier A, Martinez-Llordella M, et al. Comparison of transcriptional and blood cell-phenotypic markers between operationally tolerant liver and kidney recipients. Am J Transplant. 2011;11:1916–1926. doi: 10.1111/j.1600-6143.2011.03638.x. [DOI] [PubMed] [Google Scholar]
- 22.Bohne F, Martínez-Llordella M, Lozano J-J, et al. Intragraft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122:368–382. doi: 10.1172/JCI59411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Llordella M, Lozano JJ, Puig-Pey I, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brouard S, Mansfield E, Braud C, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007;104:15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danger R, Pallier A, Giral M, et al. Upregulation of miR-142-3p in peripheral blood mononuclear cells of operationally tolerant patients with a renal transplant. J Am Soc Nephrol. 2012;000:597–606. doi: 10.1681/ASN.2011060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 27.Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14:1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+ T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 29.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Rond S, Azéma C, Krawice-Radanne I, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J. Immunol. 1950;2006(176):3266–3276. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 31.Naji A, Le Rond S, Durrbach A, et al. CD3 + CD4low and CD3 + CD8low are induced by HLA-G: novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood. 2007;110:3936–3948. doi: 10.1182/blood-2007-04-083139. [DOI] [PubMed] [Google Scholar]
- 32.Zabransky DJ, Nirschl CJ, Durham NM, et al. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS ONE. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios− Cells Coexist within the Natural FOXP3 + T Regulatory Cell Subset in Humans. J Immunol. 2013;190:2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 34.Kim YC, Bhairavabhotla R, Yoon J, et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3 + human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–2818. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana A, Robles S, Russo MJ, et al. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871–879. doi: 10.1097/SLA.0b013e31817fc2b8. [DOI] [PubMed] [Google Scholar]
- 37.Mazière P, Enright AJ. Prediction of microRNA targets. Drug Discov Today. 2007;12:452–458. doi: 10.1016/j.drudis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Kittleson MM, Kobashigawa JA. Long-term care of the heart transplant recipient. Curr Opin Organ Transplant. 2014;19:515–524. doi: 10.1097/MOT.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 39.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.