Abstract
Objective
Strategies to improve cartilage repair tissue quality after bone marrow cell-based procedures may reduce later development of osteoarthritis. Doxycycline is inexpensive, well-tolerated, and has been shown to reduce matrix-metalloproteinases (MMP) and osteoarthritis progression. This study tests the hypotheses that doxycycline reduces MMP, enhances chondrogenesis of human bone marrow-derived mesenchymal stem cells (hMSC), and improves in vivo cartilage repair.
Design
Ninety hMSC pellets were cultured in chondrogenic media with either 0-, 1- or 2-μg/mL doxycycline. Pellets were evaluated with stereomicroscopy, proteoglycan assay, qRT-PCR, and histology. Osteochondral defects (OCD) were created in the trochlear grooves of 24-Sprague-Dawley rats treated with/without oral doxycycline. Rats were sacrificed at 12-weeks and repair tissues were examined grossly and histologically.
Results
hMSC pellets with 1-μg/mL (p=0.014) and 2-μg/mL (p=0.002) doxycycline had larger areas than pellets without doxycycline. hMSC pellets with 2-μg/mL doxycycline showed reduced mmp-13 mRNA (p=0.010) and protein at 21-days. Proteoglycan, DNA contents, and mRNA expressions of chondrogenic genes were similar (p>0.05). For the in vivo study, while the histological scores were similar between the two groups (p=0.116), the gross scores of the OCD repair tissues in doxycycline-treated rats were higher at 12-weeks (p=0.017), reflective of improved repair quality. The doxycycline-treated repairs also showed lower MMP-13 protein (p=0.029).
Conclusions
This study shows that doxycycline improves hMSC chondrogenesis and decreases MMP-13 in pellet cultures and within rat OCDs. Doxycycline exerted no negative effect on multiple measures of chondrogenesis and cartilage repair. These data support potential use of doxycycline to improve cartilage repair to delay the onset of osteoarthritis.
Keywords: Doxycycline, Chondrogenesis, Cartilage Repair, Osteochondral Defect, MSC, Stem Cells, Microfracture, Osteoarthritis, Gene Therapy
Introduction
Osteoarthritis is a leading cause of disability. Focal cartilage injuries are known to accelerate the degeneration of surrounding cartilage and to hasten the onset of osteoarthritis. Articular cartilage has limited capacity to repair following injury1, 2. Microfracture (bone marrow stimulation) is commonly used to treat focal cartilage injuries. This technique involves the recruitment of bone marrow derived mesenchymal stem cells (MSCs) to the defect area to participate in cartilage repair. However, the resulting repair does not restore hyaline articular cartilage, but instead results in formation of a mechanically inferior fibrocartilaginous scar tissue1, 2. Therefore, clinical strategies are needed to improve the type and quality of repair tissue following marrow stimulation procedures to potentially delay or prevent the onset of osteoarthritis.
Doxycycline is a widely available, inexpensive and well tolerated antibiotic that has a long clinical history in humans. In addition, doxycycline has been effectively used with the tetracycline-inducible gene regulation system for controlled gene therapy applications in the articular joint as well as other organ systems across multiple animal models3, 4. Besides anti-microbial and transgene-inducing functions, doxycycline may also have intrinsic benefits to articular cartilage. Prior studies have shown that the tetracycline class of medications has the ability to inhibit matrix metalloproteinases (MMP), with doxycycline being the most studied for this purpose5-10.
Matrix metalloproteinases are a family of zinc-dependent proteinases that differ slightly in substrate specificity. They function in maintaining the homeostasis of extracellular matrix (ECM) by degrading ECM molecules, such as collagens and proteoglycans11. Down-regulation of MMP increases the amount of collagens retained in the ECM9. MMP are upregulated in arthritis and following cartilage injury6, 9, 10, 12, 13. Among the MMP collagenases, MMP-1 (collagenase-1), -8 (collagenase-2), and -13 (collagenase-3) cleave interstitial collagens10, 14. MMP-13 is the most active for degradation of collagen-II, and has therefore been implicated in arthritic disease processes15, 16. In transgenic mice models, overexpression of MMP-13 has induced osteoarthritic changes16 while the absence of MMP-13 inhibited cartilage erosion17.
Oral administration of doxycycline has been postulated to have beneficial effects on articular cartilage by inhibiting MMP, specifically MMP-1, -3 (stromelysin), and -135-10, 18. Doxycycline administration in vivo has been shown to decrease MMP levels in small animal models, as well as in human osteoarthritic samples19, 20. When doxycycline was administrated prophylactically in animal models, decreased MMP activity was correlated to reduced proteoglycan loss from cartilage ECM, as well as to reduced degenerative changes in the weight-bearing areas of articular cartilage21, 22. In a randomized, double-blinded, placebo-controlled study evaluating the effects of doxycycline on the progression of joint space narrowing in mildly arthritic knees, the doxycycline treatment group had a decreased rate of narrowing by 40% at 16-months and 33% at 30-months as compared to placebo (p=0.017)6. On the other hand, other animal studies have not demonstrated any effect of doxycycline treatment on joint disease and/or MMP activities 23, 24. Hence numerous studies exist on the effect of doxycycline on cartilage degeneration, however with conflicting results.
Fewer studies have evaluated the effect of doxycycline on musculoskeletal repair processes. In a study using a rat laminectomy defect model, rats that received intra-peritoneal doxycycline showed enhanced wound healing, potentially due to reduction of scar tissue formation25. In another study, oral doxycycline was shown to inhibit MMP and improve and the strength of rotator cuff repairs in rats26. This study found that the doxycycline administered group had improved load to failure as well as greater collagen organization compared to controls and correlated these findings to a significant decrease in MMP-13 activity. In contrast, another study investigating the healing of Achilles tendon in rats showed decreased strength of the repaired tendon in the doxycycline group27. While the effects of doxycycline on musculoskeletal tissue repair processes may be variable, its effect on cartilage repair with MSCs as well as on MSC chondrogenesis is largely unknown. This study was performed to test the hypothesis that doxycycline reduces MMP expression, enhances human mesenchymal stem cell (hMSC) chondrogenesis and improves in vivo cartilage repair.
Methods
In vitro studies
hMSC chondrogenic differentiation
Ninety pellet cultures were prepared using human MSCs from a single donor cultured to passage 3 according to previously described procedures28. Briefly, 2.5×105 hMSCs were pelleted by centrifugation at 500×g for 15-minutes. The resulting pellets were cultured in 0.5-mL of pre-defined chondrogenic medium containing high-glucose Dulbecco's Modified Eagle Medium (DMEM, Gibco), with 1% penicillin-streptomycin (Gibco), 10−7-M dexamethasone (Sigma-Aldrich), 50-μg/mL l-ascorbic acid-2-phosphate (Sigma-Aldrich), 40-μg/mL proline (MP Biomedicals, Solon), and 1% BD™ ITS + Premix (Becton-Dickinson), 10-ng/mL transforming growth factor-beta 1 (TGF-β1, R&D Systems). The chondrogenic medium was supplemented with 0-, 1-, or 2-μg/mL of doxycycline: thirty pellets per each of doxycycline concentrations. The pellets were incubated at 37°C in 5% CO2 for 3-, 7-, 14-, or 21-days, and the media was refreshed every 2-3 days.
Chondrogenic hMSC pellet assessments
Pellet area and histological evaluation
At 14- and 21-days of culture in chondrogenic media supplemented with 0-, 1-, or 2-μg/mL doxycycline, three hMSC chondrogenic pellets per group were analyzed grossly and for cross-sectional areas. Macroscopic assessment was performed using stereomicroscopy (MVX-10 MacroView Systems, Olympus) equipped with a DP71 camera (Olympus). The pellet area was measured using DP2-BSW software (Olympus). The pellets were subsequently fixed in 10% formalin and embedded in paraffin for histological analyses. Five-μm cross-sections were stained with 0.1% Safranin O/0.5% Fast green for sulfated glycosaminoglycan (GAG) and alizarin red for calcium29, and immunostained for MMP-13 according to standard protocols. Briefly for MMP-13 immunohistochemistry (IHC), sections were de-paraffined and rehydrated via conventional methods. Antigen retrieval was performed by heating and cooling the slides in 10-mM sodium citrate (pH 6.0) with 0.05% Tween-20 buffer (Sigma-Aldrich) for 20-minutes each at 85°C and room temperature. The sections were then digested in 1-mg/mL hyaluronidase in 0.1-M sodium acetate buffer (both from Sigma-Aldrich) for 30-minutes in 37°C. The sections were then blocked with 10% goat serum (Vector Laboratories) and 1% bovine serum albumin (BSA, Fisher Scientific) for 1-hour. The sections were incubated with a rabbit polyclonal antibody against MMP-13 (Abcam) at 1:100 dilution in 1% BSA overnight at 4°C. Endogenous peroxidase was blocked using 0.3% hydrogen peroxide (Sigma) in tris-buffered saline (TBS, Calbiochem) for 15-minutes at room temperature. The slides were then treated with secondary goat polyclonal anti-rabbit antibody (Abcam) in 1% BSA in 1:100 dilution for an hour, then developed using ImmPACT™ AEC Peroxidase Substrate (Vector Laboratories), according to the manufacturer's instructions. The slides were counterstained with hematoxylin (Vector Laboratories). Images of all the stained sections were captured using TE-2000U Eclipse microscope (Nikon) equipped with a DP71 camera. The MMP-13 IHC images in Fig. 4 had the contrast increased by the same value in Adobe Photoshop CS4 to improve MMP-13-positive and -negative cell contrast for print.
Figure 4. Doxycycline Reduced MMP-13 Deposition In Vitro.
Representative images of (A) Safranin-O Fast Green, (B) MMP-13 immunohistochemistry, and (C) Alizarin Red stainings of hMSC pellets at 20x (larger box) and at 4x (smaller inset). There was no difference in staining intensities for Safranin-O Fast Green and Alizarin Red stainings among different doxycycline concentration groups at each timepoint. As well, no difference in MMP-13-positive cells for 14-day timepoint. However, more MMP-13-positive cells were found in the periphery of the hMSC pellets cultured in 0- and 1-μg/mL Dox compared to those cultured in 2-μg/mL Dox at 21-day timepoint.
Biochemical analyses
After 3-, 7-, 14-, and 21-days of chondrogenic culture, three hMSC pellets per group were washed with phosphate buffered saline (PBS, Gibco) and each dry pellet was frozen at −80°C until biochemical analyses. Each frozen pellet was incubated overnight in 0.25-mL papain buffer (50-mM sodium phosphate buffer (Sigma-Aldrich), 50-mM Ethylenediaminetetraacetic acid (EDTA, Gibco), 5-mM cysteine-HCl (Sigma-Aldrich), and 0.5-mg/mL papain (Sigma-Aldrich)) in 60°C shaking water bath (200-RPM). The papain-digested pellets were vortexed. GAG content was quantified using the standard dimethylmethylene blue (DMMB) assay using VersaMax UV-Vis spectrophotometer (Molecular Devices), and DNA content was quantified using Quant-iT™ PicoGreen® dsDNA Reagent and Kits (Molecular Probes) per manufacturer's protocol using a VICTOR™ X3 Multilabel Plate Reader (Perkin Elmer). The GAG/DNA ratios were calculated from the results of the two assays.
Gene expression analyses
Three hMSC pellets per group at 3-, 7-, 14-, and 21-days of chondrogenic culture were washed with PBS and frozen at −80°C until gene expression analyses. Total RNA was extracted from each frozen pellets using TRIzol (Invitrogen). Briefly, pellets in 0.3-mL TRIzol were grinded using microtube pestles (USA Scientific), and the ground pellet samples were homogenized using QIAshredder columns (Qiagen). The lysate was incubated at room temperature for 5-minutes, after which the contents were centrifuged at 12,000×g for 10-minutes at 4°C. RNease Mini Kit (Qiagen) was used for the rest of the RNA isolating process per manufacturer's protocol. The pre-designed human aggrecan, collagen-II, collagen-I, collagen-X, mmp-13 and 18S TaqMan primers were purchased from Applied Biosystems. Custom human sox-9 primers and probe30 and human transforming growth factor-beta receptor II primers (forward: TCCACGTGTGCCAACAACATCAAC; reverse: TGCCACTGTCTCAAACTGCTCTGA) and FAM-labeled probe (ACAACACAGAGCTGCTGCCCATTG) were purchased from Integrated DNA Technologies. qRT-PCR reactions were done in duplicate in 384-well plates in a total volume of 10-μL as previously described30 using 2x real-time TaqMan PCR master mix with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Relative expression levels normalized to 18S were calculated using the 2−ΔCt method.
In vivo studies
Animal surgeries
All longitudinal animal experiments were performed following a University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approved protocol (Protocol No. 1103868) using the previously established OCD model31, 32. Briefly, OCD creation on twenty-four three-month old Sprague Dawley rats (Harlan) was performed under general gaseous anesthesia (Isoflurane, Phoenix Pharmaceuticals). The knees were shaved and swabbed with betadine and ethanol. A medial parapatellar approach was utilized to access the stifle joint. The patella was dislocated laterally and the knee was flexed to greater than 90-degrees to deliver the femoral trochlea into operative view. The trochlear groove was identified and the midpoint of the trochlea, which was the midpoint in the superior to inferior dimension at the center of the trochlear groove, was marked. A 1.5-mm drill was used to create the OCD, at a depth of 1.0-mm. The subchondral bone was penetrated until bleeding bone was identified. Sterile saline was used to gently irrigate the area to remove any osteochondral fragments, and the incision was closed in layers. The animals were placed in standard cages with full access to food and water post-operatively. Subcutaneous buprenorphine (Reckitt Benckiser Healthcare) at 0.5-mg/kg dosage was injected twice daily for the first two-days following surgery as post-operative analgesia. There was one death associated with anesthesia complication. Hence, the number of animals per group in the in vivo study were N=11 for No Doxycycline Control group and N=12 for 12 Week Doxycycline group. The animals received either regular drinking water or 2-mg/mL doxycycline (Sigma-Aldrich) in the drinking water. Doxycycline water was replaced daily to ensure fresh supply of doxycycline.
Repair tissue assessments
Following sacrifice by carbon dioxide asphyxiation at 12-weeks post-OCD, the joints were dissected and the repair tissues were examined grossly and analyzed with the aforementioned stereomicroscopy and camera, with and without india ink staining for gross grading using the ICRS-Cartilage Repair Assessment system (Table 1)33. The harvested femurs were then fixed, decalcified, embedded in paraffin, and subsequently sectioned. The sections were stained using traditional hematoxylin and eosin (H&E) staining, as well as immunostained using rabbit polyclonal antibody against MMP-13. The stained sections were imaged using DP71 camera and Eclipse TE2000-U microscope, and evaluated using modified Holland histological scoring system (Table 2)31. Three independent blinded individuals graded both the gross and sectioned histologic specimens, and the scores were averaged for statistical analysis. For the estimate percentages of MMP-13-positive cells, the sections from four selected knees from each treatment groups were imaged at high-power fields (magnification, x20). Positive and negative cells were counted using ImageJ software (NIH), and percentages were calculated by dividing the numbers of MMP-13-stained cells by the sum of the numbers of MMP-13-stained and unstained cells. On average, 304 cells were counted per OCD repair tissue. The MMP-13 IHC images in Fig. 6C had the contrast increased by the same value in Adobe Photoshop CS4 to improve MMP-13-positive and -negative cell contrast for print and cell counting.
Table 1.
ICRS Cartilage Repair Scoring System
| Criteria | Points | Scores |
|---|---|---|
| Degree of defect repair | In level with surrounding cartilage | 4 |
| 75% repair of defect depth | 3 | |
| 50% repair of defect depth | 2 | |
| 25% repair of defect depth | 1 | |
| 0% repair of defect depth | 0 | |
| Integration to border zone | Complete integration with surrounding cartilage | 4 |
| Demarcating border < 1 mm | 3 | |
| ¾ of repair tissue integrated, ¼ with a notable border > 1 mm | 2 | |
| ½ of repair tissue integrated with surrounding cartilage, ½ with a notable border > 1mm | 1 | |
| From no contact to ¼ of repair tissue integrated with surrounding cartilage | 0 | |
| Macroscopic appearance | Intact smooth surface | 4 |
| Fibrillated surface | 3 | |
| Small, scattered fissures or cracks | 2 | |
| Several, small or few but large fissures | 1 | |
| Total degeneration of defect area | 0 |
Table 2.
Modified Holland Histological Scoring System
| Criteria | Points | Scores |
|---|---|---|
| Overall defect evaluation (throughout the entire defect depth) | ||
| Percentage filling with neoformed tissue (%) | 100 | 3 |
| >50 | 2 | |
| <50 | 1 | |
| 0 | 0 | |
| Subchondral bone evaluation | ||
| Percentage filling with neoformed tissue (%) | 100 | 3 |
| >50 | 2 | |
| <50 | 1 | |
| 0 | 0 | |
| Subchondral bone morphology | Normal trabecular bone | 4 |
| Trabecular, with some compact bone | 3 | |
| Compact bone | 2 | |
| Compact bone and fibrous tissue | 1 | |
| Only fibrous tissue or no tissue | 0 | |
| Extent of neotissue bonding with adjacent bone | Complete on both edges | 3 |
| Complete on one edge | 2 | |
| Partial on both edges | 1 | |
| Without continuity on either edge | 0 | |
| Cartilage evaluation | ||
| Morphology of neoformed surface tissue | Extensively articular cartilage | 4 |
| Mainly hyaline cartilage | 3 | |
| Fibrocartilage (spherical morphology observed with >75% of cells) | 2 | |
| Only fibrous tissue (spherical morphology observed with <75% of cells) | 1 | |
| No tissue | 0 | |
| Thickness of neoformed cartilage | Similar to the surrounding cartilage | 3 |
| Greater than surrounding cartilage | 2 | |
| Less than the surrounding cartilage | 1 | |
| No cartilage | 0 | |
| Joint surface regularity | Smooth, intact surface | 3 |
| Surface fissures (<25% neosurface thickness) | 2 | |
| Deep fissures (25-99% neosurface thickness) | 1 | |
| Complete disruption of the neosurface | 0 | |
| Chondrocyte clustering | None at all | 3 |
| <25% chondrocytes | 2 | |
| 25–100% chondrocytes | 1 | |
| No chondrocytes present (no cartilage) | 0 | |
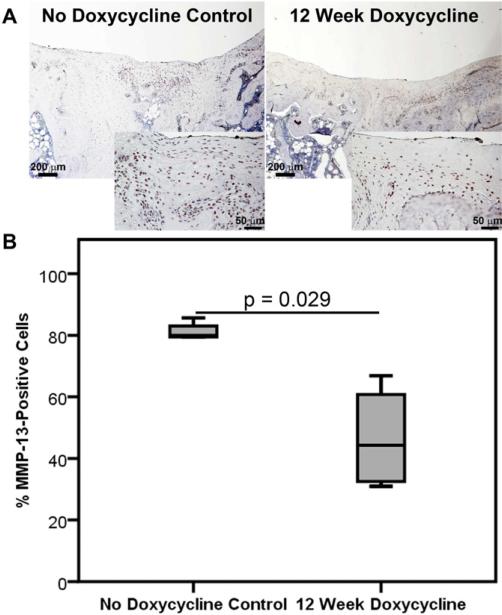
Figure 6. Doxycycline Inhibited MMP-13 Deposition In Vivo.
(A) Representative 4x (larger box) and 20x (smaller inset) images of MMP-13 immunohistochemical staining of repair tissues from No Doxycycline Control and 12 Week Doxycycline groups. (B) Median and interquartile range of percentage of MMP-13-positive cells in different treatment groups. More MMP-13-positive cells were found in the newly formed repair tissues of No Doxycycline Control group compared to those of 12 Week Doxycycline group (p=0.029). N=4/group.
Statistical analyses
All statistical analyses were conducted using Statistical Package for Social Studies (SPSS) 17.0 for Windows (IBM). Small letter “n” represents number of replicates in an independent in vitro study, while big letter “N” represents independent number of animals in the in vivo study. Assumptions of parametric data were tested for all data: normality of data distribution with Shapiro–Wilk and homogeneity of variance with Levene's tests. P-values of less than 0.05 were considered significant, unless otherwise noted. In vitro studies were repeated two times in triplicate (n=3), with data and statistical analysis with n=3 reported as line graphs with mean ± 95% confidence intervals from a representative experiment (Figs. 1-3). In vivo studies were conducted and data observed using independent animals, with N=11 for No Doxycycline Control group and N=12 for 12 Week Doxycycline group. All the data are reported as boxplots with median as the horizontal bar, interquartile range calculated using Tukey Hinge as the box, and the lowest and highest values represented as whiskers (Figs. 5B, 5C, and 6B). For comparison among three groups and multiple timepoints for the in vitro data, two-way independent analysis of variance (ANOVA) was used with doxycycline concentration and timepoints as main independent variables. For variables that were considered significant, post-hoc Tukey was used to further evaluate the data. For comparisons between two groups for the in vivo data, Student's t-test was used for parametric data (Figs. 5B and C) and Mann-Whitney U test was used for non-parametric data (Fig. 6B).
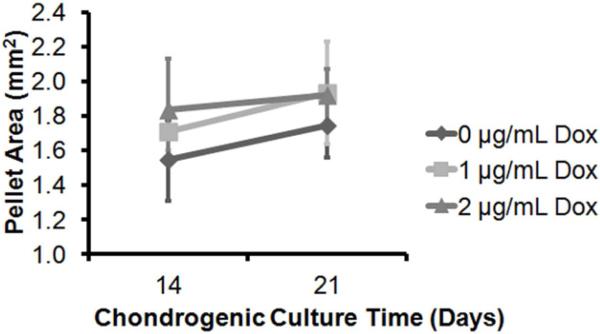
Figure 1. Doxycycline Increased MSC Pellet Area.
Cross-sectional areas were measured from the hMSC pellets cultured in chondrogenic media with 0-, 1-, or 2-μg/mL Dox for 14- and 21-days. Pellet area increased from 14- to 21-days in chondrogenic culture period (p=0.002). As well, the pellet area was significantly affected by the doxycycline concentration in the chondrogenic media regardless of the timpoints (p=0.002): 0-μg/mL Dox pellets were smaller than the 1-μg/mL (p=0.014) and 2-μg/mL Dox pellets (p=0.002). There was no difference in sizes between the 1- and 2-μg/mL Dox pellets (p=0.535). n=3/group.
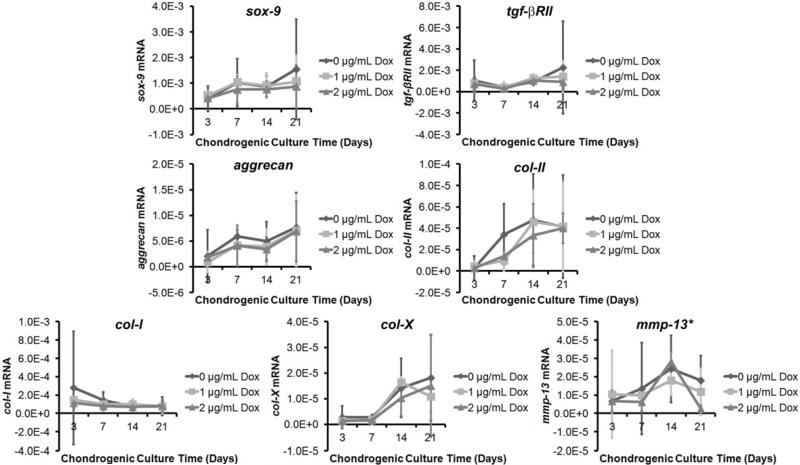
Figure 3. Doxycycline Inhibited MMP-13 Gene Expression In Vitro.
Messenger RNA levels of chondrogenic inducers sox-9 and tgf-βRII, chondrogenic genes aggrecan and col-II, fibrocartilage marker col-I, hypertrophic chondrocyte marker col-X, and catabolic gene mmp-13. There was an increasing trend in sox-9, tgf-βRII, aggrecan, col-II, and col-X levels (ps<0.01), and a decreasing trend in col-I level (p=0.028) over chondrogenic culture time. There was no significant effect of doxycycline concentration on these gene expressions (p>0.05). For mmp-13 level, there was an upward trend until day-14, with mRNA level in the 14-day pellets significantly higher than those of the 3-day (p<0.001), and 7-day (p<0.001) pellets. However, on day-21, there is a reduction in the mRNA level from day-14 (p<0.001). The mmp-13 mRNA levels on day-21 pellets were also significantly different among different doxycycline concentrations: 2-μg/mL Dox pellets had significantly lower mmp-13 levels compared to 0-μg/mL Dox pellets (p=0.01). n=3/group.
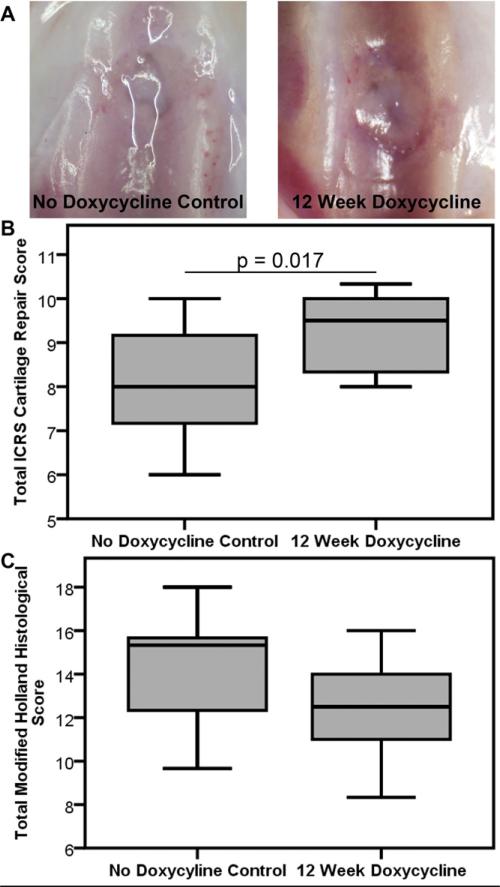
Figure 5. Doxycycline Did Not Interfere with In Vivo Cartilage Repair.
(A) Representative images of repair tissues from No Doxycycline Control and 12 Week Doxycycline groups evaluated using stereomicroscopy. (B) Gross grading of OCD repair tissues using ICRS Cartilage Repair Assessment grades showed better repair tissue for the 12 Week Doxycycline group (p=0.017), while (C) histological grading of OCD repair tissues using the modified Holland's scoring showed no difference between the two groups. N=11-12/group.
Results
Gross Evaluation of hMSC Chondrogenic Pellets (Fig. 1)
The areas of hMSC chondrogenic pellets increased with increasing concentrations of doxycycline (p=0.002) and with increasing time in chondrogenic culture (p=0.002). The post-hoc Tukey test revealed that the pellet areas at both 14- and 21-day timepoints were significantly larger for those cultured in 1-μg/mL (p=0.014) and 2-μg/mL (p=0.002) of doxycycline compared to those cultured without any addition of doxycycline. There was no significant difference in pellet areas between those cultured in 1- and 2-μg/mL doxycycline. The interaction effect between the two main variables, doxycycline concentration and culture time, was also non-significant (p=0.377).
Biochemical Analyses of hMSC Chondrogenic Pellets (Fig. 2)
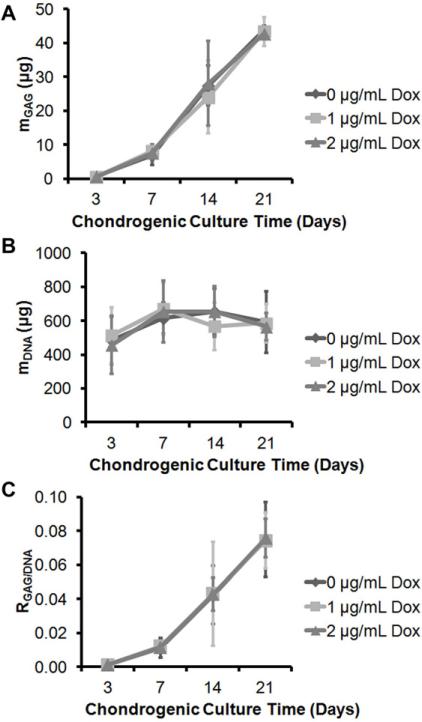
Figure 2. Doxycycline Did Not Interfere with GAG Synthesis or MSC Proliferation In Vitro.
(A) Total GAG content per hMSC pellets showed increasing GAG content with longer chondrogenic culture time (p<0.001), with GAG content of the 7-day pellets significantly greater than those of the 3-day pellets (p<0.001), those of 14-day pellets significantly greater than those of the 7-day pellets (p<0.001), and those of 21-day pellets significantly greater than those of the 14-day pellet (p<0.001). There was no significant effect of doxycycline concentration on the GAG content (p=0.412). (B) Total DNA content per hMSC pellets showed significant effect of chondrogenic culture time on the DNA contents (p<0.001): DNA content of the 3-day pellets were significantly lower than those of the 7- (p<0.001), 14- (p<0.001) and 21-day (p=0.009) pellets. There was no difference in DNA content among 7-, 14-, and 21-day pellets. As well, there was no significant effect of doxycycline concentration on the DNA content (p=0.314). (C) Normalized GAG content with respect to the DNA content showed that there is significant effect of chondrogenic culture time in the normalized GAG content (p<0.001); however, the effect of doxycycline concentration was not significant (p=1.000). n=3/group.
Total GAG (Fig. 2A) and DNA (Fig. 2B) content per pellet samples and their ratio (Fig. 2C) were measured and calculated on 3-, 7-, 14-, and 21-day old chondrogenic pellets to analyze the effect of doxycycline on hMSC chondrogenesis and proliferation/viability. For all three outcome measures, there was a significant main effect of the time in chondrogenic culture (p<0.001); however, the main effect of doxycycline concentration and the interaction effects were non-significant. The post-hoc Tukey tests for total GAG content and GAG/DNA ratio revealed significant differences of these outcome variables between all the different timepoints (p<0.001). On the other hand, the post-hoc Tukey test for total DNA content showed significant differences between day-3 and day-7 (p<0.001), -14 (p<0.001), and -21 (p=0.009).
Gene Expression Analyses of hMSC Chondrogenic Pellets (Fig. 3)
Various mRNA levels were measured on 3-, 7-, 14-, and 21-day old chondrogenic pellets to analyze the effect of doxycycline on hMSC chondrogenesis. The time in chondrogenic culture had significant effect on gene expressions for all the assayed genes: sox-9 (p=0.001), tgf-βRII (p=0.003), aggrecan (p<0.001), collagen-II (p<0.001), collagen-I (p=0.028), collagen-X (p<0.001), and mmp-13 (p<0.001). The main effect of doxycycline concentration as well as interaction effects between the two main independent variables were non-significant for sox-9, tgf-βRII, aggrecan, collagen-II, collagen-I, and collagen-X mRNA levels. For the mmp-13 gene expression, on the other hand, there was a non-significant main effect of doxycycline concentration but a significant interaction effect between the time in chondrogenic culture and doxycycline concentration (p=0.025). This indicates that the doxycycline concentration affected mmp-13 mRNA level differently on various chondrogenic culture timepoints. Specifically, mmp-13 levels for all three doxycycline concentrations were similar for day 3, 7, and 14 hMSC pellets; however, they were different for day-21 pellets (p=0.012). The 2-μg/mL Dox pellets had significantly lower mmp-13 mRNA level compared to 0-μg/mL Dox pellets (p=0.010).
Histological Analyses of hMSC Chondrogenic Pellets (Fig. 4)
Safranin O-Fast Green staining of sulfated GAGs showed increased amount of staining for 21-versus 14-day hMSC pellets (Fig. 4A). However, no differences in staining intensities were appreciable among the three different doxycycline concentrations. These results confirm the biochemical analysis of total GAG content (Fig. 2A). The MMP-13 IHC showed similar MMP-13-positive cells for all the groups at day-14 (Fig. 4B). However, the MMP-13 staining intensities decreased for all three groups by day-21. In addition, more MMP-13-positive cells were found in the 0- and 1-μg/mL doxycycline pellets. These MMP-13 IHC results at 14- and 21-day timepoints are in accordance with the mmp-13 mRNA result in figure 3. As upregulation of MMP-13 in MSC chondrogenesis has also been linked to terminal differentiation of hypertrophic chondrocytes23, 34, 35, alizarin red staining was performed. However, there were no positively stained pellets at either doxycycline concentration at both 14- and 21-day chondrogenic culture timepoints (Fig. 4C).
Evaluation of OCD Repair Tissues (Fig. 5)
The OCD repair tissues from the 12 Week Doxycycline group showed more complete repair tissue within the femoral trochlear groove by gross inspection with stereomicroscopy (Fig. 5A). The animals that received doxycycline for 12-weeks post-op had higher mean total ICRS Cartilage Repair Scores via gross stereomicroscopy examination, reflecting better quality repairs than the No Doxycycline Control group (Fig. 5B, p=0.017). Qualitative evaluation using the modified Holland Histological scoring of the H&E-stained OCD repair tissue showed similar scores between the two groups (Fig. 5C, p=0.116).
MMP-13 of OCD Repair Tissue (Fig. 6)
The repair tissues from No Doxycycline Control group showed more MMP-13-positive cells compared to those of 12 Week Doxycycline group (Fig 6A): No Doxycycline Control group had 80% MMP-13-positive cells, whereas 12 Week Doxycycline group had 44% MMP-13-positive cells (Fig 6B, p=0.029). This in vivo OCD repair tissue MMP-13 content result correlates to the in vitro hMSC pellet data (Fig. 4B).
Discussion
This study showed that doxycycline enhanced cartilage tissue formation and decreased MMP-13 both in human MSC pellet cultures and within rat osteochondral defects. Using multiple measures of chondrogenesis and cartilage repair, doxycycline also did not appear to exert any negative effects on either human MSC chondrogenesis in vitro or on rat osteochondral defect repair in vivo. These data support further work in the potential use of doxycycline to improve cartilage repair or as an adjunct to other treatment strategies to delay or prevent the onset of osteoarthritis.
As a strategy to enhance MSC chondrogenesis to improve cartilage repair, our laboratory has previously investigated gene therapy using adeno-associated virus (AAV) to deliver therapeutic proteins of interest to articular joints31. Intra-articular injection of AAV has been used in clinical trials36, and our previous study showed improved cartilage repair with use of human mesenchymal stem cells transduced with AAV-TGF-β1. Nonetheless, there are potential safety issues concerning long-term or high-dose expression of transgenes. For this reason, controllable vectors have been developed to limit treatment time/dosage to the minimal needed to achieve therapeutic efficacy. One novel system is the Tet-on promoter, in which control is facilitated by using tetracycline-class drugs as activators of the tetracycline response element (TRE)-promoter, stimulating transgene expression only in the presence of the drug. Previous studies in our laboratory have successfully modulated AAV-transgene expression utilizing this system with oral administration of 2-mg/mL doxycycline3. The data from the current study showing a potential positive effect of doxycycline without evidence of negative effects on in vitro and in vivo chondrogenesis support continued investigations into the use of TRE promoters for localized gene therapy to enhance cartilage repair.
The doses of doxycycline used in this study were based on evidence for bioavailability and bioactivity in previous studies by several groups. Oral administration of doxycycline at 2-mg/mL has been shown to result in significant upregulation of doxycycline-controlled transgene expression in various rodent models3, 37. In our previous study, 1-μg/mL doxycycline added to the culture media was adequate to induce transgene expression for AAV2-TRE-Luciferase vectors from the transduced intra-articular cells3. The 1- and 2-μg/mL doxycycline for the current in vitro and the 2-mg/mL doxycycline in drinking water for the current in vivo studies were based on these studies. Additionally, these concentrations approximate the intra-articular synovial fluid doxycycline concentration achieved following oral doxycycline. One study, in which male Sprague Dawley rats of similar weights were fed comparable dose of oral doxycycline in drinking water for 5-days to 4-weeks, determined the serum doxycycline level to be 1.8-μg/mL26. In a study where serum and synovial fluid concentrations of doxycycline were measured following oral administrations of 5-mg/kg twice a day in horses, it was determined that doxycycline distributes well from serum into synovial fluid38. Therefore, we expect the synovial fluid doxycycline concentration following oral administration of 2-mg/mL doxycycline to be not too far away from the 0 – 2-μg/mL range.
Addition of doxycycline to the drinking water is a simple and effective method of drug administration3, 26, 27, 37, 39. However, there is inherent variability with this method because free water intake by individual animals is not well-controlled. It is also difficult to monitor total water intake to determine actual doxycycline intake. The difficulty in controlling doxycycline intake through inclusion of the medication in the drinking water may account for the variable observations on the effects of doxycycline seen in this and other studies. Future studies with intra-peritoneal injection of doxycycline or oral administration as a pill or measured suspension will likely improve understanding of doxycycline on musculoskeletal tissue repair processes.
The in vitro studies where dosage could be controlled showed that doxycycline has no toxic/inhibitory effect on multiple of measures of MSC chondrogenesis. The in vitro data also showed that doxycycline exhibited a dose dependent reduction of MMP-13 gene expression as well as protein expressions by day-21 of pellet culture. This is consistent with the MMP-13 suppressing activity of doxycycline found in other differentiated tissue types, such as chondrocytes and synoviocytes7, 9. However, doxycycline-mediated inhibition of mmp-13 expression occurred earlier after 24-hours of doxycycline exposure in other studies on chondrocytes9, 10. This effect was observed at 21-days in our hMSC chondrogenic pellet cultures. The inhibitory effect of doxycycline on mmp-13 gene expression may potentially be specific to differentiated cell types, and not in multi-lineage state MSCs. The hMSC pellets are not of chondrogenic phenotype initially. As such, inhibition of MMP-13 may have been observable only after chondrogenic differentiation of the MSC cells.
In regards to in vitro chondrogenesis of human MSC, the pellet areas in the 2-μg/mL doxycycline group were larger than those in no doxycycline treatment group. Because we did not find any differences in aggrecan and col-II mRNA expression levels among pellets cultured in different doxycycline concentrations, this effect is more likely due to improved matrix retention through inhibition of MMP-13 production. As MMP-13 primarily functions to break down collagen-II7, 23, MMP-13 downregulation would ultimately lead to reduction of excessive collagen-II degradation or enhancement of collagen-II accumulation. MMP-13 is also a marker of terminal differentiation of MSCs into hypertrophic chondrocytes23, 34, 35. However, lack of differences in col-X gene expression level and lack of alizarin-red positive staining among hMSC pellets cultured in various doxycycline concentrations suggest that doxycycline is not principally involved in inhibition of MSC hypertrophy.
Our in vivo data additionally suggests that oral administration of doxycycline has no harmful effect but may improve cartilage repair. While we performed in vitro studies using human MSC to improve translational relevance, we performed in vivo studies in rats in order to investigate the effects of doxycycline on host bone marrow repair cells accessed through osteochondral defect creation. This was performed to enhance the clinical relevance by more closely mimicking the expected cellular repair processes occurring in bone marrow stimulation procedures, such as microfracture, than what can be achieved through implantation of hMSC into osteochondral defects created in athymic rodents31. The OCD repair tissues from 12 Week Doxycycline group were of similar quality histologically and higher quality grossly versus that from No Doxycycline Control group. As well, MMP-13 expression in OCD repair tissue samples was significantly greater in No Doxycycline Control than in 12 Week Doxycycline groups. This can potentially explain the higher quality repairs reflected in the ICRS scores in the doxycycline treated rats. Furthermore, this in vivo finding is correlated to the in vitro findings of MMP-13 levels in control versus doxycycline-treated MSC pellets.
In summary, we showed in both in vitro and in vivo studies that doxycycline had no toxic or inhibitory effect on MSC chondrogenesis and cartilage repair. Furthermore, doxycycline inhibited MMP-13 and potentially improved in vitro chondrogenesis and in vivo cartilage repair. These findings suggest that doxycycline can be used for external control of transgene expression in cartilage repair studies incorporating AAV-mediated gene therapy strategies without interfering with the repair process. Doxycycline inhibition of MMP-13 may also serve to potentially improve in vivo cartilage repair when used alone. As such, continued evaluation of doxycycline effects on articular cartilage and MSC based tissue repair processes may lead to readily implementable new treatment strategies to delay the development of osteoarthritis through improving cartilage repair processes.
Acknowledgements
The authors would like to thank Dr. Karin A. Payne for general advice and Michele Mulkeen for technical assistance.
Funding Source:
This work was funded by the National Institutes of Health (NIH) RO1 AR051963 (CRC), RC2 AR058929 (CRC) and T32-EB001026 (HHL/SPM), the Albert Ferguson Endowed Chair (CRC), and the Albert B. Ferguson, M.D. Orthopaedic Fund of the Pittsburgh Foundation (MJO/CRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: HHL and CRC contributed to conception and design of the study, analysis and interpretation of the data, drafting and critical revision of the article. MJO contributed to conception and design of the study, collection, assembly, analysis, and interpretation of the data. NAF contributed to analysis and interpretation of the data, and critical revision of the article. CRC is responsible for the integrity of the work as a whole.
Conflict of interest statement:
The authors declare no conflict of interest.
References
- 1.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;(423):7–16. [PubMed] [Google Scholar]
- 2.Nakajima H, Goto T, Horikawa O, Kikuchi T, Shinmei M. Characterization of the cells in the repair tissue of full-thickness articular cartilage defects. Histochem Cell Biol. 1998;109(4):331–8. doi: 10.1007/s004180050233. [DOI] [PubMed] [Google Scholar]
- 3.Payne KA, Lee HH, Haleem AM, Martins C, Yuan Z, Qiao C, et al. Osteoarthritis and cartilage / OARS. 8. Vol. 19. Osteoarthritis Research Society; 2011. Single intra-articular injection of adeno-associated virus results in stable and controllable in vivo transgene expression in normal rat knees. pp. 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stieger K, Belbellaa B, Le Guiner C, Moullier P, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Advanced Drug Delivery Reviews. 2009;61(7–8):527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekman B, Verzijl N, de Roos JADM, Koopman JL, Tekoppele JM. Doxycycline Inhibits Collagen Synthesis by Bovine Chondrocytes Cultured in Alginate. Biochemical and Biophysical Research Communications. 1997;237(1):107–110. doi: 10.1006/bbrc.1997.7088. [DOI] [PubMed] [Google Scholar]
- 6.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effects of doxycycline on progression of osteoarthritis: Results of a randomized, placebo-controlled, double-blind trial. Arthritis & Rheumatism. 2005;52(7):2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 7.Fortier LA, Motta T, Greenwald RA, Divers TJ, Mayr KG. Synoviocytes are more sensitive than cartilage to the effects of minocycline and doxycycline on IL-1α and MMP-13-induced catabolic gene responses. Journal of Orthopaedic Research. 2010;28(4):522–528. doi: 10.1002/jor.21006. [DOI] [PubMed] [Google Scholar]
- 8.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines Inhibit Connective Tissue Breakdown: New Therapeutic Implications for an Old Family of Drugs. Critical Reviews in Oral Biology & Medicine. 1991;2(3):297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 9.Shlopov BV, Smith GN, Jr, Cole AA, Hasty KA. Differential patterns of response to doxycycline and transforming growth factor β1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis & Rheumatism. 1999;42(4):719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Shlopov BV, Stuart JM, Gumanovskaya ML, Hasty KA. Regulation of cartilage collagenase by doxycycline. The Journal of Rheumatology. 2001;28(4):835–842. [PubMed] [Google Scholar]
- 11.Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354(4):846–51. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 12.Mehraban F, Lark MW, Ahmed FN, Xu F, Moskowitz RW. Increased secretion and activity of matrix metalloproteinase-3 in synovial tissues and chondrocytes from experimental osteoarthritis. Osteoarthritis and Cartilage. 1998;6(4):286–294. doi: 10.1053/joca.1998.0122. [DOI] [PubMed] [Google Scholar]
- 13.Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical Injury and Cytokines Cause Loss of Cartilage Integrity and Upregulate Proteins Associated with Catabolism, Immunity, Inflammation, and Repair. Molecular & Cellular Proteomics. 2009;8(7):1475–1489. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GN, Jr, Mickler EA, Hasty KA, Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: Relationship to structure of the enzyme. Arthritis & Rheumatism. 1999;42(6):1140–1146. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Sabatini M, Lesur C, Thomas M, Chomel A, Anract P, de Nanteuil G, et al. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52(1):171–80. doi: 10.1002/art.20900. [DOI] [PubMed] [Google Scholar]
- 16.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis & Rheumatism. 2009;60(12):3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinmeyer J, Daufeldt S, Taiwo YO. Pharmacological effect of tetracyclines on proteoglycanases from interleukin-1 -treated articular cartilage. Biochemical Pharmacology. 1998;55(1):93–100. doi: 10.1016/s0006-2952(97)00383-3. [DOI] [PubMed] [Google Scholar]
- 19.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 38(2):308–17. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 20.Smith GN, Yu LP, Brandt KD, Capello WN. Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritic cartilage. 1998;25(3):532–5. [PubMed] [Google Scholar]
- 21.Yu LP, Smith GN, Brandt KD, Myers SL, O'Connor BL, Brandt DA. Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis & Rheumatism. 1992;35(10):1150–1159. doi: 10.1002/art.1780351007. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald RA. Treatment of Destructive Arthritic Disorders with MMP Inhibitors. Annals of the New York Academy of Sciences. 1994;732(1):181–198. doi: 10.1111/j.1749-6632.1994.tb24734.x. [DOI] [PubMed] [Google Scholar]
- 23.Borzí RM, Olivotto E, Pagani S, Vitellozzi R, Neri S, Battistelli M, et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis & Rheumatism. 2010;62(8):2370–2381. doi: 10.1002/art.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bri E, Lei W, Svensson O, Chowdhury M, Moak SA, Greenwald RA. Effect of an Inhibitor of Matrix Metalloproteinases on Spontaneous Osteoarthritis in Guinea Pigs. Advances in Dental Research. 1998;12(1):82–85. doi: 10.1177/08959374980120012601. [DOI] [PubMed] [Google Scholar]
- 25.Olmarker K. Reduction of adhesion formation and promotion of wound healing after laminectomy by pharmacological inhibition of pro-inflammatory cytokines: an experimental study in the rat. Eur Spine J. 2010;19(12):2117–21. doi: 10.1007/s00586-010-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38(2):308–17. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 27.Pasternak B, Fellenius M, Aspenberg P. Doxycycline impairs tendon repair in rats. Acta Orthop Belg. 2006;72(6):756–60. [PubMed] [Google Scholar]
- 28.Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis and Cartilage. 2010;18(5):705–713. doi: 10.1016/j.joca.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson FL, Hladik C. Histotechnology: A Self-Instructional Text. 3 ed. American Society for Clinical Pathology; Hong Kong: 2009. [Google Scholar]
- 30.Lee HH, Haleem AM, Yao V, Li J, Xiao X, Chu CR. Release of Bioactive Adeno-Associated Virus from Fibrin Scaffolds: Effects of Fibrin Glue Concentrations. Tissue Engineering Part A. 2011;17(15-16):1969–1978. doi: 10.1089/ten.tea.2010.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14(10):804–13. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 32.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled In Vivo Degradation of Genipin Crosslinked Polyethylene Glycol Hydrogels within Osteochondral Defects. Tissue Engineering. 2006;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 33.ICRS Cartilage Evaluation Package 2000. (1) 2011 Dec; Available from: http://www.cartilage.org/_files/contentmanagement/ICRS_evaluation.pdf.
- 34.Bertram H, Boeuf S, Wachters J, Boehmer S, Heisel C, Hofmann MW, et al. Matrix Metalloprotease Inhibitors Suppress Initiation and Progression of Chondrogenic Differentiation of Mesenchymal Stromal Cells In Vitro. Stem Cells and Development. 2009;18(6):881–892. doi: 10.1089/scd.2008.0306. [DOI] [PubMed] [Google Scholar]
- 35.Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian Hedgehog Promotes Chondrocyte Hypertrophy and Upregulation of MMP-13 in Human Osteoarthritic Cartilage. Osteoarthritis and Cartilage. 2012;20(7):755–63. doi: 10.1016/j.joca.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, et al. Safety, Tolerability, and Clinical Outcomes after Intraarticular Injection of a Recombinant Adeno-associated Vector Containing a Tumor Necrosis Factor Antagonist Gene: Results of a Phase 1/2 Study. The Journal of Rheumatology. 2010;37(4):692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- 37.Hebda PA, Whaley D, Kim H-G, Wells A. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Repair and Regeneration. 2003;11(5):373–379. doi: 10.1046/j.1524-475x.2003.11510.x. [DOI] [PubMed] [Google Scholar]
- 38.Schnabel LV, Papich MG, Watts AE, Fortier LA. Orally administered doxycycline accumulates in synovial fluid compared to plasma. Equine Veterinary Journal. 2010;42(3):208–212. doi: 10.2746/042516409X478514. [DOI] [PubMed] [Google Scholar]
- 39.Sheng Y, Lin CC, Yue J, Sukhwani M, Shuttleworth JJ, Chu T, et al. Generation and characterization of a Tet-On (rtTA-M2) transgenic rat. BMC Dev Biol. 2010;10:17. doi: 10.1186/1471-213X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]