Abstract
Gene profiling studies have indicated that in vitro differentiated human megakaryocytes express the receptor for IL-21 (IL-21R), an immunostimulatory cytokine associated with inflammatory disorders and currently under evaluation in cancer therapy. The aim of this study was to investigate whether IL-21 modulates megakaryopoiesis. We first checked the expression of IL-21 receptor on human bone marrow and in vitro differentiated megakaryocytes. We then investigated the effect of IL-21 on the in vitro differentiation of human blood CD34+ progenitors into megakaryocytes. Finally, we analyzed the consequences of hydrodynamic transfection-mediated transient expression of IL-21, on megakaryopoiesis and thrombopoiesis in mice. The IL-21Rα chain was expressed in human bone marrow megakaryocytes and was progressively induced during in vitro differentiation of human peripheral CD34+ progenitors, while the signal transducing γ chain was down-regulated. Consistently, the STAT3 phosphorylation induced by IL-21 diminished during the later stages of megakaryocytic differentiation. In vitro, IL-21 increased the number of colony-forming unit megakaryocytes generated from CD34+ cells and the number of megakaryocytes differentiated from CD34+ progenitors in a JAK3- and STAT3-dependent manner. Forced expression of IL-21 in mice increased the density of bi-potent megakaryocyte progenitors and bone marrow megakaryocytes, and the platelet generation, but increased platelet clearance with a consequent reduction in blood cell counts. Our work suggests that IL-21 regulates megakaryocyte development and platelet homeostasis. Thus, IL-21 may link immune responses to physiological or pathological platelet-dependent processes.
Introduction
Megakaryopoiesis is mainly controlled by thrombopoietin (TPO). In vitro, TPO is essential to differentiate hematopoietic progenitors into megakaryocytes, a differentiation enhanced by cytokines such as IL-6, IL-1β, IL-3, IL-9 and IL-11.1 In vivo, megakaryopoiesis occurs in the bone marrow (BM), a complex environment in which innate and adaptive immune cells produce cytokines regulating this process, some positively (such as IL-6, TNF-α and IL-1β2,3), others negatively (IL-10, IL-4 and TGF-β4–6). This influence is exemplified by reactive thrombocytosis, attributed to infections or inflammatory diseases7 and largely mediated by IL-6.8 More recently, IL-1α was shown to enhance thrombopoiesis.9 Whether other cytokines are involved in the regulation of megakaryopoiesis requires further investigation in order to better understand inflammatory thrombocytosis. To address this issue, we compared published gene profiles of CD34+ progenitors and in vitro differentiated megakaryoblasts.10 This analysis indicated the presence of IL-21 receptor (IL-21R) on megakaryocytes.
IL-21R is a heterodimer composed of a specific alpha chain (IL-21Rα) and the common gamma chain (IL-2Rγ) required for signal transduction.11 IL-21 is produced by subsets of natural killer (NK) T cells and helper CD4+ T cells, in particular follicular Th cells and Th17 cells. In healthy individuals, the BM contains T cells producing IL-21,12 probably follicular Th cells whose frequency may increase in pathological states.13 IL-21 controls a variety of responses of different immune cells such as B, NK and T lymphocytes, macrophages and dendritic cells, and also vascular endothelial cells.11 IL-21 is associated with the development of autoimmune diseases and inflammatory disorders, and hence, like IL-6, could play a role in reactive thrombocytosis. Notably, IL-6 induces the production of IL-21 by CD4+ T cells. Recently, IL-21 was found to be expressed by mouse hematopoietic stem cells and progenitors when stimulated by TLR activators released by apoptotic cells.14
The aim of this study was to investigate the role of IL-21 on megakaryopoiesis, using in vitro assays with human cells and in vivo experiments in mice.
Methods
Techniques are described in detail in the Online Supplementary Appendix.
Antibodies
Mouse anti-human CD41/CD61, CD42b and CD42d and rat anti-mouse and human CD42c antibodies were prepared in our laboratory; other antibodies were available commercially.
In vitro differentiation of human MKs
Peripheral CD34+ progenitors from healthy blood donors were isolated and cultured in serum free media and appropriate cytokine combinations.
Colony-forming unit megakaryocyte assays
Colony-forming unit megakaryocyte (CFU-MK) assays were performed using MegaCult™-C Kits with cytokines (StemCell Technologies) according to the manufacturer’s instructions. Three independent experiments were performed in quadruplicate.
Quantification of proplatelet-bearing human MKs
On day 13 of culture, images of ten different wide fields in 24-well plates were acquired using an inverted microscope coupled to a camera (Zeiss). Round and proplatelet-bearing megakaryocytes were counted.
RT-PCR analyses
Semi-quantitative RT-PCRs were performed using total RNA of CD34+ progenitors and cultured CD41/CD61+ cells. The identity of the RT-PCR products was confirmed by DNA sequencing.
Immunofluorescence microscopy
Human BM specimens from the iliac crest were obtained from individuals having a normal megakaryocytic lineage. Mouse femora, spleens and livers were harvested, fixed, then decalcified (femora). Samples were embedded in OCT compound and cryosectioned at 8 μm. On day 13 of culture, megakaryocytes were fixed and cytospun onto poly L-lysine-coated slides. IL-21Rα was revealed using a tyramide amplification technique (TSA PLUS Fluorescence Kit, Perkin Elmer). MKs and macrophages were counterstained with anti-CD42c or F4/80 antibody, respectively, before analysis by confocal microscopy (TCS SP5, Leica Microsystems).
Flow cytometry
For flow cytometry (FC), cells were labeled as described in the Online Supplementary Appendix and analyzed on a Gallios or a BD LSRFortessa cytometer; data were analyzed with Kaluza (Beckman Coulter) or FACSDiva (BD Biosciences) software, respectively.
Hydrodynamic transfections
Murine IL-21 cDNA was cloned into the pLIVE expression vector (Mirus Bio LLC). Empty or recombinant plasmids were intravenously injected into mice.15 Plasma samples were stored at −80°C before quantification of IL-21 concentration.
Mouse platelets
The percentage of reticulated platelets was checked by FC after staining with Thiazole Orange (TO) and anti-CD42c mAb. To measure platelet survival, washed EGFP+ platelets were retroorbitally injected into mice five days after hydrodynamic transfection. The ratio of EGFP+ transfused to EGFP-endogenous platelets was determined by FC.
Statistical analysis
All values are reported as the mean±Standard Error of Mean (SEM). Statistical analyses were performed with GraphPad software (Prism v.5.0) using Student t-test, or one-way or two-way ANOVA and a Bonferroni post-hoc test.
Results
Human megakaryocytes express the IL-21 receptor
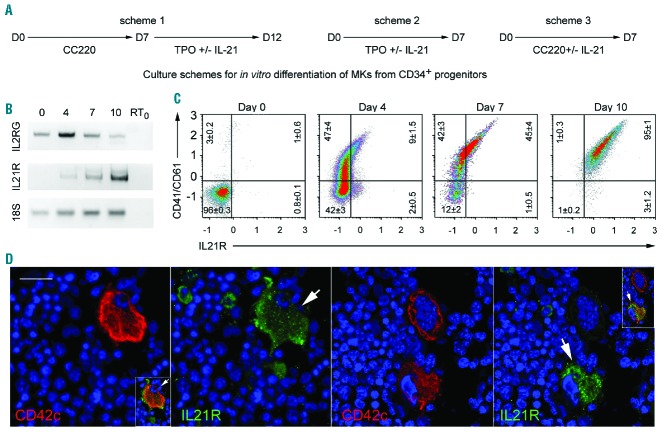
Peripheral blood CD34+ progenitors were differentiated into megakaryocytes using a 2-phase protocol optimized to generate large numbers of megakaryocytes: firstly, seven days of culture in the presence of TPO, IL-6, IL-9 and SCF, to allow the expansion of megakaryocyte progenitors, and secondly, six days of culture in the presence of TPO alone, to generate mature megakaryocytes. RNA was extracted from freshly isolated CD34+ cells and from purified CD41/CD61+ cells, isolated on days 4, 7 and 10 of culture (Figure 1A, Scheme 1, without IL-21). Semi-quantitative RT-PCR analyses showed that IL21R was not expressed in freshly isolated CD34+ cells but was progressively induced during their megakaryocytic differentiation. In contrast, IL2RG transcripts were detected in the progenitor cells, their numbers relatively increased during the first days of culture, and then decreased (Figure 1B). FC analysis of in vitro differentiated megakaryocytes confirmed the progression of IL-21Rα chain expression on CD41/CD61+ cells (Figure 1C). In human BM samples, IL-21Rα was expressed on a subpopulation of mature megakaryocytes identifiable owing to their large size (> 20 μm) and CD42c and DAPI staining (Figure 1D). Among 211 megakaryocytes from 3 individuals, 37%±7% expressed IL-21R.
Figure 1.
Human in vitro differentiated and bone marrow megakaryocytes (BM MKs) express the IL-21 receptor. (A) Human CD34+ cells were isolated from adult peripheral blood and cultured in a serum-free medium, in the presence of Megakaryocyte Expansion Supplement (CC220 cocktail, containing TPO, SCF, IL-6 and IL-9) for the first 7-day phase and of thrombopoietin (TPO) alone for the second 6-day phase. (B) Gel electrophoresis analysis of the RT-PCR products of IL2RG and IL21R mRNA and 18S rRNA (internal control) extracted from freshly isolated CD34+ cells (day 0) and CD41/CD61+ sorted cells on days 4, 7 and 10 of culture. A representative gel from three independent experiments is shown. RT0 is the negative control without cDNA. (C) Cells were labeled with anti-CD41/CD61-Alexa 488, -IL-21Rα-APC antibodies, 7AAD and analyzed by flow cytometry (FC). Representative FC dot plot analyses of the CD41/CD61 and IL-21Rα distribution among live cells (7AAD-) from day 0, 4, 7 and 10 cultures are shown. The percentages of live cells are depicted in each quadrant as the mean±Standard Error of Mean (SEM) in three independent experiments. (D) Human BM samples were fixed and labeled with an anti-IL-21Rα antibody, stained with FITC using a tyramide-based amplification method (green) and counterstained with an anti-CD42c-Alexa 555 antibody (red). Nuclei were stained with DAPI (blue). Scale bar: 20 μm. The Figure shows two representative images of samples from 3 individuals. Arrows denote IL-21Rα+ MKs. Inserts represent merged views (0.5-fold smaller scale).
The final steps of megakaryopoiesis are characterized by proplatelet formation followed by platelet release. At day 13 of culture, fixed and cytospun megakaryocytes were analyzed by immunofluorescence microscopy after labeling with anti-CD42c and anti-IL-21R mAbs (Online Supplementary Figure S1A). IL-21Rα was detected on the cellular body of about 65% of the cells but not on proplatelets. Accordingly, IL-21Rα could not be revealed on the surface of human blood platelets, in the resting state as previously described16 nor after thrombin-stimulation, nor intracellularly (Online Supplementary Figure S1B).
Altogether, these results confirm the expression of IL-21 receptor on human megakaryocytes and suggest a role of IL-21 during megakaryopoiesis but not in platelet functions.
IL-21R activity changes during the in vitro differentiation of megakaryocytes
In order to check the functionality of IL-21R in CD34+ cell-derived megakaryocytes, we focused on STAT3 and STAT5 phosphorylation, which occurs after stimulation of T cells by IL-2111 and of megakaryocytes by TPO.17 At days 7 and 12 of megakaryocyte differentiation, corresponding to the ends of the two culture phases, the cells were starved of cytokines for 5 hours, then incubated for 15 minutes with IL-21 and/or TPO, before fixation and immunostaining with anti-CD41/CD61, -pSTAT3 and -pSTAT5 antibodies. FC analysis revealed that IL-21 induced the phosphorylation of only STAT3 in CD41/CD61+ cells, the ratio of responding cells being higher on day 7 (46%±4.9%, n=3) than on day 12 (15%±2.9%, n=3) (Figure 2). TPO induced mainly STAT5 phosphorylation on day 7 of culture, and both STAT3 and STAT5 on day 12. On day 7, IL-21 and TPO signaling were additive. To confirm that STAT3 phosphorylation was mediated by IL-21R, we used the neutralizing anti-IL-21R recombinant monoclonal antibody ATR-107.18 As expected, this antibody completely inhibited the IL-21-induced phosphorylation of STAT3 in CD41+ cells at day 7 of culture when under our experimental conditions signal transduction was maximal (Online Supplementary Figure S2).
Figure 2.
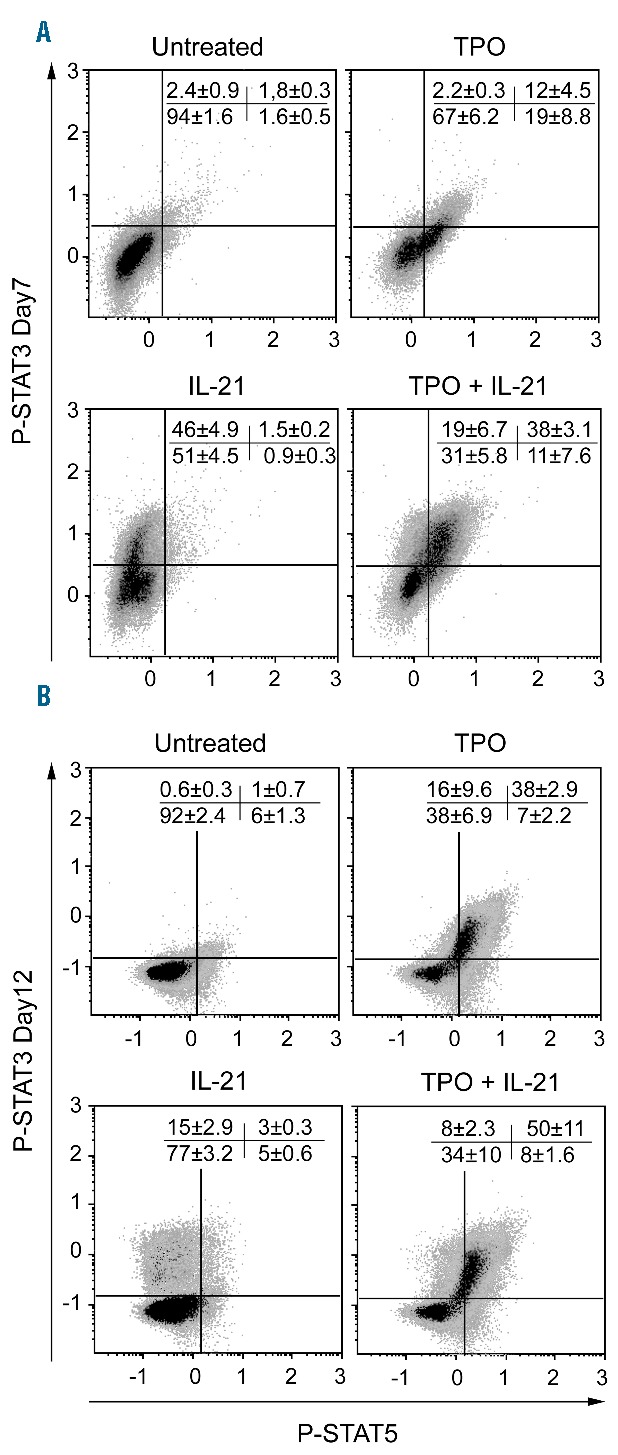
IL-21R activity changes during in vitro differentiation of megakaryocytes (MKs). CD34+ cells were cultured as described in Figure 1, scheme 1. On day 7 or 12 of culture, the cells were pre-incubated for five hours in serum-free medium without cytokines, before stimulation with thrombopoietin (TPO) and/or IL-21. The cells were then fixed, permeabilized and labeled with anti-CD41/CD61-ECD, -pSTAT3-Alexa 647 and -pSTAT5-Alexa 488 antibodies. Representative flow cytometry (FC) dot plots showing STAT3 and STAT5 phosphorylation in CD41/CD61+ cells after stimulation as indicated on days 7 and 12 of culture. Values indicate the mean±Standard Error of Mean (SEM) of the percentage of events in each quadrant in three independent experiments.
Thus, IL-21R is functional during in vitro differentiation of megakaryocytes but, probably due to downregulation of the γ chain, tends to lose its functionality during their maturation.
IL-21 promotes the in vitro proliferation of megakaryocyte progenitors through the JAK3/STAT3 pathway
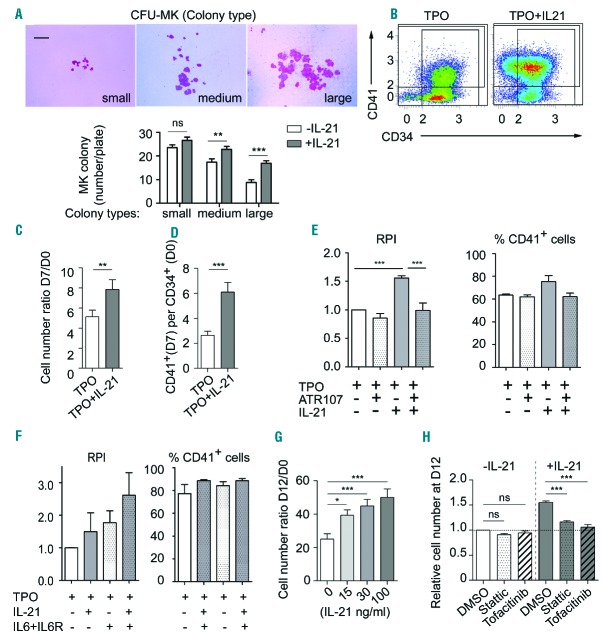
We first measured the effect of IL-21 on the megakaryocytic potential of CD34+ progenitor cells in collagen-based medium containing TPO, IL-3 and IL-6. IL-21 increased the number of medium-sized (21–50 cells per colony) and large (>50 cells per colony) colonies, on average by approximately 30% and 90%, respectively (n=3) (Figure 3A). We then investigated the effects of IL-21 on the in vitro differentiation of CD34+ progenitors into megakaryocytes. When blood CD34+ cells were cultured for seven days in the presence of TPO alone (Figure 1A, scheme 2), addition of IL-21 increased the total number of viable cells by 50% on average and doubled the number of CD41+ cells (Figure 3B–D). Specificity of the IL-21-induced cellular responses was confirmed by blocking IL-21R with anti-IL-21R antibody ATR-107: addition of this antibody on days 1 and 3 completely blocked IL-21-induced increase in the yield of the total number of cells and the percentage of CD41+ cells obtained at day 7 (Figure 3E). IL-6 in combination with soluble IL-6R has been shown to increase the yield of the in vitro differentiation of CD34+ progenitors into megakaryocytes in the presence of TPO alone.19 IL-21 increased this effect without impacting the ratio of CD41+, CD42+ or GPV+ cells as assessed in 7-day culture assays (Figure 3F and data not shown). However, when CD34+ cells were cultured in the presence of the Megakaryocyte Expansion Supplement cocktail (Figure 1A, scheme 3, CC220), addition of IL-21 in the first step did not improve the number of viable cells and of CD41+ cells obtained at day 7 (data not shown).
Figure 3.
IL-21 promotes in vitro proliferation of megakaryocyte (MK) progenitors through the JAK3/STAT3 pathway. CD34+ cells were grown in the presence (filled bars) or absence (empty bars) of IL-21 (100 ng/mL). (A) 2500 CD34+ cells were plated in quadruplicate on collagen-based medium containing thrombopoietin (TPO), IL-3 and IL-6 for ten days. (Top) Representative CD41+ MK colony types. Scale bar: 200 μm. (Bottom) Numbers of MK colony types per plate; n=3 in quadruplicates. (B–D) CD34+ cells were cultured for seven days in the presence of TPO, with or without IL-21 (Figure 1A, scheme 2). (B) Representative FACS plots of CD41 and CD34 distribution among 7AAD− cells. (C) Total live cells were counted at day 7 and reported to the number of seeded CD34+ cells (n=11). (D) Numbers of CD41+ live cells per seeded CD34+ cells were deduced after flow cytometry (FC) analysis (n=11). (E) CD34+ cells were cultured in the presence of TPO (100 ng/mL), 100 μg/mL polyclonal human immunoglobulins, in the absence or the presence of IL-21 (100 ng/mL) and ATR-107 mAb (100 ng/mL) (n=3, in duplicates). Viable cells were counted on day 7, and results were normalized relatively to the mean number of cells obtained at day 7 in the presence of TPO alone (relative proliferation index, RPI). Then, the cells were labeled with anti-CD41 mAb and 7AAD to exclude dead cells. The percentage of CD41+ cells among viable cells was determined by FC. (F) CD34+ cells were cultured in duplicates for seven days in the presence of different combinations of TPO (40 ng/mL), IL-6 (100 ng/mL) and soluble IL-6R (200 ng/mL) and IL-21 (100 ng/mL). On day 7, the RPI and the ratio of CD41+ cells among viable cells were calculated by FC (n=3, in duplicates). (G and H) CD34+ cells were cultured for 12 days as described in Figure 1A, scheme 1. (G) IL-21 was added at the indicated concentrations and analyzed on day 12 as in (C) (n=6). (H) On day 7 of culture, the cells were washed, pre-incubated for 1 hour in cytokine-free medium containing Stattic (300 nM), Tofacitinib (1500 nM) or vehicle (DMSO, 0.25%) and then cultured in the presence of TPO, with or without IL-21 (n=5). Values are reported as the mean±Standard Error of Mean (SEM). *P<0.05, **P<0.01, ***P<0.001; (A, C–E) t-tests, (F) one-way and (G) two-way ANOVA followed by a Bonferonni post-hoc test.
In order to obtain larger numbers of megakaryocyte progenitors, CD34+ cells were differentiated in the presence of CC220 cocktail according to cell culture scheme 1. Cells obtained after seven days of culture were cultured in the presence of TPO for six additional days in the presence of increasing concentrations of IL-21. Under these experimental conditions, IL-21 increased the number of megakaryocytes in a dose-dependent manner, reaching 1.9±0.1-fold increase at 100 ng/mL (n=6) (Figure 3G). The cell viability was not modified by the presence of IL-21 (data not shown).
Among the Janus kinases, JAK3 is the only molecule linked to IL-2Rγ, mediating signal transduction via STAT3 phosphorylation. Since IL-21 induced STAT3 phosphorylation in differentiating megakaryocytes, we checked whether its effect on megakaryocyte generation depended on the JAK3/STAT3 pathway. CD34+ progenitors were cultured according to scheme 1; on day 7, several concentrations of Tofacitinib (a JAK3 inhibitor) and Stattic (a STAT3 inhibitor), or their vehicle (DMSO, 0.25%), were added to the cultures, supplemented or not with IL-21 (100 ng/mL). Analysis of cells five days later revealed that 300 nM Stattic or 1500 nM Tofacitinib had no impact on the number of megakaryocytes obtained in the presence of TPO and the vehicle (DMSO) (Figure 3H). The combination of TPO+IL-21 afforded a 1.5±0.03-fold (n=5) increase in the number of megakaryocytes, as compared to TPO with the vehicle. Addition of Stattic or Tofacitinib abolished this enhancement (1.16±0.03- and 1.06±0.05-fold, respectively; n=5). FC analysis revealed that the inhibitors, at these concentrations, did not affect the differentiation of megakaryocytes since in all conditions 90% of the cells were CD41+ CD42+ (data not shown). Altogether, these results indicate that IL-21 promotes the in vitro proliferation of megakaryocyte progenitors through the JAK3/STAT3 pathway.
Megakaryocyte phenotype and platelet production are not modified by IL-21
At the end of the second phase of culture (scheme 1), we evaluated the influence of IL-21 on the megakaryocyte phenotype (Online Supplementary Figure S3). IL-21 did not significantly affect the surface expression of CD41, CD42c or CD42d on viable cells, their ploidy, the proportion of proplatelet-bearing megakaryocytes, or the number of platelets released per megakaryocyte. Thus, IL-21 had no obvious effect on the phenotype of mature megakaryocytes or on platelet production.
IL-21 stimulates megakaryopoiesis in vivo
To appraise the in vivo relevance of these observations, additional experiments were performed in mice. Firstly, we confirmed that IL-21 increased the number of megakaryocytes differentiated in vitro from murine BM Lin− cells in the presence of TPO (1.4±0.15-fold increase; n=5), without affecting the phenotype of the cells (expression of CD41/CD61 heterodimer and CD42c, and polyploidy) (Online Supplementary Figure S4). This positive effect could be inhibited by the ATR-107 mAb, which also antagonizes mouse IL-21R. IL-21 was then transiently expressed in mice using hydrodynamic transfection of an IL-21 expression vector (pLIVE-IL-21). In negative control mice receiving the empty vector (pLIVE), the plasma concentration of IL-21 remained below the detection limit (<64 pg/mL). In contrast, one day after transfection of pLIVE-IL-21, the plasma concentration of IL-21 reached 3227±952 pg/mL, then progressively declined to 494±97 pg/mL at day 7, reaching 124±17 pg/mL by day 16 post transfection (n=7) (Online Supplementary Figure S5). Animals were analyzed during the first seven days following the hydrodynamic transfer.
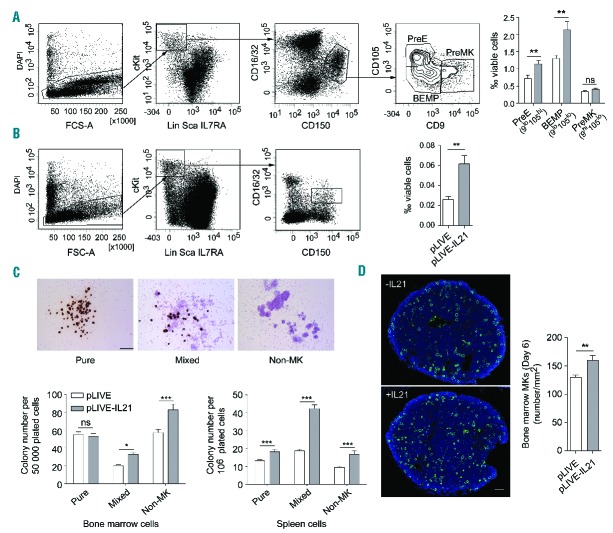
We first analyzed the numbers of megakaryocyte progenitors in the BM of the transfected mice on day 6. In the mouse, these cells belong to the Lin−Sca−IL7Rα− cKit+CD150+CD16/32lo population. Within this subset, CD9hiCD105lo and CD9loCD105hi cells represent megakaryocyte (PreMK) and erythroid (PreE) precursors, respectively, while CD9loCD105lo cells are bi-potent erythroid-megakaryocyte progenitors (BEMP).20 The percentages of PreEs and BEMPs were significantly increased in pLIVE-21 transfected animals, while PreMKs were not significantly expanded (Figure 4A). In the spleen, an important secondary hematopoietic organ, the Lin−Sca−IL7Rα− cKit+CD150+ CD16/32lo progenitors appeared to be rarer; nevertheless their number was increased by IL-21 expression (Figure 4B).
Figure 4.
Hydrodynamic gene transfer of IL-21 promotes megakaryopoiesis in vivo. The IL-21 expression vector (pLIVE-IL21) or control empty vector (pLIVE) were hydrodynamically transferred into mice. Six days later, bone marrow (BM) and spleens were recovered and hematopoietic precursor cells were analyzed by flow cytometry (FC). Representative dot plot analyses of bi-potent erythroid-megakaryocyte progenitors (BEMP), megakaryocytic (PreMK) and erythroid (PreE) BM precursors (A) and corresponding spleen cells (B). Values are the mean±Standard Error of Mean (SEM) of the percentages of gated cells among total viable cells: (A) n=9, data were analyzed using a t-test; (B) n=3. (C) BM cells and spleen cells of hydrodynamically transfected mice were plated in quadruplicate in a collagen-based medium in the presence of TPO, IL-6 and IL-3. Megakaryocytes (MK) were identified by cytochemical staining of acetylcholinesterase activity. (Top) Representative images of BM-derived pure MK colonies, mixed MK-containing colonies and non-MK colonies. (Bottom) Number of colonies of each type per 50,000 plated BM cells (left), and 106 plated spleen cells (right) (n=6). Values are the mean±Standard Error of Mean (SEM). Data were analyzed using a t-test; *P<0.05, **P<0.01, ***P<0.001. (D) Femurs were harvested six days after hydrodynamic transfection. (Left) Representative DAPI (blue) and CD42c staining (green) of transversal sections of femoral BM. Bar: 100 μm. (Right) Quantification of the number of MKs per mm2 of BM cross-section. Three 120 μm spaced whole transversal sections per femur from 6 mice per group were analyzed. Data were analyzed using a t-test.
To complete the FC analysis, we compared BM and spleen cells in CFU-MK assays using a collagen-based medium containing TPO, IL-3 and IL-6. These assays showed that the forced expression of IL-21 resulted in increased numbers of CFUs of MK-containing mixed colonies and non-megakaryocyte colonies in the BM and the spleen, while numbers of CFUs of pure MK colonies were increased only in the spleen (Figure 4C). The ratios of CFU-MKs (pure and mixed) to the number of seeded cells from control and IL-21 expressing mice were approximately 47- and 28-fold higher for BM than for spleen cells, respectively, indicating the rarity of megakaryocyte progenitors in the spleen and suggesting a minor contribution of spleen to megakaryopoiesis under these experimental conditions.
Finally, immunofluorescence analysis of BM sections stained with an anti-CD42c mAb revealed an increased MK density in mice expressing IL-21 as compared to control animals (160±8 vs. 129±4 MKs per mm2, respectively; n=6) (Figure 4D).
Altogether, these data showed that IL-21 expression increases the number of MK progenitors in the BM and the spleen and enhances megakaryopoiesis, confirming the in vivo relevance of our in vitro observations.
IL-21 expression increases platelet clearance
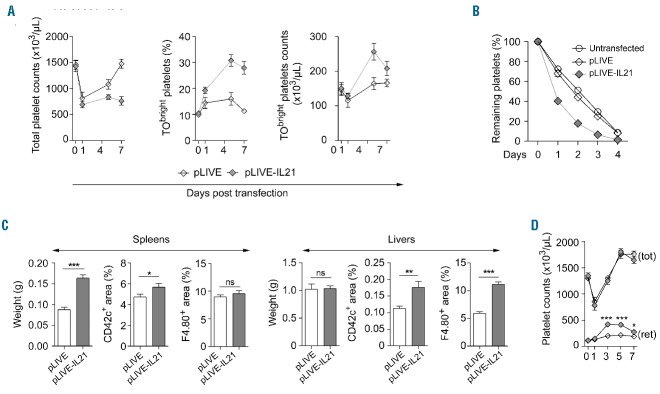
One day after hydrodynamic transfection, total platelet counts decreased by 50% (Figure 5A) and a similar decrease was observed after injection of the transfer solution or saline alone (data not shown). Thereafter, in pLIVE-receiving control animals, platelet counts progressively increased to recover their baseline value six days later (n=6) (Figure 5A, left). Conversely, in pLIVE-IL-21 transfected mice, platelet counts unexpectedly remained reduced. However, in agreement with the observed higher megakaryocyte density in the BM, the number of TObright young platelets was increased, compared to control mice (Figure 5A, middle and right). On day 6, the phenotype of the platelets from pLIVE and pLIVE-IL-21 transfected mice and their responses to thromboxane and PAR4 agonists were similar; the responses of youngest (TObright) and older (TOdim) platelets to agonists were also identical (Online Supplementary Figure S6).
Figure 5.
IL-21 promotes platelet clearance in vivo. The IL-21 expression vector (pLIVE-IL21) or control empty vector (pLIVE) were hydrodynamically transferred into mice. (A) On the indicated days after transfer, the total platelet count (left), the percentage of TObright platelets (middle) were measured and the absolute count of reticulated TObright platelets was deduced (right). (B) Five days after hydrodynamic transfection, EGFP+ platelets were transfused and their clearance was monitored by flow cytometry (FC). (C) Six days after hydrodynamic transfection of pLIVE-IL21 or pLIVE, the spleens (left) and livers (right) were harvested, weighed, fixed, immunostained and analyzed by immunofluorescence microscopy and CD42c+ platelet and F4/80+ macrophage areas were measured. Three sections per organ and a minimum of three micrographs per section were analyzed from 3 animals per group. Data were analyzed using a t-test; *P<0.05, **P<0.01, ***P<0.001. (D) Mice were splenectomized and seven days after surgery were hydrodynamically transfected with pLIVE (open symbols, n=6) or pLIVE-IL-21 (gray symbols, n=7). Total (tot) and reticulated (ret) platelet counts were determined at indicated days. Differences in counts of reticulated platelet were analyzed using a t-test; *P<0.05, ***P<0.0001.
This prolonged thrombocytopenia in mice transfected with pLIVE-IL21 could result from a peripheral mechanism, probably an increased clearance. To test this hypothesis, washed EGFP+ platelets from untreated mice were transfused into untransfected, control transfected or pLIVE-IL-21 transfected EGFP− mice and their counts were followed for five days. Two days after transfusion, the percentage of circulating EGFP+ platelets was lower in mice expressing IL-21 (n=6) as compared to control transfected (n=7) or untransfected animals (n=6) [17%±2% vs. 45±2% (P<0.001), and vs. 52%±1% (P<0.001), respectively] (Figure 5B), confirming that IL-21 expression increased platelet clearance.
Since the spleen can retain a pool of platelets which increases with splenomegaly,21 we weighed the spleens and found that six days after transfection, the spleens of mice expressing IL-21 were larger than those of control animals (163±8 mg vs. 88±6 mg, respectively; n=9) (Figure 5C, Spleens panel, left). Additional experiments showed that splenomegaly was already maximal by day 3 and stable for at least nine additional days (data not shown). The distribution of macrophages and platelets in the spleen were analyzed by immunofluorescence microscopy (Figure 5C, Spleens panel, right, and Online Supplementary Figure S7A). The areas of F4/80+ macrophages in spleen cryosections from control mice and animals expressing IL-21 were similar. The area of platelets (CD42c+) was slightly but significantly increased in mice expressing IL-21 (Figure 5C, Spleens panel, middle), indicating an increased accumulation of platelets.
To confirm the participation of the spleen in IL-21-induced persistent thrombocytopenia, complementary experiments were performed with splenectomized mice. In these animals, IL-21 expression also increased the number of circulating TObright platelets (Figure 5D), confirming that IL-21 enhanced BM megakaryopoiesis. Nevertheless, total platelet counts increased similarly in mice expressing IL-21 and control animals, with 1802±63 × 109 (n=7) versus 1755±78 × 109 (n=6) platelets/L respectively, five days after transfection. This latter observation, together with the findings in non-splenectomized animals, indicated that the spleen participates in IL-21-induced platelet clearance.
In hydrodynamically transfected splenectomized mice, control mice and those expressing IL-21 displayed similar total platelet counts, despite the increased TObright platelets, suggesting increased platelet clearance by liver macrophages. We thus also analyzed the livers in transfected animals. IL-21 expression did not impact the weight of the livers, but significantly increased the numbers of macrophages and platelets present in this tissue (Figure 5C, Livers panel, and Online Supplementary Figure S7B). Altogether, these observations suggest that macrophages in the spleen and the liver could be involved in the increase platelet clearance mediated by IL-21.
Discussion
We here showed that IL-21R is expressed when human CD34+ progenitors are cultured in the presence of TPO. The receptor was expressed on megakaryocytic CD41+ cells, but not on CD41− cells (Figure 1C). Similar observations have likewise been described.10,16 TPO alone also induced the expression of IL-21R on 40%–50% of CD41+ within seven days of culture (data not shown). The progressive IL-21Rα expression during megakaryocyte differentiation and its presence on human BM megakaryocytes strongly suggest that this expression results from commitment of the progenitor cells to the megakaryocytic lineage. On the other hand, IL-21 increased the numbers of medium and large megakaryocyte colonies obtained in CFU-MK assays with blood CD34+ progenitors, which suggests that this cytokine could also promote the expansion of committed cells.
IL-21Rα was not detected on human and mouse platelets. Another example of discordance between megakaryocytes and platelets in the expression of a membrane-associated protein is the tyrosine phosphatase receptor CD45, which is likewise present on human BM megakaryocytes but not on platelets.22 IL-21 induced the phosphorylation of STAT3 in CD41+ cells, the percentage of responding cells being higher on day 7 than on day 12, in agreement with the downregulation of the common γ chain. TPO induced the phosphorylation of only STAT5 on day 7 of in vitro human megakaryocyte differentiation, but both STAT3 and STAT5 phosphorylation was observed during the later stages of differentiation. This finding is consistent with studies documenting the TPO-induced phosphorylation of STAT3 and STAT5 in megakaryocytic cell lines23,24 and blood platelets.17,25 Thus, at late stages of megakaryocyte differentiation, the downregulation of IL-21R-mediated signaling could be compensated by TPO activity. In vitro, in the presence of TPO alone, IL-21 increased in a dose-dependent manner the number of megakaryocytic cells generated. This effect was inhibited using the blocking anti-IL21R ATR-107 mAb and was dependent on IL-21R-mediated activation of JAK3/STAT3 pathway. These observations are in line with the documented role of STAT3 in megakaryopoiesis.26,27 In the presence of TPO, IL-21 increased the capacity of IL-6 and soluble IL-6R combination to improve the in vitro differentiation of CD34+ cells into megakaryocytes, indicating complementary physiological functions.
IL-21 and IL-21R deficiencies cause immunodeficiency syndromes in man,28,29 disturb B, T and NK lymphocyte functions in mice11 but do not impact platelet counts, indicating that IL-21 is not essential for megakaryopoiesis and thrombopoiesis under healthy conditions. Our observations suggest that IL-21 could compensate for increased platelet clearance in pathologies characterized by an increased production of IL-21. Thus, a hydrodynamic transfection method was used to over-express IL-21 in vivo, in hepatocytes.15 Plasmatic IL-21 concentrations was enhanced for two weeks, while IL-1α, IL-1β and IL-6 remained undetectable between day 1 and 7 (i.e. <16 pg/mL), in agreement with previous studies,30,31 and no significant TPO variations were noticed (pLIVE vs. pLIVE-IL-21 transfected animals, n=3 per day) (data not shown).
In IL-21-expressing mice, among the BM Lin−Sca−IL7Rα− cKit+CD150+CD16/32lo progenitors, the ratio of BEMPs was expanded. Consistently, clonogenic assays revealed that IL-21 enhanced the number of megakaryocytic mixed colonies obtained from BM cells. MK are thought to derive from PreMKs, which differentiate from BEMPs.32 Because the density of BM megakaryocytes was increased, the observed numbers of BEMPs and PreMKs may reflect differences in the dynamics of the maturation of these two types of progenitors.
In the spleen, the numbers of Lin−Sca−IL7Rα− cKit+CD150+ CD16/32lo progenitors and of pure and mixed CFU-MK were increased by IL-21 expression. The number of CFU-MK per seeded cell was 30–50-fold lower in the spleen than in the BM, suggesting that in our experimental conditions IL-21 has a minor effect on spleen megakaryopoiesis. Our results are in agreement with and complement previous work, where splenomegaly and increased numbers of Lin−cKit+Sca1+ hematopoietic stem cells and CFU-GEMMs (granulocyte-erythrocyte-megakaryocyte-macrophages) were observed in the BM and spleen of mice expressing IL-21 after hydrodynamic transfection.33
Accordingly, thrombopoiesis was enhanced in transfected mice expressing IL-21, as indicated by their increased counts of young reticulated platelets. However, total platelet counts did not follow this trend. The day after hydrodynamic transfection thrombocytopenia was noticed, probably due to vascular damage and consequently, platelet consumption.15 Normal platelet count was restored between days 4 and 7 in mice transfected with the empty plasmid, but not in pLIVE-IL-21 transfected animals. An increased clearance of platelets could explain this absence of recovery (Figure 5A and B). On day 6, greater numbers of macrophages and platelets were found in the liver of mice expressing IL-21. On cryosections of the enlarged spleen of these animals, macrophages and platelets occupied similar and higher surface areas, respectively (Figure 5C). Thus, increased clearance of platelets could occur in the spleen, or in the liver, another major site for the elimination of old or activated platelets.34 IL-21 expression in splenectomized animals was accompanied with increased thrombopoiesis, but no thrombocytopenia, indicating that: i) IL-21-mediated enhanced megakaryopoiesis mainly occurs in the BM; and ii) that a major part of the thrombocytopenia provoked by IL-21 expression is mediated by the spleen. The absence of increased platelet counts in IL-21 splenectomized animals, although thrombopoiesis is increased, could be explained by increased clearance by liver macrophages, since IL-21 expression increased the density of platelets and macrophages in this organ. Thus, additional investigations are necessary to elucidate the mechanisms responsible for the increased platelet clearance induced by IL-21.
This study in the mouse is reminiscent of a pre-clinical study in macaques, which periodically received non-glycosylated recombinant IL-21 and experienced cycles of moderate anemia and thrombocytopenia followed by polycythemia and thrombocythemia.31 Because the clearance of the recombinant IL-21 was rapid, it is not possible to compare these observations with ours, which are based on the in vivo expression of IL-21. Of note, cancer patients receiving IL-21 could also develop reversible thrombocytopenia.35 It is tempting to speculate that in humans receiving recombinant IL-21, megakaryopoiesis and platelet production are increased, but platelet clearance is also enhanced and the net result is thrombocytopenia.
This study demonstrates that IL-21R is progressively induced during human megakaryopoiesis. IL-21 appears to enhance the proliferation of megakaryocyte progenitors through the JAK3/STAT3 pathway, resulting in increased in vivo megakaryopoiesis. Since IL-21 is mainly produced by T cells, our work provides new insights into the regulation of megakaryopoiesis through adaptive immunity. Our observations also suggest that the IL-21-mediated increased megakaryopoiesis could be a compensatory mechanism to counteract enhanced platelet clearance during immune responses characterized by increased IL-21 expression.
Supplementary Material
Acknowledgments
The authors would like to thank Catherine Ziessel for expert technical assistance, Arnaud Dupuis (Hôpital Universitaire de Strasbourg) for providing human BM samples, Nathalie Brouard for helpful discussions and technical advice, and Juliette Mulvihill for reviewing the English of the manuscript.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/4/637
References
- 1.Cortin V, Garnier A, Pineault N, Lemieux R, Boyer L, Proulx C. Efficient in vitro megakaryocyte maturation using cytokine cocktails optimized by statistical experimental design. Exp Hematol. 2005; 33(10):1182–1191. [DOI] [PubMed] [Google Scholar]
- 2.Dan K, Gomi S, Inokuchi K, et al. Effects of interleukin-1 and tumor necrosis factor on megakaryocytopoiesis: mechanism of reactive thrombocytosis. Acta Haematol. 1995; 93(2–4):67–72. [DOI] [PubMed] [Google Scholar]
- 3.Lotem J, Shabo Y, Sachs L. Regulation of megakaryocyte development by interleukin-6. Blood. 1989;74(5):1545–1551. [PubMed] [Google Scholar]
- 4.Catani L, Amabile M, Luatti S, et al. Interleukin-4 downregulates nuclear factor-erythroid 2 (NF-E2) expression in primary megakaryocytes and in megakaryoblastic cell lines. Stem Cells. 2001;19(4):339–347. [DOI] [PubMed] [Google Scholar]
- 5.Mitjavila MT, Vinci G, Villeval JL, et al. Human platelet alpha granules contain a nonspecific inhibitor of megakaryocyte colony formation: its relationship to type beta transforming growth factor (TGF-beta). J Cell Physiol. 1988;134(1):93–100. [DOI] [PubMed] [Google Scholar]
- 6.Sosman JA, Verma A, Moss S, et al. Interleukin 10-induced thrombocytopenia in normal healthy adult volunteers: evidence for decreased platelet production. Br J Haematol. 2000;111(1):104–111. [DOI] [PubMed] [Google Scholar]
- 7.Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245(3):295–300. [DOI] [PubMed] [Google Scholar]
- 8.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001; 98(9): 2720–2725. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura S, Nagasaki M, Kunishima S, et al. IL-1alpha induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209(3):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari F, Bortoluzzi S, Coppe A, et al. Genomic expression during human myelopoiesis. BMC Genomics. 2007;8:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–395. [DOI] [PubMed] [Google Scholar]
- 12.Hodge LS, Ziesmer SC, Yang ZZ, et al. IL-21 in the BM microenvironment contributes to IgM secretion and proliferation of malignant cells in Waldenstrom macroglobulinemia. Blood. 2012; 120(18):3774–3782. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Zhang J, Fu R, et al. Increased frequency of BM T follicular helper cells in patients with immune-related pancytopenia. Clin Dev Immunol. 2013;2013:730450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CI, Zhang L, Datta SK. Hematopoietic stem and multipotent progenitor cells produce IL-17, IL-21 and other cytokines in response to TLR signals associated with late apoptotic products and augment memory Th17 and Tc17 cells in the BM of normal and lupus mice. Clin Immunol. 2016;162:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15(12):2063–2069. [DOI] [PubMed] [Google Scholar]
- 16.Sun S, Wang W, Latchman Y, Gao D, Aronow B, Reems JA. Expression of plasma membrane receptor genes during megakaryocyte development. Physiol Genomics. 2013;45(6):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyakawa Y, Oda A, Druker BJ, et al. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood. 1996;87(2):439–446. [PubMed] [Google Scholar]
- 18.Zhu M, Pleasic-Williams S, Lin TH, Wunderlich DA, Cheng JB, Masferrer JL. pSTAT3: a target biomarker to study the pharmacology of the anti-IL-21R antibody ATR-107 in human whole blood. J Transl Med. 2013;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sui X, Tsuji K, Ebihara Y, et al. Soluble interleukin-6 (IL-6) receptor with IL-6 stimulates megakaryopoiesis from human CD34(+) cells through glycoprotein (gp)130 signaling. Blood. 1999;93(8):2525–2532. [PubMed] [Google Scholar]
- 20.Ng AP, Kauppi M, Metcalf D, Di Rago L, Hyland CD, Alexander WS. Characterization of thrombopoietin (TPO)-responsive progenitor cells in adult mouse BM with in vivo megakaryocyte and erythroid potential. Proc Natl Acad Sci USA. 2012;109(7):2364–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penny R, Rozenberg MC, Firkin BG. The splenic platelet pool. Blood. 1966;27(1):1–16. [PubMed] [Google Scholar]
- 22.Tomer A. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood. 2004; 104(9):2722–2727. [DOI] [PubMed] [Google Scholar]
- 23.Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL, O’Shea JJ. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995;370(1–2):63–68. [DOI] [PubMed] [Google Scholar]
- 24.Drachman JG, Kaushansky K. Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain. Proc Natl Acad Sci USA. 1997;94(6):2350–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majka M, Ratajczak J, Villaire G, et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, megakaryocytes, and platelets. Exp Hematol. 2002;30(7):751–760. [DOI] [PubMed] [Google Scholar]
- 26.Kirito K, Osawa M, Morita H, et al. A functional role of Stat3 in in vivo megakaryopoiesis. Blood. 2002;99(9):3220–3227. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins BJ, Roberts AW, Greenhill CJ, et al. Pathologic consequences of STAT3 hyperactivation by IL-6 and IL-11 during hematopoiesis and lymphopoiesis. Blood. 2007;109(6):2380–2388. [DOI] [PubMed] [Google Scholar]
- 28.Kotlarz D, Zietara N, Uzel G, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210(3):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzer E, Kansu A, Sic H, et al. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J Allergy Clin Immunol. 2014;133(6):1651–1659 e1612. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Tschoi M, Spolski R, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003; 63(24):9016–9022. [PubMed] [Google Scholar]
- 31.Waggie KS, Holdren MS, Byrnes-Blake K, et al. Preclinical safety, pharmacokinetics, and pharmacodynamics of recombinant human interleukin-21 in cynomolgus macaques (Macaca fascicularis). Int J Toxicol. 2012; 31(4):303–316. [DOI] [PubMed] [Google Scholar]
- 32.Tsang AP, Fujiwara Y, Hom DB, Orkin SH. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12(8):1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozaki K, Hishiya A, Hatanaka K, et al. Overexpression of interleukin 21 induces expansion of hematopoietic progenitor cells. Int J Hematol. 2006;84(3):224–230. [DOI] [PubMed] [Google Scholar]
- 34.Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17(6):585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert Opin Biol Ther. 2010; 10(5):807–817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.