Abstract
Gaucher disease, the inherited deficiency of lysosomal glucocerebrosidase, is characterized by the presence of glucosylceramide-laden macrophages resulting from impaired digestion of aged erythrocytes or apoptotic leukocytes. Studies of macrophages from patients with type 1 Gaucher disease with genotypes N370S/N370S, N370S/L444P or N370S/c.84dupG revealed that Gaucher macrophages have impaired efferocytosis resulting from reduced levels of p67phox and Rab7. The decreased Rab7 expression leads to impaired fusion of phagosomes with lysosomes. Moreover, there is defective translocation of p67phox to phagosomes, resulting in reduced intracellular production of reactive oxygen species. These factors contribute to defective deposition and clearance of apoptotic cells in phagolysosomes, which may have an impact on the inflammatory response and contribute to the organomegaly and inflammation seen in patients with Gaucher disease.
Introduction
Gaucher disease (GD) is a lysosomal storage disease caused by inherited deficiency of the lysosomal hydrolase glucocerebrosidase, which leads to accumulation of glucosylceramide and glucosylsphingosine in lysosomes. Macrophages are the primary cell type affected in GD, and inefficient breakdown of glycolipid-rich membranes of phagocytosed cells leads to accumulation of lipid-laden GD macrophages in organs of the reticuloendothelial system.1 The accumulation of these GD macrophages is thought to contribute to chronic inflammation in patients with GD, but the mechanism underlying this link has remained largely undetermined. Recently, using primary macrophages derived from patients with GD, we demonstrated that lipid storage and subsequent lysosomal dysfunction leads to impaired macroautophagy in GD macrophages, contributing to dysregulation of pro-inflammatory cytokine processing and release by these cells.2 These findings led us to explore whether phagocytosis, a process vital to macrophages and mechanistically similar to autophagy, may also be affected in GD macrophages.
While phagocytosis is important for the response of phagocytes to pathogens, phagocytosis of apoptotic cells – a process known as efferocytosis – is vital for tissue homeostasis.3 In this process, apoptotic cells release signaling molecules that recruit tissue-resident macrophages to the site of cell death4,5 and prompt phagocytosis of the apoptotic cell.6,7 Following phagocytosis, the nascent phagosome undergoes a process of maturation, facilitating digestion of the apoptotic cell.8 Clearance of apoptotic cells via efferocytosis is required to maintain immunological homeostasis, and the breakdown of this process is associated with inflammatory disease and autoimmunity.9 Hence, impairment of apoptotic cell engulfment by GD macrophages could contribute to chronic inflammation and organomegaly in GD. While studies using primary macrophages from patients with GD have indicated that GD macrophages have defects in the digestion of phagocytosed microbes, no studies have examined efferocytosis by GD macrophages.10
Here, we used primary macrophages from patients with GD to assess efferocytosis in GD. We found that while recognition and uptake of apoptotic cells by macrophages is not impaired in GD, digestion of engulfed cells is severely affected in the disease. This is caused by aberrant recruitment of phagosome-associated proteins, leading to substantially impaired phagosome maturation and phagosome-lysosome fusion. Failure of efferocytosis may, therefore, contribute to storage in GD macrophages and exacerbate the inflammatory and hematologic aspects of this disease.
Methods
Peripheral blood mononuclear cell collection and isolation and differentiation of macrophages
GD macrophages were derived from peripheral blood monocytes isolated from 25 patients with type 1 GD seen at the National Institutes of Health (Bethesda, MD, USA). GBA1 genotyping, performed as described elsewhere,11 revealed that two patients had genotype N370S/L444P, one had N370S/c.84dupG and 22 had N370S/N370S. Informed consent was obtained in accordance with a National Human Genome Research Institute Internal Review Board-approved clinical protocol. Thirty-two control macrophage samples were derived from monocytes isolated from blood from healthy donors provided by the Blood Bank at the National Institutes of Health Clinical Center. Peripheral blood mononuclear cells were isolated as described previously.12 Briefly, peripheral blood mononuclear cells were isolated using a Ficoll gradient, and monocytes were enriched via CD16 depletion. Macrophages were differentiated from purified monocytes using macrophage colony-stimulating factor (10 ng/mL) (R&D Systems, Minneapolis, MN, USA) in RPMI 1640 medium (ThermoFisher Scientific, Waltham, MA, USA), supplemented with 10% fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA) and 1% Pen Strep (ThermoFisher Scientific). On days 3 and 6, the medium was replaced, and experiments were performed on day 7. Jurkat cells were a gift from Dr. Martin Playford (National Heart, Lung, and Blood Institute, National Institutes of Health). Erythrocyte ghosts, added to the cultured cells as a source of lipid, were prepared from blood collected from a patient with GD as previously described.12
Quantification of efferocytosis by flow cytometry
Monocyte-derived macrophages were cultured in 12-well plates. Jurkat cells were incubated with CytoTracker Green (Molecular Probes, Eugene, OR, USA) at 37°C with 5% CO2 for 30 min. To induce apoptosis in Jurkat cells, cells were washed twice in RPMI without FCS. They were irradiated with ultraviolet light at 30 mJ/cm2 at a wavelength of 254 nm using a StrataLinker UV cross-linker (Stratagene, La Jolla, CA, USA) and then incubated at 37°C with 5% CO2 for 4 h. Macrophages were transferred to RPMI without FCS. Apoptotic Jurkat cells were added at a ratio of five apoptotic Jurkat cells: one macrophage and incubated together at 37°C with 5% CO2 for 1 h. Cells were then quickly washed with cold phosphate-buffered saline (PBS) to remove non-efferocytosed apoptotic Jurkat cells and scraped in PBS with 10% FCS. For antibody staining, allophycocyanin-conjugated CD11b antibodies (BD Biosciences, San Jose, CA, USA) were added to cells at a 1:200 dilution for 20 min. Cells were subsequently spun down and fresh PBS with 10% FCS was added. Flow cytometry was performed using a FACSCalibur (BD Biosciences).
Lipid coating of beads
This procedure was adapted from a published protocol.13 1,2-dioleoyl-sn-glycero-3-[phospho-L-serine] (PtdSer) (18:1 PS) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (PtdCho) (18:1 PC) dissolved in chloroform were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). 100 mol% PC and an 80 mol% PC:20 mol% PS mixture were dried under N2, suspended in Dulbecco PBS without calcium to a concentration of 200 nM, and sonicated for 2 min. 6.4 μm glass beads or 2 μm green microsphere beads (Bangs Laboratory, Inc., Fishers, IN, USA) were centrifuged and washed three times, suspended in Dulbecco PBS and sonicated for 2 min to form a single-bead suspension. Beads were then added to the lipid suspensions (1×105 beads/nmol lipid), vortexed vigorously for 2 min, and incubated at room temperature for 10 min. The resulting lipid-coated beads were centrifuged, washed three times and stored at 4°C in Dulbecco PBS. The coated beads were stained with annexin V (Roche Life Science, Indianapolis, IN, USA) and analyzed using a FACSCalibur (BD Biosciences).
Immunocytochemistry
For microsphere bead assays, cells were plated on glass cover-slips in 12-well plates. Lipid-coated beads were added to the macrophages. The plates were spun at 4°C at 200 rpm for 2 min and incubated for 10 min at 4°C to allow the beads to bind to macrophages. Cells were washed with cold PBS and incubated at 37°C for the indicated times. Cells were fixed for 1 min with ice-cold methanol and then blocked in PBS containing 0.1% saponin, 100 μM glycine, and 2% donkey serum. For the assays of apoptotic Jurkat cells, macrophages were plated on glass chamber slides. Cells were incubated with apoptotic Jurkat cells labeled with CytoTracker green (Molecular Probes) for 60 min, fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100 for 10 min and blocked.
Cells were then incubated with the primary antibody diluted in PBS containing 0.1% saponin and 0.1% bovine serum albumin. The following primary antibodies were used for immunocytochemistry: goat α-p67phox antibody (1:200, Santa Cruz Biotechnology, Dallas, TX, USA), rabbit α-Rab5 (1:100, Abcam, Cambridge, UK), mouse α-Rab7 (1:100; Santa Cruz Biotechnology), rabbit α-Rab7 (1:100; Abcam), mouse α-Lamp1 (1:200, hybridoma), mouse α-Lamp2 (1:200, hybridoma) and goat α-Cathepsin D (1:200, R&D Systems). Cells were washed and incubated with donkey α-mouse, α-rabbit, or α-goat secondary antibodies conjugated to Alexa Fluor® 488, Alexa Fluor® 555, Alexa Fluor® 647 (Invitrogen) or Alexa Fluor® 647 phalloidin (Molecular Probes). Cells were mounted with ProLong® Gold antifade reagent with DAPI (Molecular Probes), and Z-stack images were acquired with a Zeiss 510 META laser scanning microscope (Carl Zeiss MicroImaging, Inc., Jena, Germany using a 488-nm argon, a 543-nm HeNe and an ultraviolet laser. Images were acquired using a Plan Neofluar 63×/1.42 oil DIC objective (Carl Zeiss MicroImaging, Inc.). The intensity of each protein in the phagosomes was analyzed using IMARIS software (Bitplane, Belfast, Ireland).
Measurement of intracellular reactive oxygen species
Macrophages were cultured in 96-well, black, clear-bottom plates in the presence and absence of erythrocyte ghosts.12 Apoptotic Jurkat cells were then added for 60 min. In addition, cells were incubated with apoptotic Jurkat cells supplemented with ATP (0.5 mM). Cells were stained with CellROX™ Deep Red Reagent (Molecular Probes) at a final concentration of 5 μM, and incubated for 30 min at 37°C. Cells were washed, and fluorescence was evaluated at 640/665 nm using a FlexStation 3 microplate reader (Molecular Devices).
Protein isolation and immunoblotting
Cells were harvested and sonicated at 4°C in RIPA lysate buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% Na deoxycholate, 0.1% SDS and protease inhibitor]. Protein quantification was performed using a BCA protein assay (Thermo Scientific, Waltham, MA, USA). Lysates were run on 4% – 20% polyacrylamide gels (BioRad, Hercules, CA, USA) and transferred to polyvinylidene difluoride membranes using the Trans-Blot Turbo system (BioRad). Blots were dried, reactivated in methanol and blocked using a mixture of one part of PBS with one part of Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature. The primary antibodies used were: α-p67phox (Abcam), α-Rab5 (Abcam), α-Rab7 (Abcam and Santa Cruz Biotechnology), α-β-actin (Abcam) and α-GAPDH (GeneTex, Irvine, CA, USA). Primary antibodies were diluted 1:1000, except for β-actin, which was diluted 1:4000. Dilution was done in blocking buffer containing 0.1% Tween-20. Blots were incubated with the primary antibody dilution overnight at 4°C, followed by 5 min washes in PBST (0.1% Tween-20). IRDye® 680RD goat anti-rabbit and IRDye® 800CW donkey anti-mouse secondary antibodies (Li-COR Bioscience) were diluted 1:10,000 in blocking buffer containing 0.1% Tween-20 and 0.01% SDS. Blots were incubated with secondary antibody for 1 h at room termperature, followed by 5 min washes in PBST. Blots were imaged using the Li-COR Odyssey imaging system (Li-COR Bioscience) and quantified using Image Studio (Li-COR Bioscience).
Statistical analysis
Statistical analyses were performed using GraphPadPrism 6.0 software. Significance was determined by a Student t-test. Data from two groups or more than two independent variables were analyzed by ANOVA, followed by the Bonferroni post hoc test. Data are presented as mean values ± standard deviation. Significance levels for differences between controls and patients’ macrophages were set when P<0.05(*), P<0.01(**), and P<0.001(***) for different conditions.
Results
Gaucher macrophages engulf apoptotic cells
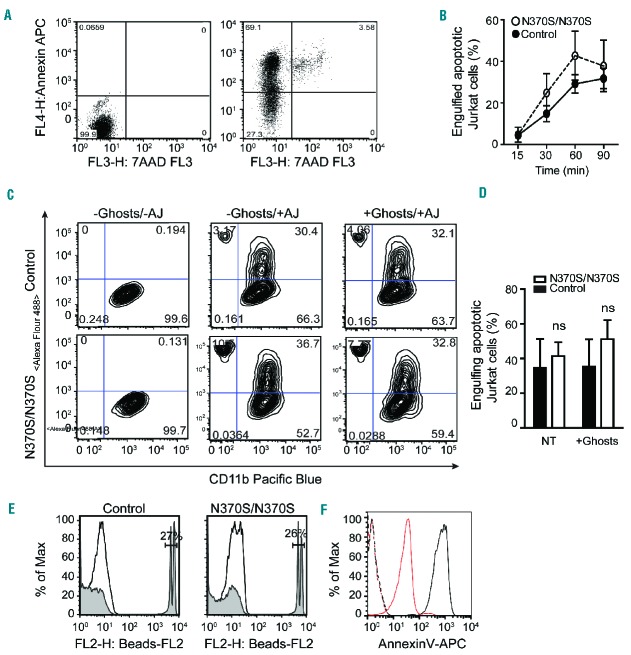
Phagocytic clearance of apoptotic cells is initiated by the migration of macrophages to the site where apoptotic cells are located in the response to specific attraction signals released by the cells as they undergo apoptosis. The second signal directing efferocytosis is the juxtacrine “eat me” signal, which is displayed on the surface of apoptotic cells in order to initiate the process of their internalization and digestion. The primary “eat me” signal is exposed phosphatidylserine (PtdSer), a phospholipid typically found on the cytosolic surface of the plasma membrane, but which diffuses to the outer surface during apoptosis. To evaluate the response of macrophages to this juxtacrine signaling, GD and control macrophages were incubated with apoptotic Jurkat cells at a high confluence (Figure 1A). Macrophages were detached, stained for CD11b, and the engulfment of apoptotic cells at different time points was evaluated by flow cytometry (Figure 1B,C). The engulfment of both apoptotic Jurkat cells and 2 μm beads coated with PtdSer (Figure 1E) did not differ significantly between control and GD macrophages, and pre-incubation with erythrocyte ghosts (to mimic the in vivo condition)12 had no impact on the macrophages’ efferocytic ability (Figure 1D).
Figure 1.
Efferocytosis in Gaucher macrophages. (A) Jurkat cells were UV-irradiated, incubated at 37°C for 4 h, stained for annexin V/7AAD and analyzed by flow cytometry. Early apoptotic cells represent 66.1% of the population. (B) Control and Gaucher macrophages were co-cultured with GFP-labeled apoptotic Jurkat cells for different time intervals. Non-efferocytosed apoptotic Jurkat cells were removed by washing with PBS, and macrophages were detached and analyzed by flow cytometry. Three independent experiments were performed on samples from three different patients with Gaucher disease (GD). (C–D) GFP-labeled apoptotic Jurkat cells (AJ) were added to macrophages both with and without the addition of erythrocyte ghosts for 60 min. They were detached after extensive washing, stained for CD11b, and analyzed by flow cytometry. The graph represents data from six independent experiments. (E) 2 μm FluoSpheres sulfate beads, coated with PtdSer, were added to macrophages for 60 min and analyzed by flow cytometry. (F) 5 μm glass beads were coated with PtdSer and PtdCho, stained with annexin V and analyzed by flow cytometery. Dashed red and black lines represent unstained coated beads, while solid red and black lines indicate stained beads coated with PtdCho and PtdCho-PtdSer.
Impaired efferocytosis is due to defective phagosome maturation in Gaucher macrophages
Both impaired recognition and clearance of apoptotic cells may contribute to inflammation in GD.2 Following engulfment, deposition of ingested cells within phagosomes is achieved by gradual acidification of the phagosome and fusion with lysosomes. Rab5 and Rab7 play coordinated roles in the process of phagosome maturation. Rab5 accumulates at the cytosolic surface of the nascent phagosome during engulfment of apoptotic cells, and mediates phagosome maturation.14,15 We studied phagosome maturation at different time points after adding glass beads coated with a mixture of PtdSer and PtdCho. Glass beads were used to rule out the participation of ligands other than PtdSer that might be present on the surface of apoptotic cells. Another advantage of these glass beads is that they do not introduce additional proteins and they are not broken down or degraded within the phagosome. The presence of PtdSer-coated beads was confirmed by annexin V (Figure 1F). Control and GD1 macrophages were incubated with PtdSer-coated beads for different time intervals, and immunofluorescence staining was performed to identify proteins associated with specific stages of phagosome maturation. Rab5 and Rab7 are indicators of early and late endosomes, respectively, while cathepsin D (CathD) and Lamp2 were used as lysosomal markers.
Rab5 is responsible for the recruitment of Rab7 to the surface of phagosomes, and Rab7, in turn, mediates the fusion of phagosomes with lysosomes.16,17 Figure 2A shows that Rab5 accumulated on phagosomes 5 min after phagocytosis, and this accumulation decreased gradually in both control and GD macrophages. The recruitment of Rab5 to the phagosome occurred similarly in control and GD macrophages (Figure 2A). However, in control macrophages, Rab7 appeared 10 min after phagocytosis, remaining for 120 min, while the amount of Rab7 on phagosomes was significantly reduced in GD macrophages compared to controls (Figure 2A–D). In addition, levels of CathD and Lamp2, two different lysosomal markers, increased in the phagosomes of control macrophages 40 min after engulfing PtdSer-coated beads, while they remained low for 120 min in GD macrophages (Figure 2B,E,F). Macrophages were also stained for Rab5, Lamp2 and CathD 120 min after efferocytosis, demonstrating the absence of Rab5 on the phagosome 120 min after engulfing the beads (Figure 2B, bottom panel).
Figure 2.
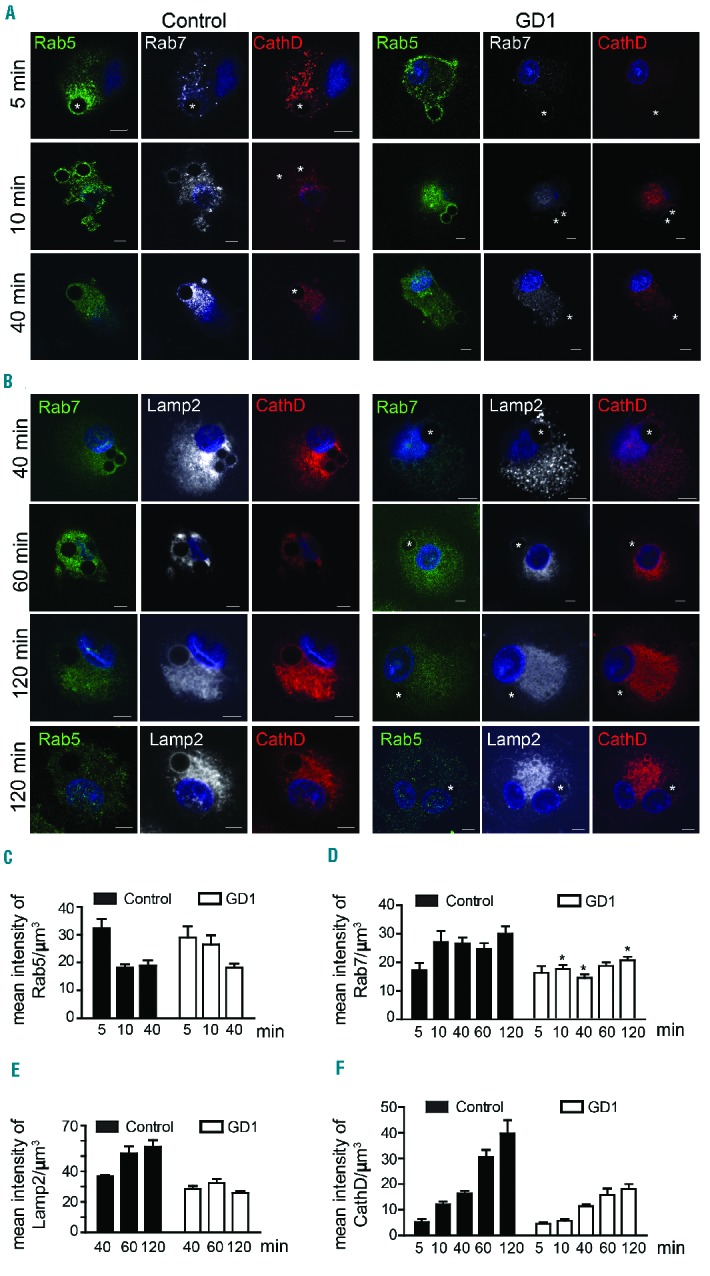
Phagosome maturation is impaired in Gaucher macrophages. (A,B) Control and Gaucher macrophages were co-cultured with PtdSer-coated glass beads (shown by*) for different time intervals. Cells were fixed and were stained with (A) Rab5 (green), Rab7 (white) and CathD (red) or (B) Rab7 (green), Lamp2 (white) and CathD (red). (C–F) Using IMARIS software, the intensity of Rab5 (C), Rab7 (D), Lamp2 (E) and CathD (F) was measured on the phagosomes. Graphs represent data from 20 images at each time point. This experiment was performed using cells from four different individuals with genotype N370S/N370S, two with genotype N370S/L444P and six controls.
We next studied the expression of Rab7 in macrophages fed with apoptotic Jurkat cells. Rab7 levels were lower in GD macrophages than in control macrophages both 30 min (Figure 3A) and 60 min (Figure 3B) after efferocytosis of apoptotic Jurkat cells. When macrophages were fed with erythrocyte ghosts prior to efferocytosis, Rab7 levels were significantly lower in GD macrophages (48%±0.09) than control macrophages (Figure 3B,C).
Figure 3.
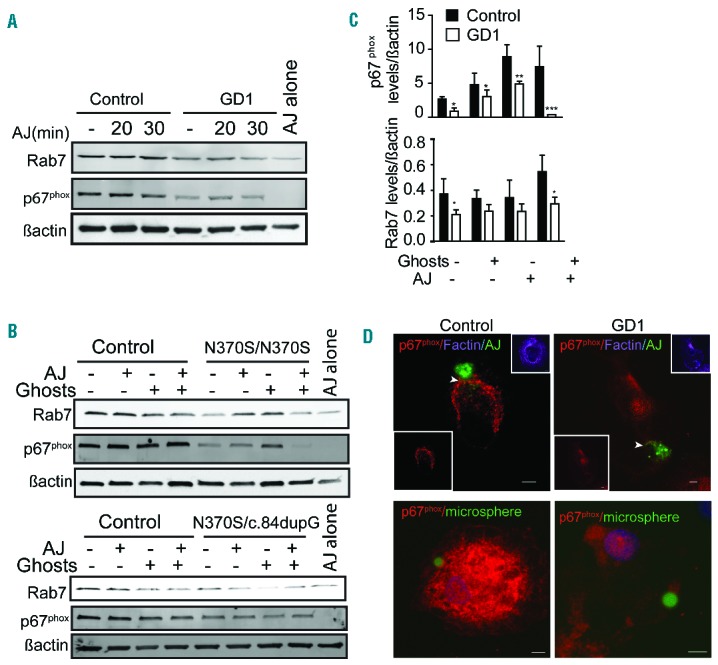
Rab7 and p67phox are reduced in Gaucher macrophages. (A, B) Control and Gaucher macrophages were co-cultured with apoptotic Jurkat cells (AJ). Macrophages were lysed after engulfing the apoptotic Jurkat cells for (A) 20, 30 min or (B) 60 min in the presence and absence of erythrocyte ghosts. Lysates were probed for Rab7 and p67phox. In (B) the upper blot shows cells from a patient with genotype N370S/N370S and the lower blot cells from a patient with genotype N370S/c.84dupG. (C) Quantification of band intensity 60 min after efferocytosis. Data represent the average ± standard deviation from three individuals with genotype N370S/N370S and two with N370S/L444P. (D) GFP-labeled apoptotic Jurkat cells (AJ) or green microbeads-PtdSer were added to control and Gaucher macrophages for 60 min, washed, fixed, and stained for p67phox (red), F-actin (purple), apoptotic Jurkat cells (AJ) or PtdSer-coated microsphere beads (green) and DAPI (blue). Z-stack images were acquired using a Zeiss 510 confocal microscope. Scale bars, 5 μm. Images represent five different independent experiments using N370S/N370S macrophages.
The respiratory burst is diminished in Gaucher macrophages
We previously demonstrated that the digestion of erythrocyte ghosts was defective in GD macrophages due to reduced production of reactive oxygen species (ROS).12 During phagocytosis, phagosomes actively recruit elements of the NADPH-oxidase complex (NOX2), which generates ROS within the phagosomal lumen and mediates the degradation of phagosomal contents. Among the five components of NOX2, gp91phox and p22phox are present in the membrane, and, upon phagocytosis, they recruit the cytosolic components p47phox and p67phox.18 We found that at baseline, GD macrophages had reduced p67phox levels compared to control macrophages. Twenty minutes after efferocytosis, p67phox levels increased marginally in control macrophages, but decreased further in GD macrophages (Figure 3A). Feeding GD macrophages with ghosts prior to efferocytosis resulted in an even more profound reduction in p67phox (Figure 3B,C).
Following efferocytosis, in control macrophages, p67phox translocated to phagosomes containing apoptotic Jurkat cells or beads coated with PtdSer (Figure 3D), while in GD macrophages no significant translocation was observed. Next we studied the recruitment of p67phox to phagosomes containing PtdSer-coated glassbeads. Macrophages were fixed at different time points after engulfing PtdSer-coated beads and were co-stained for p67phox and Rab5 or Rab7. Immunostaining showed that 10 min after engulfing beads coated with PtdSer, p67phox was present on the early phagosome (stained with Rab5) in control, but not GD macrophages (Figure 4A–C). In addition, the level of p67phox increased significantly in control macrophages during phagosome maturation when co-stained with Rab7 (120 min), while no significant translocation of p67phox to the phagosome was observed in GD macrophages (Figure 4A–C). Moreover, immunostaining showed that after engulfing apoptotic Jurkat cells, p67phox was present and co-localized with Rab7 on late phagosomes in control, but not GD macrophages (Figure 4D).
Figure 4.
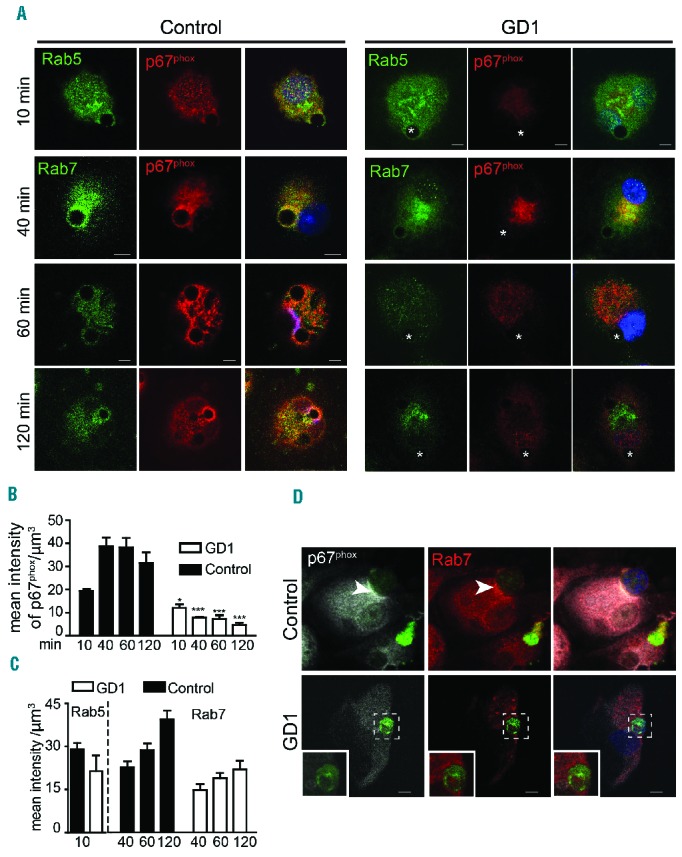
p67phox recruitment to phagosomes is diminished in Gaucher macrophages. (A) Control and Gaucher macrophages were co-cultured with PtdSer-coated beads and fixed at different time points. Cells were co-stained with p67phox (red) and Rab5 (green) or Rab7 (green). DAPI (blue) staining demonstrates the position of the nucleus. Images are representative of 25 pictures taken at each time point in four different individuals with genotype N370S/N370S, two with N370S/L444P mutations and six controls. (B, C) The intensity of each protein in the phagosome was measured using IMARIS software. (D) GFP-labeled apoptotic Jurkat cells were added to control and Gaucher macrophages for 60 min, washed, fixed and stained for p67phox (white), Rab7 (red) and apoptotic Jurkat cells (green). Images represent 40 pictures taken in four independent experiments (63× magnification, scale bars: 5 μm). Insets show higher magnifications of the areas outlined in the images.
The nucleotide ATP is a strong “find-me” signal, and small amounts of ATP are released during early apoptosis. Extracellular ATP stimulates ROS production by activating NADPH oxidase.19,20 We, therefore, studied the translocation of p67phox to phagosomes in the presence of ATP by costaining for p67phox and Rab5 or Rab7 at different time points. A significant increase in translocation of p67phox to the early phagosomes (Figure 5A) was observed in the presence of ATP. Recruitment of p67phox to late phagosomes containing coated beads (co-stained with Rab7) increased significantly in control, but not GD macrophages (Figure 5B). Figure 5C also shows that in the presence of ATP, cytosolic p67phox protein levels increased notably in control macrophages, while only slightly increased levels were observed in GD macrophages.
Figure 5.
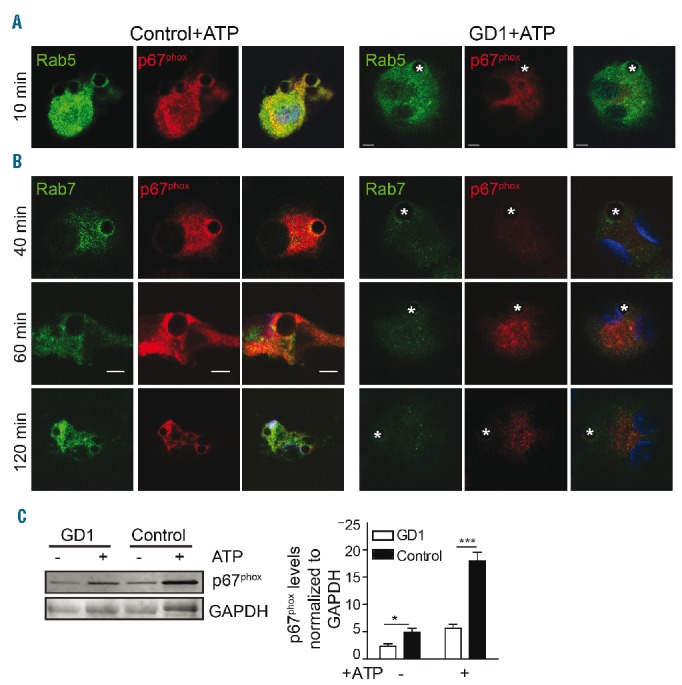
Impaired p67phox translocation to the phagosome in the presence of ATP in Gaucher macrophages. (A, B) In the presence of ATP (5 mM for 30 min), PtdSer-coated glass beads were added to control and Gaucher macrophages for different time intervals. Cells were washed, fixed and stained with p67phox (red), Rab5 or Rab7 (green) and DAPI (nuclear stain, blue), and imaged by confocal microscopy. Scale bars, 5 μm. Images represent 15 pictures at each time point from three independent experiments. (C) Cytosolic fractions from Gaucher macrophages (N370S/N370S) and control macrophages stained for p67phox in the presence and absence of ATP (5 mM for 30 min). The graph presents the results of three independent experiments from patients with genotype N370S/N370S.
It has been reported that efferocytosis leads to increased ROS production in macrophages.21 Intercellular ROS was measured using a fluorescent redox sensitive dye. Hydrogen peroxide levels increased marginally in control macrophages after engulfing apoptotic Jurkat cells, and increased significantly after the addition of ATP. This elevation was more profound when macrophages were fed with ghosts prior to efferocytosis or in the presence of ATP. However, ROS production did not change in GD macrophages (Figure 6). Thus, in GD macrophages the failure of p67phox to translocate to the phagosome after phagocytosing PtdSer coated-beads, and in response to ATP, led to less ROS production. These data suggest that digestion of apoptotic cells is impaired in GD macrophages.
Figure 6.
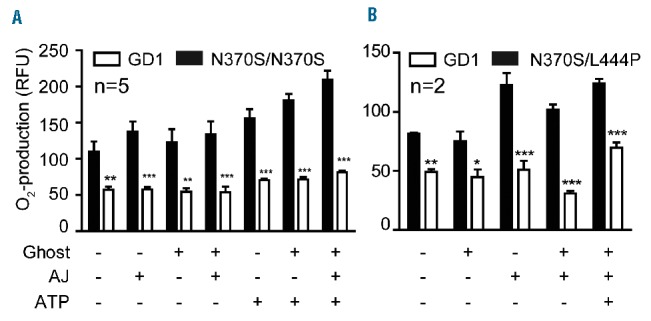
The respiratory burst is impaired in Gaucher macrophages. (A, B). The production of reactive oxygen species (ROS) production was measured in Gaucher macrophages [(A) genotype N370S/N370S or (B) N370S/L444P] after engulfing apoptotic Jurkat cells (AJ) in the presence or absence of erythrocyte ghosts. In addition, 5 mM ATP was added to serum-free media (after centrifugation to remove media from the apoptotic Jurkat cells) in the presence or absence of added apoptotic Jurkat cells.
Discussion
The most common feature of GD is splenomegaly, which at times can be massive. It has been shown that the amount of glucosylceramide accumulation in this organ does not account for the extensive degree of enlargement, and thus other factors must be involved. In this study we show that macrophages from patients with GD manifest impaired efferocytosis (clearance of apoptotic cells), as reflected by delayed phagosome-lysosome fusion. Impaired clearance of apoptotic cells can result in splenomegaly due to an imbalance between apoptosis and phagocytosis.22 Apoptosis is vital for the removal of problematic cells. Despite the constant turnover of cells through apoptosis, apoptotic cells are rarely seen under physiological conditions. This suggests that at steady state, the rate of removal of apoptotic cells is high, and the dying cells are adequately removed by tissue-resident professional phagocytes such as macrophages.23 Apoptotic cells recruit macrophages by releasing chemotactic factors, and the nucleotide ATP serves as a key mediator for this recruitment. This process requires caspase-mediated activation of pannexin 1 channels to release ATP from apoptotic cells.24 Subsequently, nucleotides are detected by purogenic receptors (such as P2Y2) on monocytes and macrophages.25
The process of efferocytosis depends on recognition of apoptotic cells by phagocytes. Phagocytes target PtdSer, a ubiquitous hallmark of apoptosis independent of the cell type and the form of cell death. PtdSer can mediate tethering of apoptotic cells to phagocytes, delivering a “tickle” signal to phagocytes to stimulate the internalization of apoptotic cells by engaging different receptors.26 However, no significant differences were observed in the ability of control and GD macrophages to engulf apoptotic Jurkat cells or microspheres coated with PtdSer (Figure 2).
While recognition and engulfment of apoptotic cells have been extensively studied, little is known about how apoptotic cells are degraded. Since the efficiency of engulfment appeared intact, we studied phagosome maturation in Gaucher macrophages, both in the presence and absence of erythrocyte ghosts. After engulfing apoptotic cells, nascent phagosomes fuse with early endosomes, late endosomes and ultimately lysosomes, the terminal degradative compartment.7 These fusion events change the composition of the phagosome membrane and lumen, load digestive enzymes and trigger phagosome acidification.27 Rab GTPases target and tether specific organelles to phagosomes.28 Rab5 is essential for tethering the surface of early endosomes to nascent phagosomes29 and Rab7, located on late endosomes, mediates the tethering and fusion between late endosomes and lysosomes.7,30 It has been shown that when Rab7 is absent, apoptotic Jurkat cells are engulfed normally, but are not degraded.15 Our study shows that recruitment of Rab7 to late phagosomes containing glass microsphere beads coated with PtdSer was impaired significantly in GD macrophages (Figure 2A–D). Moreover, while the translocation of cathepsin D to the phagosome increased gradually in control macrophages 40 min after efferocytosis, it was significantly delayed in GD macrophages (Figure 2B,F). When GD macrophages were fed with erythrocyte ghosts prior to efferocytosis, Rab7 levels remained low (Figure 3). These findings indicate impaired lysosome-phagosome fusion in GD macrophages, resulting in delayed degradation of phagocytes.
A clinical observation substantiating the inefficient efferocytosis observed31 is the finding that many patients with GD exhibit autoantibodies reactive to autoantigens. Testing for autoantigens in sera from 43 patients with type 1 GD revealed autoreactivity in all but ten patients. Eleven of the subjects reacted to eight to 12 antigens, although the antibodies were not generally associated with immune manifestations.32 Other studies have found antiphospholipid, lupus anticoagulant and anticardiolipid in patients with GD. Impaired efferecytosis by GD macrophages could result in undigested material spilling into the blood, where they induce autoantibody formation.33,34
We previously demonstrated that GD macrophages manifest the M1 (activated macrophage) phenotype, as indicated by the secretion of inflammatory cytokines including interleukin-1β and interleukin-6 in the presence of the TLR4 ligand lipopolysaccharide.2 In fully differentiated M1 macrophages treated with lipopolysaccharide, the kinetics of phagosome maturation are delayed,35 while in M2 macrophages (alternatively activated macrophages), early phagosomes undergo a rapid transition to phagolysosomes with enhanced proteolytic activity.36 There is evidence that the differential rates of maturation are influenced by the luminal pH of the phagosome. Phagosome acidification is mainly mediated by V-ATPases, which translocate protons from the cytosol into the lumen of phagosomes and lysosomes. In general, M1 macrophages display a slower rate of acidification, and have a less acidic phagosomal pH,37 while in M2 macrophages, phagosomes are acidified immediately in order to clear apoptotic bodies rapidly and effectively.36 Once the recycling proteins have been removed, macrophages recruit the NADPH-oxidase complex to ensure the acidification of phagosomes and the killing of the phagocyte products through the production of oxygen radicals.38 The production of superoxide by NOX2 in the lumen of phagosomes affects phagosomal pH, as protons are used when superoxide dismutates into hydrogen peroxide. NOX2 assembly and expression increase significantly in M1 macrophages treated with lipopolysaccharide, which allows rapid and robust production of ROS in response to inflammatory stimuli,39 while M2 macrophages show reduced NOX2 expression.36 Although ROS production by NOX2 limits acidification of phagosomes in dendritic cells, it does not affect phagosome acidification in macrophages, due to extensive recruitment of V-ATPases and limited ROS production.40 We found that p67phox is significantly reduced in GD macrophages, even with the addition of erythrocytes ghosts prior to efferocytosis (Figure 4). Moreover, in GD macrophages this subunit fails to translocate to the phagosome, which leads to impaired NOX2 assembly (Figures 4 and 5) and reduced ROS production (Figure 6). Together these findings confirm impaired efferocytosis and degradation of apoptotic cells in GD macrophages. These aspects of the disease pathogenesis may contribute to inflammation and splenomegaly in patients with GD.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/4/656
References
- 1.Lee RE. The fine structure of the cerebroside occurring in Gaucher’s disease. Proc Natl Acad Sci USA. 1968;61(2):484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aflaki E, Moaven N, Borger DK, et al. Lysosomal storage and impaired autophagy lead to inflammasome activation in Gaucher macrophages. Aging Cell. 2016;15(1):77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. [DOI] [PubMed] [Google Scholar]
- 4.Franz S, Gaipl US, Munoz LE, et al. Apoptosis and autoimmunity: when apoptotic cells break their silence. Curr Rheumatol Rep. 2006;8(4):245–247. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203(12):2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadok VA, Henson PM. Apoptosis: giving phosphatidylserine recognition an assist–with a twist. Curr Biol. 2003;13(16):R655–657. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124(5):677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366(Pt 3):689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. [DOI] [PubMed] [Google Scholar]
- 10.Marodi L, Kaposzta R, Toth J, Laszlo A. Impaired microbicidal capacity of mononuclear phagocytes from patients with type I Gaucher disease: partial correction by enzyme replacement therapy. Blood. 1995;86(12):4645–4649. [PubMed] [Google Scholar]
- 11.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15(2):181–188. [DOI] [PubMed] [Google Scholar]
- 12.Aflaki E, Stubblefield BK, Maniwang E, et al. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6(240): 240ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buranda T. Biomimetic molecular assemblies on glass and mesoporous silica microbeads for biotechnology. Langmuir. 2003;19(5):1654–1663. [Google Scholar]
- 14.Kitano M, Nakaya M, Nakamura T, Nagata S, Matsuda M. Imaging of Rab5 activity identifies essential regulators for phagosome maturation. Nature. 2008;453(7192):241–245. [DOI] [PubMed] [Google Scholar]
- 15.Kinchen JM, Doukoumetzidis K, Almendinger J, et al. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10(5): 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 2003;23(18):6494–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X, Lu N, Zhou Z. Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells. PLoS Biol. 2008;6(3):e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergnaud S, Paclet MH, El Benna J, Pocidalo MA, Morel F. Complementation of NADPH oxidase in p67-phox-deficient CGD patients p67-phox/p40-phox interaction. Eur J Biochem. 2000;267(4):1059–1067. [DOI] [PubMed] [Google Scholar]
- 19.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009; 183(5):3302–3308. [DOI] [PubMed] [Google Scholar]
- 21.Yvan-Charvet L, Pagler TA, Seimon TA, et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106(12):1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang A, Dai J, Xie Z, et al. High molecular weight kininogen binds phosphatidylserine and opsonizes urokinase plasminogen activator receptor-mediated efferocytosis. J Immunol. 2014;192(9):4398–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chekeni FB, Elliott MR, Sandilos JK, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155(4):501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Yu X. Phagosome maturation during the removal of apoptotic cells: receptors lead the way. Trends Cell Biol. 2008;18(10): 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. [DOI] [PubMed] [Google Scholar]
- 29.Duclos S, Diez R, Garin J, et al. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J Cell Sci. 2000;113(Pt 19):3531–3541. [DOI] [PubMed] [Google Scholar]
- 30.Vieira OV, Bucci C, Harrison RE, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23(7):2501–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machaczka M, Klimkowska M, Regenthal S, Hagglund H. Gaucher disease with foamy transformed macrophages and erythrophagocytic activity. J Inherit Metab Dis. 2011;34(1):233–235. [DOI] [PubMed] [Google Scholar]
- 32.Shoenfeld Y, Beresovski A, Zharhary D, et al. Natural autoantibodies in sera of patients with Gaucher’s disease. J Clin Immunol. 1995;15(6):363–372. [DOI] [PubMed] [Google Scholar]
- 33.Barone R, Giuffrida G, Musso R, Carpinteri G, Fiumara A. Haemostatic abnormalities and lupus anticoagulant activity in patients with Gaucher disease type I. J Inherit Metab Dis. 2000;23(4):387–390. [DOI] [PubMed] [Google Scholar]
- 34.Granel B, Serratrice J, Swiader L, et al. [Antiphospholipid antibodies and Gaucher’s disease. A case report]. Rev Med Interne. 2002;23(12):1037–1039. [DOI] [PubMed] [Google Scholar]
- 35.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8(3):241–250. [DOI] [PubMed] [Google Scholar]
- 36.Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, Yates RM. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood. 2011;118(15):4199–4208. [DOI] [PubMed] [Google Scholar]
- 37.Ghigo E, Capo C, Tung CH, Raoult D, Gorvel JP, Mege JL. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J Immunol. 2002;169(8): 4488–4495. [DOI] [PubMed] [Google Scholar]
- 38.Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J Biol Chem. 2002;277(6):4512–4518. [DOI] [PubMed] [Google Scholar]
- 39.Amezaga MA, Bazzoni F, Sorio C, Rossi F, Cassatella MA. Evidence for the involvement of distinct signal transduction pathways in the regulation of constitutive and interferon gamma-dependent gene expression of NADPH oxidase components (gp91-phox, p47-phox, and p22-phox) and high-affinity receptor for IgG (Fc gamma R-I) in human polymorphonuclear leukocytes. Blood. 1992;79(3):735–744. [PubMed] [Google Scholar]
- 40.Savina A, Jancic C, Hugues S, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.