Abstract
Hematopoietic–specific microRNA-142 is a critical regulator of various blood cell lineages, but its role in erythrocytes is unexplored. Herein, we characterize the impact of microRNA-142 on erythrocyte physiology and molecular cell biology, using a mouse loss-of-function allele. We report that microRNA-142 is required for maintaining the typical erythrocyte biconcave shape and structural resilience, for the normal metabolism of reactive oxygen species, and for overall lifespan. microRNA-142 further controls ACTIN filament homeostasis and membrane skeleton organization. The analyses presented reveal previously unappreciated functions of microRNA-142 and contribute to an emerging view of small RNAs as key players in erythropoiesis. Finally, the work herein demonstrates how a housekeeping network of cytoskeletal regulators can be reshaped by a single micro-RNA denominator in a cell type specific manner.
Introduction
microRNAs (miRNAs) are genome encoded small non-coding RNAs that post-transcriptionally regulate gene expression. The hematopoietic–specific microRNA-142 (miR-142) gene, and particularly its mature miR-142-3p product, has emerged as a critical regulator of various blood lineages. The miR-142 gene locus was historically associated with the (8;17) translocation, in B-cell leukemia,1 several years before miRNAs were discovered. Recent experimental evidence uncovered miR-142 involvement in the differentiation and function of hemangioblasts,2,3 lymphocytes,4–7 neutrophils8 and macrophages.7,9,10 Furthermore, we have recently uncovered a key role for miR-142 in the maintenance of CD4+ dendritic cells11 by characterizing a novel mouse model with deletion of the miR-142 allele.12
Using this model, we also discovered that miR-142 is expressed in the bipotent MK-erythroid precursors (PreMegEs) and demonstrated that miR-142 function is essential for the differentiation of megakaryocytes (MKs) and that its loss results in pronounced thrombocytopenia.12 Several other groups independently developed miR-142 mouse alleles, including Kramer et al.7 and Shrestha et al.13
Erythrocytes are the most abundant cell type in the circulation. Erythrocyte numbers in peripheral blood are tightly controlled, and new red blood cells are generated to congregate and compensate for conditions of stress, such as in the case of traumatic blood loss.14,15 The maintenance of normal mechanical properties is essential to permit erythrocytes to withstand shear forces in the circulation. The flexibility and strength of the erythrocyte membrane is conferred by stereotyped pentagonal and hexagonal arrays of short ACTIN filament, which are cross-linked by long, flexible spectrin molecules.16 ANKYRIN, BAND 3/SLC4A1 and PROTEIN 4.1/EPB41A also play important roles in the cytoskeleton scaffold, and their dysfunction is associated with various types of hemolytic anemia.17,18
High oxygen carrying capacity, hemoglobin autoxidation and high polyunsaturated fatty acid content in the lipid bilayer make mature erythrocytes particularly susceptible to oxidative damage. Accordingly, oxidative damage inflicts cytoskeletal reorganization and shortens erythrocyte life span, which is ~40 days in Mus musculus, when adequate antioxidant response is manifested.19
miRNAs, and the effector protein cofactor ARGONAUTE,20 play important roles in erythropoiesis. For example, miR-451/144, whose biogenesis depends on ARGONAUTE and not on Dicer activity, promotes efficient erythropoiesis by inhibiting 14-3-3zeta, Rab14 and Gata2,21–27 and miR-191 regulates erythrocyte enucleation.28
In the work herein, we test the hypothesis that miR-142 activity is pivotal in controlling erythrocyte survival, cytoskeleton and function. miR-142 regulates the erythrocyte cytoskeleton morphology and biomechanical properties and its capacity to cope with oxidative stress, affecting the survival of red blood cells in vivo.
Methods
Mouse genetics and cell biology
The miR-142 null allele is descrcribed by Chapnik and colleagues.12 Mouse strains were housed and handled in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Weizmann Institute of Science. Phenylhydrazine (PHZ) protocol follows that of Gutierrez and colleagues.29 Red blood cell (RBC) life span analysis was performed with EZ-Link Sulfo-NHS-Biotin (Thermo Scientific). Deformability and osmotic fragility was determined as in Relevy et al.,30 Barshtein et al.31 and Beutler and colleagues.32
Microscopy
Erythrocyte ultrastructural analysis was perfomed with secondary electron (SE) detector in high resolution Ultra 55 (Zeiss) or ESEM (FEI) microscopes. Super resolution micrographs captured on Vutara SR200 STORM microscope with 647 nm laser excitation power density of about 7 kW/cm2. Z-stack image was measured by acquiring 700 frames at 50 Hz for each z position at 0.1 um steps, and 405-nm activation laser power was ramped slowly to maintain optimal single-molecule density. Single-molecule fitting was performed with Vutara software. Fluorescence-activated cell sorting (FACS) and high-speed cell imaging analysis in flow with ImageStreamX protocols are described by Chapnik et al.12 and George and colleagues.33
A detailed description of the Material and Methods, including primers and antibodies used, is provided in the Online Supplementary Materials.
Results
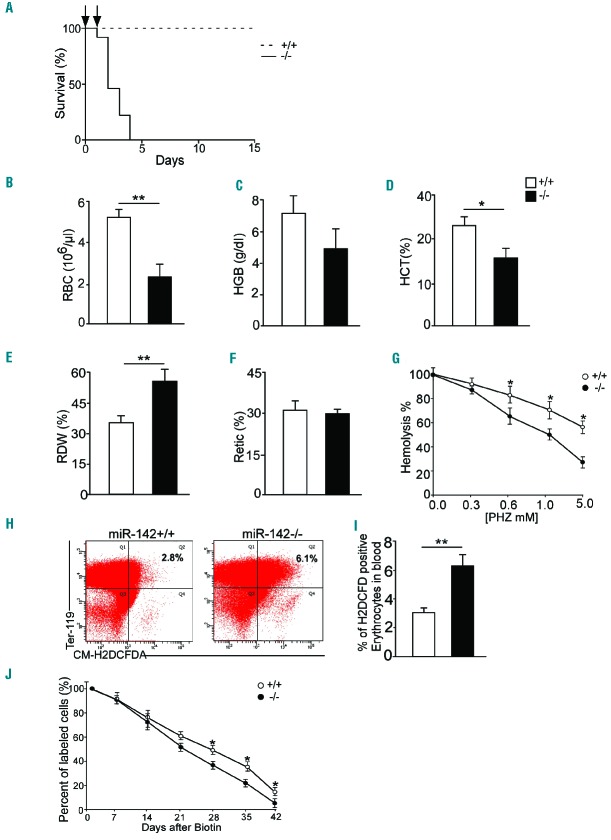
In screening for hematopoietic phenotypes of the miR-142 knockout model,12 we performed complete blood counts (Figure 1A–C) that revealed a reduction of erythrocyte numbers, hemoglobin levels and hematocrit concentration. Red blood cell distribution width (RDW) and the percentage of circulating reticulocytes increased significantly (Figure 1D,E). Microscopic analysis of methylene blue stained smears demonstrated more reticulocytes in miR-142−/− peripheral blood than in controls. The fraction of irregular cell morphologies was also higher in miR-142-deficient peripheral blood, relative to controls (Figure 1F).
Figure 1.

Ineffective erythropoiesis in miR-142−/− animals, erythroid hyperplasia and anemia. Bar graphs are shown for measures of (A) red blood cell numbers (RBC), (B) hemoglobin (HGB), (C) hematocrit (HCT) and (D) red cell distribution width (RDW) values in six-month-old miR-142−/− mice and controls. (E) Reticulocytes (Retic) levels in three-month-old miR-142−/− mice and controls. (F) Upper panels, micrographs of miR-142−/− and WT peripheral blood smears, stained with Methylene blue, demonstrating residual RNA in reticulocytes (black arrows) and of May-Grunwald-Giemsa staining (lower panels), which depict abnormal morphology of miR-142−/− erythrocytes. Scale bars, 5 μm. Representative results from one of two independent experiments are shown (mean ± SEM) with at least five animals in each group. *P<0.05; **P<0.005; ***P<0.0005.
Because loss of miRNA gene phenotypes are often better characterized under sensitized stress conditions,34,35 we studied recovery after the introduction of a hemolytic challenge with PHZ, a chemical that induces hemolytic anemia.21 The administration of two PHZ injections on successive days is a standard protocol from which wildtype (WT) animals fully recovered. However, it resulted in the death of all miR-142−/− mice (Figure 2A). We then used a reduced PHZ dose by employing a single injection. Under this regimen, miR-142−/−mice exhibited anemia that was significantly more severe than in WT controls (Figure 2B–F). Recovery from the milder protocol of PHZ-induced anemia was delayed in miR-142−/− mice, however, all mutant mice survived. A complete blood count, 11 days after insult, revealed that mutant animals recover more slowly than controls. For example, hemoglobin levels after 11 days were 14 g / dl and 8 g / ld in WT and miR-142 null animals, respectively. These data demonstrate that hematopoietic-specific miR-142 activity is required for normal erythrocyte levels under steady state, and for adequately coping with the hemolytic stress induced by PHZ.
Figure 2.
Vulnerability of miR-142−/− erythrocytes to oxidative challenge and increased ROS levels. (A) Kaplan-Meier survival plot of WT (n=12) and miR 142−/−(n=13) mice, treated by two intraperitoneal injections of PHZ (48 mg/kg) on the first two days of the study (arrows). Bar graph of erythrocyte measures in peripheral blood of animal subjected to reduced PHZ dosing (single injection of 48 mg/kg, n=4 per group), including (B) blood cell numbers (RBC), (C) hemoglobin (HGB), (D) hematocrit (HCT), (E) red cell distribution width (RDW) values and (F) percentage of circulating reticulocytes (Retic). (G) In vitro hemolysis, measured as percentage of live RBCs in glass hemocytometer, 4 hrs after incubation with increasing phenylhydrazine (PHZ) concentrations. (H) ROS quantified by FACS of cells treated with CM-H2DCFDA and co-gated as Ter119HI and (I) bar graph depicting the percentage of erythroid cells with relatively high ROS, taken from top right plot quadrant and averaged for three independent experimental repeats from WT and miR-142−/− mice (n=3 per genotype). (J) In vivo eryhtorcyte lifespan, gated by streptavidin-Cy5 and Ter119HI antibodies in control and miR-142−/− mice, pulse-chased by Sulfo-NHS-Biotin labeling (n=4). *P<0.05; **P<0.005.
To test if miR-142 knockout erythrocytes exhibit cell-autonomous sensitivity to PHZ we performed an in vitro study by incubating circulating erythrocytes with different PHZ doses. Methylene blue staining revealed that miR-142 null erythrocytes are more susceptible to PHZ-induced hemolysis than control erythrocytes (Figure 2G). PHZ is known to induce oxidative stress, and reactive oxygen species (ROS) are reported to play a part in the toxic mechanism.36 Therefore, we measured a fluorescent indicator for ROS, CM-H2DCFDA, by flow cytometry in untreated WT and miR-142 null circulating erythrocytes. We observed a two-fold upregulation of ROS species in Ter-119HI erythrocytes (Figure 2H,I). Finally, to test miR-142 null erythrocyte lifespan in vivo, we performed a pulse-chase study of erythrocytes by Sulfo-NHS-Biotin labeling. miR-142 erythrocyte lifespan was approximately 10% shorter than control cells, when calculated on days 21–42 after PHZ treatment (Figure 2J). Therefore, it seems that miR-142-deficient erythrocytes exhibit a shorter than expected lifespan in vivo and synthesize more ROS, which plausibly contributes to their death, resulting in the observed anemia.
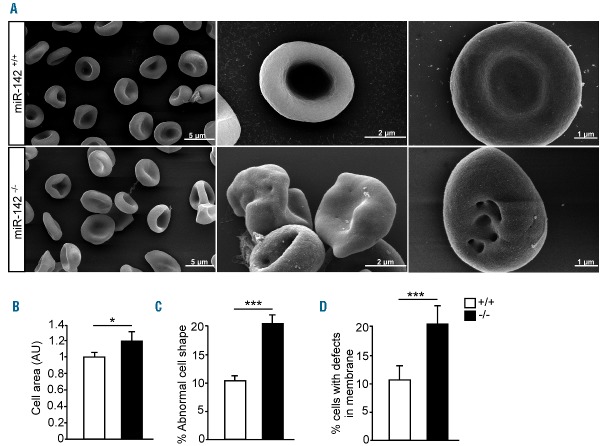
Oxidative damage has been shown to cause changes to RBC morphology, biomechanical properties and cytoskeletal reorganization.37 We therefore sought to characterize the morphology of miR-142−/− erythrocytes by scanning electron microscopy (SEM). The study revealed that miR-142−/− erythrocytes were larger (radial dimension) on average than control cells and that abnormal forms, such as leptocytes and knizocytes, were more prevalent than in controls (Figure 3A–C). We also observed a higher proportion of miR-142−/− erythrocytes with prominent membrane defects. These deformations included hole-like structures that were observed only in miR-142−/− erythrocytes but not in control cells (Figure 3D). Taken together, these data demonstrate that miR-142 is required for normal erythrocyte morphology.
Figure 3.
miR-142−/− erythrocytes exhibit defective morphology. (A) Scanning electron microscopy reveals the stereotypic biconcave form of WT erythrocytes and atypical morphology of miR-142−/− erythrocytes (knizocytes, leptocytes). Micrograph scales noted in panels. (B) Quantification of cell surface area, (C) percentage of cells with abnormal morphology or (D) structural membrane changes in miR-142−/− (n=3) and WT cells (n=3). Representative results from one of two independent experiments (mean ± SEM) with >100 cells per group. *P<0.05; ***P<0.0005. AU: arbitrary unit.
Next, we explored ACTIN distribution, via ImageStreamX flow cytometry. miR-142−/− erythrocytes displayed decreased ACTIN polarity and were less circular than control RBCs (Figure 4A–D), consistent with changes in the organization of the erythrocyte cytoskeleton. Therefore, F-ACTIN assembly may be a key mechanism, whereby miR-142 controls erythroid cell development.
Figure 4.
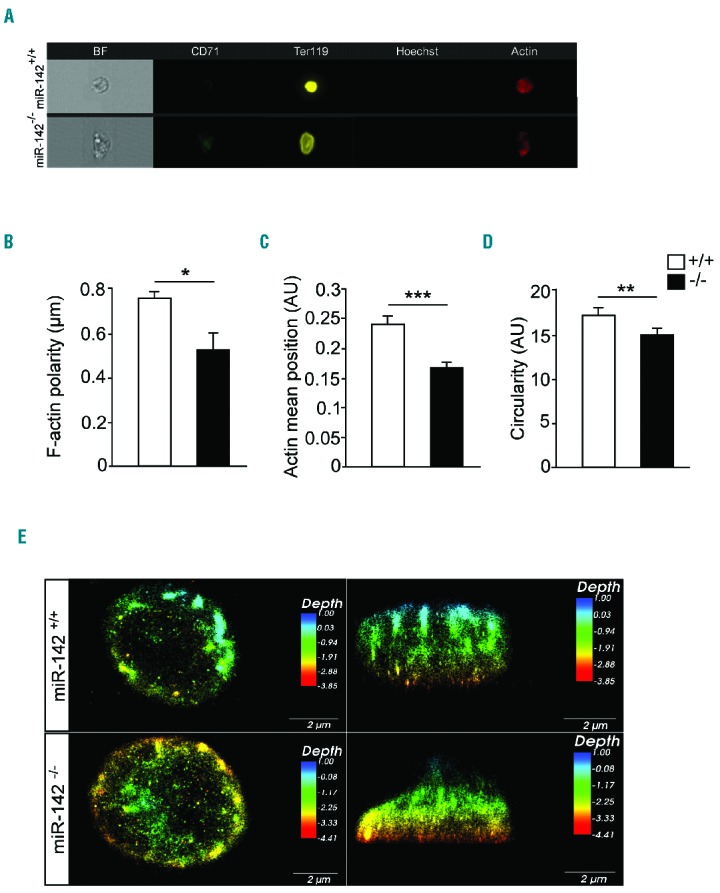
Disturbed cytoskeletal architecture in the absence of miR-142. (A) Representative single-cell images of RBCs, obtained by ImageStreamX flow cytometer. Bright-field (BR, gray), Ter119HI (yellow), Alexa Fluor 647-Phalloidin (F-actin, red), CD71Neg (green) and HoechstNeg (purple). Scale bar, 7 μm. (B) Decreased ACTIN polarity, (C) ACTIN position in the cell and (D) low circularity in miR-142−/− RBCs relative to WT controls revealed by ImageStreamX analysis. (E) Super-resolution microscopy images of F-ACTIN depth in RBCs, top and side view. Scale bar, 2 μm. Three animals per group (mean ± SEM). *P<0.05; **P<0.005; ***P<0.0005. AU: arbitrary unit.
The analysis was substantiated by super-resolution stochastic optical reconstruction microscopy (STORM), which demonstrated the typical dense submembranous ACTIN meshwork in WT erythrocytes. However, aberrant F-ACTIN polarity was observed in miR-142-deficient cells, in accordance with ImageStreamX measurements. These structural changes in ACTIN distribution are observed in erythrocytes deficient of miR-142, even when the cellular morphology was seemingly normal (Figure 4E).
Therefore, in contrast to the symmetrically-rounded WT red blood cell, the ACTIN meshwork of miR-142-deficient erythrocytes drive irregularities to the cytoskeleton.
Erythrocyte ability to pass through the microvasculature of the splenic sinuses is defined by their deformability, a characteristic that depends on cytoskeletal properties. The assessment of deformability may therefore expose changes in the ACTIN cytoskeleton functions. We quantified miR-142−/− erythrocyte deformability, under flow-induced shear stress (3.0 Pa), by measuring the major and minor axes elongation ratio of slide-adherent erythrocytes, as in the work of Relevy and colleagues.30 Intriguingly, miR-142−/− cells were more deformable than control erythrocytes (Figure 5A). In addition, an independent analysis of osmotic fragility revealed that miR-142−/− cells are more resistant to osmotic lysis than WT control cells (Figure 5B). We concluded that miR-142 activity is necessary for maintaining normal membrane mechanical properties, and its deficiency leads to abnormal cellular deformability.
Figure 5.
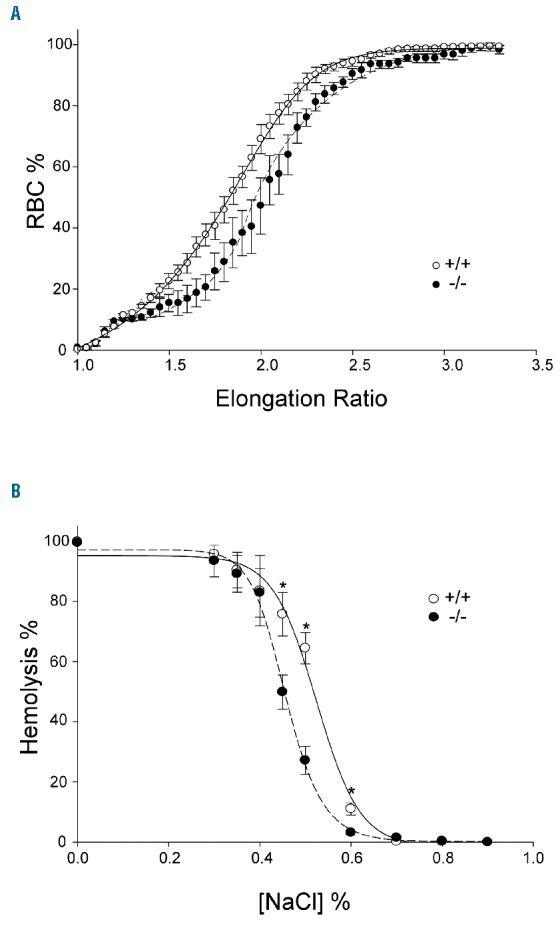
Disturbed RBC deformability and osmotic fragility of miR-142−/−. (A) RBC deformability is described by the elongation ratio (ER) distribution in miR-142−/− (n=4) and WT (n=4) cells under shear stress of 3.0 Pa. miR-142−/− curve is shifted to the right, meaning that mutant cells are more deformable than control erythrocytes. (B) miR-142−/− osmotic fragility curve is shifted to the left of a typical WT curve. miR-142−/− RBCs are less susceptible to decreased osmotic pressure and undergo lysis only after greater reduction in NaCl concentrations, relative to WT cells. n=4 WT and 4 miR-142−/− experimental repeats. *P<0.05. RBC: red blood cell; NaCI: sodium chloride.
The typical shape of the RBC and its elasticity are attributed to cell membrane proteins, surface charge and phospholipid composition, whose interactions reinforce the erythrocyte membrane with a deformable cytoskeleton network. Key steps in understanding the composition and function of the erythrocyte cytoskeleton were taken in the 1980’s with the revelation of a designated meshwork composed of ACTIN, SPECTRIN, ANKYRIN, BAND 3/SLC4A1 and PROTEIN 4.1/EPB41.16–18,38 miR-142 deficiency downregulated SPECTRIN and upregulated ADDUCTIN and ANKYRIN levels, relative to controls (Figure 6A–I). Since the canonical erythrocyte cytoskeleton proteins do not harbor binding sites for miR-142, these effects were probably indirect, and may be related to the derepression of COFILIN, GRLF1 or other immediate targets of miR-142.
Figure 6.
miR-142 activity is required for normal RBC protein expression. (A) Western blots of RBC proteins probed with the indicated antibodies. Each panel is representative of results from >5 blots. (B) Bar graph represents the ratio of SPECTRIN/GAPDH. (C) GRLF1/GAPDH. (D) ADUCTIN/GAPDH. (E) ACTIN/GAPDH. (F) BAND3/GAPDH. (G) ANKYRIN/GAPDH. (H) COFILLIN/GAPDH. (I) WASL/GAPDH. *P<0.05.
K-562 is a human erythroleukemia line, derived from chronic myelogenous leukemia in terminal blast crises.39 K-562 cells can be driven to erythrocyte differentiation with hemin treatment.40 To test if the main observations with miR-142 loss-of-function are extendable to human RBCs, we knocked down miR-142-3p by transfecting K-562 cells with a miRZip-142-3p plasmid and induced erythroid differentiation with hemin. miR-142-3p was knocked down to 40% of the expression levels in K-562 cells that were transfected with miRZip-control vector (Figure 7A) and accordingly, miR-142-3p targets were derepressed, including CFL2, GRLF1 and WASL (Figure 7B). ImageStreamX cytometry revealed an increased average cell area, reduced circularity and decreased F-ACTIN polarity, consistent with observations in mouse miR-142 knockout erythrocytes (Figure 7C–F). Therefore, knockdown of human miR-142-3p in K-562 cells recapitulates several phenotypes observed in vivo in the mouse miR-142 null allele.
Figure 7.
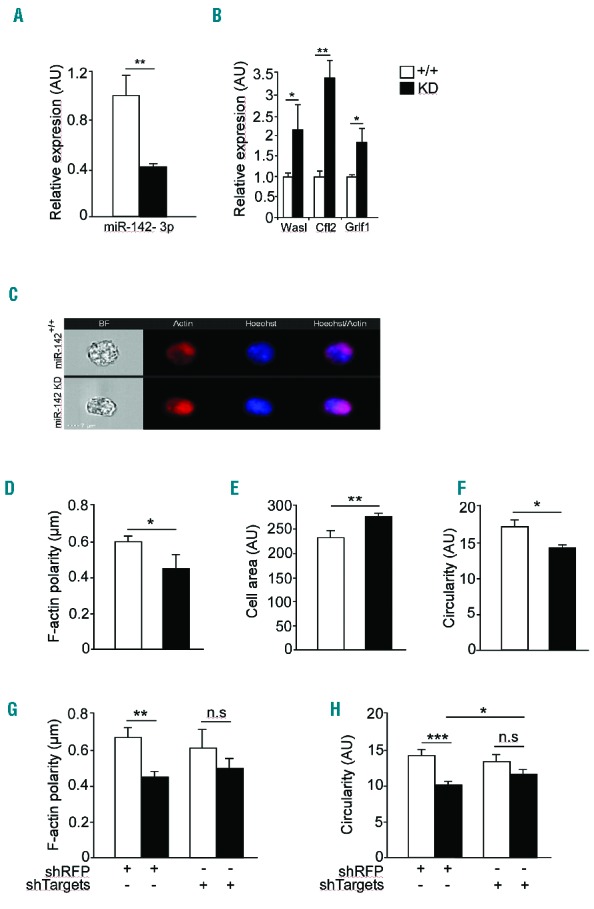
miR-142 regulates ACTIN cytoskeletal architecture and dynamics in K-562 cells. Expression of miR-142 (A) and its targets (B) in K-562 cells transfected with a knockdown (KD) miR-ZIP-142 vector. Representative micrographs, obtained with ImageStreamX flow cytometer and stained with Alexa Fluor 647-Phalloidin (F-actin, red), and Hoechst (blue, C). Scale bar, 7 μm. Decreased F-ACTIN polarity (D), increased cell area (E) and decreased circularity (F) in K-562 cells after miR-142 knockdown, relative to WT controls. K-562 transduced with miR-ZIP-142 knockdown vector and concomitantly with a lentivirus encoding a shRNA directed against RFP (shRFP) or a set of shRNA vectors as indicated. ImageStreamX flow cytometery that was performed 72 hrs later revealed that knockdown of targets partially restored F-ACTIN polarity (G) and circularity (H). Representative results from one of two independent experiments (mean ± SEM), three experimental repeats per group. *P<0.05; **P<0.005; ***P<0.0005. AU: arbitrary unit; n.s: non significant; sh: short hairpin; RFP: red fluorescent protein.
To test the relevance of miR-142 targets we next designed a rescue experiment, whereby we knocked down miR-142 in K-562 cells and then targeted derepressed targets by short hairpin RNA (shRNA). The shRNAs, expressed from lentiviral vectors, effectively reduced WASL, CFL2 and ARHGAP35/GRLF1 expression. ImageStreamX flow cytometry of miR-142-3p knockdown K-562 cells, in which WASL, CFL2 and ARHGAP35/GRLF1 were silenced, partially recovered the circularity phenotype defect imposed by miR-142 deficiency, relative to a lentivirus encoding a shRNA directed against red fluorescent protein, (shRFP), that was employed as control (Figure 7G,H). These data are consistent with the activity of ACTIN-binding proteins as effectors of miR-142-3p in control of the erythroid cell cytoskeleton. In summary, our study characterizes a new regulator of erythrocytes. miR-142 is an interesting regulator as it controls the cytoskeleton in several hematopoietic cell lines. Furthermore, miR-142 regulates the erythrocytes capacity to cope with ROS. One of the intriguing conclusions emerging from the current study is that a housekeeping network of cytoskeletal regulators can be reshaped by a single miRNA denominator in a cell type specific manner.
Discussion
Our study demonstrates that miR-142 controls critical facets of erythrocyte maturity, playing roles in the regulation of RBC size, mechano-physical properties, lifespan and numbers, in vivo. Specifically, miR-142 functions help erythrocytes to cope with oxidative stress, and regulate the cytoskeleton. Accordingly, irregular morphology, abnormal mechanical properties and elevated ROS levels prevail in miR-142-deficient erythrocytes.
Erythrocytes are sensitive to oxidative stress because of the high physiological hemoglobin level.41,42 Accordingly, the consequences of oxidative damage are often manifested as changes in erythrocyte deformability, splenic sequestration and a decreased lifespan.43–46 The sensitivity of miR-142 null cells to ROS is consistent with hypersensitivity to PHZ-induced oxidative stress, as compared with WT littermates. However, the mechanisms by which miR-142 regulates oxidative stress are still unknown.
Several proteins that are known to maintain the RBC structure are dysregulated in miR-142-deficient erythrocytes. However, since aductin, ankyrin and spectrin mRNAs do not harbor cis binding sites (miRNA recognition elements) for miR-142 in their 3′ untranslated regions, plausibly they are indirectly controlled by the miRNA. Direct targets, whose expression is derepressed and hence upregulated in miR-142-deficient adult erythrocytes, include Cofilin and Grlf1, which are regulated by the same miRNA also in MKs.12 Consistently, the knockdown of WASL, CFL2 and ARHGAP35 / GRLF1 in K-562 cells partially rescued the typical circular architecture of erythroid cells. These data provide initial evidence that targets that were originally characterized in MKs also function downstream of miR-142-3p in the erythroid lineage. However the exact contribution of specific targets should be explored in future works, and it is also likely that additional miR-142-3p targets participate in the regulation of the ACTIN network and cytoskeleton organization.
Abnormal erythrocyte structure or membrane deformability are observed in many clinical red cell disorders,47 therefore, the new link to miR-142 suggests that this miRNA gene might be an essential component in erythrocyte pathologies. Furthermore, based on the study of the human K-562 cell line, it may be that miR-142 functions are conserved to humans.
In summary, our analysis suggests a critical role for miR- 142 in controlling a network of erythrocyte proteins that are required for the unique cellular morphology, function, and for oxidative defense. More generally, the use of mouse genetics to uncover the functions of a lineage-specific miRNA helps to explain how ubiquitous regulatory networks can be reshaped to meet with the specialized requirements of a unique cell type.
In the future, it will be important to investigate additional functions of miR-142, including its potential ability to regulate erythropoiesis, the mechanisms by which 142 contributes to anti-oxidative activity, and whether the cytoskeleton network downstream of miR-142, that functions in megakaryocytes and erythrocytes, is present in other hematopoietic cells wherein miR-142 is expressed.
Supplementary Material
Acknowledgments
The authors would like to thank Ofira Higfa and Yehudah Melamed for veterinary services and husbandry and Dr. Joseph Lotem for helpful discussions. The work is funded by the Minerva foundation and Minna-James-Heineman Stiftung through Minerva. Work at the Hornstein lab is further funded by an ERC consolidator program, Israel Science Foundation, the Legacy-heritage program, The Bruno and Ilse Frick Foundation for Research on ALS, the ALS Therapy Alliance, The Motor Neurone Disease Association (UK), the Thierry Latran Foundation for ALS research, the ERA-Net for Research Programmes on Rare Diseases (FP7), A. Alfred Taubman through IsrALS, Teva Pharmaceutical Industries Ltd as part of the Israeli National Network of Excellence in Neuroscience (NNE), Yeda-Sela, Yeda-CEO, Israel Ministry of Trade and Industry, Y. Leon Benoziyo Institute for Molecular Medicine, The Kekst Family Institute for Medical Genetics, The David and Fela Shapell Family Center for Genetic Disorders Research, The Crown Human Genome Center, the Nathan, Shirley, Philip and Charlene Vener New Scientist Fund, Julius and Ray Charlestein Foundation, The Fraida Foundation, The Wolfson Family Charitable Trust, The Adelis Foundation, MERCK (UK), Maria Halphen, and the Estates of Fannie Sherr, Lola Asseof and Lilly Fulop. Electron microscopy studies were partially founded by The Moskowitz Center for Nano and Bio-Nano Imaging at WIS. E.H. is Head of The Nella and Leon Benoziyo Center for Neurological Diseases, and the lab is further supported by Dr. Sydney Brenner.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/4/676
References
- 1.Gauwerky CE, Huebner K, Isobe M, Nowell PC, Croce CM. Activation of MYC in a masked t(8;17) translocation results in an aggressive B-cell leukemia. Proc Natl Acad Sci USA. 1989;86(22):8867–8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nimmo R, Ciau-Uitz A, Ruiz-Herguido C, et al. MiR-142–3p controls the specification of definitive hemangioblasts during ontogeny. Dev Cell. 2013;26(3):237–249. [DOI] [PubMed] [Google Scholar]
- 3.Lu X, Li X, He Q, et al. miR-142–3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res. 2013;23(12):1356–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Haupt S, Prots I, et al. miR-142–3p is involved in CD25+ CD4 T cell proliferation by targeting the expression of glycoprotein A repetitions predominant. J Immunol. 2013;190(12):6579–6588. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Oravecz-Wilson K, Mathewson N, et al. Mature T cell responses are controlled by microRNA-142. J Clin Invest. 2015; 125(7):2825–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer NJ, Wang WL, Reyes EY, et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood. 2015; 125(24):3720–3730. [DOI] [PubMed] [Google Scholar]
- 8.Fan HB, Liu YJ, Wang L, et al. miR-142–3p acts as an essential modulator of neutrophil development in zebrafish. Blood. 2014; 124(8):1320–1330. [DOI] [PubMed] [Google Scholar]
- 9.Lagrange B, Martin RZ, Droin N, et al. A role for miR-142–3p in colony-stimulating factor 1-induced monocyte differentiation into macrophages. Biochim Biophys Acta. 2013;1833(8):1936–1946. [DOI] [PubMed] [Google Scholar]
- 10.Sonda N, Simonato F, Peranzoni E, et al. miR-142–3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38(6):1236–1249. [DOI] [PubMed] [Google Scholar]
- 11.Mildner A, Chapnik E, Manor O, et al. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood. 2013;121(6):1016–1027. [DOI] [PubMed] [Google Scholar]
- 12.Chapnik E, Rivkin N, Mildner A, et al. miR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. Elife. 2014;3:e01964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha A, Carraro G, El Agha E, et al. Generation and Validation of miR-142 Knock Out Mice. PloS one. 2015; 10(9):e0136913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood. 1996;88(1):75–81. [PubMed] [Google Scholar]
- 15.Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13(22): 2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byers TJ, Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci USA. 1985;82(18):6153–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarides E, Woods C. Biogenesis of the red blood cell membrane-skeleton and the control of erythroid morphogenesis. Annu Rev Cell Biol. 1989;5:427–452. [DOI] [PubMed] [Google Scholar]
- 18.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. [DOI] [PubMed] [Google Scholar]
- 19.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Carroll D, Mecklenbrauker I, Das PP, et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21(16):1999–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen KD, Simmini S, Abreu-Goodger C, et al. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 2010;207(7):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M, Tan YS, Cheng WC, Kingsbury TJ, Heimfeld S, Civin CI. MIR144 and MIR451 regulate human erythropoiesis via RAB14. Br J Haematol. 2015;168(4):583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrick DM, Zhang CC, Tao Y, et al. Defective erythroid differentiation in miR-451 mutant mice mediated by 14–3–3zeta. Genes Dev. 2010;24(15):1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu D, dos Santos CO, Zhao G, et al. miR-451 protects against erythroid oxidant stress by repressing 14–3–3zeta. Genes Dev. 2010;24(15):1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dore LC, Amigo JD, Dos Santos CO, et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc Natl Acad Sci USA. 2008;105(9):3333–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pase L, Layton JE, Kloosterman WP, Carradice D, Waterhouse PM, Lieschke GJ. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113(8):1794–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Flygare J, Wong P, Lim B, Lodish HF. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev. 2011;25(2):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez L, Tsukamoto S, Suzuki M, et al. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood. 2008;111(8):4375–4385. [DOI] [PubMed] [Google Scholar]
- 30.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48(1):136–146. [DOI] [PubMed] [Google Scholar]
- 31.Barshtein G, Gural A, Manny N, Zelig O, Yedgar S, Arbell D. Storage-induced damage to red blood cell mechanical properties can be only partially reversed by rejuvenation. Transfus Med Hemother. 2014;41(3): 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beutler E, Kuhl W, West C. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood. 1982;59(6):1141–1147. [PubMed] [Google Scholar]
- 33.George TC, Fanning SL, Fitzgerald-Bocarsly P, et al. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311(1–2):117–129. [DOI] [PubMed] [Google Scholar]
- 34.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38 Suppl:S20–24. [DOI] [PubMed] [Google Scholar]
- 35.Emde A, Hornstein E. miRNAs at the interface of cellular stress and disease. EMBO J. 2014;33(13):1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo M, Itoh S, Kusaka T, Imai T, Isobe K, Onishi S. The ability of neonatal and maternal erythrocytes to produce reactive oxygen species in response to oxidative stress. Early Hum Dev. 2002;66(2):81–88. [DOI] [PubMed] [Google Scholar]
- 37.Sinha A, Chu TT, Dao M, Chandramohanadas R. Single-cell evaluation of red blood cell bio-mechanical and nano-structural alterations upon chemically induced oxidative stress. Sci Rep. 2015;5: 9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Branton D, Cohen CM, Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981;24(1):24–32. [DOI] [PubMed] [Google Scholar]
- 39.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- 40.Rutherford TR, Clegg JB, Weatherall DJ. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979;280(5718):164–165. [DOI] [PubMed] [Google Scholar]
- 41.Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990;76(5):1015–1020. [PubMed] [Google Scholar]
- 42.Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8(7):609–619. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285(26):19976–19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder LM, Fortier NL, Trainor J, et al. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76(5):1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980;66(3):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cimen MY. Free radical metabolism in human erythrocytes. Clinica Chim Acta. 2008;390(1–2):1–11. [DOI] [PubMed] [Google Scholar]
- 47.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141(3): 367–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.