FLT3 mutations represent the most frequent molecular aberrations in acute myeloid leukemia (AML), and are associated with a higher probability of relapse and worse outcome. Activating mutations of FLT3 predominantly comprise internal tandem duplications (FLT3-ITDs), and can be detected in up to 30% of AML patients.1 FLT3-ITDs are patient-specific, and the individual location and varying length of the duplicated genomic sequence results in a broad variability which is translated into highly individual peptide motifs arising from duplicated amino acid sequences within the FLT3 gene.
Here, a retrospective single center analysis of patient-specific sequence motifs of FLT3-ITDs, including a detailed description of putatively relevant amino acid motifs, is presented. Furthermore, the clinical impact of FLT3-ITD diversity on both the response to induction chemotherapy and leukemia-free survival (LFS) in this cohort of patients with AML is shown.
Several studies have addressed the question whether the diversity of FLT3-ITD affects clinical outcome of AML patients. Stirewalt and colleagues demonstrated an impact of ITD length (< 40 versus > 40 base pairs) on LFS in 151 elderly AML patients who received intensive chemotherapy.2 Kayser and colleagues showed that AML patients harboring FLT3-ITD located in the first tyrosine kinase domain (TKD1) of the FLT3 gene have a worse prognosis as compared to patients with FLT3-ITD within the juxtamembrane domain (JMD).3
In contrast, a retrospective analysis of 260 FLT3-ITD positive AML patients, separating FLT3-ITD into three different groups according to its localization, could not confirm this observation but demonstrated a statistical trend towards an association between a more C-terminal location of FLT3-ITD and decreased survival without affecting event-free survival (EFS). No association between ITD length, distinct sequence motifs, and clinical outcome of AML patients was observed in this study.4
A more detailed analysis of FLT3-ITD sequence diversity in pediatric AML revealed a regular duplication of certain peptide motifs in children with activated FLT3. In detail, FLT3-ITD sequence analysis in 77 children revealed that all patients harbored a duplication of seven amino acids, comprising the YVDFREY sequence (position 591 to 597), while in about one third (31%) of pediatric patients, FLT3-ITD contained a duplicated STAT5 binding motif, including tyrosine residues at position 589 and 591.5
FLT3-ITD patients analysed in this retrospective study were treated with conventional induction chemotherapy using cytarabine and either idarubicine or mitoxantrone according to standard protocols in our department. Clinical characteristics of AML patients harboring single FLT3-ITD, response criteria, mutant-to-wild-type ratio, and experimental procedures of FLT3-ITD sequence analyses are provided in the Online Supplementary Material.
FLT3-ITD screening, in our cohort of intensively treated AML patients, was part of the central diagnostic procedures within clinical studies of the Ostdeutsche Studiengruppe für Hämatologie und Onkologie, as listed in the Online Supplementary Material. Screening revealed 50 patients with FLT3-ITD-positive AML, including 6 (12%) and 1 (2%) out of 50 patients with two or three different FLT3-ITDs, respectively, suggesting distinct AML clones with different activating mutations of FLT3 (Figure 1A). These results were in line with previously published analyses.4,5 Detailed information about the amino acid sequences of all FLT3-ITDs, including highlighting tyrosine, serine and threonine residues, is provided in Online Supplementary Figure S1.
Figure 1.
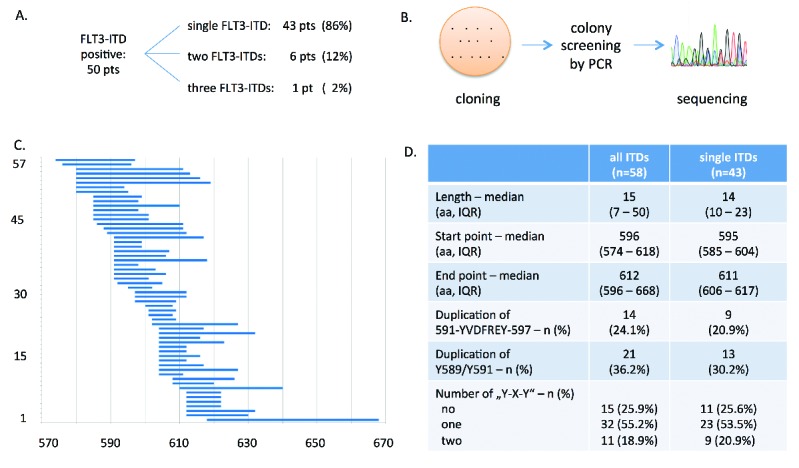
Synopsis of sequence analysis of FLT3-ITD in AML patients. A. Number of patients with single FLT3-ITD and patients with two or more internal tandem duplications. B. Principle of cloning, colony screening, and sequence analysis to determine the exact sequence of each individual FLT3-ITD. C. Illustration of all 57 identified FLT3-ITDs indicating starting point, length and end point of each of each ITD according to the amino acid sequence of FLT3. The x-axis indicates the corresponding codons of FLT3 protein sequence. All applied methods are comprehensively described in Online Supplementary Material. D. Sequence analyses of FLT3-ITDs demonstrating its localization and length as well as the proportion of patients presenting a duplication of distinct peptide motifs, e.g., YVDFREY (position 591 to 597), the STAT5 binding site (Y589/Y591) or “Y-X-Y” motifs in general. IQR, interquartile range; aa, amino acid.
Sequence analysis was performed on all identified FLT3-ITDs in order to correlate patient-specific ITD characteristics with clinical data, such as response to induction chemotherapy, or survival of the 43 AML patients harboring single FLT3-ITD (Figure 1B and C).
Analysis of duplicated amino acid sequences demonstrated that 9 out of 43 patients (21%) presented with a duplication of the YVDFREY peptide motif, while 13 out of 43 patients (30%) harbored an additional STAT5 binding site. These data are, in part, conflicting with sequence analyses published by Meshinchi and colleagues.5 Other “Y-X-Y” sequence motifs of FLT3-ITD, such as Y589/591, were also analysed. Up to two different “Y-X-Y” motifs could be identified, while the majority of these sequences was represented by the STAT5 docking site (Figure 1D).
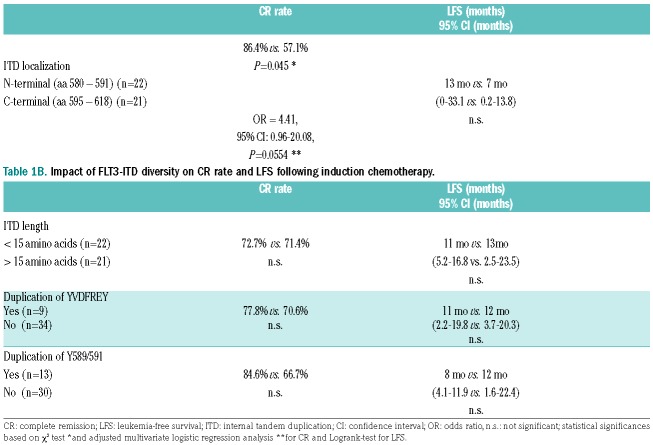
Localization of FLT3-ITD, defined as the starting point of the individual tandem duplication, affects both the response to induction chemotherapy and the LFS (Table 1A). In detail, dichotomization of FLT3-ITD sequences according to its starting points, resulted in two subgroups: 22 patients with N-terminal FLT3-ITD (aa 580 – 591), and 21 patients with C-terminal FLT3-ITD (aa 595 – 618), respectively. By means of χ2 test, FLT3-ITD localized towards the N-terminus resulted in a significantly higher rate of complete remission (CR) compared to patients with FLT3-ITD downstream (86.4% versus 57.1%, P=0.045). Adjusted multivariate logistic regression analysis of CR, considering age and gender as further covariates, could confirm this observation (OR = 4.41, 95% CI: 0.96 – 20.08, P=0.0554) (Table 1A).
Table 1.
A. Impact of FLT3-ITD localization on CR rate and LFS following induction chemotherapy.
B. Impact of FLT3-ITD diversity on CR rate and LFS following induction chemotherapy.
Although no statistical significance was demonstrated when comparing LFS of the two subgroups, there might be a potential impact of FLT3-ITD localization (13 mo versus 7 mo) (Table 1A, Figure S2).
FLT3-ITD allelic ratio was calculated as the ratio under the curve of mutant and wild-type alleles.6 The allelic burden of FLT3-ITD was equally distributed between the two subgroups (median: 0.60 versus 0.61, not significant). Sequence data of each individual FLT3-ITD is indicated in Online Supplementary Figure S1.
Cytogenetic analysis revealed a balanced distribution of patients harbouring a normal karyotype between both subgroups: 15 out of 22 patients (68%) and 14 out of 21 patients (67%), respectively. No impact of ITD length, the YVDFREY motif, or additional STAT5 binding sites on CR rate or LFS was detected (Table 1B).
Mutations of FLT3 represent the most frequent molecular aberration in AML.7 In a prospective clinical trial, the combined treatment of AML with intensive chemotherapy and the FLT3 inhibitor sorafenib lead to improved event-free survival, independently of the presence of FLT3-ITD.8
Treatment of FLT3-ITD positive AML requires further improvement, and several questions concerning the risk stratification of this subgroup of AML patients remain unanswered, e.g., regarding the clinical decision of subsequent allogeneic stem cell transplantation in first complete remission. Even though clinical data indicates a worse prognosis for FLT3-ITD- positive AML in the case of a high allelic ratio or localization of the internal tandem duplication within the TKD1 domain of FLT3, the clinical impact of FLT3-ITD diversity remains a challenging issue in AML treatment.3,9
As a result of two retrospective studies evaluating allogeneic stem cell transplantation as a strategy for FLT3-ITD positive patients in first complete remission, this treatment approach is still a matter of debate.10,11 Thus, additional factors concerning FLT3-ITD need to be identified in order to achieve an even more subtle risk classification of FLT3-ITD-positive AML patients. Besides the impact of the FLT3 allelic ratio, the FLT3-ITD diversity (e.g., localization of the start point) should be evaluated in a prospective manner regarding the impact of allogeneic stem cell transplantation.12
Distinct sequence motifs evolving from FLT3-ITD can be associated with a different response of constitutively activated FLT3 towards tyrosine kinase inhibitors (TKI).13 Heidel and colleagues recently demonstrated that TKI sensitivity does not only vary due to FLT3-ITD localization. In particular, they observed a differential responsiveness of FLT3-ITD towards TKI depending on in vivo or in vitro condition, and hypothesized a functional role of differential activation of DNA-damage response pathways.14
The present data support the hypothesis that FLT3-ITDs located closer to the C-terminus of the FLT3 gene are associated with worse prognosis. It has been clearly demonstrated that localization of the ITD impacts remission rate following AML induction chemotherapy, independently of the mutant allelic burden. Though not statistically significant, a more downstream localization of FLT3-ITD may also be correlated with impaired LFS in our cohort of AML patients. Impact on duplication of the STAT5 docking site, or other distinct sequence motifs, was not observed.
In summary, our data may contribute to the design of prospective clinical trials investigating the clinical impact of FLT3-ITD diversity. Furthermore, a systematic evaluation of patient-specific ITDs could also help to explain the differing sensitivity of FLT3-ITD-positive AML to treatment with TKI.15
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding: this work was in part supported by a grant from Gilead (Munich, Germany).
References
- 1.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. [DOI] [PubMed] [Google Scholar]
- 2.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107(9):3724–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–2392. [DOI] [PubMed] [Google Scholar]
- 4.Schnittger S, Bacher U, Haferlach C, Alpermann T, Kern W, Haferlach T. Diversity of the juxtamembrane and TKD1 mutations (exons 13–15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosomes Cancer. 2012;51(10):910–924. [DOI] [PubMed] [Google Scholar]
- 5.Meshinchi S, Stirewalt DL, Alonzo TA, et al. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood. 2008;111(10):4930–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratcorona M, Brunet S, Nomdedeu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738. [DOI] [PubMed] [Google Scholar]
- 7.Miller CA, Wilson RK, Ley TJ. Genomic landscapes and clonality of de novo AML. N Engl J Med. 2013;369(15):1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015; 16(16):1691–1699 [DOI] [PubMed] [Google Scholar]
- 9.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. [DOI] [PubMed] [Google Scholar]
- 10.Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109(5):2264–2265; author reply 2265. [DOI] [PubMed] [Google Scholar]
- 11.Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–3665. [DOI] [PubMed] [Google Scholar]
- 12.Ho AD, Schetelig J, Bochtler T, et al. Allogeneic stem cell transplantation improves survival in patients with acute myeloid leukemia characterized by a high allelic ratio of mutant FLT3-ITD. Biol Blood Marrow Transplant. 2016;22(3):462–469. [DOI] [PubMed] [Google Scholar]
- 13.Breitenbuecher F, Markova B, Kasper S, et al. A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood. 2009;113(17):4063–4073. [DOI] [PubMed] [Google Scholar]
- 14.Arreba-Tutusaus P, Mack TS, Bullinger L, et al. Impact of FLT3-ITD location on sensitivity to TKI-therapy in vitro and in vivo. Leukemia. 2016;30(5):1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konig H, Levis M. Targeting FLT3 to treat leukemia. Expert Opin Ther Targets. 2015;19(1):37–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.