Central nervous system (CNS) relapse is a principal cause of treatment failure among patients with acute lymphoblastic leukemia (ALL). Isolated CNS relapse occurs in approximately 3%–8% of children with leukemia and accounts for 30%–40% of initial relapses in some clinical trials.1 Furthermore, CNS-directed therapies are associated with seizures, secondary neoplasms, encephalopathy, and long-term endocrine, developmental, neurovascular, and cognitive deficits.2 Protection from leukemia relapse in the CNS is crucial to both long-term survival and quality of life and thus there is a significant need to develop more effective and less toxic therapies for the treatment of CNS leukemia. While many studies have demonstrated that interactions between leukemia cells and components of the bone marrow (BM) microenvironment influence leukemia development, maintenance and chemo-resistance, the role of the CNS microenvironment in leukemia has not received sufficient attention.3 However, in support of a role for a functionally significant CNS niche in leukemia, high rates (approx. 50%–75%) of CNS leukemia are observed in: i) patients in the absence of adequate CNS-directed therapies;4 and ii) mice transplanted with human, primary B-cell precursor leukemia cells.5 Furthermore, Akers et al. demonstrated that leukemia cells co-cultured adherent to CNS-derived cells exhibited enhanced chemo-resistance relative to the cells in suspension.6 Together these observations suggest that direct effects of the CNS niche on leukemia cells may contribute more to relapse and therapy resistance than the ability of leukemia cells to migrate from the BM to the immunologically privileged CNS.5
To investigate the influence of the CNS niche in leukemia, we asked whether the CNS niche could impart unique and functionally important gene expression changes in leukemia cells. We transplanted NALM-6 human, pre-B leukemia cells into NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) immune-compromized mice without prior irradiation to avoid perturbing the BM and CNS niches. After systemic leukemia development (Figure 1A) the mice were euthanized, leukemia cells were isolated from the BM and CNS microenvironments, and gene expression profiling of approximately 700 cancer-associated genes (NanoString® Technology) was performed. This approach identified 36 leukemia genes differentially expressed (30 up-regulated and 6 down-regulated; fold change ≥2 and FDR<0.05) in leukemia cells in the CNS microenvironment relative to the BM (Figure 1B and Online Supplementary Table S1). Furthermore, functional annotation revealed the up-regulated genes were involved in multiple pathways important for leukemia biology, including MAPK, RAS, and apoptosis (Online Supplementary Table S2). These data support our hypothesis that the CNS provides a unique leukemia niche that may influence leukemia biology. We elected to further examine the gene PBX1 as it is a transcription factor with known roles in hematopoiesis, leukemia, and cancer biology. PBX1 regulates the self-renewal of long-term hematopoietic stem cells by maintaining their quiescence as well as preventing myeloid maturation and maintaining lymphoid potential in hematopoietic progenitors.7 PBX1 also contributes to Evi-1-mediated leukemia development in a murine model of leukemia and is a partner with TCF3 in the t(1;19) translocation that occurs in approximately 5% of pre-B ALL.8 Interestingly, this translocation is also associated with an increased risk for CNS relapse, which may, in part, be due to Mer kinase upregulation in patients with the t(1;19).9,10 However, while this is an intriguing clinical observation, the mechanisms by which the TCF3-PBX1 translocation contribute to leukemia development and CNS relapse are undoubtedly more complex than simply PBX1 upregulation.11
Figure 1.
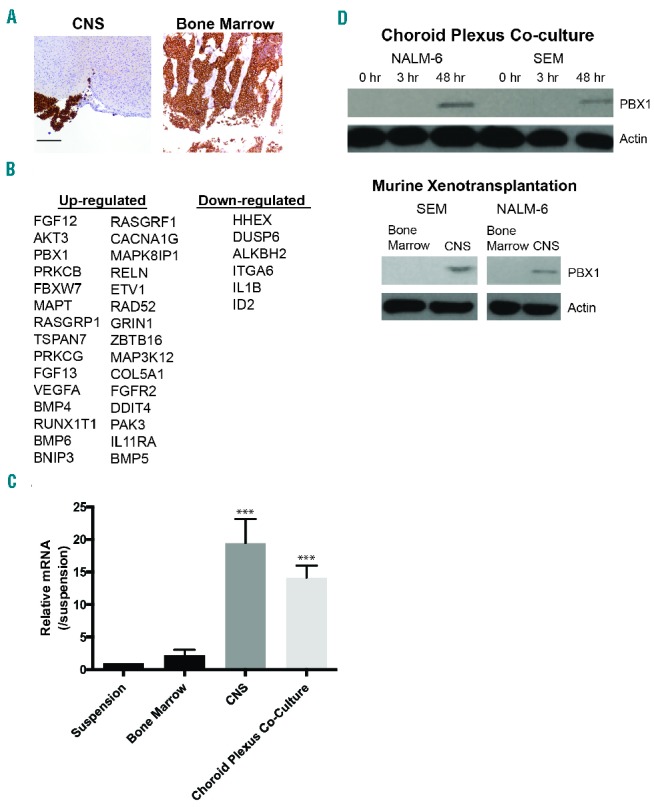
The central nervous system (CNS) and bone marrow (BM) microenvironments uniquely influence leukemia gene expression patterns. (A) Mouse brain and BM demonstrating NALM-6 leukemia involvement by human CD10 IHC staining approximately four weeks after injection with leukemia cells (10X magnification, 200 micron scale bar). Leukemia cells appear brown. (B) NALM-6 genes differentially regulated (fold change ≥2 and FDR<0.05) in the CNS niche relative to the BM identified with the Nanostring PanCancer Pathway Panel. (C) Expression of PBX1 mRNA in leukemia cells isolated from the mouse BM, CNS, or co-cultured with choroid plexus cells relative to cells in suspension determined by quantitative RT-PCR. PBX1 mRNA levels were normalized to GAPDH expression. ***P<0.0005 when leukemia cells isolated from the CNS or Z310 co-culture are compared to the other conditions. (D) Western blot showing PBX1 protein levels in NALM-6 and SEM leukemia cells grown in suspension, adherent to choroid plexus cells, or isolated from mouse CNS or BM.
We next confirmed PBX1 mRNA upregulation in leukemia cells either isolated from the CNS or in leukemia cells co-cultured with CNS-derived, murine choroid plexus cells by quantitative PCR (Figure 1C).12 We selected choroid plexus cells for co-culture experiments because both postmortem CNS examinations of ALL patients and murine leukemia xenotransplantation studies suggest that leukemia cells initially transit and localize at the blood-CSF barrier that is comprised of the choroid plexus epithelium.5,13 Supporting the generalizability of these results, additional human B-cell leukemia lines SEM and REH also exhibited PBX1 mRNA upregulation in leukemia cells isolated from the murine CNS niche (Online Supplementary Figure S1A). Western blots of NALM-6 and SEM leukemia cells isolated from the mouse CNS as well as from leukemia cells co-cultured ex vivo with choroid plexus cells also showed PBX1 upregulation at the protein level (Figure 1D). Furthermore, the co-culture experiments showed a time-dependent increase in PBX1 protein levels (Figure 1D) which, in combination with flow cytometry data demonstrating the purity of the leukemia cells following CD19 microbead isolation (Online Supplementary Figure S1B), indicate the increase in PBX1 levels is not due to contamination by non-leukemia cells. In agreement with the in vivo results, co-culture of leukemia cells with HS-5 BM stromal cells did not increase PBX1 protein expression in leukemia cells (Online Supplementary Figure S1C). Finally, culture of leukemia cells in either choroid plexus cell-conditioned media or human cerebral spinal fluid (CSF) had no discernible effects on PBX1 protein levels (Online Supplementary Figure S1D and E). These data suggest that direct cell contact with choroid plexus cells, rather than a soluble factor(s), causes PBX1 upregulation in leukemia cells.
Following confirmation of PBX1 upregulation in leukemia cells within the CNS microenvironment, we next ectopically expressed PBX1, or GFP as a control, in leukemia cells to identify functional consequences of PBX1 expression in leukemia cells. Supporting the likelihood of this, as previously described, PBX1 has known roles in hematopoietic stem cell biology,7 leukemia,8,9 and, in the context of ovarian cancer, both stem cell biology and chemo-resistance.14 Leukemia cells expressing PBX1 exhibited decreased sensitivity to cytarabine, a chemotherapeutic commonly used in the treatment of CNS leukemia,1 relative to control cells as measured by both proliferation and apoptosis assays (Figure 2A and B). Furthermore, shRNA targeting PBX1, but not control shRNA, prevented PBX1 upregulation in leukemia cells when co-cultured with choroid plexus cells and significantly attenuated the ability of choroid plexus cells to protect leukemia cells from chemotherapy-induced apoptosis (Figure 2C and Online Supplementary Figure S1F). The relatively modest effect of PBX1 knockdown on apoptosis resistance suggests that additional PBX1-independent mechanisms also significantly contribute to choroid plexus mediated leukemia chemo-resistance. Moreover, although PBX1 had no effect on leukemia proliferation, it caused enhanced colony-forming ability in semi-solid media, consistent with increased leukemia self-renewal properties (Figure 2D and E and Online Supplementary Figure S1G).15 Finally, mice xenotransplanted with NALM-6 leukemia cells expressing PBX1 exhibited modestly increased CNS disease compared to mice xenotransplanted with NALM-6 or NALM6-GFP cells (Figure 2F). Together these results provide a strong foundation for future in vivo experiments to further define how PBX1 upregulation in leukemia cells may contribute to both leukemia self-renewal properties and chemo-resistance in the CNS niche.
Figure 2.
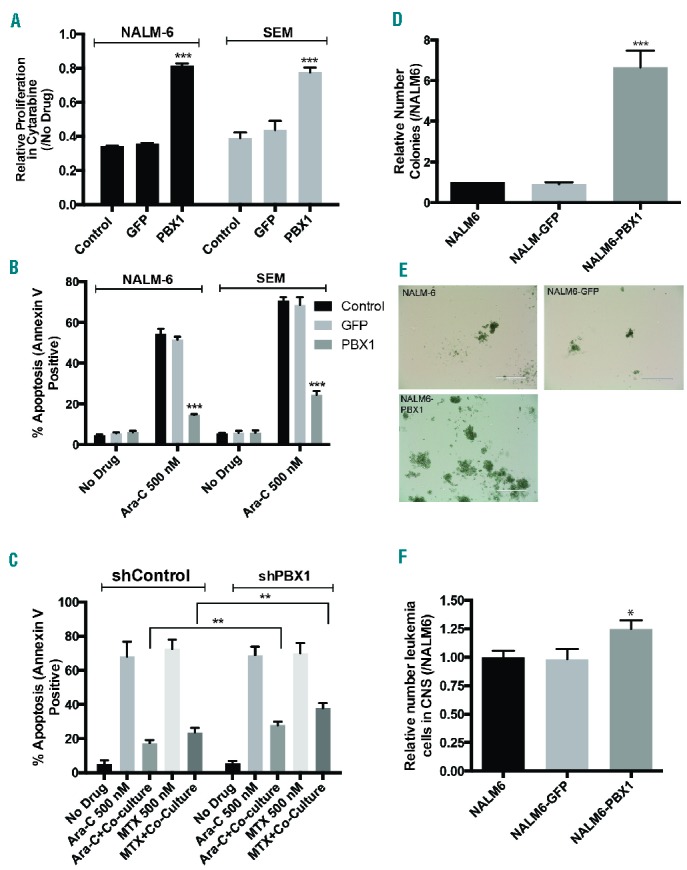
Expression of PBX1 in leukemia cells enhances chemo-resistance and colony formation. (A) Proliferation of NALM-6 and SEM leukemia cells transduced with GFP, PBX1, or un-transduced control cells was measured in the absence or presence of cytarabine 500 nM using the CellTitre-Glo Assay (Promega). ***P<0.0005 when comparing cells expressing PBX1 with either GFP or control cells. (B) Apoptosis of NALM-6 and SEM leukemia cells transduced with GFP, PBX1, or un-transduced control cells was measured in the absence or presence of cytarabine 500 nM using annexin-V staining. ***P<0.0005 when comparing cells expressing PBX1 with either GFP or control cells. (C) SEM cells expressing shControl or shPBX1 were cultured in suspension or adherent to choroid plexus cells and the effect of either methotrexate 500 nM or cytarabine 500 nM on apoptosis was measured by annexin-V staining and flow cytometry. **P<0.005. (D) Colony formation of NALM-6, NALM6-GFP, and NALM6-PBX1 leukemia cells ten days after plating in methylcellulose. Values are expressed as the number of colonies relative to the control NALM-6 cells. ***P<0.0005 when comparing NALM6-PBX1 with either NALM6-GFP or NALM-6. (E) Representative images (10X) of colonies ten days after plating in methylcellulose. (F) The number of leukemia cells in the mouse CNS were quantified by flow cytometry 21 days after injecting mice with NALM-6, NALM6-GFP, or NALM6-PBX1 leukemia cells (n=4 mice for each cell line). Data were normalized to the NALM-6 cells. *P<0.05 when comparing the NALM6-PBX1 cells with either NALM6-GFP or NALM-6.
In summary, we believe this work illustrates the unique and functionally important effect that the CNS microenvironment has on leukemia cells. More comprehensive analyses of the leukemia transcriptome, genome, and proteome in the CNS niche will build upon this foundation and provide a more detailed understanding of the role of the CNS niche in leukemia as well as new insights into CNS relapse and leukemia biology. Finally, this approach may enhance both the durability and quality of the cure for ALL patients by identifying targetable CNS niche-mediated mechanisms of leukemia chemo-resistance and self-renewal, or leukemia cell vulnerabilities unique to the CNS niche.
Supplementary Material
Acknowledgments
The authors would like to thank the University of Minnesota Pathology, Genomics, and Flow Cytometry Cores for assistance with immunohistochemistry, NanoString, and FACS-sorting, respectively. We thank Dr. Michael Cleary (Stanford University) for the pMYs-PBX1B-IRES-GFP vector and Dr. Wei Zheng (Purdue University) for the Z310 cell line. PMG is supported by NIH Grant 5K08CA154782-02. This work was funded in part by a Hyundai Hope on Wheels New Investigator Award and the Timothy O’Connell Foundation.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pui C-H, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LB. Balancing cure and long-term risks in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. Hematology Am Soc Hematol Educ Program. 2014;2014(1):190–197. [DOI] [PubMed] [Google Scholar]
- 3.Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126(22):2443–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans AE, Gilbert ES, Zandstra R. The increasing incidence of central nervous system leukemia in children. (Children’s Cancer Study Group A). Cancer. 1970;26(2):404–409. [DOI] [PubMed] [Google Scholar]
- 5.Williams MTS, Yousafzai YM, Elder A, et al. The ability to cross the blood-cerebrospinal fluid barrier is a generic property of acute lymphoblastic leukaemia blasts. Blood. 2016;127(16):1998–2006. [DOI] [PubMed] [Google Scholar]
- 6.Akers SM, Rellick SL, Fortney JE, Gibson LF. Cellular elements of the subarachnoid space promote ALL survival during chemotherapy. Leuk Res. 2011;35(6):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ficara F, Crisafulli L, Lin C, et al. Pbx1 restrains myeloid maturation while preserving lymphoid potential in hematopoietic progenitors. J Cell Sci. 2013;126(Pt 14):3181–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimabe M, Goyama S, Watanabe-Okochi N, et al. Pbx1 is a downstream target of Evi-1 in hematopoietic stem/progenitors and leukemic cells. Oncogene. 2009;28(49):4364–4374. [DOI] [PubMed] [Google Scholar]
- 9.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause S, Pfeiffer C, Strube S, et al. Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central ner vous system (CNS). Blood. 2015;125(5):820–830. [DOI] [PubMed] [Google Scholar]
- 11.Aspland SE, Bendall HH, Murre C. The role of E2A-PBX1 in leukemogenesis. Oncogene. 2001;20(40):5708–5717. [DOI] [PubMed] [Google Scholar]
- 12.Monnot AD, Zheng W. Culture of choroid plexus epithelial cells and in vitro model of blood-CSF barrier. Methods Mol Biol. Totowa, NJ: Humana Press; 2013;945(Chapter 2):13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price RA, Johnson WW. The central nervous system in childhood leukemia. I. The arachnoid. Cancer. 1973;31(3):520–533. [DOI] [PubMed] [Google Scholar]
- 14.Jung J-G, Kim T-H, Gerry E, et al. Abstract A32: PBX1, a transcriptional regulator, promotes stemness and chemoresistance in ovarian cancer. Clin Cancer Res. 2016;22(2 Supplement):A32–2. [Google Scholar]
- 15.Churchman ML, Low J, Qu C, et al. Efficacy of Retinoids in IKZF1-Mutated BCR-ABL1 Acute Lymphoblastic Leukemia. Cancer Cell. 2015;28(3):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


