Chronic lymphocytic leukemia (CLL) is the most common leukemia in adulthood with an estimated incidence of 5 cases/100,000/year and a median overall survival (OS) of almost 21 years.1 CLL is a very heterogeneous disease characterized by patients with slowly increasing lymphocytes to patients with rapidly progressive and sometimes life-threatening disease.2 CLL is also associated with several immunological abnormalities3 that may predispose to autoimmune4 as well as to infectious5 diseases. Neurological complications are uncommon, but may involve both central and peripheral nervous system.6,7 The pathogenesis of these complications varies from the spread of CLL, opportunistic infections, immune-mediated acute or chronic polyradiculoneuropathies, iatrogenic neuropathies, or may simply be coincidental.
While central nervous system (CNS) complications of CLL have recently been investigated,8 peripheral nervous system illnesses are generally overlooked or considered secondary to chemotherapies or infections.
The aim of our longitudinal cohort study was to assess the prevalence and characteristics of peripheral neuropathy (PN) in a wide population of CLL patients, and their correlation with CLL-specific clinical and biological prognostic markers [cytogenetic analyses, TP53 aberrations (17p deletion and/or TP53 mutation), immunoglobulin heavy chain variable region (IGHV) mutational status, CD38 and ZAP70 expressions].4–5
Time to peripheral neuropathies (TTPN) was calculated from the date of CLL diagnosis to PN occurrence (event) or last available follow up (censored). Detailed information on prognostic marker evaluation and statistical methods are reported in the Online Supplementary Appendix. All the patients who reported symptoms suggestive of PN underwent neurological and neurophysiological evaluations. Sera from patients with PN were tested for the presence of antibodies to peripheral nerve antigens (gangliosides and sulfatides) as previously reported.9 The occurrence of varicella zoster virus (VZV) reactivation, which causes radiculopathies, as well as peripheral facial nerve palsy, a mononeuropathy, has also been recorded. The role of chemo-immunotherapies, infections reactivation or genetic associations will also be discussed.
The study was approved by the local ethic committee and was carried out according to the Declaration of Helsinki. Informed consent was obtained from all patients.
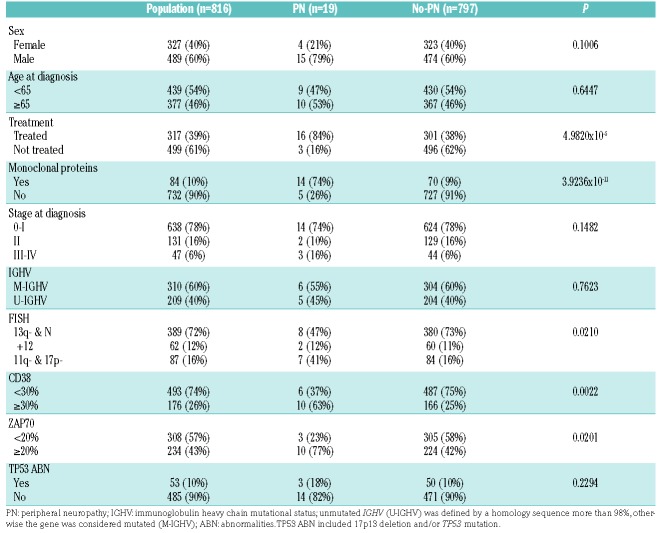
Eight hundred and sixteen patients affected with CLL and regularly followed at the Hematology and Clinical Immunology Unit of the University of Padova were recruited and their characteristics are summarized in Table 1.
Table 1.
Clinical and biological characteristics of the whole population, patients with and without peripheral neuropathies (PN and no-PN, respectively).
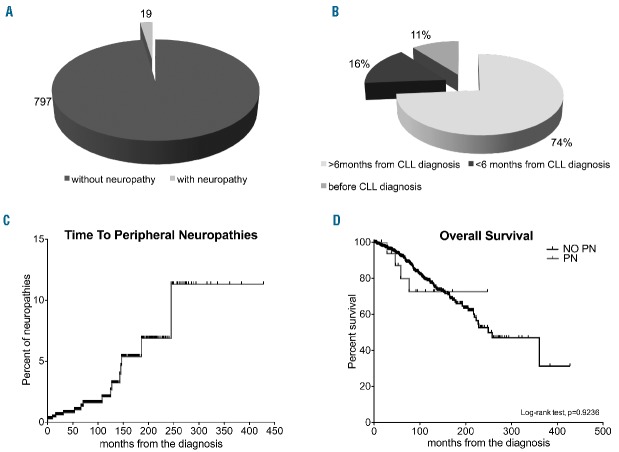
Nineteen (2.2%) (Figure 1A) out of 816 patients, mainly men (63%), suffered from PN during a median follow up of 99 months, as confirmed by extensive neurophysiological studies (Online Supplementary Appendix); 4 other subjects reported symptoms suggestive of PN, that were, however, not confirmed by neurological and neurophysiological evaluation. The median age at PN occurrence was 69±12 years and 4 patients (22%) had a Rai stage of 2 or over. Ninety percent of the PN cases were identified after CLL diagnosis and in 3 patients PN occurred within the first six months from CLL diagnosis (Figure 1B). Of the 19 patients with PN, the majority (10 of 19) had sensory axonal PN, 5 sensory-motor axonal PN, one had a multiple mononeuropathy, and 3 fulfilled the clinical and neurophysiological criteria of chronic inflammatory polyradiculoneuropathy (CIDP). For 3 patients (one affected by CIDP, one with sensory axonal PN and the patient with multiple mononeuropathy), the neuropathy represented the symptom at onset of CLL. Serum antibodies to peripheral nerve antigens were absent in all 19 subjects.
Figure 1.
Prevalence, onset, time to disease onset and overall survival in study subjects. (A) Prevalence of neuropathy among the whole population; 19 subjects suffered from peripheral neuropathy (PN). (B) Distribution of onset of PN according to the diagnosis of chronic lymphocytic leukemia (CLL): 2 of 19 patients (11%) developed PN before CLL, 3 (16%) and 13 (74%) subjects before and after six months from CLL diagnosis, respectively. (C) Kaplan-Meier curve for time to PN. In this analysis, we did not include the 3 patients who developed PN before CLL diagnosis. (D) Kaplan-Meier curves for overall survival between patients with and without PN (P=0.9236).
When investigating comorbidities that might have caused or contributed to the PN [i.e. diabetes mellitus, hepatitis C virus (HCV) infection], we observed that predisposing conditions were present in 5 patients (3 diabetes mellitus and 2 HCV). In 2 patients, the sensory axonal neuropathy was present before CLL diagnosis and likely secondary to type 2 diabetes mellitus. In 3 patients, an iatrogenic cause of neuropathy has been identified, namely lenalidomide for 2 patients and ibrutinib10 for the third patient. In all the 3 cases, neuropathic symptoms occurred after the beginning of chemotherapy. After excluding diabetic and iatrogenic PN, in 12 of 816 patients with PN (1.5% of all the cohort) other identifiable causes of PN (e.g. infections, vitamin deficiency) had been ruled out. Population-based data for PN are lacking, but the overall prevalence of chronic PN in the general population is thought to be around 1%. Interestingly, the presence of CIDP in our sample is high (0.37%) compared to the general population,11 as already described in a large European study on lymphoma-associated paraneoplastic neuropathies.12
Since the risk of developing PN is a time-dependent variable, we performed Kaplan-Meier analysis (Figure 1C). The risk of developing PN increases over time, with an estimated TTPN of 2.1% after ten years from the diagnosis and 6.9% after 20 years of follow up.
We also investigated the association with clinical and biological prognostic markers in patients with and without PN. Interestingly, CLL subjects who developed PN harbored high cytogenetic risk by FISH (i.e. 11q and 17p deletion; P=0.0210) and at diagnosis CD38 (P=0.0055) and ZAP70 (P=0.0360), which are known to be negative prognostic markers, were more often expressed. A higher percentage of monoclonal proteins (P=3.9236×10−11) in patients with PN (Table 1) was also found. The median level of M proteins among patients with PN was 2.44g/L; 6 were IgM/κ, 4 IgG/κ, 2 IgG/λ, one IgM/λ, and one exclusively λ. The occurrence of monoclonal protein may be coincidental. However 10% of patients with idiopathic PN have a monoclonal serum protein prevalence,13 which is much higher than that in the general population. Conversely, the prevalence of PN in patients with monoclonal protein is close to 5% for IgG paraproteins, 15% for IgA, and 30%–50% for IgM, the latter being often associated with antibody reactivity to peripheral nerve antigens.14
It is interesting to note that PN was more common in previously treated patients (Table 1) (P=4.9820×10−5), although CLL chemo-immunotherapeutic agents (fludarabine, bendamustine, cyclophosphamide, clorambucil, rituximab, etc.) are not known to cause iatrogenic PN. It is likely that duration and severity of the disease may play a role.
Furthermore, it is important to point out that 2 of 12 subjects (17%) treated with lenalidomide developed PN after a median treatment of 28 months (range 6–32 months). This percentage is high when compared with the incidence of PN in lenalidomide-treated multiple myeloma patients.15,16
The Kaplan-Meier analysis showed that the estimated 10-year OS for patients with and without PN was 73% and 78% (Log-rank test, P=0.9236), respectively (Figure 1D). As a consequence, patients with PN do not have any greater risk of death than patients without PN.
Among our cohort of patients, 36 (4.4%) experienced VZV re-activation causing zoster radiculopathy. Interestingly, in one of these 36 cases, both sensory and motor fibers were involved, as documented by severe muscle weakness and electromyography. Involvement of motor fibers in VZV radiculopathy is a rare event, which might have been favored by the derangements in the immune regulation that characterize CLL patients. Furthermore, we also identified 4 cases (0.5%) of idiopathic facial nerve palsy.
Neurological manifestations in patients with CLL may be numerous and various (gait disorder, paresthesia, headache, etc.). Recently, a group from the Mayo Clinic extensively described the central nervous system (CNS) complications in CLL patients, showing that the most common etiologies were infections (1%), autoimmune/inflammatory diseases (0.7%), direct CNS involvement by CLL (0.4%), CNS Richter syndrome (0.3%), and other cancers (0.2%). The Authors concluded that in almost 80% of patients neurological symptoms are due to causes other than CLL.3
However, the exact relationship between neurological manifestations and CLL remains to be elucidated, and it may be difficult to establish whether they are exclusively neoplastic, paraneoplastic, inflammatory, iatrogenic or simply incidental.17 It is likely that different pathogenic mechanisms take part in the genesis of neurological diseases. Here we provide evidence that PN is not such a rare complication in patients with CLL. After ruling out PN due to concomitant diseases or iatrogenic, a percentage of CLL-associated PN still remains. Its incidence increases during follow up and occurs more commonly in subjects with unfavorable biological prognostic makers, especially in those who present a monoclonal protein. The high percentage of CIDP in our cohort (25% of the patients with neuropathy) deserves consideration and is consistent with the frequent occurrence of demyelinating polyradiculoneuritis in patients with non-Hodgkin lymphoma.12
Supplementary Material
Acknowledgments
The authors would like to thank Susanna Ruggero and Elisabetta Toffanin for antibody testing and Monica Facco for immunophenotypic and molecular analysis of CLL cells.
Footnotes
Funding: this work was supported by funds from A.I.R.C. to GS (IG-15286) and LT (IG-15397), Ministero dell’Istruzione dell’Università e della Ricerca (PRIN 2008, 2010–2011 from LT, FIRB 2010 from GS), AIRC Regional Project with Fondazione CARIPARO and CARIVERONA, and Regione Veneto on Chronic Lymphocytic Leukemia.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visentin A, Facco M, Frezzato F, et al. Integrated CLL Scoring System, a New and Simple Index to Predict Time to Treatment and Overall Survival in Patients With Chronic Lymphocytic Leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(10):612–620 e615. [DOI] [PubMed] [Google Scholar]
- 3.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. [DOI] [PubMed] [Google Scholar]
- 4.Zent CS, Kay NE. Autoimmune complications in chronic lymphocytic leukaemia (CLL). Best Pract Res Clin Haematol. 2010;23(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visentin A, Compagno N, Cinetto F, et al. Clinical profile associated with infections in patients with chronic lymphocytic leukemia. Protective role of immunoglobulin replacement therapy. Haematologica. 2015;100(12):e515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JH, Hammack JE, McDonnell SK, Tefferi A. The neurologic complications of B-cell chronic lymphocytic leukemia. Neurology. 1997;48(2):407–412. [DOI] [PubMed] [Google Scholar]
- 7.Lopes da Silva R. Spectrum of neurologic complications in chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2012; 12(3):164–179. [DOI] [PubMed] [Google Scholar]
- 8.Strati P, Uhm JH, Kaufmann TJ, et al. Prevalence and characteristics of central nervous system involvement by chronic lymphocytic leukemia. Haematologica. 2016;101(4):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagnolo M, Ferrari S, Dalla Torre C, et al. Polyneuropathy with anti-sulfatide and anti-MAG antibodies: clinical, neurophysiological, pathological features and response to treatment. J Neuroimmunol. 2015;281:1–4. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014; 371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanewinckel R, Ikram MA, Van Doorn PA. Peripheral neuropathies. Handb Clin Neurol. 2016;138:263–282. [DOI] [PubMed] [Google Scholar]
- 12.Briani C, Vitaliani R, Grisold W, et al. Spectrum of paraneoplastic disease associated with lymphoma. Neurology. 2011;76(8):705–710. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JJ, Jr, Kyle RA, O’Brien PC, Dyck PJ. Prevalence of monoclonal protein in peripheral neuropathy. Neurology. 1981;31(11):1480–1483. [DOI] [PubMed] [Google Scholar]
- 14.Raheja D, Specht C, Simmons Z. Paraproteinemic neuropathies. Muscle Nerve. 2015;51(1):1–13. [DOI] [PubMed] [Google Scholar]
- 15.Briani C, Berno T, Campagnolo M, Zambello R. Lenalidomide for bortezomib-resistant multiple myeloma. Nat Rev Clin Oncol. 2010; 7(9). [DOI] [PubMed] [Google Scholar]
- 16.Briani C, Torre CD, Campagnolo M, et al. Lenalidomide in patients with chemotherapy-induced polyneuropathy and relapsed or refractory multiple myeloma: results from a single-centre prospective study. J Peripher Nerv Syst. 2013;18(1):19–24. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan BC, Price RS, Chen KS, Feldman EL. The importance of rare subtypes in diagnosis and treatment of peripheral neuropathy: a review. JAMA Neurol. 2015;72(12):1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.