Approximately 20% of all non-Hodgkin lymphoma (NHL) and 60% of indolent NHL (iNHL) cases are follicular lymphoma (FL),1,2 a disease considered treatable, but not curable, with currently available therapeutic options.3 While survival in patients with FL has improved with the availability of more effective treatment options over the past 20 years,4,5 a need remains for treatments that can be used repeatedly or continuously and, ideally, with minimal risk for cumulative toxicities. Idelalisib, a first-in-class, orally bioavailable, selective phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitor, is approved for patients with relapsed/refractory FL who have received ≥2 prior systemic therapies.6,7
In a phase 2, multicenter, single-arm, open-label study of 125 patients with relapsed or refractory iNHL (clinicaltrials.gov Identifier:01282424), idelalisib monotherapy demonstrated antitumor activity and acceptable tolerability across 4 histologic subtypes, including 72 patients (58%) with FL.8 To better characterize the efficacy and safety of idelalisib treatment for patients with refractory FL, we performed a post hoc subgroup analysis of patients with FL (grade 1, 2, or 3a) enrolled in this study. Details of study design, study assessments and endpoints, and statistical methods have been published previously.8 Patients must have received ≥2 prior chemotherapy- or immunotherapy-based regimens for FL, with refractoriness to both rituximab as well as an alkylating agent. Main exclusion criteria were histologic transformation, central nervous system lymphoma, a history of hepatic dysfunction, and active systemic infection. Patients received idelalisib 150 mg twice daily until progressive disease (PD) or unacceptable toxicity (Online Supplementary Methods).
Demographics and baseline characteristics for the FL patients are summarized in Table 1. The overall response rate (ORR) was 55.6% (n=40/72; 95% confidence interval [CI], 43.4–67.3; P<0.001 for testing against the null hypothesis) in patients with FL overall and did not differ when stratified by FL grade (Figure 1). Idelalisib was effective across evaluated patient categories, regardless of the number of prior therapies, refractoriness to previous regimens, bulky disease, and age (Figure 1). At data cutoff (June 11, 2014), complete response (CR) was achieved in 10 patients (13.9%), partial response (PR) in 30 patients (41.7%), and stable disease (SD) in 23 (31.9%) for an overall disease control rate of 87.5%. Eight patients (11.1%) had PD and 1 patient was not evaluable. Fifty-seven percent of patients achieved ≥50% reduction in the sum of the products of the diameters of the index lymph node (Online Supplementary Figure S1).
Table 1.
Baseline Characteristics and Patient Disposition.*
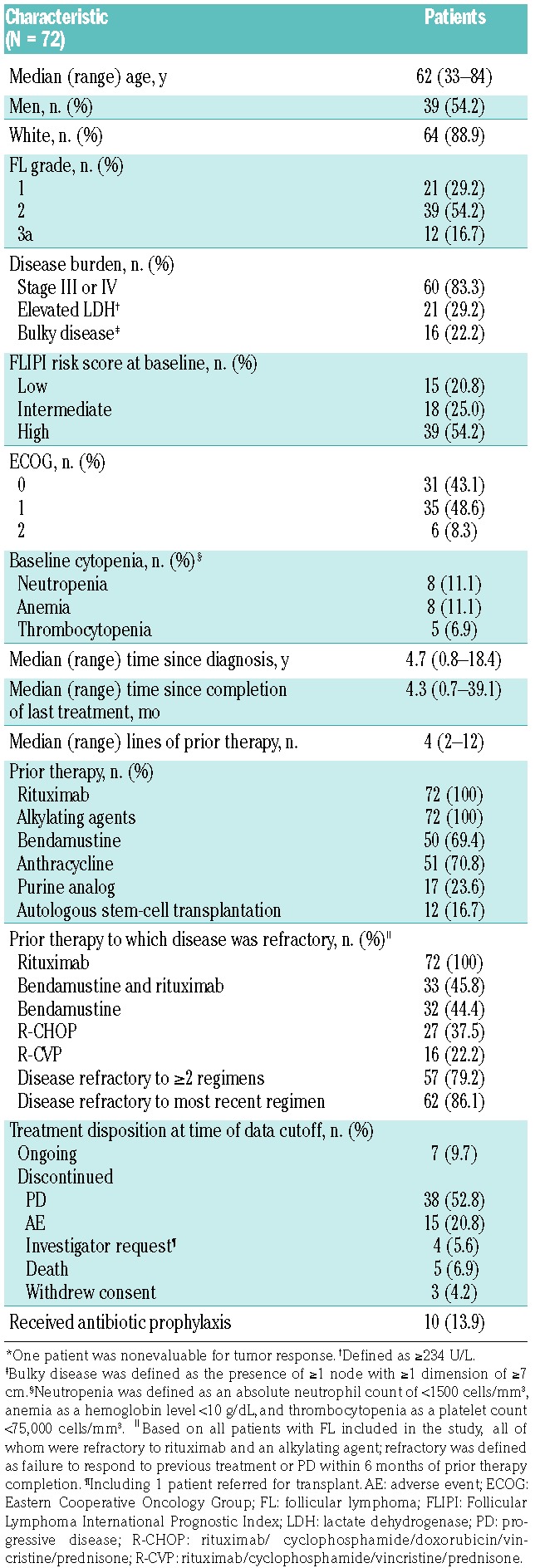
Figure 1.
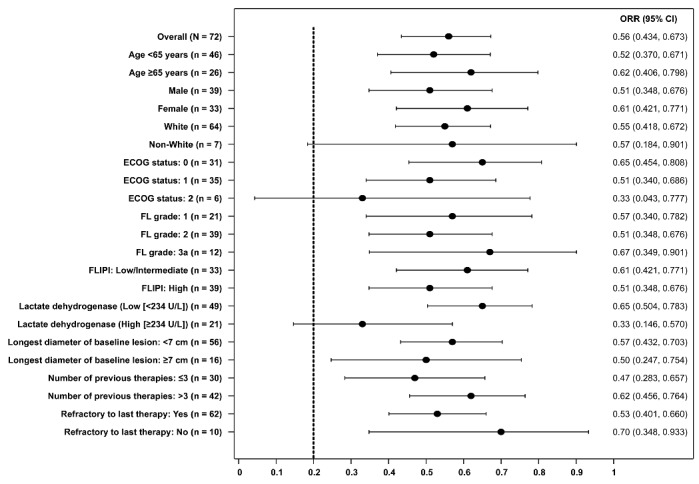
Forest plot of ORR. Response rate was assessed by an independent review committee. The dashed line denotes the null hypothesis response rate of 0.20. CI: confidence interval; ECOG: Eastern Cooperative Oncology group; FL: follicular lymphoma; FLIPI: Follicular Lymphoma International Prognostic Index; ORR: overall response rate.
Overall, median time to first response (TTR) was 2.6 months (range, 1.6–11.0), and the median duration of response was 10.8 months (range, 0–26.9). For patients with a CR as first response, TTR ranged from 1.8 to 8.4 months, whereas time to CR as best response ranged from 1.9 to 19.2 months (Online Supplementary Table S1).
Median PFS with idelalisib was 11.0 months (95% CI, 8.0–14.0) overall (Figure 2A); 43.0% of patients remained progression-free at 12 months. In contrast, median PFS for patients’ most recent regimens before study entry was 5.1 months (95% CI, 4.4–6.0, unadjusted analysis [Figure 2B]). Median PFS in patients with CR, PR, and SD were 30.6 (95% CI, 5.6–30.6), 11.0 (95% CI, 8.1–14.2), and 8.2 (95% CI, 3.7–13.1) months, respectively (Figure 2A). In patients with PD, median PFS was 1.6 months (95% CI, 0.8–1.9). In patients with grade 3a FL, median PFS was 11.0 months (95% CI, 3.6–30.6), with 48.5% of patients remaining progression-free at 12 months.
Figure 2.
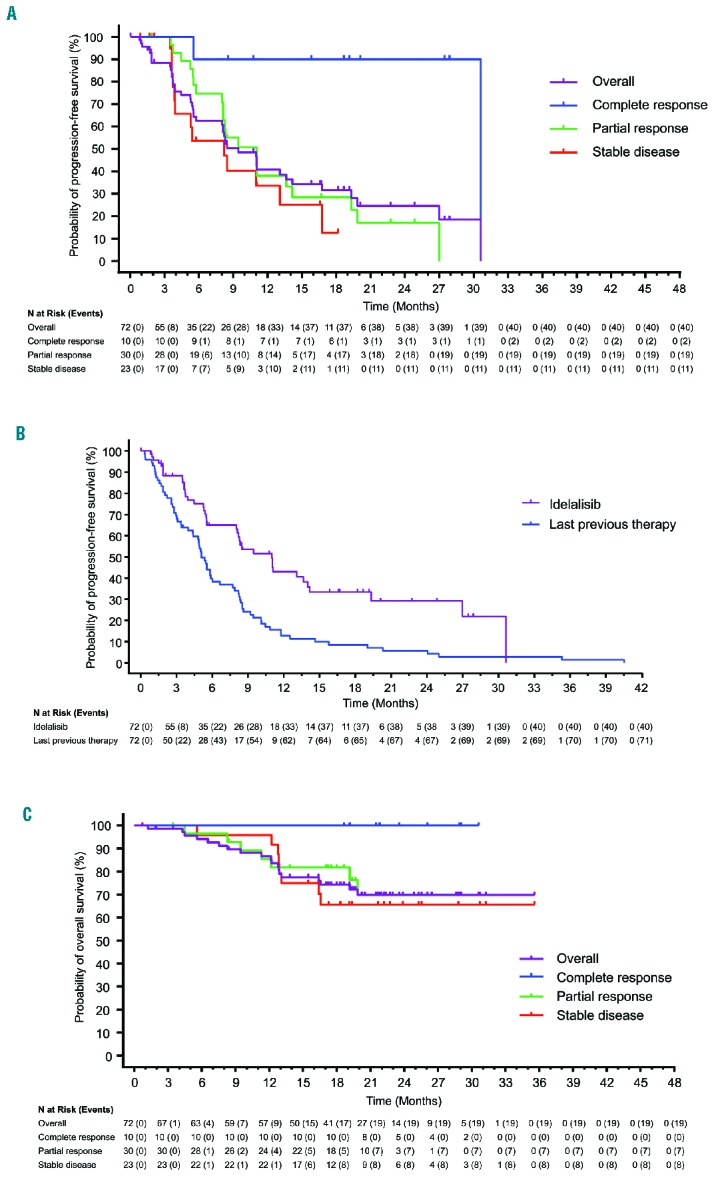
Progression-free and overall survival. (A) Kaplan Meier estimated progression-free survival of overall follicular lymphoma cohort and in patients with complete response, partial response, or stable disease. (B) Kaplan Meier estimated progression-free survival of overall follicular lymphoma cohort vs. progression-free survival with previous line of therapy before study inclusion (unadjusted analysis). (C) Kaplan Meier estimated overall survival of overall follicular lymphoma cohort and in patients with complete response, partial response, or stable disease.
At the time of data cutoff, median OS had not been reached. At 24 months, OS was estimated to be 69.8%, and all patients achieving a CR had survived (Figure 2C). The unadjusted OS at 24 months was 71.3% in patients with a PR, 64.1% in patients with SD, and 21.9% in patients with PD. In patients with grade 3a FL, OS at 24 months was 60.0%.
PD led to idelalisib discontinuation in 38 patients; 23 initiated another treatment within 1 to 131 days. Ten of these 38 patients died (median post-idelalisib OS not reached (NR); 95% CI [11.1 – NR]; range, 0.3–11.1 months).
Twenty-two patients discontinued idelalisib for a reason other than PD or death and 13 initiated their next treatment within 8 to 439 days. Four of these patients subsequently died (median post-idelalisib OS not reached; 95% CI [NR]; range, 1.7–13.7 months; time between idelalisib discontinuation and death, 26–417 days). For these 4 patients, reasons for discontinuation were investigator request (n=2), withdrawal of consent (n=1), and an adverse event (AE) of septic shock (n=1); causes of death were PD (n=2), acute respiratory distress syndrome and septic shock (n=1), and septic shock and pneumonia in the context of an antecedent medical history notable for methicillin-resistant Staphylococcus aureus (MRSA) and Streptococcus viridans bacteremia as well as MRSA necrotizing pneumonia (n=1).
The median (range) duration of idelalisib exposure was 6.5 (0.6–31.0) months. The most common treatment-emergent AEs (TEAEs) were diarrhea (51.4%), cough (31.9%), pyrexia (29.2%), fatigue (27.8%), and nausea (27.8%). Diarrhea, pneumonia, and pyrexia were the most frequently reported grade ≥3 TEAEs (13.9%, 6.9%, and 4.2%, respectively). The most common grade ≥3 laboratory abnormalities included neutropenia (22.2%) and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevations (13.9%). Serious AEs included pyrexia (12.5%), diarrhea (6.9%), pneumonia (5.6%), and colitis (4.2%). Nine patients experienced bleeding events of any grade, with 3 patients having grade ≥3 events; all bleeding events resolved without a change in dose (Online Supplementary Table S2). Thirty-seven patients experienced any grade neutropenia; 21 with grade <3 and 16 with grade ≥3. Of these, 9 (43%) and 12 (75%) also experienced infections.
Upper respiratory infections and pneumonia were observed in 11 (15.3%) and 8 (11.1%) patients, respectively (Online Supplementary Table S2). Most occurrences were grade 1 or 2; grade 3 pneumonia occurred in 5 patients (6.9%). One case of cytomegalovirus (CMV) pneumonia and no cases of Pneumocystis jirovecii pneumonia were reported.
Pneumonitis was reported in 3 patients (4.2%). In 1 patient (event onset at 61.1 weeks), the event resolved with steroid medication and dose interruption, and the patient was reexposed to idelalisib 100 mg, with no recurrence. In a second patient (event onset at 16.1 weeks), the event resolved after drug discontinuation. The third patient developed pneumonitis after 54 weeks of treatment and died from ongoing pneumonitis 23 days after drug discontinuation.
Thirty-eight patients (52.8%) experienced diarrhea (n=37) and/or colitis (n=4) of any grade; 35 (48.6%) had grade 1/2 diarrhea (n=33) and/or colitis (n=3), and 12 (16.7%) had grade ≥3 diarrhea (n=10) or colitis (n=2). Grade ≥3 diarrhea or colitis tended to occur later during treatment than grade 1/2 diarrhea or colitis (median time to onset of first event, 24.7 weeks [range, 3.7–77.3] vs. 15.7 weeks [range, 0.1–84.9], respectively), but gastrointestinal events resolved in 3 to 6 weeks regardless of severity. Of these 12 patients with grade ≥3 diarrhea or colitis, the study drug was interrupted and rechallenged in 5 patients; 4 did not experience a recurrence (Online Supplementary Table S3). Ten patients experienced grade ≥3 ALT/AST elevations, all of which were asymptomatic. The study drug was interrupted and rechallenged in 7 of these 10 patients; 5 did not have a recurrence.
At the data cutoff, 4 patients had died due to AEs considered unlikely to be related to idelalisib treatment (heart failure, septic shock, splenic infarct/acute abdomen, and death of unknown cause), and 2 from AEs considered possibly (acute cardiac arrest) or probably (drug-induced pneumonitis) related to idelalisib treatment.
Idelalisib treatment was interrupted in 32 patients (44.4%), primarily because of AEs (n=24). Twenty-two patients (30.6%; including 17 patients who had dose interruptions) underwent dose reduction (due to AEs [n=21]) from 150 mg BID to 100 mg BID (n=21) or 75 mg BID (n=7, including 6 previously dose-reduced to 100 mg BID). Eighteen patients reported AEs leading to discontinuation of idelalisib. Details are provided in the Online Supplementary Table S4.
The current analysis included more high-risk patients than other studies.9–12 In addition, this analysis reflects a considerably longer median follow-up period compared with other phase 2 studies in relapsed/refractory FL (19.4 vs. 4.4–6.5 months).12,13 The clinical benefits of idelalisib monotherapy were consistent regardless of patient characteristics such as age, FL grade, and number of prior therapies. Furthermore, the baseline characteristics of most patients in this subgroup analysis were consistent with those previously shown to define a high-risk FL population likely to have early progression of disease and associated poor outcomes.14
In these heavily pretreated patients with relapsed/refractory FL, idelalisib monotherapy demonstrated an acceptable and manageable safety profile consistent with that for the overall iNHL study population.8 Diarrhea, colitis, and transaminase elevations were generally manageable with dose interruption/reduction or drug discontinuation. A substantial proportion of patients with diarrhea and/or colitis or transaminase elevations were reexposed to idelalisib without recurrence of the event. The incidence of hematologic toxicities was lower than previously reported for bendamustine monotherapy.15 Rates of CMV and Pneumocystis jirovecii infection were low (1.3% and 0%, respectively), but appropriate monitoring and prophylaxis are still recommended based on recent safety data. However, given the unmet need in high-risk patients with relapsed/refractory FL, the overall benefit of idelalisib in such patients outweighs these safety concerns.
Supplementary Material
Acknowledgments
This study was supported by Gilead Sciences, Inc. Editorial assistance for the development of this manuscript was provided by Meryl Gersh, PhD, of AlphaBioCom, LLC, King of Prussia, PA, and funded by Gilead Sciences, Inc., Foster City, CA.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Glass AG, Karnell LH, Menck HR. The National Cancer Data Base report on non-Hodgkin’s lymphoma. Cancer. 1997;80(12):2311–2320. [PubMed] [Google Scholar]
- 2.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunning MA, Vose JM. Management of indolent lymphoma: where are we now and where are we going. Blood Rev. 2012;26(6):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23(22):5019–5026. [DOI] [PubMed] [Google Scholar]
- 5.Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122(6):981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ZYDELIG® (idelalisib) tablets, for oral use. Prescribing information. Gilead Sciences, Inc; Foster City, CA, USA: 2014. [Google Scholar]
- 7.ZYDELIG (idelalisib tablets). Summary of Product Characteristics, Gilead Sciences International Ltd, Cambridge, UK: 2015. [Google Scholar]
- 8.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014; 370(11):1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuczman MS, Fayad L, Delwail V, et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119(16):3698–3704. [DOI] [PubMed] [Google Scholar]
- 10.Tinmouth A, Zanke B, Imrie KR. Fludarabine in alkylator-resistant follicular non-Hodgkin’s lymphoma. Leuk Lymphoma. 2001;41(1–2):137–145. [DOI] [PubMed] [Google Scholar]
- 11.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108(10):3295–3301. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett NL, LaPlant BR, Qi J, et al. Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a phase 2 consortium (P2C) trial. Blood. 2014;124(21):800. [Google Scholar]
- 13.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol. 2009;27(32):5404–5409. [DOI] [PubMed] [Google Scholar]
- 14.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015; 33(23):2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010; 116(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


