Abstract
Background
Adenomyosis, defined as the invasion of endometrial glands and stroma into the myometrium, is a common gynecological disorder. In the present study we report on the effect of leonurine on ICR mice with adenomyosis induced by neonatal tamoxifen.
Material/Method
After being treated with tamoxifen for 4, 8, and 12 weeks, we assessed body weight and pain modulation in mice in hotplate tests. The mice were divided into 5 groups: a low-dose leonurine treatment group, a high-dose leonurine treatment group, a valproic acid (VPA) treatment group, a vehicle only treatment group, and a blank control group. We evaluated body weight, pain modulation in hotplate tests, and the depth of myometrial infiltration. Immunoreactivity staining of progesterone receptor (PR), nuclear factor-κB phosphorylated-p65 (p-p65), cyclooxygenase-2 (COX-2), and oxytocin receptor (OTR) was evaluated by immunohistochemistry.
Results
The measurement of the body weight, myometrial infiltration, and pain modulation showed that neonatal tamoxifen treatment led to adenomyosis. Leonurine treatment appeared to decrease hyperalgesia and myometrial infiltration. Immunoreactivity staining showed decreased p-p65, COX-2, and OTR protein expressions.
Conclusions
Our results indicate that leonurine attenuates hyperalgesia in mice with induced adenomyosis via down-regulating expressions of p-P65, COX-2, and OTR, and could be beneficial for treating adenomyosis.
MeSH Keywords: Adenomyosis, Hyperalgesia, Leonurus
Background
Adenomyosis is fairly prevalent in multiparous premenopausal women ages 35–50 years [1,2]. Hysterectomy is the definitive treatment for adenomyosis in severe cases. Conservative treatment is important for patients who desire to preserve fertility. The traditional medical therapy of adenomyosis is mainly based on hormonal dependency, including GnRH-a, oral contraceptives, testosterone derivatives, progestin [3], and nonsteroidal anti-inflammatory drugs. However, hormonal treatment has some disadvantages, including adverse effects, relapse after withdrawal, short course, and lesion survival after medication [4,5]. Since no currently available medical treatment is generally effective, further drug development studies are needed.
Patients with adenomyosis more frequently present dysmenorrhea as a chronic pelvic pain. Leonurine is an alkaloid typically found in the plant Leonuri herba, which has been used to treat dysmenorrhea for hundreds of years in China. Furthermore, the molecular bases of hyperalgesia in patients with adenomyosis are complicated and not well understood. PRs are synthesized in the endometrial stromal, which are responsible for proliferation and remodeling of the endometrium. Altered expression of PR-A and PR-B was reported in endometriosis patients with eutopic endometrium [6]. Another study suggested that glutamate decarboxylase 65 (GAD65)-mediated GABA synthesis play a partial role in hyperalgesia through regulation of histone deacetylase (HDAC) [7,8]. COX-2, known to be a target of nonsteroidal drugs, was reported to be involved in hyperalgesia induced by streptozotocin [9,10]. Previous evidence also indicated that OTR and transient receptor potential vanilloid type 1 (TRPV1) might be involved in dysmenorrhea [11].
In this study, we focused on the effect of leonurine on treating ICR mice with induced adenomyosis. We hypothesized that leonurine would reduce hyperalgesia and suppress the myometrial infiltration of ectopic endometrium in mice. Our previous study demonstrated that VPA, a histone deacetylase inhibitor, could resolve dysmenorrhea and consequently reduce the uterus size [12], and was used as a positive indicator in this experiment. To illuminate the molecular basis, the protein expressions of PR, p-p65, COX-2, and OTR in mice were assessed.
Material and Methods
Chemicals
Leonurine and VPA (valproic acid sodium salt, P4543) were purchased from Sigma-Aldrich (St. Louis, MO). All the drugs were dissolved in 2% DMSO in 0.1 mol·L-1 Na2SO4 (pH 5.0). Tamoxifen citrate was purchased from Fudan Forward Pharmaceutical Company (Shanghai, China).
Animals and treatments
We purchased 35 newborn (day 1 after birth) female ICR mice and the mother mice from Shanghai Laboratory Animal Corporation (Shanghai, China). Each mouse was housed in its own cage under controlled conditions (20°C, 12: 12 light/dark cycle with lights on at 6: 00 am, and free access to food and fresh water). All experiments were performed under the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the Institutional Experimental Animal Review Board of Shanghai Obstetrics and Gynecology Hospital, Shanghai College of Medicine, Fudan University.
All mice were randomly divided into 5 groups. Following the methods of Parrott [13], 4 groups of female neonatal mice were orally dosed with 1 mg/kg tamoxifen suspended in a peanut oil/lecithin/condensed milk mixture (2: 0.2: 3, by volume) at a dose volume of 5 μl/g body weight from day 2, and the untreated group received no treatment as a blank control. At 4 weeks of age, all the female neonatal mice were weaned and separated from their mothers. The body weight, hotplate, and tail-flick tests were evaluated every 4 weeks. At 16 weeks of age, 4 groups of mice received daily treatment for 3 weeks: Group 1 received a low-dose (30 mg/kg body weight) leonurine treatment; Group 2 received a high-dose (60 mg/kg body weight) leonurine treatment; Group 3 received VPA treatment (10 mg/kg body weight); and Group 4 received the vehicle (2% DMSO in 0.1 mol·L-1 Na2SO4 adjusted to pH5.0) only and served as a control. Body weights were assessed and hotplate tests were performed before and after drug treatment.
Hotplate test
The hotplate test is a standard method for measuring nociception in rodents. It is a well-known test that is suitable for use in studying reiteration leading to a modification of response latencies. The test was performed using a Hot Plate Analgesia Meter (BME-480, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Tianjin, China) consisting of a 25×25 cm metal plate. The temperature set at 55.0±0.1°C. A plastic cylinder (18 cm high ×20 cm diameter) was placed on the hotplate. The time interval (in seconds) between placement and the licking of its hind paws was measured as the latency to respond to thermal stimulus. Each mouse was tested once per session.
Histological examination of myometrial infiltration
The depth of myometrial infiltration of ectopic endometrium was determined following the criteria of Bird [12]. For histological examination, serial 4-mm sections were obtained from each paraffin-embedded tissue block, with 3 randomly selected sections were chosen for HE staining. If endometrial glands and stroma were found in myometrium, the diagnosis of adenomyosis was made.
Immunohistochemistry analysis
Routine deparaffinization and rehydration procedures were performed. The primary antibody against target antigens were: rabbit polyclonal antibody against PR (SC-539; Santa Cruz, CA; 1: 50), p-p65 (3037; Cell Signaling Technology, Boston, MA; 1: 50), rabbit monoclonal antibody against COX-2 (4212-1; Epitomics, San Francisco, CA; 1: 100) and OTR (251676; Abbiotec, CA; 1: 100). The slides were heated at 98°C in an EDTA buffer (pH 9.0) for 30 min and cooled at room temperature. Sections were incubated overnight with the respective antibody at 4°C. The slides were subsequently incubated with the biotinylated secondary antibody, Supervision TM Universal (anti-rabbit) Detection Reagent (HRP) (GK500705, Shanghai Gene Tech Company, Shanghai, China), at room temperature for 30 min. Finally, the tissue sections were counterstained with hematoxylin and mounted.
A series of 5 random fields of 1 section were examined under a microscope (Olympus BX51, Olympus, Tokyo, Japan) equipped with a digital camera (Olympus DP70, Olympus, Tokyo, Japan). Immunohistochemical parameters evaluated in the area were: (i) integrated optical density (IOD), (ii) total stained area (S), and (iii) mean optical density (MOD) defined as MOD=IOD/S, equivalent to the intensity of staining in all positive cells. Immunoreactivity staining was quantitatively characterized using Image Pro-Plus 6.0 (Media Cybernetics, Inc., USA).
Statistical analysis
The results are expressed as means ± standard deviation (SD) from at least 3 independent experiments. The paired sample t test was used to analyze before-after comparison using SPSS 13.0 statistical software. The Kruskal-Wallis test was performed to investigate multiple comparisons of ranked data. Measurement data were compared with LSD method of analysis of variance among multiple groups. Pearson’s correlation coefficient was used to determine the correlation between myometrial infiltration depth and treatment. Significance was set as p<0.05.
Results
Induction of adenomyosis in mice and pathological findings
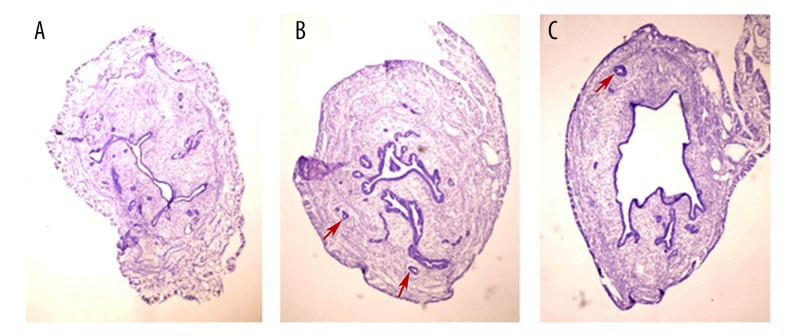
No mice died during this experiment. The H-E staining indicated that adenomyosis occurred in all tamoxifen-treated female neonatal mice. As shown in Figure 1, a well-demarcated endometrium could be clearly seen in sample A, which was surrounded by regular layers of smooth muscles and connective tissue. Invasions of endometrial glands into the myometrium were discovered in samples B and C.
Figure 1.
Light microscopy of uterus of control group and tamoxifen-treated group. (A) Endometrium from an untreated mouse showed endometrial lumen lined by columnar cells with well-defined layers of endometrial stroma and smooth muscle. (B) and (C) Untreated mice showed adenomyosis with endometrial glands penetrating deeply into myometrium (red arrows). H-E: original magnification: ×200.
Effect on body weight following drug treatment
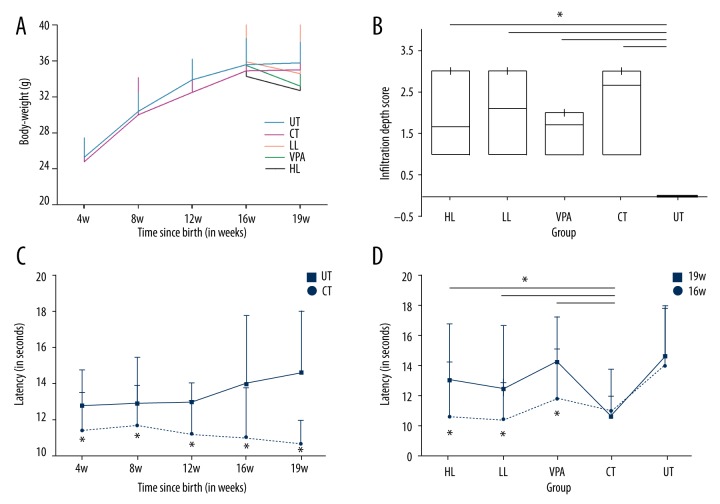
The body weight of all the mice at 4, 8, 12, 16, and 19 weeks were evaluated. Neonatal dosing with tamoxifen resulted in a consist weight loss in mice compared to untreated mice. Dosing for 3 weeks with high- and low-dose leonurine and VPA caused weight loss compared to the control group at week 19, but the difference was not significant. High- and low-dose leonurine and VPA treatment caused weight loss compared to the untreated group, but the difference was not significant (Figure 2A).
Figure 2.
(A) The average body weight of groups. (B) Assessment of the depth of myometrial infiltration in groups. (C) Hotplate latency in untreated group (control) and tamoxifen-treated group. (D) Hotplate latency of multiple groups. UT – untreated; CT – control; LL – low-dose leonurine; HL – high-dose leonurine.
Infiltration of myometrium followed by drug treatment
The effect of drug treatment on the myometrial infiltration was assessed by the infiltration depth scores. The boxplot in Figure 2B shows that dosing with neonatal tamoxifen resulted in significantly higher infiltration depth score than in untreated mice (p<0.05). High- and low-dose leonurine and VPA treatment reduced the infiltration of myometrium induced by tamoxifen, but the difference was not significant (Figure 2B). There was a negative correlation between dosing with leonurine and myometrial infiltration (r=−0.735, p<0.05).
Hotplate response latency
To identify the thermal pain modulation in mice, the hotplate test was conducted by evaluating the response to thermal stimuli (Figure 2C, 2D). Hotplate response latency of the mice dosed with tamoxifen significantly decreased in a general and long-term way (p<0.05). The latency of pro-treat mice at 19 w appeared to decrease significantly (p<0.05), compared to pre-treat mice at 16 w. Treatment with high- and low-dose leonurine and VPA significantly (p<0.05) prolonged the response latency compared to the control group.
Expressions of PR, p-p65, COX-2, and OTR proteins affected by drug treatment
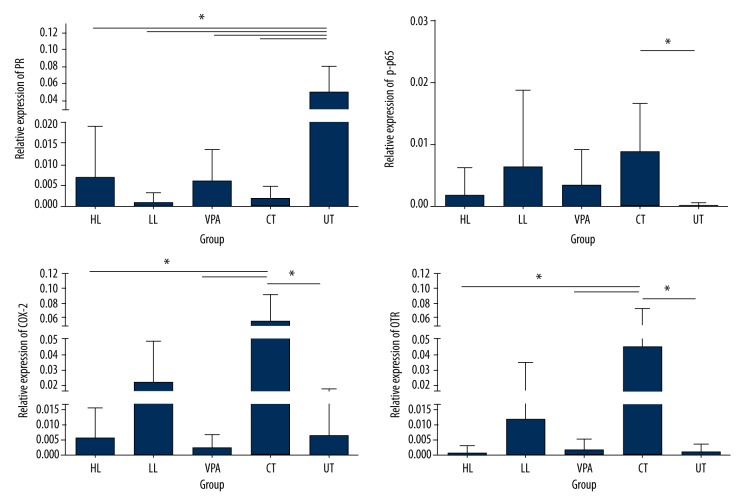
Tissue-specific regulation of PR, p-p65, COX-2, and OTR proteins in the uterus was determined by immunohistochemistry as described (Figure 3). A significant decline was shown in PR protein levels in HL, LL, VPA, and CT groups, compared to the UT group (p<0.05). There was no difference between the drug-treated group and the CT group (p>0.05). An obvious elevation of p-P65, COX-2, and OTR protein levels was shown in the CT group compared with the UT group (p< 0.05). Treatment with high- and low-dose leonurine and VPA resulted in decreased p-P65 expression compared to the CT group, but the difference was not significant. High-dose leonurine and VPA treatment significantly decreased the expression of COX-2 compared to the UT group (p<0.05). Expression of OTR was significantly up-regulated in the CT group compared to the UT group (p<0.05). Treatment with high-dose leonurine and VPA significantly down-regulated the OTR expression compared to the CT group (p<0.05).
Figure 3.
Expression levels of PR, p-p65, COX-2, and OTR proteins as affected by drug treatment. IHC was performed for the protein level assays.
Discussion
Adenomyosis is a common gynecological disorder in patients with postmenopausal breast cancer who were treated with the selective estrogen receptor modulator (SERM) tamoxifen [14]. In the present study, hyperalgesia was induced by dosing with tamoxifen in neonatal ICR mice sensitive to xenobiotic estrogens. Pathological examination of uterine tissues showed myometrial infiltration. Continuous weight loss in tamoxifen-induced mice was observed, but the change was not significantly different compared to untreated mice. We also found thermal hyperalgesia in tamoxifen-induced mice, as shown by a reduction of hotplate response latency. Only 50% of patients with adenomyosis who receive hormonal treatments or surgery have pain relief [15]. Therefore, further studies on novel and effective therapies are needed.
Previously, we reported that VPA, a histone deacetylase inhibitor, suppressed proliferation of adenomyosis stromal cells [16]. Leonurine is a well-known Chinese traditional medicine for treating dysmenorrhea. Here, we showed the effect of leonurine in treating mice with adenomyosis. We demonstrated that drug treatment with leonurine and VPA caused a weight loss in mice, but the change was not significant. The pathology findings identified that leonurine and VPA partially inhibited the penetration of endometrial glands into the myometrium. The results of hotplate testing showed that treatment with leonurine and VPA prolonged the response latency to thermal stimuli, suggesting that leonurine protects against hyperalgesia.
However, the mechanism responsible for alleviating pain in mice with adenomyosis of leonurine is not readily apparent. It was proved that leonurine exerted beneficial effects in treating osteoclast-related diseases or acute kidney injury through inhibiting NF-κB signaling [17,18]. P65 is the most common form of NF-κB, which has been implicated in inflammation and tumorigenesis. Also, NF-κB regulates the synthesis of COX-2. In this study, we found a lower expression of PR and elevated expressions of p-p65, COX-2, and OTR. We confirmed that leonurine played a role in suppressing phosphorylation of P65 and expression of COX-2 and OTR. The data from our immunohistochemical evaluation suggested that leonurine attenuates generalized hyperalgesia in mice through regulating the protein levels of p-p65, COX-2, and OTR.
Conclusions
Taken together, these findings suggest that leonurine could be an attractive agent for improving generalized hyperalgesia. This study is the first to provide evidence that leonurine can prevent hyperalgesia and myometrial infiltration, and elucidates the mechanism involved.
Acknowledgement
We sincerely thank Professor Sun-wei Guo for with data analysis and experimental design.
Footnotes
Disclosure of conflict of interest
None.
Source of support: This research was supported by grant no. 81571416 (Jichan Nie) from the National Science Foundation of China
References
- 1.Kitawaki J. Adenomyosis: the pathophysiology of an oestrogen-dependent disease. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):493–502. doi: 10.1016/j.bpobgyn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4(4):312–22. doi: 10.1093/humupd/4.4.312. [DOI] [PubMed] [Google Scholar]
- 3.Levgur M. Therapeutic options for adenomyosis: A review. Arch Gynecol Obstet. 2007;276(1):1–15. doi: 10.1007/s00404-006-0299-8. [DOI] [PubMed] [Google Scholar]
- 4.Nothnick W, Alali Z. Recent advances in the understanding of endometriosis: The role of inflammatory mediators in disease pathogenesis and treatment. F1000Res. 2016;5(2):23–29. doi: 10.12688/f1000research.7504.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–75. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 6.Wolfler MM, Kuppers M, Rath W, et al. Altered expression of progesterone receptor isoforms A and B in human eutopic endometrium in endometriosis patients. Ann Anat. 2016;206:1–6. doi: 10.1016/j.aanat.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Kubo K, Nishikawa K, Ishizeki J, et al. Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. J Pharmacol Exp Ther. 2009;331(1):162–69. doi: 10.1124/jpet.109.156034. [DOI] [PubMed] [Google Scholar]
- 8.Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-alpha (TNF-alpha) in major depressive disorder: A systematic review. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050733. pii: E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujubu DA, Fletcher BS, Varnum BC, et al. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266(20):12866–72. [PubMed] [Google Scholar]
- 10.Matsunaga A, Kawamoto M, Shiraishi S, et al. Intrathecally administered COX-2 but not COX-1 or COX-3 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia in rats. Eur J Pharmacol. 2007;554(1):12–17. doi: 10.1016/j.ejphar.2006.09.072. [DOI] [PubMed] [Google Scholar]
- 11.Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol. 2010;202(4):346e341–48. doi: 10.1016/j.ajog.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Guo SW. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J Obstet Gynaecol Res. 2011;37(7):696–708. doi: 10.1111/j.1447-0756.2011.01655.x. [DOI] [PubMed] [Google Scholar]
- 13.Green AR, Styles JA, Parrott EL, et al. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol. 2005;56(4–5):255–63. doi: 10.1016/j.etp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Cohen I, Beyth Y, Shapira J, et al. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol Obstet Invest. 1997;44(3):200–5. doi: 10.1159/000291520. [DOI] [PubMed] [Google Scholar]
- 15.Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: A critical analysis of the evidence. Fertil Steril. 1997;68(3):393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Guo SW. A pilot study on the off-label use of valproic acid to treat adenomyosis. Fertil Steril. 2008;89(1):246–50. doi: 10.1016/j.fertnstert.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Yuan FL, Xu RS, Jiang DL, et al. Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-kappaB and PI3K/Akt signaling pathways. Bone. 2015;75:128–37. doi: 10.1016/j.bone.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Chen M, Ren X, et al. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia. 2014;97:148–55. doi: 10.1016/j.fitote.2014.06.005. [DOI] [PubMed] [Google Scholar]