Abstract
Importance
Increased dietary potassium intake is thought to be associated with low blood pressure (BP). Whether potassium supplementation may be used as an antihypertensive agent is a question that should be answered.
Objective
To assess the effect of oral potassium supplementation on blood pressure in patients with primary hypertension.
Search methods
We searched Medline, Web of Science, Scopus, Cochrane Central Register of Controlled Trials until October 2016. We also screened reference lists of articles and previous reviews. We applied no language restrictions.
Selection criteria
We included randomized placebo-controlled clinical trials addressing the effect of potassium supplementation on primary hypertension for a minimum of 4 weeks.
Data collection and analysis
We extracted data on systolic and diastolic BP (SBP and DBP) at the final follow-up. We explored the heterogeneity across studies using Cochran's test and I2 statistic and assessed the probability of publication bias using Begg's and Egger's tests. We reported the mean difference (MD) of SBP and DBP in a random-effects model.
Results
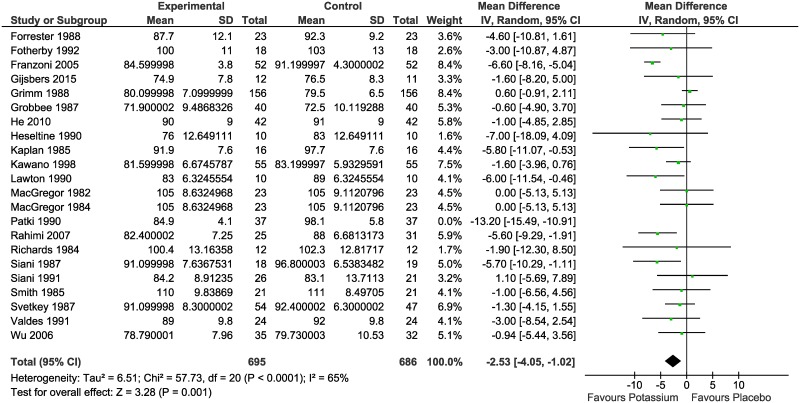
We found a total of 9059 articles and included 23 trials with 1213 participants. Compared to placebo, potassium supplementation resulted in modest but significant reductions in both SBP (MD -4.25 mmHg; 95% CI: -5.96 to -2.53; I2 = 41%) and DBP (MD -2.53 mmHg; 95% CI: -4.05 to -1.02; I2 = 65%). According to the change-score analysis, based on 8 out of 23 trials, compared to baseline, the mean changes in SBP (MD -8.89 mmHg; 95% CI: -13.67 to -4.11) and DBP (MD -6.42 mmHg; 95% CI: -10.99 to -1.84) was significantly higher in the intervention group than the control group.
Conclusions
Our findings indicated that potassium supplementation is a safe medication with no important adverse effects that has a modest but significant impact BP and may be recommended as an adjuvant antihypertensive agent for patients with essential hypertension.
Introduction
Evidence has shown that high potassium intake can reduce blood pressure (BP), decrease the risk of developing cardiovascular disease, and mitigate the adverse effects of salt on blood pressure [1]. The World Health Organization (WHO) recommends a potassium intake of at least 90 mmol/day (3.5 g/day) from food for adults to reduce BP and risk of cardiovascular disease, cerebrovascular events, and coronary heart disease. Current evidence has shown no significant difference between the flavor and taste of potassium-enriched salt and regular salt [2]. The WHO also recommends a potassium intake of at least 90 mmol/day from food for children to control BP [3]. However, there is no need to give a supplement or specially formulated products because most people can replace needed potassium through food consumption [4,5].
Using potassium supplementation as an antihypertensive agent is a question that should be answered. Clinical trials to date have reported conflicting results on the BP-lowering effect of potassium supplementation. One previous meta-analysis conducted in 1997 demonstrated that potassium supplementation was associated with a remarkable reduction in mean systolic and diastolic BP (SBP and DBP) in people with or without hypertension. The authors suggested potassium intake for prevention and treatment of raised blood pressure, particularly in people who are not able to reduce their intake of sodium [6]. A systematic review was conducted in 1999 to provide evidence-based recommendations on dietary consumption and supplementation of potassium in the prevention and treatment of hypertension. The authors concluded that potassium supplementation above the recommended daily dietary intake should not be recommended as a treatment for hypertension [5]. A Cochrane meta-analysis, performed in 2006, reported no effect of potassium supplementation on primary hypertension. The authors recommended further investigation based on evidence from high quality long-term randomized controlled trials (RCTs) to explore whether potassium supplementation may reduce blood pressure and improve health outcomes [7]. Another meta-analysis, conducted in 2013, including RCTs and cohort studies, reported that increased potassium intake can reduce blood pressure in people with or without hypertension without side effect on blood lipid and catecholamine concentrations, or renal function. The authors suggested high dietary potassium intakes to prevent and control hypertension and stroke [8].
In this meta-analysis we just focused on the effect of potassium supplementation versus placebo on blood pressure in patients with essential hypertension. Furthermore, we planned to explore the dose-response relationship between potassium intake and blood pressure. We also displayed the temporal trends of evidence and how the conclusion may shift over a period of time. Therefore, we performed this updated meta-analysis to summarize the evidence from current randomized controlled trials to explore the benefits and harms of potassium intake for patients with essential hypertension and provide recommendations on the consumption of potassium supplementation as an adjuvant agent for management of hypertension.
Methods
The Vice-chancellor of Research and Technology, Hamadan University of Medical Sciences, approved and funded this review. We wrote the report based on the PRISMA checklist of items for reporting systematic reviews and meta-analyses [9]. The supporting PRISMA checklist of this review is available as supporting information; see S1 PRISMA Checklist.
Eligibility criteria
We included randomized controlled trials that reported the effect of potassium supplementation on SBP and DBP among patients with essential hypertension (SBP ≥140 mmHg and DBP ≥90 mmHg) [10]. We considered a minimum of 4 weeks of therapy to ensure that the intervention had sufficient time to produce an effect. Having a placebo group was necessary for inclusion in the review. Placebo included inert materials such as cellulose. We excluded trials that used potassium-enriched salts or potassium supplementation in combination with other minerals such as calcium or magnesium. We also excluded trials that assessed the prophylactic antihypertensive effect of potassium supplementation in normotensive people. The main outcome of interest was the variation in measured SBP and DBP readings at the final follow up.
Information sources and search
We searched PubMed, Web of Science, Scopus, and the Cochrane Central Register of Controlled Trials until December 2015. We also searched the reference lists of included trials and previous relevant reviews. We applied no language limitations. We included the following search terms: (hypertension or hypertensive or blood pressure) and (potassium) and (clinical trial or controlled trial).
Study selection
We pooled search results using EndNote software and removed duplicate records of the same report. Two of us (AM and EH) independently screened titles and abstracts and excluded the ineligible studies. Any disagreements were resolved through consensus. We retrieved and evaluated the full text of the potentially eligible trials for further evaluation. In cases where we found multiple reports of the same trail, we used the latest report.
Data extraction
Two of us (AM and EH) independently extracted data from all included studies using an electronic datasheet prepared in Stata software. Disagreements were resolved by consensus. We extracted the following data from the eligible trials: first author’s name, year of publication, country, language, sex, age, study design (parallel, cross-over), sample size, dose (mmol/day), and mean (SD) SBP and DBP.
Methodological quality
We assessed the methodological quality of the included studies using the Delphi checklist [11]. The checklist includes a set of items as follows. (1) Was a standard randomization performed? (2) Was the allocation of intervention concealed? (3) Was the patient blinded? (4) Was the care provider blinded? (5) Was the outcome assessor blinded? (6) Were the two groups similar at baseline? (7) Were the eligibility criteria well-defined? (8) Was the variability of the outcome presented? (9) Was an intention-to-treat analysis performed? On the basis of this checklist, we allocated a maximum score of nine to each study.
Heterogeneity and publication biases
We explored the statistical heterogeneity across studies by chi-squared (Chi2) test [12] and measured its quantity by the I2 statistic [13] at the 5% significance level (P<0.05). We assessed the between-study variance using tau-squared (Tau2) statistic [12]. We investigated the possibility of publication bias by the Egger's and Begg's tests [14,15] and Trim & Fill method [16].
Summary measures
We performed a meta-analysis to obtain a summary measure of the mean difference of BP between the intervention (receiving potassium supplementation) and control (receiving placebo) groups at the final follow-up using a random-effects model [17]. For assessing the intervention effect, a negative valued denoted a reduction in BP among the intervention group compared with the placebo group. All statistical analyses were performed at a significance level of 0.05 using Stata software, version 11 (StataCorp, College Station, TX, USA) and Review Manager, version 5.3.5.
Sensitivity analysis
We used sequential algorithm [18] to achieve the minimum final I2 below the desired 50% threshold. For this purpose, for 23 trails included in this meta-analysis, we performed 23 new meta-analyses, while one trial was excluded from the calculations each time. The trail that was responsible for the largest reduction in I2 was dropped and a new set of 23−1 trials was created. When two or more trials caused exactly the same reduction in I2 by their exclusion, we dropped the trial with the largest reduction in Cochran's test. We continued this process until I2 decreased below the desired pre-set threshold. In the last step, if there was a possibility that more than one omitted trial could result in I2 dropping below the desired threshold, we reported the minimum I2.
Results
Description of studies
The results of the search process are shown in Fig 1. We found a total of 9059 trials, including 8512 articles through searching the electronic databases until October 2016 and 547 trials through screening reference list of the included trials. We excluded 1413 duplicates using EndNote software and 7520 ineligible trials through reading titles and abstracts. We also excluded 103 trials after checking the full-text reports, because they did not meet the inclusion criteria of this systematic review. Finally, 23 trials remained for meta-analysis, including 9 parallel and 14 crossover randomized placebo-controlled clinical trials involving 1213 participants [19–41]. All trials were published in English. The characteristics of the included trials are presented in Table 1.
Fig 1. Flow of information through the different phases of the systematic review.
Table 1. Summary of studies results.
| First author, yr | Country | Mean age (yr) | Sex | Study design | Dose (mmol/d) | Sample size | Follow-up period (w) | Quality score a |
|---|---|---|---|---|---|---|---|---|
| Forrester et al, 1988[19] | Jamaica | No data | Both | Parallel | 48 | 46 | No data | Abstract |
| Fotherby et al, 1992[20] | UK | 75.0 | Both | Crossover | 60 | 18 | 4 | 6 |
| Franzoni et al, 2005[21] | Italy | 52.0 | Both | Parallel | 30 | 104 | 4 | 3 |
| Gijsbers et al, 2015[41] | Netherlands | 65.8 | Both | Crossover | 66 | 23 | 4 | 7 |
| Grimm et al, 1988[22] | USA | 58.0 | Male | Parallel | 96 | 312 | 12 | 5 |
| Grobbee et al, 1987[23] | Netherlands | 24.0 | Both | Crossover | 72 | 40 | 12 | 7 |
| He et al, 2010[24] | UK | 51.0 | Both | Crossover | 64 | 84 | 4 | 7 |
| Heseltine et al, 1990[25] | UK | >65.0 | Both | Crossover | 60 | 10 | 4 | 7 |
| Kaplan et al, 1985[26] | South Western | 48.8 | Both | Crossover | 60 | 16 | 6 | 6 |
| Kawano et al, 1998[27] | Japan | 62.3 | Both | Crossover | 64 | 55 | 4 | 4 |
| Lawton et al, 1990[28] | USA | 24.0 | Male | Crossover | 100 | 10 | 4 | 4 |
| MacGregor et al, 1982[29] | London | 45.0 | Both | Crossover | 60 | 23 | 8 | 6 |
| MacGregor et al, 1984[30] | England | 45.0 | Both | Crossover | 64 | 23 | 4 | 7 |
| Obel et al, 1989[31] | Kenia | 40.0 | Both | Parallel | 64 | 48 | 16 | 7 |
| Patki et al, 1990[32] | India | 49.9 | Both | Crossover | 60 | 37 | 8 | 7 |
| Rahimi et al, 2007[33] | Iran | 48.8 | Both | Parallel | 102 | 56 | 4 | 3 |
| Richards et al, 1984[34] | New Zealand | 19–59 | Both | Crossover | 200 | 12 | 4 | Abstract |
| Siani et al, 1987[36] | Italy | 45.0 | Both | Parallel | 48 | 37 | 15 | 7 |
| Siani et al, 1991[35] | Italy | 30–65 | Both | Parallel | 30 | 47 | 52 | 6 |
| Smith et al, 1985[37] | UK | 53.0 | Both | Crossover | 64 | 20 | 4 | 4 |
| Svetkey et al, 1987[38] | Singapore | 51.0 | Both | Parallel | 40 | 101 | 8 | 8 |
| Valdes et al, 1991[39] | Chile | 50.0 | Both | Crossover | 64 | 24 | 4 | 7 |
| Wu et al, 2006[40] | China | 53.0 | Both | Parallel | 6 | 67 | 4 | 4 |
a Assessment of methological quality of studies on the basis of Delphi checklist
Main outcome measures
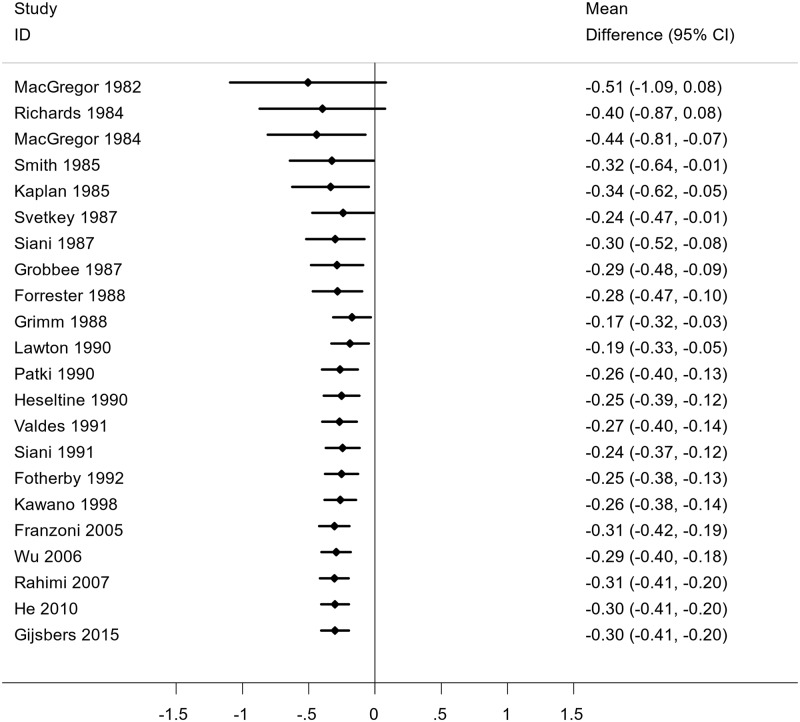
Meta-analysis of the difference in mean BP between the intervention and control groups resulted in statistically significant reductions in both SBP (MD -4.25 mmHg; 95% CI: -5.96 to -2.53; I2 = 41%) (Fig 2) and DBP (MD -2.53 mmHg; 95% CI: -4.05 to -1.02; I2 = 65%) (Fig 3).
Fig 2. Meta-analysis of the randomized controlled trials reporting the effect of potassium supplementation on systolic blood pressure.
Fig 3. Meta-analysis of the randomized controlled trials reporting the effect of potassium supplementation on diastolic blood pressure.
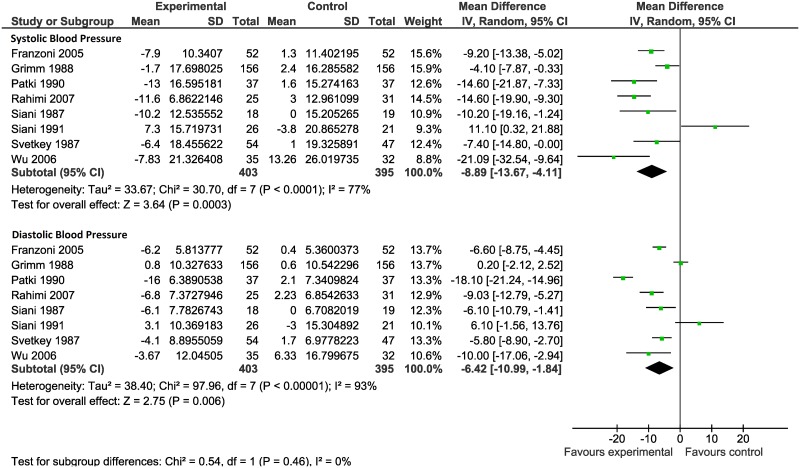
We performed change-score analysis based on 8 out of 23 trials that reported the changes in SBP and DPB in the two groups compared to baseline (Fig 4). As shown in this figure, compared to baseline, the mean changes in SBP (MD -8.89 mmHg; 95% CI: -13.67 to -4.11) and DBP (MD -6.42 mmHg; 95% CI: -10.99 to -1.84) was significantly higher in the intervention group than the control group.
Fig 4. Meta-analysis of the randomized controlled trials reporting the mean change scores from baseline in systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the intervention and control groups.
There was an extreme value (outlier) among the included studies [31]. This trial consisted of 48 black patients with mild hypertension who received 64 mmol/day potassium supplementation for 16 weeks. According the results of this trial, potassium supplements resulted in significant decrease in mean SBP (MD -38 ±2.32 mmHg) and DBP (MD -18 ±1.15 mmHg) compared to control group. To establish homogeneity among the studies, we excluded this outlier from the meta-analysis.
Among the included studies, actually only two studies [22,38] including 428 patients, reported mild systemic adverse effect in a small number of patients as follows. Overall, among the patients who received potassium supplementation, two reported nausea or vomiting, 14 reported change in bowel habits (diarrhea, constipation), 15 reported abdominal pain, 12 reported gas (belching or flatulence), 2 reported headache, one reported anxiety, and one reported lethargy. Among the patients who received placebo, two reported nausea or vomiting, 15 reported change in bowel habits (diarrhea, constipation), 8 reported abdominal pain, 6 reported gas (belching or flatulence), 3 reported headache, one reported palpitation, one reported skin rash, one reported anxiety, one reported dizziness, and one reported lethargy.
Publication bias
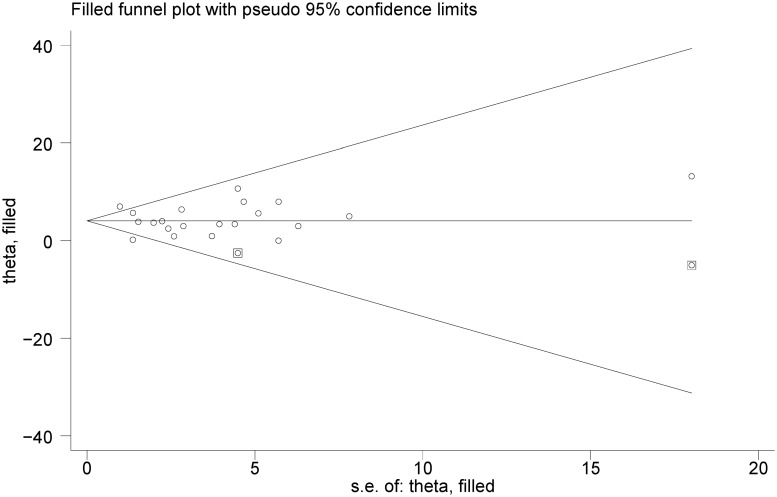
We explored publication bias using Begg's and Egger's tests. According to these statistical tests, there was no evidence of significant publication bias among trials reporting the effect of potassium supplementation on SBP (P = 0.398 and P = 0.921) and DBP (P = 0.239 and P = 0.998), respectively. We also explored the possibility of publication bias using Trim and Fill method (Fig 5). This statistical method is a rank-based data augmentation technique that estimates the number and outcomes of missing studies and corrects the results of meta-analysis by incorporating the theoretical missing studies [21]. Based on this method, we found two potentially missing studies. However there was no statistically significant difference between the mean difference of SPB resulted from original meta-analysis -4.25 (95% CI: -5.96, -2.53) and the mean difference of SPB resulted from Trim and Fill method -4.07 (95% CI -5.32, -2.82). This method confirmed the absence of publication bias.
Fig 5. The filled funnel plot of the randomized controlled trials reporting the effect of potassium supplementation on systolic blood pressure.
Heterogeneity and sensitivity analysis
There was low statistical heterogeneity among trials reporting the effect of potassium supplementation on SBP (I2 = 41%) but there was evidence of moderate heterogeneity among trials addressing the effect of potassium intake on DBP (I2 = 65%). We performed a meta-regression to determine the causes of heterogeneity among results of studies (Table 2). We considered mean difference of SBP and DBP as the dependent variable and covariates such as potassium supplementation dosage, duration of follow-up, and participants' mean age as predictors. The result of meta-regression revealed that the association between potassium dosage, follow-up period and mean age were not statistically significant, therefore they did not play an important role in the heterogeneity across studies.
Table 2. Result of meta-regression analysis for exploring sources of heterogenity considerring systolic and diastolic blood pressure as the dependent variables.
| Variables | Coefficeint | SE | t | P value | 95% CI | |
|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | ||||||
| Potassium dosage (mmol/day) | -0.04501 | 0.06096 | -0.74 | 0.472 | -0.17575 | 0.08572 |
| Follow-up period (week) | -0.00348 | 0.02621 | -0.13 | 0.896 | -0.05969 | 0.05273 |
| Age mean (year) | -0.39616 | 0.20750 | -1.91 | 0.077 | -0.84121 | 0.04890 |
| Constant | 9.31997 | 4.06457 | 2.29 | 0.038 | 0.60233 | 18.03760 |
| Diastolic blood pressure (mmHg) | ||||||
| Potassium dosage (mmol/day) | -0.05228 | 0.06440 | -0.81 | 0.433 | -0.19260 | 0.08804 |
| Follow-up period (week) | -0.00028 | 0.02470 | -0.01 | 0.991 | -0.05410 | 0.05353 |
| Age mean (year) | -0.16892 | 0.19584 | -0.86 | 0.405 | -0.59562 | 0.25777 |
| Constant | 6.42272 | 4.28737 | 1.50 | 0.160 | -2.91866 | 15.76410 |
We also performed sensitivity analysis on the basis of the sequential algorithm to achieve between-study homogeneity. We achieved the minimum I2 below desired threshold (50%) by omitting two trials [21,32] from the meta-analysis (MD -1.83; 95% CI: -2.93 to -0.73; I2 = 22%).
Subgroup analysis
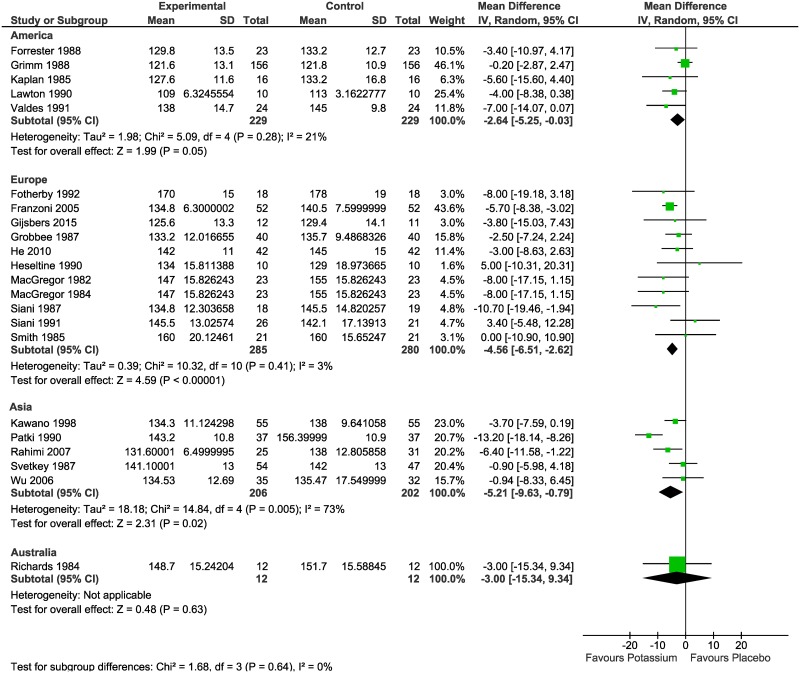
We conducted a subgroup analysis to explore the possibility of heterogeneity in the effect of potassium supplementation on SBP and DBP by continent. As shown in Fig 6, the mean difference in SBP was -2.64 (95% CI: -5.25 to -0.03) mmHg in America; -4.56 (95% CI: -6.51 to -2.62) mmHg in Europe; -5.21 (95% CI: -9.63 to -0.79) mmHg in Asia; and -3.00 (95% CI: -15.34 to 9.34) mmHg In Australia (based on one trial).
Fig 6. Meta-analysis of the randomized controlled trials reporting the effect of potassium supplementation on systolic blood pressure (SBP) by continent.
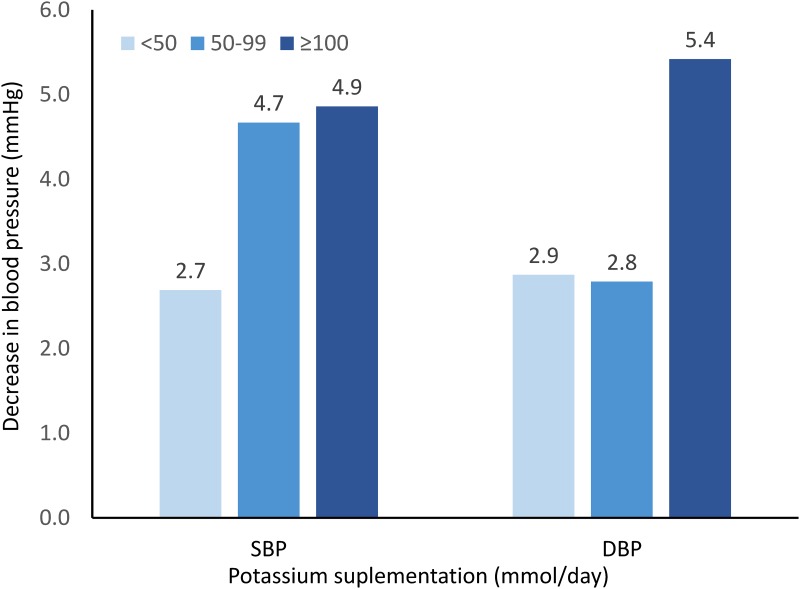
We conducted a subgroup analysis to explore the dose-response relationship between potassium intake and blood pressure. According to the potassium supplementation dosage, we divided the trials into low-dose (<50 mmol/day), moderate-dose (50–99 mmol/day), and high-dose (≥100 mmol/day) and then performed meta-analysis for each category. According to the results presented in Fig 7, there was a dose-response relationship between potassium intake and reduction in systolic and diastolic BP.
Fig 7. The dose-response relationship between potassium intake and reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Further analysis
We sorted studies chronologically and performed a cumulative meta-analysis to detect temporal trends of evidence and to see how the evidence has shifted over time. A cumulative meta-analysis is a meta-analysis run first with one study, then repeated with a second study added, then a third, and so on. Accordingly, the first horizontal line indicates the effect based on one trial, the second line indicates the cumulative effect based on two trials, and so on. The results of cumulative meta-analysis is given in Fig 8. As we move down the plot, we see a consistency in the results of consecutive experiments and the effect size tends to stabilize. The presence of consistency between the results means that the results continually favor the treatment effect of potassium and thus no further experiment is required to make a conclusion about the impact of potassium supplementation on BP.
Fig 8. Cumulative meta-analysis of the randomized controlled trials reporting the effect of potassium supplementation on systolic blood pressure.
Discussion
Our findings indicated that potassium supplementation had a statistically significant effect on both SBP and DBP. Subgroup analyses revealed an evidence of dose-response relationship between potassium intake and BP reduction. Accordingly, potassium supplementation had a clinically modest impact on essential hypertension and thus may be used as an adjuvant antihypertensive agent.
In addition to conventional comparing SBP and DBP in the intervention and control groups, we performed change-score analysis to explore the changes in SBP and DPB in the two groups compared to baseline. Based on this method, the mean differences between the intervention and control groups became more obvious. The reason was that change-score analysis with baseline adjustment provided a better estimate of the effect of potassium supplementation on blood pressure.
There was evidence of heterogeneity (low P value and a large Chi2 statistic) among the trials addressing the effect of potassium supplementation and DBP. However care should be taken in the interpretation of the Chi2 test, since it has low statistical power in the situation of a meta-analysis when studies have small sample size or are few in number. On the other hand, when there are many studies in a meta-analysis, the test has high power to detect a small amount of heterogeneity that may be clinically unimportant [42]. In such situation I2 statistic is useful method because this statistic has been developed for quantifying inconsistency across studies. It moves the focus away from testing whether heterogeneity is present to assessing its impact on the meta-analysis [13]. According to I2 statistic there was low to moderate heterogeneity across studies that may be due to clinical and methodological diversity among studies.
Potassium is an essential nutrient that plays a key role in maintenance of body fluid, acid-base balance, and normal cell structure and function [43]. There is a significant inverse correlation between dietary potassium intake and BP [44,45]. Even the dietary sodium/potassium ratio is more closely associated with BP than either sodium or potassium alone [45]. Dietary potassium intake appears to cause natriuresis and prevent retention of sodium and thus lower BP [46]. Other physiological mechanisms underlying the blood pressure lowering effect of potassium supplementation are as follows: endothelial vascular cells and macrophages inhibit the formation of free radicals by inhibiting the proliferation of the smooth vascular muscle cells, and reducing the vascular resistance [46].
Our findings are consistent with two previous meta-analyses [6,8], but inconsistent with a third one [7]. Whelton et al [6] conducted a meta-analysis in 1997 and included 33 RCTs performed before 1995 investigating the effect of potassium supplementation on BP. They demonstrated that potassium supplementation was correlated with a remarkable reduction in the mean (95% CI) SBP and DBP of -3.11 (-1.91 to -4.31) and -1.97 (-0.52 to -3.42) mmHg, respectively. The authors recommended potassium intake for prevention and treatment of hypertension. Aburto et al [8] performed a meta-analysis in 2013, including 22 RCTs and 11 cohort studies addressing the effects of potassium supplementation on blood pressure, renal function, blood lipids, catecholamine concentrations. They reported that an increase in potassium intake could reduce SBP by 3.49 (95% CI: 1.82 to 5.15) and DBP by 1.96 (0.86 to 3.06) mmHg in adults with raised blood pressure with no important side effect on blood lipid and catecholamine concentrations or renal function. The authors suggested high dietary potassium intakes to prevent and control hypertension and stroke. Dickinson et al [7] conducted a Cochrane meta-analysis in 2006, including six RCTs comparing the effect of oral potassium supplements with placebo on primary hypertension. They reported no significant reductions in SBP (MD: -11.2, 95% CI: -25.2 to 2.7) and DBP (MD: -5.0, 95% CI: -12.5 to 2.4). They concluded that potassium supplementation has no significant effect on blood pressure. However, due to small number of RCTs, included in this meta-analysis, they suggested further investigation should be done on the basis of high quality RCTs of longer duration.
This review had some limitations as follows. We planned to perform change score analysis rather than comparing post-treatment mean BP. However, only 8 out of 23 trials reported the baseline SPB and DBP. Therefore, we performed change-score analysis just based on eight trials. The review did not include studies enrolling individuals with normal BP. Thus, the results of this review should not be interpreted to include normotensive people. We could not differentiate the effect of various types of potassium provided in supplements because all studies used potassium chloride except one that used potassium aspartate [21]. Finally, we could not assess the possibility of difference in BP by gender, because 21 out of 23 RCTs were in mixed populations of males and females.
Conclusion
This meta-analysis provided evidence based on RCTs of the effect of potassium intake on BP in hypertensive patients. Our findings indicated that potassium supplementation is a safe medication with no important adverse effects that has a modest but significant impact BP and may be recommended as an adjuvant antihypertensive agent for patients with essential hypertension.
Supporting information
(DOC)
Acknowledgments
This was part of the MSc thesis in Epidemiology. We would like to appreciate the Vice-Chancellor for Research and Technology of the Hamadan University of Medical Sciences for supporting this work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Vice-Chancellor for Research and Technology, the Hamadan University of Medical Sciences.
References
- 1.Dietary Guidelines Advisory Committee. The report of the dietary guidelines advisory committee on dietary guidelines for Americans, 2005 Washington, D.C: Department of Health and Human Services; 2005 [cited 13 June 2016]. http://www.health.gov/dietaryguidelines/dga2005/report/default.htm.
- 2.Maleki A, Soltanian AR, Zeraati F, Sheikh V, Poorolajal J. The flavor and acceptability of six different potassium-enriched (sodium reduced) iodized salts: A single-blind, randomized, crossover, placebo-controlled trial. Clin Hypertens. 2016; 22: 18 10.1186/s40885-016-0054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Guideline: Potassium intake for adults and children. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 4.Sawka MN, Montain SJ. Fluid and electrolyte supplementation for exercise heat stress. Am J Clin Nutr. 2000; 72(2 Suppl): 564s–72s. [DOI] [PubMed] [Google Scholar]
- 5.Burgess E, Lewanczuk R, Bolli P, Chockalingam A, Cutler H, Taylor G, et al. Lifestyle modifications to prevent and control hypertension. 6. Recommendations on potassium, magnesium and calcium. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ. 1999; 160(9 Suppl): S35–45. [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997; 277(20): 1624–1632. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006(3): Cd004641 10.1002/14651858.CD004641.pub2 [DOI] [PubMed] [Google Scholar]
- 8.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013; 346: f1378 10.1136/bmj.f1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8(5): 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42(6): 1206–1252. 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 11.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998; 51(12): 1235–1241. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.0.2 [Updataed: September 2009, Access date: 25 June 2016]. London: The Cochrane Collaboration; 2009. http://handbook.cochrane.org/v5.0.2/. [Google Scholar]
- 13.Higgins JPT, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003; 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50(4): 1088–1101. [PubMed] [Google Scholar]
- 15.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duval S, Tweedie R. A nonparametric "trim and fill" method of accounting for publication bias in meta-analysis. JASA. 2000; 95(449): 89–98. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008; 37(5): 1148–1157. 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrester TE, Grell GA. Changes in red cell sodium content and blood pressure levels with potassium supplementation in black hypertensive patients. West Indian Med J. 1988; 37(2): 92–96. [PubMed] [Google Scholar]
- 20.Fotherby MD, Potter JF. Potassium supplementation reduces clinic and ambulatory blood pressure in elderly hypertensive patients. J Hypertens. 1992; 10(11): 1403–1408. [DOI] [PubMed] [Google Scholar]
- 21.Franzoni F, Santoro G, Carpi A, Da Prato F, Bartolomucci F, Femia FR, et al. Antihypertensive effect of oral potassium aspartate supplementation in mild to moderate arterial hypertension. Biomedicine & Pharmacotherapy. 2005; 59(1–2): 25–29. [DOI] [PubMed] [Google Scholar]
- 22.Grimm RH, Kofron PM, Neaton JD, Svendsen KH, Elmer PJ, Holland L, et al. Effect of potassium supplementation combined with dietary sodium reduction on blood pressure in men taking antihypertensive medication. J Hypertens Suppl. 1988; 6(4): S591–S593. [DOI] [PubMed] [Google Scholar]
- 23.Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens. 1987; 5(1): 115–119. [DOI] [PubMed] [Google Scholar]
- 24.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, et al. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010; 55(3): 681–688. 10.1161/HYPERTENSIONAHA.109.147488 [DOI] [PubMed] [Google Scholar]
- 25.Heseltine D, Thomas T, Wilkinson R, James OFW, Potter JF. Potassium supplementation in the treatment of idiopathic postural hypotension. Age and Ageing. 1990; 19(6): 409–414. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan NM, Carnegie A, Raskin P, Heller JA, Simmons M. Potassium supplementation in hypertensive patients with diuretic-induced hypokalemia. N Engl J Med. 1985; 312(12): 746–749. 10.1056/NEJM198503213121203 [DOI] [PubMed] [Google Scholar]
- 27.Kawano Y, Minami J, Takishita S, Omae T. Effects of potassium supplementation on office, home, and 24-h blood pressure in patients with essential hypertension. Am J Hypertens. 1998; 11(10): 1141–1146. [DOI] [PubMed] [Google Scholar]
- 28.Lawton WJ, Fitz AE, Anderson EA, Sinkey CA, Coleman RA. Effect of dietary potassium on blood pressure, renal function, muscle sympathetic nerve activity, and forearm vascular resistance and flow in normotensive and borderline hypertensive humans. Circulation. 1990; 81(1): 173–184. [DOI] [PubMed] [Google Scholar]
- 29.MacGregor GA, Smith SJ, Markandu ND, Banks RA, Sagnella GA. Moderate potassium supplementation in essential hypertension. Lancet. 1982; 2(8298): 567–570. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor GA, Smith SJ, Markandu ND, Sagnella GA. Does increasing potassium intake lower blood pressure in essential hypertension? J Cardiovasc Pharmacol. 1984;6(Suppl 1): S244–S249. [DOI] [PubMed] [Google Scholar]
- 31.Obel AO. Placebo-controlled trial of potassium supplements in black patients with mild essential hypertension. J Cardiovasc Pharmacol. 1989; 14(2): 294–296. [DOI] [PubMed] [Google Scholar]
- 32.Patki PS, Singh J, Gokhale SV, Bulakh PM, Shrotri DS, Patwardhan B. Efficacy of potassium and magnesium in essential hypertension: a double-blind, placebo controlled, crossover study. BMJ. 1990; 301(6751): 521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahimi ARO, Mahmoodpoor A, Sanaie S. The effect of high-calcium and high-potassium diet on grade-I hypertension and high normal blood pressure. Pakistan Journal of Medical Sciences. 2007; 23(4): 589–592. [Google Scholar]
- 34.Richards AM, Espiner EA, Nicholls MG, Ikram H, Maslowski A, Hamilton E, et al. Blood-pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet. 1984; 323(8380): 757–761. [DOI] [PubMed] [Google Scholar]
- 35.Siani A, Strazzullo P, Giacco A, Pacioni D, Celentano E, Mancini M. Increasing the dietary potassium intake reduces the need for antihypertensive medication. Ann Intern Med. 1991; 115(10): 753–759. [DOI] [PubMed] [Google Scholar]
- 36.Siani A, Strazzullo P, Russo L, Guglielmi S, Iacoviello L, Ferrara LA, et al. Controlled trial of long term oral potassium supplements in patients with mild hypertension. Br Med J. 1987; 294(6585): 1453–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SJ, Markandu ND, Sagnella GA, MacGregor GA. Moderate potassium chloride supplementation in essential hypertension: is it additive to moderate sodium restriction? Br Med J. 1985; 290(6462): 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svetkey LP, Yarger WE, Feussner JR, DeLong E, Klotman PE. Double-blind, placebo-controlled trial of potassium chloride in the treatment of mild hypertension. Hypertension. 1987; 9(5): 444–450. [DOI] [PubMed] [Google Scholar]
- 39.Valdes G, Vio CP, Montero J, Avendano R. Potassium supplementation lowers blood pressure and increases urinary kallikrein in essential hypertensives. J Hum Hypertens. 1991; 5(2): 91–96. [PubMed] [Google Scholar]
- 40.Wu G, Tian H, Han K, Xi Y, Yao Y, Ma A. Potassium magnesium supplementation for four weeks improves small distal artery compliance and reduces blood pressure in patients with essential hypertension. Clin Exp Hypertens. 2006; 28(5): 489–497. 10.1080/10641960600798705 [DOI] [PubMed] [Google Scholar]
- 41.Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJ, Geleijnse JM. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens. 2015; 29(10): 592–598. 10.1038/jhh.2015.3 [DOI] [PubMed] [Google Scholar]
- 42.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.0.2 [updated September 2009]. London: The Cochrane Collaboration; 2009. http://handbook.cochrane.org/v5.0.2/. [Google Scholar]
- 43.Young DB. Role of potassium in preventive cardiovascular medicine. Boston: Kluwer Academic Publishers; 2001. [Google Scholar]
- 44.Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2015;33(8):1509–20. 10.1097/HJH.0000000000000611 [DOI] [PubMed] [Google Scholar]
- 45.Khaw KT, Barrett-Connor E. The association between blood pressure, age, and dietary sodium and potassium: a population study. Circulation. 1988;77(1):53–61. [DOI] [PubMed] [Google Scholar]
- 46.Buemi M, Senatore M, Corica F, Aloisi C, Romeo A, Tramontana D, et al. Diet and arterial hypertension: is the sodium ion alone important? Med Res Rev. 2002;22(4):419–28. 10.1002/med.10013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.