Abstract
Background
The aim of this study is to investigate the clinical significance of neutrophil gelatinase–associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) for acute kidney injury (AKI) in patients with scrub typhus.
Methods
From 2014 to 2015, 145 patients were diagnosed with scrub typhus. Of these, we enrolled 138 patients who were followed up until renal recovery or for at least 3 months. We measured serum and urine NGAL and KIM-1 levels and evaluated prognostic factors affecting scrub typhus–associated AKI.
Results
Of the 138 patients, 25 had scrub typhus–associated AKI. The incidence of AKI was 18.1%; of which 11.6%, 4.3%, and 2.2% were classified as risk, injury, and failure, respectively, according to RIFLE criteria. Compared with patients in the non-AKI group, patients in the AKI group were older and showed higher total leukocyte counts and hypoalbuminemia or one or more comorbidities such as hypertension (72% vs 33%, p<0.01), diabetes (40% vs 14%, p<0.01), or chronic kidney disease (32% vs 1%, p<0.01). In addition, serum NGAL values (404± 269 vs 116± 78 ng/mL, P<0.001), KIM-1 values (0.80± 0.52 vs 0.33± 0.68 ng/mL, P<0.001), urine NGAL/creatinine values (371± 672 vs 27± 39 ng/mg, P<0.001) and urine KIM-1/creatinine values (4.04± 2.43 vs 2.38± 1.89 ng/mg, P<0.001) were higher in the AKI group than in the non-AKI group. By multivariate logistic regression, serum NGAL and the presence of chronic kidney disease were significant predictors of AKI.
Conclusion
Serum NGAL might be an additive predictor for scrub typhus–associated AKI.
Introduction
Scrub typhus, which is caused by Orientia tsutsugamushi and is transmitted by chigger bite, is an acute febrile disease that can involve many vital organs including the kidneys [1–3]. The incidence of acute kidney injury (AKI) in scrub typhus has been reported to range from 21 to 43% [4–6], and old age and co-morbidity are associated with the development of AKI [6]. The possible mechanisms of AKI associated with scrub typhus include pre-renal failure, septic shock, vasculitis, rhabdomyolysis, and direct renal invasion of O. tsutsugamushi [7–11].
Acute kidney injury is a common disorder that is strongly linked to short- and long-term morbidity and mortality [12, 13]. More timely diagnosis would allow for earlier intervention and improve patient outcomes. Many investigations have sought to identify markers that can facilitate the early diagnosis, differential diagnosis, and short- and long-term prognosis of AKI. Several novel AKI biomarkers have been identified including neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), cystatin C, interleukin (IL)-18, and liver-type fatty acid–binding protein [14–17]. Of these, NGAL and KIM-1 are the most widely studied biomarkers of AKI. However, there is no report on the relationship between scrub typhus-associated AKI and biomarkers such as NGAL and KIM-1.
Therefore, we investigated the clinical significances of neutrophil gelatinase–associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) for AKI in patients with scrub typhus.
Methods
Patient selection
Between 2014 and 2015, 145 patients were diagnosed with scrub typhus, which was confirmed by a positive IgM ELISA for scrub typhus (InBios International Inc., Seattle, WA, USA) in patients with acute febrile illness and rash. Patients who were transferred to another hospital during treatment or had concomitant infections such as leptospirosis, malaria, or dengue fever were excluded from the study. We also excluded patients who were not followed up until complete recovery of renal function or for at least 3 months after discharge. After exclusions, a total of 138 patients were enrolled in this study after obtaining written informed consent from each participant. This study was approved by the Institutional Review Board of the Presbyterian Medical Center, Jeonju, South Korea.
All patients underwent a detailed clinical history and examination, a standard set of investigations including complete blood counts, liver function tests, serum creatinine, urea, electrolytes, chest radiograph, three peripheral blood smears for malaria, urinalysis, and two blood cultures. The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria [18], and patients were categorized into the R, I or F categories. The estimated glomerular filtration rate (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation [19]. When the baseline serum creatinine was not available, it was calculated using the standard four-variable MDRD formula assuming eGFR of 75 mL/min/1.73 m2. The RIFLE class was determined based on the worst among serum creatinine levels, eGFR, and urine output criteria. Renal replacement therapy was initiated using the standard indications.
Sandwich enzyme-linked immunosorbent assay analysis of NGAL and KIM-1 expression in serum and urine samples
Blood and urine samples were collected at the first presentation, before specific treatment, and at follow-up (3 days after taking the initial sample). Peripheral venous blood was drawn into pyrogen-free vacuum blood collection tubes with EDTA as anticoagulant, centrifuged within 30 minutes at 2,000 g for 5 minutes to obtain platelet-poor plasma, and the obtained samples were stored in multiple aliquots at 280uC until analysis. The samples were then stored at –80°C until assayed. Random spot urine samples were also centrifuged at 2,000 g for 5 min and the supernatants were stored in aliquots at –80°C. Plasma and urine NGAL and KIM-1 levels were measured using the human NGAL enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) and the KIM-1 ELISA kit (R&D Systems, Minneapolis, MN, USA). The urinary creatinine (Cr) concentration was used to normalize NGAL and KIM-1 measurements to account for the influence of urinary dilution on concentration. Urinary levels of the biomarkers were expressed as uNGAL/Cr and uKIM-1/Cr ratio as ng/mg creatinine. Interassay and intraassay coefficient variations were 5% to 10% for batched samples analyzed on the same day.
Multiplex assays
Interferon (IFN) γ, tumor necrosis factor [TNF]-α, and TH2 (IL-10) cytokines were measured with multiplex bead flow cytometry (Flowcytomix; Bender Medsystems, Vienna, Austria). Briefly, patient serum was added to the bead mixtures that were coated with antibodies to human cytokines. A biotin-conjugated mixture was added to the serum antibody-bead mixture complex. After incubation, a streptavidin-phycoerythrin (PE) solution was added to bind the biotin-conjugate. Following further incubation, unbound streptavidin-PE was removed during a subsequent wash step and the analyte was coupled with bead mixtures, biotinylated antibody, and streptavidin-PE emitting fluorescent signals. A standard curve was prepared from serial dilutions of a standard mixture. The concentrations of human cytokines were analyzed by FACScanto flow cytometry (Becton Dickinson Biosciences, San Jose, CA, USA) and Flowcytomix Pro 2.2 software (Bender Medsystems).
Statistical analysis
All data are presented as mean ± standard deviation unless otherwise specified. The baseline characteristics of patients in the non-AKI and AKI groups were compared using t tests, chi-square test, or Fisher’s exact test, as appropriate. Clinically relevant parameters or variables that were significantly associated with the presence of AKI in the univariate analysis were included in the multivariate analysis. Receiver operating characteristics (ROC) analysis was used to calculate the area under the curve (AUC) for NGAL and KIM-1 and to find the best cutoff values for the diagnosis of AKI. A P-value <0.05 was considered statistically significant. Statistical analysis was carried out using SPSS version 22.0.
Results
Baseline characteristics
The baseline characteristics of the 138 study subjects are presented in Table 1.
Table 1. The clinical and laboratory findings of the 138 patients with scrub typhus.
| Characteristics | |
|---|---|
| Age, years | 65 ± 13 |
| Male, n (%) | 49 (36) |
| Eschar, n (%) | 130 (94) |
| Co-morbidity, n (%) | 64 (46) |
| Diabetes, n (%) | 26 (19) |
| Hypertension, n (%) | 55 (40) |
| CKD, n (%) | 9 (7) |
| Duration of hospital stay, days | 6.4 ± 4.3 |
| Fever, n (%) | 132 (96) |
| Systolic BP (<90 mmHg), n (%) | 8 (6) |
| ICU care, n (%) | 5 (4) |
| Serum creatinine (mg/dl) | 1.07 ± 0.58 |
| eGFR adm, ml/min/1.73m2 | 64 ± 28 |
| Serum ALT (IU/L) | 82 ± 124 |
| Total leukocyte count (× 103/ mL) | 7.73 ± 5.96 |
| Platelet count (× 103/ mL) | 134 ± 55 |
| Patient with baseline renal function, n (%) | 73 (53) |
| Acute kidney injury, n (%) RIFLESCr RIFLEUO RIFLESCr+UO |
25 (18) 24 (17) 7 (5) 25 (18) |
The patients included 49 (36%) men and 89 (64%) women with a mean age of 65 years (range, 20–86). Eschars were found in 130 (94%) patients. Sixty-four patients had comorbidities such as hypertension, diabetes, or chronic kidney disease. The mean duration of hospital stay was 6.4 days, and 132 (96%) patients had fever during the hospitalization period. Eight (6%) patients were hypotensive (systolic blood pressure <80 mmHg) at admission, and five (4%) required admission to the intensive care unit. The mean plasma alanine aminotransferase (ALT) concentration was 82 IU/L, and the mean total leukocyte count was 7.73×103/mL. The mean eGFR was 64mL/min/1.73m2. Of the 138 patients enrolled in this study, 25 (18%) experienced AKI. All patients were treated with anti-rickettsia agents such as doxycycline or azythromycin. Upon admission, blood and urine samples were collected from all patients before treatment. Second blood and urine samples (3 days after the initial sample) were analyzed in 37 patients.
Comparison of clinical characteristics between non-AKI group and AKI group
Compared with patients in the non-AKI group, patients in the AKI group were older (74 ± 9 vs. 63 ± 12 years, P<0.01) and more likely to have one or more comorbidity such as diabetes (40% vs. 14%, P<0.01), hypertension (72% vs. 33%, P<0.01), or chronic kidney disease (32% vs. 1%, P<0.01) (Table 2).
Table 2. Comparison of baseline characteristics between non-AKI and AKI group.
| AKI (n = 25) |
Non-AKI (n = 113) |
P-value | |
|---|---|---|---|
| Age | 74 ± 9 | 63± 12 | < 0.01 |
| Male, n(%) | 12 (48) | 37 (33) | NS |
| Duration of hospital stay, days | 5.6 ± 2.5 | 10.2 ± 7.7 | < 0.01 |
| Comorbidity, n(%) Diabetes, n (%) Hypertension, n (%) CKD, n (%) |
18 (72) 10 (40) 18 (72) 8 (32) |
46 (41) 16 (14) 37 (33) 1 (1) |
< 0.01 < 0.01 < 0.01 < 0.01 |
| Systolic BP (<90 mmHg), n (%) | 5 (20) | 3 (3) | < 0.01 |
| Hemoglobin (mg/dl) | 11.20 ± 1.80 | 13.12 ± 1.60 | < 0.01 |
| Leukocyte (× 103/ mL) | 11.00 ± 4.78 | 7.00 ± 5.97 | < 0.01 |
| Platelet count (× 103/ mL) | 123 ± 67 | 137 ± 52 | NS |
| Total bilirubin level | 0.81 ± 0.45 | 0.76 ± 0.36 | NS |
| Serum albumin (mg/dl) | 3.27 ± 0.50 | 3.78 ± 0.56 | < 0.01 |
| Serum ALT (IU/L) | 56 ± 31 | 88 ± 136 | NS |
| C-reactive protein (mg/dl) | 9.28 ± 5.71 | 5.73 ± 4.04 | < 0.01 |
| Creatinine (mg/dl) | 1.94 ± 0.91 | 0.87 ± 0.17 | < 0.01 |
| eGFR ml/min/1.73m2 | 28 ± 13 | 72 ± 24 | < 0.01 |
| Serum NGAL (ng/mL) | 404 ± 269 | 116 ± 78 | < 0.01 |
| Urine NGAL/Cr (ng/mg) | 371 ± 672 | 27± 39 | < 0.01 |
| Serum KIM-1 (ng/mL) | 0.80 ± 0.52 | 0.33 ± 0.68 | < 0.01 |
| Urine KIM-1/Cr (ng/mg) | 4.04 ± 2.43 | 2.38 ± 1.89 | < 0.01 |
| IL-10 (pg/mL) | 152 ± 163 | 62 ± 103 | < 0.01 |
| TNF-α (pg/mL) | 55 ± 58 | 15 ± 15 | < 0.01 |
| IFN (pg/mL) | 169 ± 297 | 156 ± 278 | NS |
Patients in the AKI group had worse renal function (28 ± 13 vs. 72 ± 24 mL/min/1.73m2, P<0.01) at admission, and a higher incidence of hypotension while in the hospital. Inflammatory markers such as total leukocyte count were higher in patients with AKI than in those without AKI (11.00 × 103/ mL vs. 7.00 × 103/mL, P<0.01). Plasma ALT concentrations and total bilirubin levels were not different between the two groups. Serum NGAL (404 ± 269 vs. 116 ± 78 ng/mL, P<0.01), KIM-1 (0.80 ± 0.52 vs. 0.33± 0.68 ng/mL, P<0.01), urine NGAL/creatinine values (371± 672 vs. 27± 39 ng/mg, P<0.01), and urine KIM-1/creatinine values (4.04 ± 2.43 vs. 2.38 ± 1.89 ng/mg, P<0.01) were higher in the AKI group than in the non-AKI group. Plasma levels of IL-10 (152 ± 163 vs. 62 ± 103 pg/mL, P<0.01) and TNF-α (55 ± 58 vs. 15 ± 15 pg/mL, P<0.01) were higher in the AKI group than in the non-AKI group, but there was no difference in IFN-γ levels between the two groups. Although there was no difference in NGAL and KIM-1 levels between the first and second samples in the non-AKI group (n = 26), the values decreased during treatment in the AKI group (n = 11).
Clinical course of acute kidney injury in patients with scrub typhus
According to the RIFLE criteria, 16 (64%), 6 (24%), and 3 (12%) patients in our study fell into the R, I, and F categories, respectively (Table 3).
Table 3. Clinical characteristics of 25 patients with AKI.
| Risk (n = 16) | Injury (n = 6) | Failure (n = 3) | ||
|---|---|---|---|---|
| RIFLE criteria | RIFLESCr (n = 24) | 16 (67) | 5 (21) | 3 (12) |
| RIFLEUO (n = 7) | 2 (29) | 2 (29) | 3 (42) | |
| RIFLESCr+UO (n = 25) | 16 (64) | 6 (24) | 3 (12) | |
| NGAL | Serum NGAL (ng/mL) | 328± 270 | 530 ± 266 | 559 ± 125 |
| Urine NGAL/Cr (ng/mg) | 170 ± 392 | 280 ± 190 | 1629 ± 1206 | |
| KIM-1 | Serum KIM-1 (ng/mL) | 0.69 ± 0.57 | 0.88 ± 0.35 | 1.23 ± 0.37 |
| Urine KIM-1/Cr (ng/mg) | 3.36 ± 0.57 | 4.05 ± 0.35 | 7.68 ± 0.37 | |
| Cytokines | IL-10 (pg/mL) | 86 ± 91 | 228 ± 174 | 338 ± 224 |
| TNF-α (pg/mL) | 50 ± 58 | 44 ± 42 | 104 ± 82 | |
| IFN (pg/mL) | 154 ± 303 | 214 ± 362 | 162 ± 192 | |
| FENa < 1%, n (%) | 15 (94) | 3 (50) | 1 (33) | |
| Recovery of renal function within 72 h, n (%) | 16 (100) | 3 (50) | 0 | |
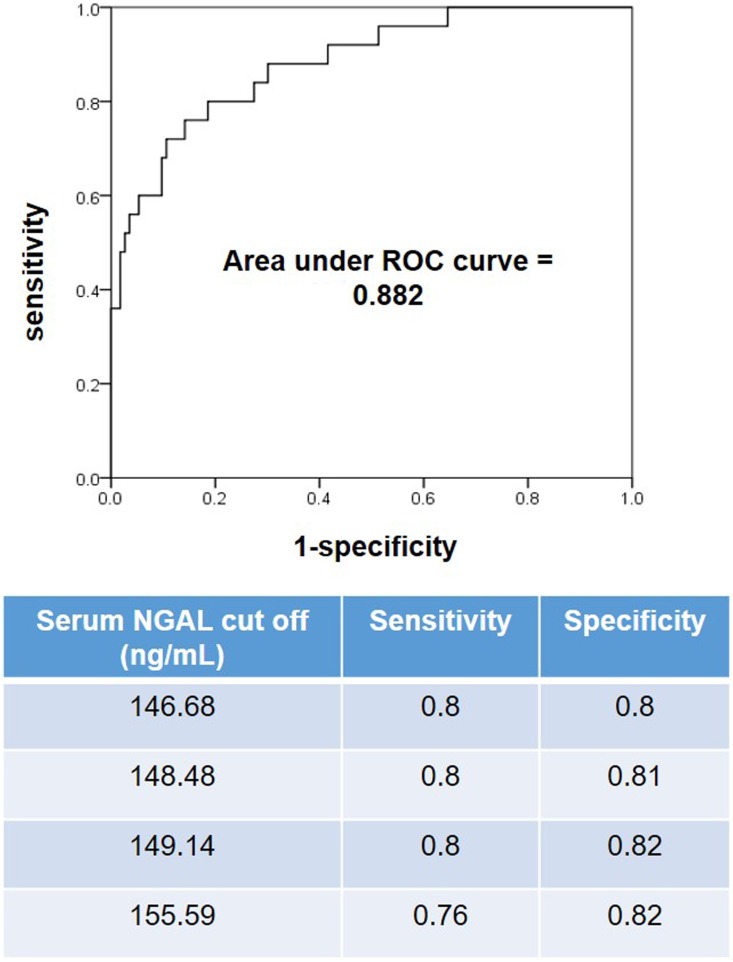
The NGAL and KIM-1 levels in serum and urine increased progressively with AKI severity based on the RIFLE criteria. Twenty-two patients had AKI prior to admission and three patients experienced AKI during their hospitalization. Nineteen patients recovered from AKI within 72 hours. Twenty-four patients with AKI recovered within 3 months without renal replacement therapy and one patient died due to septic shock from pneumonia. Using univariate analysis, age, diabetes, hypertension, chronic kidney disease, occurrence of at least one episode of hypotension, hemoglobin, total leukocyte count, serum albumin, serum NGAL, urine NGAL/creatinine, serum KIM-1, urine KIM-1/creatinine, IL-10, and TNF-α were significant predictors of AKI. After adjustment for these factors in a multivariate logistic regression analysis, the presence of chronic kidney disease and serum NGAL were identified as the only significant predictors of AKI (Table 4). The area under the ROC curve was 0.882 for serum NGAL. Serum NGAL of 149.14 ng/mL yielded good sensitivity (80%) and specificity (82%) (Fig 1).
Table 4. Predictors of AKI (multivariative analysis).
| Variables | Relative risk | 95% confidence interval Lower Upper |
P value |
|---|---|---|---|
| Serum NGAL | 1.04 | 1.003 1.019 | 0.006 |
| Presence of CKD | 67.01 | 2.120 2118.817 | 0.017 |
Fig 1. Receiver operating characteristic curve and performance characteristics for serum NGAL upon admission.
The area under the ROC curve for the serum NGAL test is 88% (CI 0.808–0.956).
Discussion
In the present study, we show that serum and urine levels of NGAL and KIM-1 are higher in patients with AKI than in those without AKI, and that serum NGAL and the presence of chronic kidney disease are significant predictors of AKI in scrub typhus. Therefore, serum NGAL could be an additive predictor for scrub typhus-associated AKI. Our findings provide a rationale for using NGAL as a diagnostic tool for the detection of AKI in patients with scrub typhus.
Scrub typhus is endemic to geographically distinct regions, which include Japan, Taiwan, China, and South Korea. It can cause multiorgan complications including AKI, and the incidence of AKI ranges from 21 to 43% [4–6]. In this study, the incidence of AKI was 18%, which was similar to previous results. However, the incidences were different according to the serum creatinine (17%) or urine output (5%) criteria. Because the patients with initial normal serum creatinine were not placed urinary catheter in presenting study, we believe that the incidence of AKI based on RIFLEUO might be underestimated. After more than a decade of intensive research efforts, several novel AKI biomarkers have been identified and their performance has been validated for the prediction of AKI in in various settings such as pediatric and adult cardiac surgery patients, critically ill patients, and patients in the emergency room, as well as in the kidney transplant setting [20–23]. However, to date, there have been no reports of evaluation of NGAL and KIM-1 in patients with scrub typhus.
We used RIFLE classification system in the presenting study instead of the Acute Kidney Injury Network (AKIN) classification or the Kidney Disease: Improving Global Outcome (KDIGO) AKI classification system. The major strength of RIFLE classification system is that it considers the change of any measure of renal function from baseline [24]. Given the absence of readily available methods for the measurement of GFR when serum creatinine concentration is not in a steady state, changes in GFR are not included in the AKIN or KDIGO AKI classification systems [24–26]. Baseline renal function was not available in 65 (47%) patients in this study. Furthermore, the diagnostic criteria for AKI by using AKIN and KDIGO system require at least 2 creatinine values within 48 hours. However, most patients with AKI in scrub typhus have the worst renal function at admission [6], which was also shown in this study. Therefore, we used the RIFLE classification system in this study instead of the AKIN or KDIGO criteria.
In the present study, we measured serum and urine NGAL and KIM-1 of patients with scrub typhus upon admission before treatment and found that NGAL and KIM-1 levels in both serum and urine were higher in the AKI group than in the non-AKI group. The NGAL and KIM-1 levels in serum and urine seemed to be correlated with AKI severity based on RIFLE criteria (R<I<F), and reduction of NGAL and KIM-1 levels was shown during serial measurement following treatment. Furthermore, serum NGAL and the presence of chronic kidney disease were significant predictors of AKI in scrub typhus. Therefore, we believe that serum NGAL could be an additive predictor for scrub typhus–associated AKI, as shown for other types of AKI in previous studies.
However, we question whether serum NGAL is clinically useful for the detection of AKI in patients with scrub typhus due to the characteristics of AKI in this setting. In general, biomarkers such as NGAL are valuable when they can detect AKI before the increase in plasma creatinine, thus allowing for earlier intervention. However, in our study 22 out of 25 patients with AKI had worsening of renal function prior to admission, similar to previous results [6]. Thus, AKI biomarkers, including serum NGAL, might be less useful in scrub typhus than in other clinical settings, because most patients with AKI associated with scrub typhus already had AKI prior to admission. However, three patients in our study experienced AKI within 72 hours after admission, and for these patients the mean serum NGAL levels (276 vs. 116 ng/mL) on admission were higher than those were for patients in the non-AKI group. Therefore, serum NGAL might be helpful to detect AKI in patients with scrub typhus in cases that develop during hospitalization. To clarify the utility of serum NGAL, studies with a larger number of scrub typhus participants that develop AKI during hospitalization are needed.
It was previously reported that NGAL could predict death or dialysis at the time of admission [26–28]. However, in this study, only one patient died, and no patients needed renal replacement therapy. The patient who died had AKI during hospitalization, which improved within several days. The cause of death was septic shock resulting from the aggravation of hospital-acquired pneumonia on the 45th hospital day. Therefore, we cannot estimate whether NGAL is a predictor of death or dialysis at the time of admission in scrub typhus–associated AKI.
The pathogenesis of O. tsutsugamushi infection involves initial infection of endothelial cells and subsequent perivascular infiltration of T cells and monocyte/macrophages, resulting in vasculitis [29, 30]. This interaction between microbes and endothelial cells triggers a wide range of inflammatory responses, including the production of several cytokines by endothelial and non-endothelial cells. It was reported that some cytokines, including IL-8, contribute to disease severity in patients with scrub typhus [31], and cytokines such as IL-6 and IL-10 were associated with the development of AKI [32]. In our study, levels of three cytokines, IL-10, TNF, and INF, were higher in the AKI group than the non-AKI group. However, these cytokines were not identified to be predictors of AKI in patients with scrub typhus. Washburn et al. reported that urinary IL-18 concentration increases in 24–48 hours prior to AKI based on the RIFLE criteria and is correlated with AKI severity [33]. In the present study, even though the results are statistically significant, we noted a trend of the correlation of IL-10 concentration (R<I<F). To reveal the relationship between AKI severity and cytokine more clearly, studies with a larger number of blood and urine samples to measure various cytokines are needed.
Our study has some limitations. First, the number of patients in the AKI group was too small and more studies with a larger population are needed. Second, we measured AKI biomarkers serially in only 37 patients. More samples are needed to evaluate the change in NGAL and KIM-1 more precisely.
To our knowledge, this is the first report to show the relationship between AKI biomarkers and scrub typhus. In our study, the incidence of scrub typhus–associated AKI was 18%, and serum NGAL and the presence of chronic kidney disease were significant predictors of AKI in scrub typhus. Therefore, we think that serum NGAL might be a helpful biomarker for the detection of AKI in patients with scrub typhus.
Supporting information
(XLSX)
Acknowledgments
The authors wish to acknowledge financial support of the Korean Society of Nephrology and Christian Medical Research Center, Presbyterian Medical Center, Jeonju, Korea.
Abbreviations
- NGAL
neutrophil gelatinase-associated lipocalin
- KIM-1
kidney injury molecule-1
- AKI
acute kidney injury
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
- eGFR
estimated glomerular filtration rate
- eGFR adm
eGFR at the time of admission
- MDRD
Modification of Diet in Renal Disease
- ALT
alanine aminotransferase
- CKD
chronic kidney disease
- BP
blood pressure
- ICU
intensive care unit
- FENa
fractional excretion of sodium
- RIFLEScr
RIFLE diagnosis based on RIFLE serum creatinine criteria only
- RIFLEUO
RIFLE diagnosis based on RIFLE urine output criteria only
- RIFLEScr+UO
RIFLE diagnosis based on both serum creatinine and urine criteria
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors wish to acknowledge financial support of the Korean Society of Nephrology and Christian Medical Research Center, Presbyterian Medical Center, Jeonju, Korea. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML et al. Scrub typhus among hospitalized patients with febrile illness in South India: magnitude and clinical predictors. J Infect 2006; 52:56–60 doi: 10.1016/j.jinf.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Mahajan SK. Scrub typhus. J Assoc Physician India 2005; 53:954–958 [PubMed] [Google Scholar]
- 3.Mandell GL, Douglas JR, Bennett JE. Principles and practice of infectious disease. 7th ed. Philadelphia: Churchill Livingstone; 2010. P.2529–2530 [Google Scholar]
- 4.Basu G, Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, et al. Acute kidney injury in tropical acute febrile illness in a tertiary care center-RIFLE criteria validation. Nephrol Dial Transplant 2011; 26(2):524–531 doi: 10.1093/ndt/gfq477 [DOI] [PubMed] [Google Scholar]
- 5.Attur RP, Kuppasamy S, Bairy M, Nagaraju SP, Pammidi NR, Kamath V, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol 2013; 17(5):725–729 doi: 10.1007/s10157-012-0753-9 [DOI] [PubMed] [Google Scholar]
- 6.Sun IO, Kim MC, Park JW, Yang MA, Lee CB, Yoon HJ, et al. Clinical characteristics of acute kidney injury in patients with scrub typhus-RIFLE criteria validation. J Infect Chemother. 2014; 20(2):93–96 doi: 10.1016/j.jiac.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Hsu GJ, Young T, Peng MY, Chang FY, Chou MY, Sheu LF, et al. Acute renal failure associated with scrub typhus: report of a case. J Formos Med Assoc 1993; 92:475–477 [PubMed] [Google Scholar]
- 8.Yen TH, Chang CT, Lin JL, Jiang JR, Lee KF. Scrub typhus: A frequently overlooked cause of acute renal failure. Renal failure 2003; 25:397–410 [DOI] [PubMed] [Google Scholar]
- 9.Thap LC, Supanaranond W, Treeprasertsuk S, Kitvatanachai S, Phonrat B. Septic shock secondary to scrub typhus: characteristics and complications. Southeast Asian J Trop Med Public Health 2002; 33:780–786 [PubMed] [Google Scholar]
- 10.Young PC, Hae CC, Lee KH, Hoon CJ. Tsutsugamushi infection associated acute rhabdomyolysis and acute renal failure. Korean J Intern Med 2003;18:248–250 doi: 10.3904/kjim.2003.18.4.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DM, Kang DW, Kim JO, Chung JH, Kim HL, Park CY, et al. Acute renal failure due to acute tubular necrosis caused by direct invasion Oriental tsutsugamushi. J Clin Microbiol 2008; 46:1548–1550 doi: 10.1128/JCM.01040-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singbartl K, Joannidis M: Short-term Effects of Acute Kidney Injury. Crit Care Clin 2015; 31:751–762 doi: 10.1016/j.ccc.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Hsu RK, McCulloch CE, Heung M, Saran R, Shahinian VB,Pavkov ME, et al. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Exploring Potential Reasons for the Temporal Trend in Dialysis-Requiring AKI in the United States. Clin J Am Soc Nephrol 2016; 11: 14–20 doi: 10.2215/CJN.04520415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alge JL, Arthur JM. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10(1):147–155 doi: 10.2215/CJN.12191213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury-pathophysiological basis and clinical performance. Acta Physiol 2016; July 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra R, Siew ED. Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 2017; 12(1):149–173 doi: 10.2215/CJN.01300216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 2008; 1:200–208 doi: 10.1111/j.1752-8062.2008.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8: R204–12. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53:766–772 doi: 10.1373/clinchem.2006.077180 [DOI] [PubMed] [Google Scholar]
- 20.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 2007; 11(6): R127 doi: 10.1186/cc6192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh CR, Coca SG, Thiessen-Philbrrok, Li S, Kim RW, Koyner JL, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011: 22:1748–1757 doi: 10.1681/ASN.2010121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Geus HR, Bakker J, Lesaffre EM, le Noble JL. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011; 183(7):907–914 doi: 10.1164/rccm.200908-1214OC [DOI] [PubMed] [Google Scholar]
- 23.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipoclin for diagnosing acute kidney injury. Ann Intern Med. 2008; 3;148(11):810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valette X, du Cheyron D. A critical appraisal of the accuracy of the RIFLE and AKIN classifications in defining “acute kidney insufficiency” in critically ill patients. J Crit Care. 2013;28(2):116–125 doi: 10.1016/j.jcrc.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 25.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31 doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138 [Google Scholar]
- 27.Nickolas TL, Schmidt-Ott KM, Canetta P, Forester C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardial. 2012; 59(3):246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bojan M, Vicca S, Lopez-Lopez V, Mogenet A, Pouard P, Falissard B, et al. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol 2013, 9(2):285–294 doi: 10.2215/CJN.04730513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parola P, Paddock CK, Raoult D. Tick-borne rickettsioses around the world: emerging disease challenging old concepts. Clin Microbiol Rev 2005; 18:719–756 doi: 10.1128/CMR.18.4.719-756.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol 2008; 6:375–386 doi: 10.1038/nrmicro1866 [DOI] [PubMed] [Google Scholar]
- 31.Astrup E, Janardhanan J, Otterdal K, Ueland T, Prakash JA, Lekva T, et al. Cytokine Network in scrub typhus: high levels of interleukin-8 are associated with disease severity and mortality. PLOS Negl Trop Dis. 2014; 8(2):e2648 doi: 10.1371/journal.pntd.0002648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers after pediatric cardiac surgery. Pediatr Nephrol 2015; 30(9): 1519–1527 doi: 10.1007/s00467-015-3088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR, et al. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant 2008;23(2):566–572 doi: 10.1093/ndt/gfm638 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.