Abstract
Zika virus (ZIKV) is an enveloped, positive-sense, single-stranded RNA virus that belongs to the genus Flavivirus, family Flaviviridae, which includes many human and animal pathogens, such as dengue virus (DENV), West Nile virus, and Japanese encephalitis virus. In the original as well as subsequent experimental and clinical reports, ZIKV seems to have moderate neurotropism (in animal models) and neurovirulence (in human fetuses), but no neuroinvasiveness (in human adults). Intrauterine ZIKV infection (viral pathology) has been linked to an increased incidence of microcephaly, while increased Guillain-Barré syndrome (GBS) following ZIKV infection is likely immune-mediated (immunopathology). Clinically, in ZIKV infection, antibodies against other flaviviruses, such as DENV, have been detected; these antibodies can cross-react with ZIKV without ZIKV neutralization. In theory, such non-neutralizing antibodies are generated at the expense of decreased production of neutralizing antibodies (“antigenic sin”), leading to poor viral clearance, while the non-neutralizing antibodies can also enhance viral replication in Fc receptor (FcR)-bearing cells via antibody-dependent enhancement (ADE). Here, we propose three potential roles of the antibody-mediated pathogenesis of ZIKV infection: 1) cross-reactive antibodies that recognize ZIKV and neural antigens cause GBS; 2) ZIKV-antibody complex is transported transplacentally via neonatal FcR (FcRn), resulting in fetal infection; and 3) ZIKV-antibody complex is taken up at peripheral nerve endings and transported to neurons in the central nervous system (CNS), by which the virus can enter the CNS without crossing the blood-brain barrier.
Keywords: Animal Models, Experimental Autoimmune Neuritis, Gangliosides, Placenta, Retrograde Axonal Flow, Theiler’s Murine En-cephalomyelitis Virus, Yellow Fever Virus
1. Introduction
1.1. Original reports of Zika virus (ZIKV)
ZIKV was first isolated in 1947 and reported in 1952 by Dick and colleagues.1,2 In searching for yellow fever virus (YFV) in a forested area called Zika3 approximately 12 kilometers northeast of the Virus Research Institute, in Entebbe, Uganda (Fig. 1), rhesus monkeys were housed in cages on wooden platforms along the one-mile length of Zika Forest.1 The serum from Rhesus 766, one of the sentinel monkeys, which developed transient fever without other clinical signs, was injected intracerebrally into Swiss albino mice. A filterable transmissible agent isolated from brains of infected mice, designated as ZIKV, was neutralized by convalescent serum from Rhesus 766. ZIKV was also isolated from mosquitoes, Aedes africanus, caught in Zika Forest.1,4
Fig. 1.
Zika Forest, located near Lake Victoria in Uganda. Zika virus (ZIKV) was first isolated from a sentinel monkey in Zika Forest in 1947.
Mice experimentally infected with ZIKV initially developed inactivity and ruffled fur, and then began to exhibit motor weakness and hind limb paralysis, usually followed by death.2 The pathological changes in ZIKV-infected mice were confined to the central nervous system (CNS): neuronal degeneration and cellular infiltration were found in the brain and spinal cord, and ZIKV was recovered only from the CNS. Mice younger than 6 weeks of age were more susceptible to ZIKV infection. Susceptibility also depended on the route of inoculation, with the intracerebral route being more effective than the intraperitoneal route.2 The intranasal route did not infect mice efficiently. ZIKV did not induce clinical signs in cotton-rats and rabbits, but rabbits produced antibody against ZIKV.2 ZIKV seemed to infect guinea pigs, but mouse-adapted ZIKV caused no disease in guinea pigs.2 Typically, experimentally infected monkeys had no clinical signs, while viremia and anti-virus antibodies were demonstrated in all ZIKV-infected monkeys.2
1.2. ZIKV and the family Flaviviridae
ZIKV is an enveloped, positive-sense, single-stranded (ss) RNA virus that belongs to the Spondweni serocomplex within the genus Flavivirus, family Flaviviridae5 that was reclassified from the family Togaviridae in 1986.6 The family Flaviviridae is composed of four genera (Table 1): Flavivirus, Hepacivirus, Pestivirus, and Pegivirus, which share similarities in virion morphology and genome organization, e.g., all members lack a 3’-terminal poly(A) tract.6 Many viruses of the family Flaviviridae are human and/or animal pathogens. For example, the genus Flavivirus includes the human pathogens of YFV, dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV), and tick-borne encephalitis virus (TBEV). The genus Hepacivirus includes hepatitis C virus (HCV) that causes liver cirrhosis and hepatocellular carcinoma.7 The genus Pestivirus contains animal pathogens of major economic importance, including bovine viral diarrhea virus (BVDV), classical swine fever virus (CSFV),8 and Border disease virus (BDV).9 The genus Pegivirus contains Theiler’s disease-associated virus (TDAV).10,11
Table 1.
The family Flaviviridae and its diseases
| Virus | Genus | Mode of transmission |
Disease | |||
|---|---|---|---|---|---|---|
| Arthralgia | Hemorrhagic fever | Encephalitis | ||||
| DENV | ○ | ○ | ||||
| JEV | ○ | |||||
| SLEV | Flavivirus | mosquito or tick | ○ | |||
| TBEV | ○ | |||||
| WNV | ○ | ○ | ||||
| YFV | ○ | |||||
| ZIKV | mosquito human-human |
microcephaly? GBS? myelitis? | ||||
| HCV | Hepacivirus | human-human | hepatitis, liver cirrhosis, hepatocellular carcinoma | |||
| BDV | sheep-sheep | conjunctivitis, diarrhea, anorexia, abortion, tremor | ||||
| BVDV | Pestivirus | cattle-cattle | fetal infection, diarrhea infertility | |||
| CSFV | swine-swine | conjunctivitis, diarrhea, weakness, dullness | ||||
| TDAV | Pegivirus | horse disease | hepatitis | |||
BDV, Border disease virus; BVDB, bovine viral diarrhea virus; CSFV, classical swine fever virus; DENV, dengue virus; GBS, Guillain-Barré syndrome; HCV, hepatitis C virus; JEV, Japanese encephalitis virus; SLEV, St. Louis encephalitis virus; TBEV, tick-borne encephalitis virus; TDAV, Theiler’s disease-associated virus; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, Zika virus
Flaviviruses bind to the cell surface receptor via the envelope (E) protein, leading to receptor-mediated endocytosis. Fusion of the viral and host membranes occurs during endosomal trafficking, where E protein dimers dissociate and become fusogenic trimers.12 Following fusion of the viral envelope and cell membrane,13 viral RNA genomes are delivered into the cytoplasm, where the RNA genome has three roles : mRNA, template during replication, and genetic material in virion. The RNA genome is initially translated into a single polyprotein either cap-dependently (in the genus Flavivirus) or using an internal ribosome entry site (IRES) (in the other three genera).14 The polyprotein precursor is proteolytically processed to form multiple functional viral proteins.15 Virions bud into the endoplasmic reticulum (ER), and are released at the cell surface by exocytosis.16
1.3. ZIKV infection in humans
Almost all viruses of the genus Flavivirus are arthropod (mosquito or tick)-borne viruses (or arboviruses); many are human pathogens, including DENV, JEV, SLEV, TBEV, WNV, and YFV. Flaviviruses cause three types of severe diseases in humans: febrile illness with arthralgia by DENV and WNV; encephalitis by JEV, SLEV, TBEV, and WNV17; and hemorrhagic fevers by DENV and YFV. YFV and DENV are the two top viruses causing fatal hemorrhagic fever in humans annually. YFV kills 30,000 people/year and DENV-induced dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) result in 22,000 deaths/year18; these numbers of annual deaths are much higher than more widely publicized viruses, such as hantavirus (10, 000 deaths), Lassa virus (5,000 deaths), and Ebola virus (less than 100, except during the 2014 outbreak with more than 10,000 deaths). Effective vaccines have been developed for YFV,19 JEV, and TBEV, while there is a great need for vaccines against other flaviviruses, particularly DENV and WNV.
Human cases of ZIKV infection were first reported in the 1950s in Africa.20,21 However, Simpson,22 in reporting himself as the first proven human case of ZIKV infection, contended that on closer analysis the previously reported patients were infected instead with Spondweni virus, a closely related mosquito-borne flavivirus first isolated in 1955 from Mansonia uniformis mosquitoes, collected from Lake Simbu, located in the Spondweni region in South Africa.23 Outside Africa, human infection with ZIKV was subsequently reported in the 1970s in southeast Asia,24,25 and ZIKV was isolated from Aedes aegypti in Malaysia in 1969.26 However, no large-scale outbreaks of ZIKV infection occurred until 2007, when, quite unexpectedly, more than 100 inhabitants on Yap Island, in the Federated States of Micronesia, were infected.27 The principal mosquito vector was Aedes hensilii. Subsequently, a much larger ZIKV outbreak, with an attack rate of 66%, occurred in French Polynesia in 2013.28
Like other mosquito-borne flaviviruses, ZIKV infects humans mainly by mosquito bites. Mosquito-mediated transmission of ZIKV is initiated when blood-feeding female Aedes mosquito injects the virus into human skin, followed by infection of cells in the epidermis and/or dermis via virus-specific receptor binding. Although the ZIKV receptors in vivo are unknown, in vitro infection of ZIKV can be mediated by DC-SIGN and TAM receptors (Axl and Tyro3),29,30 all of which have also been reported to facilitate viral entry of DENV.
Unlike other flaviviruses, ZIKV is unusual in that it can also be transmitted vertically from mother to fetus during pregnancy,31 as well as horizontally by sexual intercourse between either male and female or male and male partners.32–34 The other striking peculiarity is that the ZIKV outbreaks in French Polynesia and Brazil have been associated with serious neurological sequelae, notably microcephaly, Guillain-Barré syndrome (GBS), and acute myelitis.35,36 As a result, on February 1, 2016, the Director-General of the World Health Organization (WHO) convened an Emergency Committee, under the International Health Regulations (IHR), and following the Committee’s advice, declared that the recent cluster of microcephaly cases and other neurological disorders reported in Brazil, following a similar cluster in French Polynesia in 2014, constitutes a Public Health Emergency of International Concern (PHEIC).37 The term PHEIC is defined in the IHR as “an extraordinary event that is to constitute a public health risk to other States through the international spread of disease and to potentially require a coordinated international response”.
The marked rise in microcephaly among neonates born to ZIKV-infected mothers, especially in French Polynesia and Brazil, cannot be ignored or dismissed.38 Preliminary data from a case-control study in Brazil indicate that the “microcephaly epidemic is a result of congenital Zika virus infection”.39 Moreover, a study by the Centers for Disease Control and Prevention concluded that a causal relationship existed between prenatal ZIKV infection and microcephaly,40 and a strong association was found between the risk of microcephaly and ZIKV infection risk in the first trimester.41 Despite these reports, other factors known to cause microcephaly and other neurological outcomes have not been completely excluded.
2. Pathogenesis of ZIKV infection
2.1. Viral pathology versus immunopathology
Tissue injury in viral infections can be induced mainly by two pathomechanisms: direct virus infection (viral pathology) and immune-mediated tissue damage (immunopathology)42 (Table 2). In viral pathology, viral replication inside host cells leads to destruction of the cell plasma membrane and release of virions as well as death of infected cells.43 Cell lysis is a common outcome of infections by most nonenveloped viruses, and some enveloped viruses. In immunopathology, uncontrolled cellular and humoral immune effector cells and molecules, such as pro-inflammatory cytokines, result in tissue damage. The pathomechanism by which virus infection causes histopathology is determined by several factors, including the virus strain, infection route, hosts’ age and genetic background, time after virus infection, and tissue tropism.43
Table 2.
Two possible pathomechanisms of virus-induced diseases
| Pathomechanism | Factor | Representative disease |
Experimental model |
ZIKV |
|---|---|---|---|---|
| Viral pathology | Viral replication and molecules |
rabies, poliomyelitis, PML |
Friend virus, TMEV GDVII |
microcephaly |
| Immunopathology | Immune cells and molecules (T cells, antibodies, cytokines, NO) |
GBS, ADEM | WNV, TMEV DA |
GBS, myelitis |
ADEM, acute disseminated encephalomyelitis; GBS, Guillain-Barré syndrome; NO, nitric oxide; PML, progressive multifocal leukoencephalopathy; TMEV DA, Daniels strain of Theiler’s murine encephalomyelitis virus; TMEV GDVII, GDVII strain of Theiler’s murine encephalomyelitis virus; WNV, West Nile virus; ZIKV, Zika virus
Clinically, some virus infections result in CNS tissue damage by viral pathology; for example, rabies virus and poliovirus directly infect and kill neurons, while direct infection of oligodendrocytes by human polyomavirus JC results in demyelination in progressive multifocal leukoen-cephalopathy (PML).44 In experimental Friend virus infection, the tissue damage is caused by viral replication (viral pathology)45; here suppression of anti-viral immune responses (antiviral inflammation) is detrimental, since viral replication is enhanced when anti-viral immunity is suppressed.45 On the other hand, GBS and acute disseminated encephalomyelitis (ADEM) are caused by pure immunopathology, and can be triggered even after the clearance of the pathogen or after vaccination with inactivated virus.44 In experimental WNV infection in mice, the tissue damage is caused by immunopathology; here, suppression of anti-viral immune responses reduces pathology.46
Some viruses cause tissue damage by either viral pathology or immunopathology as well as a combination of the two pathomechanisms, depending on extenuating conditions.47 For example, Theiler’s murine encephalomyelitis virus (TMEV), a positive-sense ssRNA virus belonging to the family Picornaviridae, has been used widely for neurovirology and neuropathology studies in mice. Intracerebral injection of the highly virulent GDVII strain of TMEV results in infection of neurons, where the virus replicates in the CNS efficiently, without induction of anti-virus immune responses, killing mice by pure viral pathology.48 A less virulent Daniels (DA) strain of TMEV also infects neurons in the brain about 1 week after infection (acute phase) with more mild viral pathology. Then, about 1 month after infection (chronic phase), the DA strain induces an inflammatory demyelinating disease, a viral model for multiple sclerosis (MS), mainly by immunopathology with limited viral replication. Host age also influences the susceptibility and pathomechanism of TMEV infection; neonatal mice develop fatal encephalopathy (viral pathology),49 young mice develop an inflammatory demyelinating disease (immunopathology > viral pathology), and older mice can clear TMEV without pathology.
On the other hand, intraperitoneal injection of TMEV can efficiently induce myocarditis, instead of CNS disease, in some mouse strains; direct viral replication in the cardiomyocytes damages the heart 4 days after infection (phase I, viral pathology), while T-cell infiltration damages the heart 1–2 weeks after infection (phase II. immunopathology).50 In ZIKV infection, we propose that two different pathomechanisms cause different neuropathology; microcephaly is likely caused by viral pathology in the brain (see section 2.3), while GBS is likely caused by immunopathology in the peripheral nervous system (PNS) (see section 3.4).
2.2. Neuroinvasiveness, tropism, and virulence
Defining three key categories is essential to understanding the pathogenesis of CNS virus infections: neuroinvasiveness, neurotropism, and neurovirulence.51 Neuroinvasiveness is the ability of the virus to enter the CNS. Neurotropism is the ability of the virus to infect neural cells, i. e., any one of four major CNS parenchymal cell types: neurons, oligodendrocytes, astrocytes, and microglia (neuronotropism means infections specifically of neurons; e.g., poliovirus efficiently infects only neurons). Neurovirulence is the ability of the virus to cause CNS disease. Mumps virus can enter the CNS easily (high neuroinvasiveness), but infect ependymal cells, not parenchymal cells (low neurotropism), and cause only mild meningitis (low neurovirulence) (Table 3). Human T-lymphotropic virus (HTLV) 1 has low neuroinvasiveness and no neurotropism (infects only CD4+ T cells) but high neurovirulence, inducing severe spinal cord damage, resulting in HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP).44,52 Rabies virus can enter the CNS from peripheral nerves, and infect CNS neurons efficiently, with a 100% case fatality rate in humans (high neuroinvasiveness, high neurotropism, and high neurovirulence). Experimental intracerebral injection of TMEV results in infection of neurons in the brain of all infected mice (high neurotropism), and causes severe neurological diseases, including polioencephalitis and demyelination (high neurovirulence), while the peripheral injection of TMEV usually does not cause CNS infection (low neuroinvasiveness).
Table 3.
Three viral abilities determine neuropathogenesis
| Viral ability | Mumps | Rabies | HTLV | WNV | TMEV | ZIKV | |
|---|---|---|---|---|---|---|---|
| Neuroinvasiveness | + + + | + + + | + | + | + | − adult* |
+ fetus** |
| Neurotropism | − | + + + | − | + + | + + + | + + mouse*** |
+ ? human |
| Neurovirulence | + | + + + | + + + | + + | + + + | − adult* |
+ + fetus** |
HTLV, human T-lymphotropic virus; TMEV; Theiler’s murine encephalomyelitis virus infection (in mice); WNV, West Nile virus; ZIKV, Zika virus
, in human adults
, in human fetuses
, in mice infected with ZIKV intracerebrally
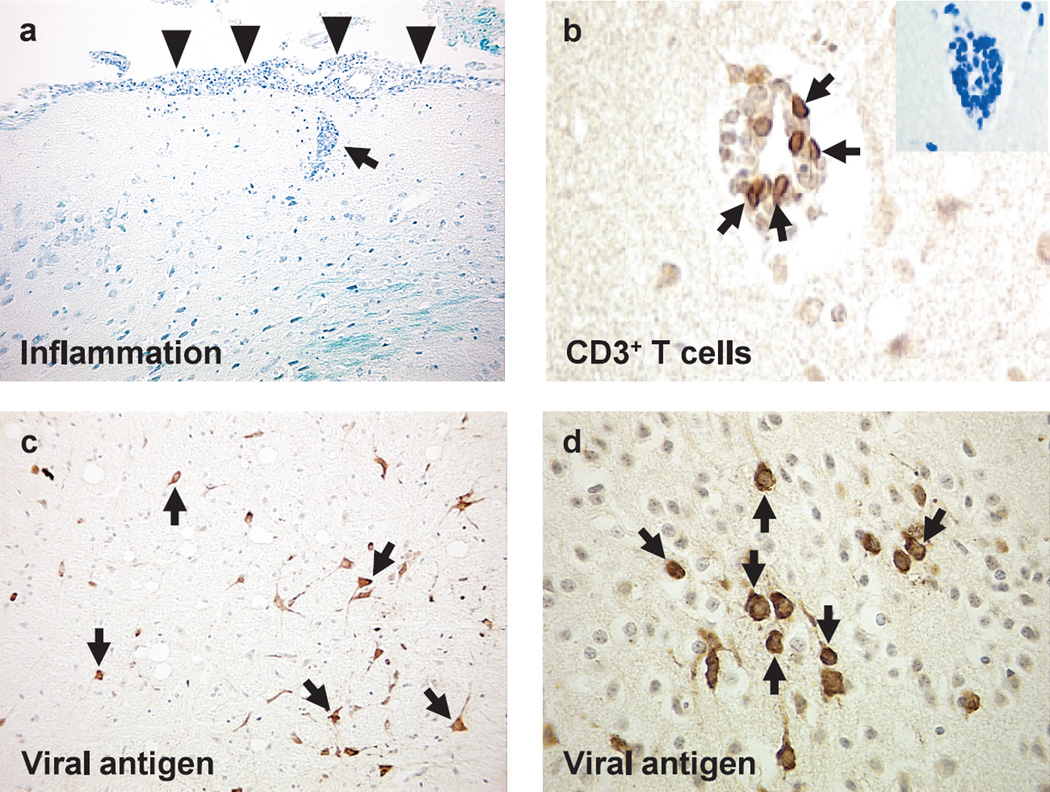
To distinguish the three categories is sometimes difficult; it is possible to dissect out three components only in animal experiments where animals can be infected by different routes of inouclation. Intracerebral innoculation is the best way to assess neurotropism, while the peripheral innoculation, including intravenous, intraperitoneal, intranasal, subcutaneous, and intramuscular injections, is used to evaluate neuroinvasiveness. For example, among flaviviruses, WNV has an animal model that can be used to determine three categories of neuropathogenesis. From animal experiments as well as epidemiological and clinical findings in humans, WNV seems to have low to moderate neuroinvasiveness, moderate neurotropism, and moderate neurovirulence, since WNV infects focal, but not diffuse, neurons in the CNS53–55 (Fig. 2), and causes WNV meningoencephalitis only in a small minority of elderly patients through mosquito bites.56
Fig. 2.
Neuropathology of West Nile virus (WNV) encephalitis. The brain was harvested from mice infected with WNV subcutaneously, and embedded in paraffin.55 (a) Meningitis (arrowheads) and perivascular cuffing (arrow) composed of mononuclear cells in the brain (Luxol fast blue stain). (b) T cells (arrows) in the perivascular cuffing by immunohistochemistry against CD3. (c, d) Viral antigens (arrows) in the cytoplasm and cell processes of neurons by immunohistochemistry with rabbit anti-WNV antibody (1 : 4,000 dilution, 81-015, BioReliance, Rockville, Maryland, USA), following trypsin treatment.
ZIKV seems to have no neuroinvasiveness in adults, since infected individuals do not develop CNS infection following viremia or intravenous injections, either clinically or experimentally. On the other hand, ZIKV may have high neurotropism because intracerebral injection in animal models and intrauterine transmission in fetuses result in active CNS viral replication. Recent studies in animal models suggest that ZIKV first infects the placenta and then preferentially neural progenitor cells of the fetal brain.57–62
Regarding ZIKV neurovirulence, several factors appear to be important, since 1) intracerebral infections with high-dose ZIKV resulted in severe neurological diseases depending on age and species, and 2) only in the past few years, ZIKV infection has been associated with neurological diseases, which may depend on virus strains, host factors, or vectors (mosquito species). Phylogenetic trees based on whole genome sequences divided ZIKV isolates into three genotypes: West African, East African, and Asian. Although all viral isolates from recent ZIKV epidemics belong to the Asian genotype,63 it is unclear whether the differences between the genotypes influence any aspects of viral characteristics.
2.3. CNS fetal virus infections and microcephaly
While intrauterine infections that lead to congenital abnormalities are relatively rare, virus can infect the fetus mainly from the blood across the placenta following viremia in the mother.64 The placental barrier excludes some substances, while other particles, including immunoglobulin (Ig) G, can pass the placenta with ease (ZIKV may use this physiological transport system to infect fetuses; see section 3.4). The placental barrier prevents virus from fetal infections, some viruses can infect the placenta, which may alter its barrier function. Among viruses that can cause human fetal infections, rubella, cytomegalovirus, and human immunodeficiency virus (HIV) infections of the fetus are commonly associated with fetal CNS diseases, including microcephaly. Here, viral infections of the fetus result in congenital abnormalities with or without cytopathic effects.
‘Microcephaly’ means small head; head circumference measurements of two or more standard deviations below the mean are abnormal.65 Because the brain and skull usually grow in parallel, the term ‘microcephaly’ is generally used to indicate smallness of the brain. Microcephaly can be classified into two groups, with or without associated malformations (including genetic disorders). Microcephaly without associated malformations can be caused by virus infections, particularly rubella virus and cytomegalovirus. In ZIKV infection, although the precise mechanism of microcephaly is unknown, ZIKV RNA has been found in the amniotic fluid and brain of fetuses and infants with microcephaly. Also, the high rates of microcephaly among infants born to ZIKV-infected mothers linked microcephaly to maternal ZIKV infection.32 Oliveira Melo and colleagues demonstrated fetal microcephaly cases with brain calcifications using ultrasound.66 The presence of brain calcifications has been reported in other fetal virus infections and suggestive of an intrauterine CNS infection. These results suggest that microcephaly is induced by direct ZIKV infection in the brain of fetuses (viral pathology).67
3. Roles of antibody in ZIKV infection
3.1. Antigenic sin and antibody-dependent enhancement (ADE)
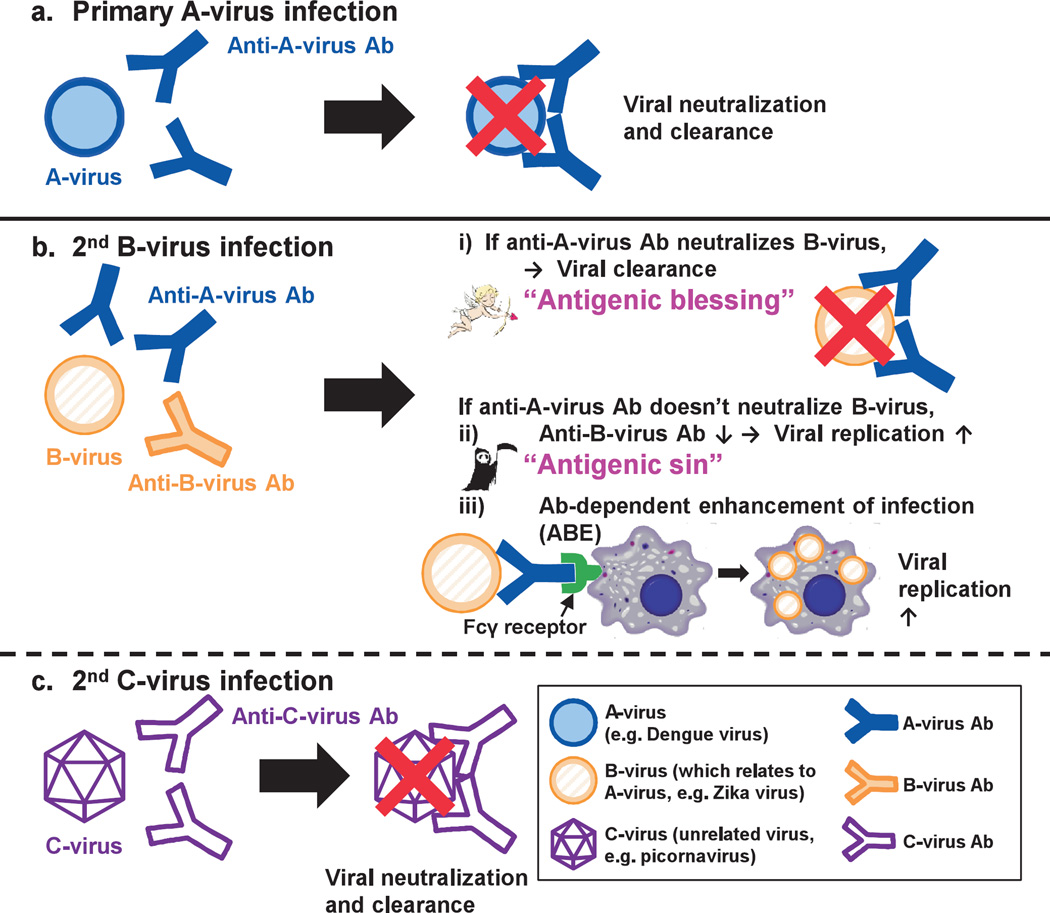
Generally, in virus infections, anti-viral antibodies bind extracellular viruses, resulting in neutralization and clearance of viruses from the host. For example, A-virus infection induces anti-A-virus antibodies that bind, neutralize, and clear the A-virus during primary infection (Fig. 3a). Then, when another irrelevant viral infection follows, the host immune system makes antibodies to the second virus, which results in viral neutralization and clearance. In Fig. 3c, the second infection with irrelevant C-virus induces anti-C-virus antibodies that neutralize and clear the C-virus. However, sequential infections with viruses, containing epitopes that are cross-reactive to related viruses, can induce higher antibody titers to the first virus than to those of the newly infecting second virus; the antibody responses to the originally infected virus are reinforced at the expense of response to the newer virus.68 In Fig. 3b, the secondary infection with B-virus that mimics A-virus antigenically induces higher antibody responses to A-virus than to B-virus.
Fig. 3.
Clearance or enhancement of viral infections mediated by anti-viral antibodies in the sequential viral infections, (a) In the primary infection with A-virus (e.g., dengue virus belonging to the genus Flavivirus), anti-A virus antibodies (Abs) are produced, resulting in viral neutralization and clearance. Here, a second infection with B-virus that is related to A-virus (e.g., Zika virus belonging to the genus Flavivirus) (b) or with irrelevant C-virus (e.g., picornavirus) (c) can occur, (c) C-virus infection induces only anti-C-virus Abs, but not anti-A-virus Ab, resulting in neutralization and clearance of the C-virus. (b) On the other hand, since B-virus mimics A-virus antigenically containing epitopes that are cross-reactive to A-virus, B-virus infection can induce higher Ab responses to A-virus than B-virus. Then, if increased anti-A-virus Abs neutralize B-virus, this results in efficient clearance of B-virus (“antigenic blessing”). On the contrary, if anti-A-virus Abs have no neutralizing activity to B-virus, decreased anti-B-virus Ab production at the expense of increased anti-A-virus Ab production results in poor clearance of B-virus (“antigenic sin”). Moreover, the Fc region of the virus-antibody complex can be captured by Fc receptor (FcR)-positive (+) cells, such as macrophages, leading to viral replication in FcR+ cells, which is termed “antibody-dependent enhancement of infection (ADE)”.
Although this phenomenon is termed “original antigenic sin”, its outcome can be a sin (detrimental) or blessing (beneficial), which may differ among virus infections. The outcome depends on whether or not the cross-reactive antibodies have neutralizing abilities. When the antibody neutralizes the secondary infecting virus, this means that sensitization (≒ vaccination) against the original virus can protect a virus infection with the second related virus. In Fig. 3bi, when increased anti-A-virus antibodies neutralize B-virus, this results in efficient clearance of B-virus (“antigenic blessing”). On the other hand, as suggested in influenza virus infections, when the antibody has no neutralizing activity to the second virus, the antibody response to the previous infecting virus is more vigorous than the response to the current one, which reduces production of the neutralizing antibody to the newly infecting virus, resulting in more active viral replication. In Fig. 3bii, decreased anti-B-virus antibody production at the expense of increased anti-A-virus antibody production results in poor clearance of B-virus.
There is another mechanism by which non-neutralizing anti-viral antibody can increase pathogenesis; in some viral infections, such as DENV, Ebola virus, and HIV, anti-viral antibody has been shown to enhance infections of virus instead of its clearance; this phenomenon is termed “antibody-dependent enhancement (ADE) of infection”69. Both protective immunity and increased pathogenesis in secondary viral infections have been observed in flavivirus studies.70 DENV belongs to the family Flaviviridae, genus Flavivirus, the same genus as ZIKV, and has four serotypes. Following a primary infection with one serotype of DENV, patients infected with another serotype of DENV have been reported to develop more severe disease, such as DHF and DSS.71,72 One explanation of the exacerbation of the secondary DENV infection is ADE, where antibodies generated during the primary DENV infection bind, but do not neutralize, the DENV of a different serotype during the secondary infection, then virus-antibody complex is captured by Fc receptor (FcR) and/or complement (C’) and C’ receptor (CR) on phagocytes, such as monocytes and macrophages, enhancing infection of FcR+ and/or CR+ phagocytes. In Fig. 3biii, anti-A-virus antibody binds B-virus, but does not neutralize B-virus, and then the Fc region of the virus-antibody complex is captured by FcR+ cells. This results in viral replication in the FcR+ cells, instead of viral clearance. It should be noted that the anti-viral antibody class that can cause ADE by itself is IgG, but not other classes, since major FcRs on phagocytes are Feγ receptors; FcμR is very rare in general and not expressed on phagocytes in humans or mice73; IgM is not involved in ADE by itself, but virus-IgM-C’-CR-mediated ADE might be possible, in theory.
3.2. Neutralizing versus non-neutralizing cross-reactive antibodies
In the original ZIKV report,1 Dick and co-workers quantified serum neutralizing titers using an “intracerebral” neutralization test, described by Max Theiler, in which mixtures of serum and serially diluted virus-infected mouse brain were intracerebrally inoculated into mice. They demonstrated that anti-serum against ZIKV did not neutralize YFV, DENV, or TMEV (FA, GDVII, and TO strains)74,75; reciprocally, anti-sera against the above three viruses and other viruses, including WNV and SLEV, did not significantly neutralize ZIKV.
On the other hand, anti-sera against ZIKV have been shown to bind (cross-react with) other flavivirus antigens, including DENV and YFV. Thus, in theory, the presence of one flavivirus antibody can lead to ADE of a secondary infection with other flaviviruses when the flavivirus antibody cross-reacts with other flavivirus epitopes, without having a viral neutralization ability. Dejnirattisai and colleagues76 demonstrated that plasma from patients infected with DENV (any four serotype) cross-reacted with ZIKV but without ZIKV neutralization. The DENV plasma increased not only ZIKV binding to the human myeloid cell line U937, detected by flow cytometry, but also viral titers in the supernatant, determined by focus forming assay (although the kinetics of intracellular viral replication was not shown). Monoclonal antibodies (mAbs) to DENV also enhanced ZIKV binding to U937, while some mAbs to DENV E protein dimer inhibited ZIKV binding. It was not shown that the anti-DENV-dimer mAbs inhibited the conformational change from dimers to trimers of E protein; the conformational change of E protein is necessary for virus-cell fusion for viral genome entry into the cytoplasm in flavivirus infections.13
The above study is somewhat inconsistent with the study of samples from French Polynesian patients with GBS by the same research group.77 In the French Polynesian patients, only 19% of anti-ZIKV IgM samples cross-reacted with DENV, while 100% of sera from ZIKV patients neutralized both ZIKV and DENV (even five DENV IgG-negative serum samples neutralized DENV). It is unclear: 1) how anti-ZIKV antibodies neutralized DENV despite the lack of cross-reactivity to DENV,77 and 2) why the majority of anti-ZIKV antibodies did not cross-react with DENV in the French Polynesian study, while anti-DENV sera and mAbs showed high cross-reactivity to ZIKV without neutralizing ZIKV in another study by the same research group.76
The considerable cross-reactivity of flavivirus antibodies should be taken into account when serologic test results are interpreted. One suggests that samples positive for anti-ZIKV IgM antibody and negative for anti-DENV IgM may be interpreted as presumptive recent ZIKV infection.32 However, this cannot rule out the possibility of other flavivirus infections, particularly YFV, which are circulating in the areas where ZIKV is endemic.
3.3. Guillain-Barré syndrome in ZIKV infection
GBS is characterized by the development of a rapidly progressive paralytic syndrome with several patterns of PNS involvement,78 including acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor sensory axonal neuropathy (AMSAN), and Miller Fisher syndrome (MFS).79 Approximately 70% of GBS cases occur 1–3 weeks after infections with various microbes including Campylobacter (C.) jejuni, Mycoplasma pneumoniae, cytomegalovirus, Epstein-Barr virus, HIV, and hepatitis viruses.80 Experimental autoimmune neuritis (EAN) has been used for an animal model for GBS; EAN can be induced by sensitization with PNS antigen, and is similar to GBS, clinically, neuropathologically, and immunologically. Thus, microbial infections have been proposed to cause GBS, for example, due to molecular mimicry between microbes and PNS antigens, which triggers generation of detrimental immune responses that recognize not only microbes but also PNS antigens, leading to PNS pathology. In GBS associated with C. jejuni infection, anti-C. jejuni antibodies, which cross-react with gangliosides present in a large quantity in nervous tissues, including nodes of Ranvier, seem to play a pathogenic role.79
Although ZIKV infection has often been described as a possible cause of GBS in the popular press, there have been only a few case reports with generally weak evidence that ZIKV is associated with GBS. Braisl and co-workers81 reported a case report of GBS in a patient who had DENV infection 5 years ealier, and developed neurological signs including muscle weakness and areflexia. Real-time PCR for ZIKV RNA was positive in serum, cerebrospinal fluid (CSF), saliva, and urine, but antibody responses to ZIKV were not tested.
There is only one case-control study that investigated the association between ZIKV infection and GBS.77 Between October 2013 and April 2014, French Polynesia (which includes Tahiti) experienced a large ZIKV outbreak with more than 32,000 patients suspected of having ZIKV infection. During the period, 42 GBS patients presented to hospital, while the annual number of cases of GBS in French Polynesia between 2009 and 2012 was 3–10. In the 42 GBS cases, of whom 74% patients were men, the patients had clinical signs/symptoms of viral infection, including conjunctivitis, rash, fever, and arthralgia, 4–10 days before the onset of neurological signs/symptoms, such as muscle weakness, areflexia, facial palsy, and paresthesia. Electrophysiological findings were compatible with AMAN, and the patients were treated with intravenous Igs (IVIG). While mechanical ventilation was required in 29% of patients, no patients died; 3 months after discharge, 57% walked without assistance.
There are several concerns about the above study. First blood samples were collected from the 42 GBS patients at hospital admission (2–8 days after the onset of neurological symptoms); 98% patients had antibodies against ZIKV, but viral RNA was not detected in any of the samples.77 In the control group samples from those with acute ZIKV infection without neurological symptoms, viral RNA was detected in all samples, while anti-ZIKV antibodies were not examined. In the other control group samples from patients with non-febrile illnesses, 36% of samples had ZIKV antibodies, while viral RNA was not examined. This study did not set up a control group composed of non-GBS patients with neurological diseases. Antibodies to glycolipids (GM1, GA1, GM2, GDla, GDlb, or GQlb) were detected in 31% of patients at the onset of GBS, in which GA1 antibody responses were the highest (19%). Cross-reactivity was not found between GA1 and ZIKV in two samples examined, while no other cross-relativities, such as between ZIKV and other glycolipids or flaviviruses, were examined. Thus, it is unclear whether anti-GAl antibodies were associated with ZIKV infection, while anti-GA1 antibody has been detected in other GBS, including MFS.82
As discussed above, the pathogenesis of ZIKV-associated GBS remains unclear. A recent report of 66 ZIKV-associated GBS cases in Colombia indicated that GBS may be a heterogeneous disease in ZIKV-infected patients.83,84 Clinically, about a half of the GBS patients developed neurologic symptoms during or immediately after ZIKV infecton; thus, this group of patients had a “parainfectious” onset, not a postinfectious onset typically seen in GBS. Nerve conduction studies showed that 78% of patients had the AIDP subtype, and only one patient had the AMAN subtype. Other notable findings included : 1) 50% patients had bilateral facial paralysis; 2) ZIKV RT-PCR was positive in 40% of urine samples, but in only three CSF samples; and 3) an anti-DENV antibody kit was used to examine anti-flavivirus antibody responses, but anti-ZIKV, YFV or glycolipid antibody responses were not tested.
3.4. Three hypothetical roles of anti-ZIKV-antibody in GBS, placental entry, and neuronal spread
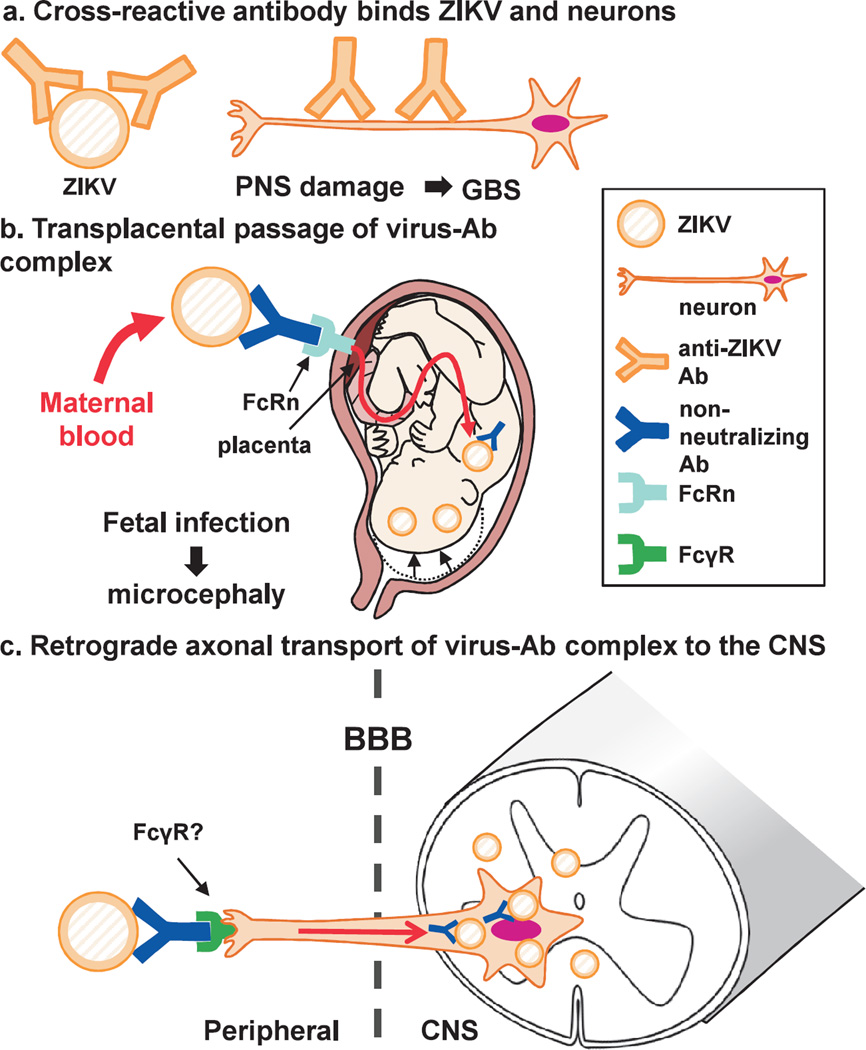
Although the precise pathomechanisms of microcephaly and GBS associated with ZIKV infection are currently unknown, we hypothesize that anti-ZIKV antibody may contribute to neurovirulence of ZIKV in three ways : 1) cross-reactive antibody recognizes both viral antigen and neuroantigen; 2) transplacental passage of the virus; and 3) axonal transport of the virus to the CNS. The second and third ways are mediated by FcR expressed on the placenta and nerve endings, respectively, resulting from the capture of the Fc region of a virus-antibody complex.
First, we hypothesize that anti-ZIKV antibody may be responsible for induction of GBS (Fig. 4a). GBS usually involves only the PNS, and neither the CNS nor other visceral organs.85 Interestingly, in ZIKV infection, although increased GBS cases have been reported, there is no increase in cases with other immune-mediated diseases or CNS demyelinating diseases, including ADEM and MS86; ADEM and MS have been associated with virus infections and autoimmune responses to CNS myelin antigens, while their precise pathomechanisms are unclear.87 Thus, we hypothesize that ZIKV infection can induce cross-reactive (autoimmune) responses specific for PNS antigens, but induce neither CNS-specific autoimmunity nor dysregulation of autoimmune responses in general.
Fig. 4.
Three hypothetical pathogenic roles of anti-Zika virus (ZIKV) antibody (Ab) in Guillain-Barré syndrome (GBS) and microcephaly, (a) Anti-ZIKV antibodies may cross-react with neural antigens specific to the peripheral nervous system (PNS), which damages PNS, leading to GBS. (b) Non-neutralizing IgG can form ZIKV-IgG immune complex, which can be captured by the neonatal Fc receptor (FcRn) on the placenta without viral neutralization. This will lead to transplacental passage of the immune complex, and infection of the fetus, causing microcephaly, (c) The immune complex, composed of ZIKV and non-neutralizing IgG antibody, is taken up at the nerve ending via Feγ receptor (FcγR) in the periphery, and then transported to the cell body of neurons in the central nervous system (CNS), using retrograde axonal flow. Here, by this neural route, the virus can enter the CNS without crossing the blood-brain barrier (BBB).
In GBS, autoantibodies against neural antigens, particularly gangliosides, are present in the sera from approximately 60% of GBS patients, and are useful diagnostic markers and possible pathogenic factors79,88; the autoantibody responses may also play a role in ZIKV-associated GBS. Although it is unknown how such autoantibody responses are induced in most GBS cases, there is strong clinical and experimental evidence that, in GBS cases with C. jejuni infection, molecular mimicry between C. jejuni and ganglioside leads to generation of antibodies that react to both C. jejuni and gangliosides, particularly GM1, which induce PNS damage.89 Such autoantibody responses may also be induced, playing a pathogenic role in induction of ZIKV-associated GBS (Fig. 4a), while, so far, there has been no report on molecular mimicry between ZIKV and PNS antigens. It should be noted that GBS following DENV infection is uncommon, while there are several DENV-associated GBS case reports.90–92 Thus, antigenic epitopes responsible for ZIKV-associated GBS may be unique in ZIKV and not common with DENV.
Second, anti-ZIKV antibody may play a role in transplacental transport of the virus from maternal blood to the fetus (Fig. 4b). Among Ig classes, only IgG can cross the placenta, allowing transport of IgG from maternal blood to the fetus, which is mediated by the neonatal Fc receptor (FcRn) on the placenta. FcRn is a major histocompatibility complex (MHC) class I-like transmembrane protein associated with β2-microglobulin.93 FcRn is one of subtypes of IgG Fc receptors (FcγRs), binds to the IgG Fc region, and is responsible for transporting antibodies of the IgG class from the mother to the fetus.94,95 While transplacental passage of IgG is beneficial in passive transfer of humoral immunity against microbes, passage of pathogenic autoantibodies can induce transient autoimmune diseases in neonates, such as neonatal myasthenia gravis and neonatal Graves’ disease.96 Thus, when neutralizing anti-ZIKV IgG is transferred transplacentally, it can protect the fetus from ZIKV infection. On the other hand, when non-neutralizing IgG binds ZIKV and forms ZIKV-IgG immune complexes in the maternal blood, these complexes can be captured by FcRn on the placenta, and transferred to the fetus without viral neutralization, leading to fetal infection and microcephaly (Fig. 4b).
Third, non-neutralizing anti-ZIKV antibody can also mediate retrograde axonal transport of the virus from the periphery to the CNS, when the antibody binds the virus without neutralization (Fig. 4c). We and others have demonstrated that antibodies can be taken up at the nerve endings of neurons, and transferred to the neuronal cell body by retrograde axonal flow.97–99 In this scenario, when one neuron has a nerve ending in the periphery and the cell body in the CNS, the IgG is transported from the periphery to the CNS without crossing the blood-brain barrier (e.g., lower motor neurons have their nerve endings at neuromuscular junctions in the periphery with their cell bodies in the anterior horn of the spinal cord). When the antibody is an autoantibody to neurons, such as anti-neuronal Yo and Hu antibodies derived from patients with paraneoplastic cerebellar degeneration, axonal transport of neurotoxic auto antibodies leads to neuronal cell death. Although the precise mechanism is unclear, the antibody seems to be taken up at the nerve ending via FcR,100 and transported to the cell bodies of neurons via retrograde axonal flow.101 In theory, if the virus-antibody complex is transported to the neuronal cell body without neutralization, the virus can use the neural route to spread from the peripheral nerve endings into the CNS within axons, and propagate in the cytoplasm of neurons. Alternatively, when virus-antibody complex is incorporated into FcR+ monocyte-macrophages, the virus can hide in the cells and is transported into the CNS (Trojan horse theory, as proposed in HIV and TMEV infection).102
4. Conclusions
In this review, the virological, immunological, and neurological aspects associated with ZIKV infection were summarized. Although the precise pathomechanism of ZIKV-associated microcephaly and GBS is unknown, we proposed three potential pathogenic roles of anti-ZIKV antibody in GBS and microcephaly. Testing our hypotheses will provide insights into ZIKV-associated pathogenesis as well as anti-viral antibody-mediated immunopathology in general.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R21NS059724), the National Institute of General Medical Sciences COBRE grants (P30GM110703 and P30GM114737), and Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research-KAKENHI, 16H07356). We thank Ms. Lucile G. M. Guion and Ms. Namie Sakiyama for excellent technical assistance.
Abbreviations
- ADE
antibody-dependent enhancement
- ADEM
acute disseminated encephalomyelitis
- AIDP
acute inflammatory demyelinating polyneuropathy
- AMAN
acute motor axonal neuropathy
- AMSAN
acute motor sensory axonal neuropathy
- BBB
blood-brain barrier
- BDV
Border disease virus
- BVDV
bovine viral diarrhea virus
- C.
Campylobacter
- C’
complement
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CR
C’ receptor
- CSFV
classical swine fever virus
- DA
Daniels
- DENV
dengue virus
- DHF
dengue hemorrhagic fever
- DSS
dengue shock syndrome
- E
envelope
- EAN
experimental autoimmune neuritis
- ER
endoplasmic reticulum
- FcγRs
IgG Fc receptors
- FcR
Fc receptor
- FcRn
neonatal Fc receptor
- GBS
Guillain-Barré syndrome
- HAM/TSP
HTLV-associated myelopathy/tropical spastic paraparesis
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HTLV
human T-lymphotropic virus
- IHR
International Health Regulations
- Ig
immunoglobulin
- IRES
internal ribosome entry site
- IVIG
intravenous Igs
- JEV
Japanese encephalitis virus
- mAbs
monoclonal antibodies
- MFS
Miller Fisher syndrome
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- PHEIC
Public Health Emergency of International Concern
- PML
progressive multifocal leukoencephalopathy
- PNS
peripheral nervous system
- SLEV
St. Louis encephalitis virus
- ss
single-stranded
- TBEV
tick-borne encephalitis virus
- TDAV
Theiler’s disease-associated virus
- TMEV
Theiler’s murine encephalomyelitis virus
- WHO
World Health Organization
- WNV
West Nile virus
- YFV
yellow fever virus
- ZIKV
Zika virus
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. Epub 1952/09/01. [DOI] [PubMed] [Google Scholar]
- 2.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–534. doi: 10.1016/0035-9203(52)90043-6. Epub 1952/09/01. [DOI] [PubMed] [Google Scholar]
- 3.Kaddumukasa MA, Mutebi J-P, Lutwama JJ, Masembe C, Akol AM. Mosquitoes of Zika Forest, Uganda : species composition and relative abundance. J Med Entomol. 2014;51(1):104–113. doi: 10.1603/me12269. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddow AJ, Williams MC, Woodall JP, Simpson DIH, Goma LKH. Twelve isolations of Zika virus from Aedes (Stegomyia) Africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ. 1964;31:57–69. Epub 1964/01/01. [PMC free article] [PubMed] [Google Scholar]
- 5.Simmonds P, Becker P, Collett MS, Gould EA, Heinz FX, Meyers G, et al. Family Flaviviridae. In: King AMQ, Lefkowitz E, Adams MJ, Carstens EB, editors. Virus Taxonomy : Ninth Report of the International Committee on Taxonomy of Viruses. 1st. London, UK: Elsevier Inc; 2012. pp. 1003–1020. [Google Scholar]
- 6.Lindenbach BD, Murray CL, Thiel H-J, Rice CM. Flaviviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2013. pp. 712–746. [Google Scholar]
- 7.Ke P-Y, Chen SS-L. Hepatitis C virus and cellular stress response: implications to molecular pathogenesis of liver diseases. Viruses. 2012;4(10):2251–2290. doi: 10.3390/v4102251. Epub 2012/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moennig V. Introduction to classical swine fever: virus, disease and control policy. Vet Microbiol. 2000;73(2–3):93–102. doi: 10.1016/s0378-1135(00)00137-1. Epub 2000/04/28. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J, Li W. Border disease virus. In: Liu D, editor. Molecular detection of animal viral pathogens. Boca Raton, FL: CRC Press; 2016. pp. 211–222. [Google Scholar]
- 10.Chandriani S, Skewes-Cox P, Zhong W, Ganem DE, Divers TJ, Van Blaricum AJ, et al. Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. Proc Natl Acad Sci U S A. 2013;110(15):E1407–E1415. doi: 10.1073/pnas.1219217110. Epub 2013/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divers TJ. The equine liver in health and disease. AAEP Proceedings. 2015;61:66–103. [Google Scholar]
- 12.Harrison SC. Principles of virus structure. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2013. pp. 52–86. [Google Scholar]
- 13.Kielian M, Rey FA. Virus membrane-fusion proteins : more than one way to make a hairpin. Nature reviews Microbiology. 2006;4(1):67–76. doi: 10.1038/nrmicro1326. Epub 2005/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spahn CM, Kieft JS, Grassucci RA, Penczek PA, Zhou K, Doudna JA, et al. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science. 2001;291(5510):1959–1962. doi: 10.1126/science.1058409. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 15.Flint SJ, Racaniello VR, Rail GF, Skalka AM, Enquist LW. Principles of virology. 4th. Vol. 2. Washington, DC: ASM Press; 2015. Barriers to infection; pp. 24–51. [Google Scholar]
- 16.Stiasny K, Heinz FX. Flavivirus membrane fusion. J Gen Virol. 2006;87(Pt 10):2755–2766. doi: 10.1099/vir.0.82210-0. Epub 2006/09/12. [DOI] [PubMed] [Google Scholar]
- 17.Kelley TW, Prayson RA, Ruiz AI, Isada CM, Gordon SM. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am J Clin Pathol. 2003;119(5):749–753. doi: 10.1309/PU4R-76JJ-MG1F-81RP. Epub 2003/05/23. [DOI] [PubMed] [Google Scholar]
- 18.Zapata JC, Cox D, Salvato MS. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl Trop Dis. 2014;8(6):e2858. doi: 10.1371/journal.pntd.0002858. Epub 2014/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theiler M, Smith HH. The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med. 1937;65(6):787–800. doi: 10.1084/jem.65.6.787. Epub 1937/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145. doi: 10.1016/0035-9203(54)90006-1. Epub 1954/03/01. [DOI] [PubMed] [Google Scholar]
- 21.Beareroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50(5):442–448. Epub 1956/09/01. [PubMed] [Google Scholar]
- 22.Simpson DIH. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335–338. Epub 1964/07/01. [PubMed] [Google Scholar]
- 23.Kokernot RH, Smithburn KC, Muspratt J, Hodgson B. Studies on arthropod-borne viruses of Tongaland. VIII. Spondweni virus, an agent previously unknown, isolated from Taeniorhynchus (Mansonioides) uniformis. S Afr J Med Sci. 1957;22(2–3):103–112. Epub 1957/10/01. [PubMed] [Google Scholar]
- 24.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, et al. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol. 1983;77(2):131–137. doi: 10.1080/00034983.1983.11811687. Epub 1983/04/01. [DOI] [PubMed] [Google Scholar]
- 25.Olson JG, Ksiazek TG. Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389–393. doi: 10.1016/0035-9203(81)90100-0. Epub 1981/01/01. [DOI] [PubMed] [Google Scholar]
- 26.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. Epub 1969/05/01. [DOI] [PubMed] [Google Scholar]
- 27.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. Epub 2009/06/12. [DOI] [PubMed] [Google Scholar]
- 28.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, et al. Zika virus, French Polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–1086. doi: 10.3201/eid2006.140138. Epub 2014/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89(17):8880–8896. doi: 10.1128/JVI.00354-15. Epub 2015/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18(5):591–596. doi: 10.1016/j.stem.2016.03.012. Epub 2016/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Besnard M, Lastère S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro surveill. 2014;19(13) Epub 2014/04/12. [PubMed] [Google Scholar]
- 32.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. Epub 2016/03/31. [DOI] [PubMed] [Google Scholar]
- 33.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus--Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65(14):372–374. doi: 10.15585/mmwr.mm6514a3. Epub 2016/04/15. [DOI] [PubMed] [Google Scholar]
- 34.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(28):716–717. doi: 10.15585/mmwr.mm6528e2. Epub 2016/07/22. [DOI] [PubMed] [Google Scholar]
- 35.Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481. doi: 10.1016/S0140-6736(16)00644-9. Epub 2016/03/08. [DOI] [PubMed] [Google Scholar]
- 36.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastère S, Valour F, et al. Zika virus infection complicated by Guillain-Barrè syndrome—case report, French Polynesia, December 2013. Euro surveill. 2014;19(9) doi: 10.2807/1560-7917.es2014.19.9.20720. Epub 2014/03/15. [DOI] [PubMed] [Google Scholar]
- 37.Chan M. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [cited 2016 February 1];2016 Available from: http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/
- 38.Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, et al. Zika virus as a cause of neurologic disorders. N Engl J Med. 2016;374(16):1506–1509. doi: 10.1056/NEJMp1602708. Epub 2016/03/10. [DOI] [PubMed] [Google Scholar]
- 39.de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016 : preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–1363. doi: 10.1016/S1473-3099(16)30318-8. Epub 2016/09/20. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N Engl J Med. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338. Epub 2016/04/14. [DOI] [PubMed] [Google Scholar]
- 41.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375(1):1–4. doi: 10.1056/NEJMp1605367. Epub 2016/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez NE, Sato F, Kawai E, Omura S, Chervenak RP, Tsunoda I. Regulatory T cells and Th17 cells in viral infections: implications for multiple sclerosis and myocarditis. Future Virol. 2012;7(6):593–608. doi: 10.2217/fvl.12.44. Epub 2012/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flint SJ, Racaniello VR, Rail GF, Skalka AM, Enquist LW. Principles of virology. 4th. Vol. 2. Washington, DC: ASM Press; 2015. Mechanisms of pathogenesis; pp. 134–173. [Google Scholar]
- 44.Love S, Wiley CA, Lucas S. Viral infections. In: Love S, Budka H, Ironside JW, Perry A, editors. Greenfield’s Neuropathology. 9th. Boca Raton, FL: CRC Press; 2015. pp. 1087–1191. [Google Scholar]
- 45.Zelinskyy G, Dietze KK, Hüsecken YP, Schimmer S, Nair S, Werner T, et al. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114(15):3199–3207. doi: 10.1182/blood-2009-03-208736. Epub 2009/08/13. [DOI] [PubMed] [Google Scholar]
- 46.Lanteri MC, O’Brien KM, Purtha WE, Cameron MJ, Lund JM, Owen RE, et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest. 2009;119(11):3266–3277. doi: 10.1172/JCI39387. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato F, Tanaka H, Hasanovic F, Tsunoda I. Theiler’ s virus infection : pathophysiology of demyelination and neurodegeneration. Pathophysiology. 2011;18(1):31–41. doi: 10.1016/j.pathophys.2010.04.011. Epub 2010/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsunoda I, Fujinami RS. Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J Neuroimmune Pharmacol. 2010;5(3):355–369. doi: 10.1007/s11481-009-9179-x. Epub 2009/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez M, Leibowitz JL, Powell HC, Lampert PW. Neonatal infection with the Daniels strain of Theiler’s murine encephalomyelitis virus. Lab Invest. 1983;49(6):672–679. Epub 1983/12/01. [PubMed] [Google Scholar]
- 50.Omura S, Kawai E, Sato F, Martinez NE, Chaitanya GV, Rollyson PA, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet. 2014;7(4):444–454. doi: 10.1161/CIRCGENETICS.114.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson RT. Viral infections of the nervous system. 2nd. Philadelphia: Lippincott-Raven; 1998. Pathogenesis of CNS infections; pp. 35–59. [Google Scholar]
- 52.Iwasaki Y. Pathology of chronic myelopathy associated with HTLV-I infection (HAM/TSP) J Neurol Sci. 1990;96(1):103–123. doi: 10.1016/0022-510x(90)90060-z. Epub 1990/04/01. [DOI] [PubMed] [Google Scholar]
- 53.Guarner J, Shieh WJ, Hunter S, Paddock CD, Morken T, Campbell GL, et al. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Human pathology. 2004;35(8):983–990. doi: 10.1016/j.humpath.2004.04.008. Epub 2004/08/07. [DOI] [PubMed] [Google Scholar]
- 54.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, et al. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61(8):1210–1220. doi: 10.1001/archneur.61.8.1210. Epub 2004/08/18. [DOI] [PubMed] [Google Scholar]
- 55.Hasanovic F, Tsunoda I, Morrey JD, Siddharthan V, Fujinami RS. Detection of West Nile virus antigen in the brain using immunohistochemistry; Utah Conference on Underguraduate Research (UCUR); Salt Lake City, Utah. 2007. Feb 2, p. 96. 2007. [Google Scholar]
- 56.Guarner J, Shieh W-J, Hunter S, Paddock CD, Morken T, Campbell GL, et al. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35(8):983–990. doi: 10.1016/j.humpath.2004.04.008. Epub 2004/08/07. [DOI] [PubMed] [Google Scholar]
- 57.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–271. doi: 10.1038/nature18296. Epub 2016/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–818. doi: 10.1126/science.aaf6116. Epub 2016/04/12. [DOI] [PubMed] [Google Scholar]
- 59.Hofer U. Viral Pathogenesis: Tracing the steps of Zika virus. Nat Rev Microbiol. 2016;14(7):401. doi: 10.1038/nrmicro.2016.80. Epub 2016/05/24. [DOI] [PubMed] [Google Scholar]
- 60.Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19(1):120–126. doi: 10.1016/j.stem.2016.04.017. Epub 2016/05/18. [DOI] [PubMed] [Google Scholar]
- 61.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165(5):1081–1091. doi: 10.1016/j.cell.2016.05.008. Epub 2016/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. doi: 10.1016/j.cell.2016.04.032. Epub 2016/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, del Carmen Castillo Signor L. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg Infect Dis. 2016;22(5):933–935. doi: 10.3201/eid2205.160065. Epub 2016/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson RT. Viral infections of the nervous system. 2nd. Philadelphia: Lippincott-Raven; 1998. Viral infections of the developing nervous system; pp. 315–348. [Google Scholar]
- 65.Harding BN, Golden JA. Malformations. In: Love S, Budka H, Ironside JW, Perry A, editors. Greenfield’ s Neuropathology. 9th. Boca Raton, FL: CRC Press; 2015. pp. 270–398. [Google Scholar]
- 66.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. Epub 2016/01/06. [DOI] [PubMed] [Google Scholar]
- 67.Miner JJ, Diamond MS. Understanding how Zika virus enters and infects neural target cells. Cell Stem Cell. 2016;18(5):559–560. doi: 10.1016/j.stem.2016.04.009. Epub 2016/05/08. [DOI] [PubMed] [Google Scholar]
- 68.Morens DM, Burke DS, Halstead SB. The wages of original antigenic sin. Emerg Infect Dis. 2010;16(6):1023–1024. doi: 10.3201/eid1606.100453. Epub 2010/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection : molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–398. doi: 10.1002/rmv.405. Epub 2003/11/20. [DOI] [PubMed] [Google Scholar]
- 70.Johnson BW, Kosoy O, Martin DA, Noga AJ, Russell BJ, Johnson AA, et al. West Nile virus infection and serologic response among persons previously vaccinated against yellow fever and Japanese encephalitis viruses. Vector Borne Zoonotic Dis. 2005;5(2):137–145. doi: 10.1089/vbz.2005.5.137. Epub 2005/07/14. [DOI] [PubMed] [Google Scholar]
- 71.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–315. doi: 10.1016/j.virol.2010.12.020. Epub 2011/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierson TC, Diamond MS. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA: Lippincott Williams and Wilkins; 2013. pp. 747–794. [Google Scholar]
- 73.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. The long elusive IgM Fc receptor, FcmuR. J Clin Immunol. 2014;34(Suppl 1):S35–S45. doi: 10.1007/s10875-014-0022-7. Epub 2014/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler’s murine encephalomyelitis virus. Acta Neuropathol. 1996;91(6):595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- 75.Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228(2):388–393. doi: 10.1006/viro.1996.8382. Epub 1997/02/17. [DOI] [PubMed] [Google Scholar]
- 76.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17(9):1102–1108. doi: 10.1038/ni.3515. Epub 2016/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao-Lormeau V-M, Blake A, Mons S, Lastére S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia : a case-control study. Lancet. 2016;387(10027):1531–1539. doi: 10.1016/S0140-6736(16)00562-6. Epub 2016/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt RE, Bibao JM. Diseases of peripheral nerves. In: Love S, Budka H, Ironside JW, Perry A, editors. Greenfield’s Neuropathology. 9th. Boca Raton, FL: CRC Press; 2015. pp. 1413–1514. [Google Scholar]
- 79.Hauser SL, Asbury AK. Harrison’s principles of internal medicine. 17th. New York: McGraw-Hill Medical; 2008. Guillain-Barré syndrome and other immune-mediated neuropaties; pp. 2667–2672. [Google Scholar]
- 80.Johnson RT. Viral infections of the nervous system. 2nd. Philadelphia: Lippincott-Raven; 1998. Postinfectious demyelinating diseases; pp. 181–210. [Google Scholar]
- 81.Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet. 2016;387(10026):1482. doi: 10.1016/S0140-6736(16)30058-7. Epub 2016/04/27. [DOI] [PubMed] [Google Scholar]
- 82.Oyazato Y, Shiihara T, Kusunoki S, Adachi M, Ohnishi N, Taniguchi H, et al. A case of anti-GAl antibody-positive Fisher syndrome with elevated tau protein in cerebrospinal fluid. Brain Dev. 2012;34(4):329–332. doi: 10.1016/j.braindev.2011.06.007. Epub 2011/07/12. [DOI] [PubMed] [Google Scholar]
- 83.Frontera JA, da Silva IR. Zika getting on your nerves? The association with the Guillain-Barré syndrome. N Engl J Med. 2016;375:1581–1582. doi: 10.1056/NEJMe1611840. Epub 2016/10/06. [DOI] [PubMed] [Google Scholar]
- 84.Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. Epub 2016/10/06. [DOI] [PubMed] [Google Scholar]
- 85.Tsunoda I, Fujinami RS. Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin Immunopathol. 2002;24(2):105–125. doi: 10.1007/s00281-002-0105-z. Epub 2002/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato F, Omura S, Jaffe SL, Tsunoda I. Role of CD4+ T lymphocytes in pathophysiology of multiple sclerosis. In: Minagar A, editor. Multiple sclerosis: a mechanistic view. London, UK: Elsevier Inc; 2016. pp. 41–69. [Google Scholar]
- 87.Pender MP. Acute disseminated encephalomyelitis. In: Pender MP, McCombe PA, editors. Autoimmune neurological disease. Cambridge; New York: Cambridge University Press; 1995. pp. 155–165. [Google Scholar]
- 88.Kusunoki S, Kaida K. Antibodies against gang-lioside complexes in Guillain-Barré syndrome and related disorders. J Neurochem. 2011;116(5):828–832. doi: 10.1111/j.1471-4159.2010.07029.x. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 89.Yuki N. Guillain-Barré syndrome and anti-gang-lioside antibodies: a clinician-scientist’s journey. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(7):299–326. doi: 10.2183/pjab.88.299. Epub 2012/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carod-Artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. doi: 10.1016/S1474-4422(13)70150-9. Epub 2013/08/21. [DOI] [PubMed] [Google Scholar]
- 91.Shah I. Dengue presenting as Guillain Barre syndrome. Dengue Bulletin. 2007;31:166–168. [Google Scholar]
- 92.Ralapanawa DMPUK, Kularatne SAM, Jayalath WATA. Guillain-Barre syndrome following dengue fever and literature review. BMC Res Notes. 2015;8:729. doi: 10.1186/s13104-015-1672-0. Epub 2015/11/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4(2):81–89. doi: 10.1530/ror.0.0040081. Epub 1999/06/05. [DOI] [PubMed] [Google Scholar]
- 94.Sand KMK, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the interaction between FcRn and albumin : opportunities for design of albumin-based therapeutics. Front Immunol. 2014;5:682. doi: 10.3389/fimmu.2014.00682. Epub 2015/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roopenian DC, Akilesh S. FcRn : the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725. doi: 10.1038/nri2155. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 96.LaFranchi S. Congenital hyperthyroidism. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18th. Philadelphia: Saunders; 2007. pp. 2336–2337. [Google Scholar]
- 97.Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, Tsunoda I, et al. Anti-Yo antibody uptake and interaction with its intracellular target antigen causes Purkinje cell death in rat cerebellar slice cultures: a possible mechanism for paraneoplastic cerebellar degeneration in humans with gynecological or breast cancers. PLoS One. 2015;10(4):e0123446. doi: 10.1371/journal.pone.0123446. Epub 2015/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, Tsunoda I, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160. doi: 10.1186/s12974-014-0160-0. Epub 2014/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greenlee JE, Clawson SA, Hill KE, Wood BL, Tsunoda I, Carlson NG. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J Neuropathol Exp Neurol. 2010;69(10):997–1007. doi: 10.1097/NEN.0b013e3181f0c82b. Epub 2010/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Congdon EE, Gu J, Sait HBR, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013;288(49):35452–35465. doi: 10.1074/jbc.M113.491001. Epub 2013/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamamoto T, Iwasaki Y, Konno H, Iizuka H, Zhao JX. Retrograde transport and differential accumulation of serum proteins in motor neurons: implications for motor neuron diseases. Neurology. 1987;37(5):843–846. doi: 10.1212/wnl.37.5.843. Epub 1987/05/01. [DOI] [PubMed] [Google Scholar]
- 102.Tsunoda I, Fujinami RS. Theiler’s murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent viral infections. New York: John Wiley & Sons; 1999. pp. 517–536. [Google Scholar]