Abstract
Colorectal cancer (CRC) is a complex cancer disease, and approximately 40% of the surgically cured patients will experience cancer recurrence within 5 years. During recent years, research has shown that CRC treatment should be tailored to the individual patient due to the wide variety of risk factors, genetic factors, and surgical complexity. In this review, we provide an overview of the considerations that are needed to provide an individualized, patient-tailored treatment. We emphasize the need to assess the predictors of CRC, and we summarize the latest research on CRC genetics and immunotherapy. Finally, we provide a summary of the significant variations in the colon and rectal anatomy that is important to consider in an individualized surgical approach. For the individual patient with CRC, a tailored treatment approach is needed in the preoperative, operative, and postoperative phase.
Keywords: cancer, colorectal, colorectal surgery, locoregional recurrence, metastases, active immunotherapy, liquid biopsy, lymph nodes
Introduction
Up to 40% of patients experience recurrence after curative surgery for colorectal cancer (CRC), usually in the form of distant or regional metastases. Despite decades of research, the mechanisms behind the metastatic process in CRC are still not fully understood. We know that metastases to the liver, lungs, and peritoneum are most common, but recurrence is also observed in the brain, peripheral lymph nodes, bone, and thyroid gland.1 Although most recurrences (approximately 80%) occur during the first 3 years after curative resection, a CRC recurrence may manifest several years after curative surgery. On occasion, recurrence from CRC may occur as long as 10 years after the initial curative resection (ie, disseminated cancer cell dormancy).2 The mechanism behind this potentially long time span from curative surgery to the manifestation of distant metastases is poorly understood. The temporal complexity related to the recurrent growth of cancer implies an individualized treatment approach. Among colorectal surgeons and oncologists, it is now accepted that “one size does not fit all,” and there is an increasing acceptance of a more personalized treatment (ie, precision medicine) tailored for the individual patient with CRC.
Precision medicine is a new approach to disease prevention and treatment based on individual characteristics regarding the environment, genes, lifestyle, and individual risk factors. Recently, the National Institutes of Health launched a national research program on precision medicine, aiming to enroll more than 1 million US citizens, where the aim is to explore the area of precision medicine (https://www.nih.gov/research-training/allofus-research-program). Oncology is a natural part of the precision medicine initiative, as predictive genetic markers already play an important role in clinical decision making regarding choice of treatment in cancer. Cancer is a genetic disease with multiple risk factors, disease mechanisms, and treatment options. Due to intertumor heterogeneity and a wide variety of patient characteristics, precision medicine with an individualized and tailored treatment approach might be beneficial and contribute to improved survival.
The aim of this article is to present factors with a prognostic or predictive potential in CRC, factors that could be used in a tailored treatment approach for patients with CRC. First, we focus on the variance of CRC predictors and the new developments related to tumor molecular biology. Second, we address the variance in the anatomy of the colon and rectum, and how awareness of the patient’s individual anatomy provides individualized patient-tailored surgery. Finally, we provide an overview of the implications these genetic, anatomic, and surgical factors may play in the present and in the future of CRC precision medicine.
Predictors of Metastatic Spread
There are several acknowledged prognostic factors in CRC, predicting the risk of cancer recurrence. Knowledge of these factors is fundamental in individualized CRC treatment approach, precision medicine, and surgical decision making. These predictors are associated with the risk of CRC recurrence, and there exist a huge variance among the individual patients with CRC related to the prognostic significance of these predictors. The most common predictors of metastatic spread are described in the following sections.
Sex
Male sex is a poor prognostic factor of CRC. Men have a higher incidence of CRC compared with women and also have inferior survival after curative surgery. According to the data from the Norwegian Cancer Registry, women have a 12% decreased risk of cancer-specific mortality.3 There is comprehensive research performed within the field of CRC related to gender as a risk factor. It is shown that male sex has a higher incidence of CRC and, in general, has a poorer prognosis with a higher risk of recurrent disease after curative resection. Storli et al4 showed that men have a hazard ratio (HR) of 1.52 (P = .03) of death after curative colon cancer resection compared with women. Similarly, data from the Norwegian Colorectal Cancer Registry and from Japan have shown that male sex is a poor prognostic factor.3,5,6 The underlying biological mechanism for the observed gender difference is poorly understood.
Age
Age is an independent risk factor of CRC, and elderly also have decreased survival rates after curative surgery. With advancing age, cancer registry data have shown that men have a higher risk of CRC compared with women.4
TNM stage
It is well established that the TNM system is a useful prognostic marker in CRC. The “T” describes the depth of tumor growth in the colorectal wall. T1 tumors invade the submucosa, T2 tumors invade the muscularis propria, and T3 tumors invade the subserosa. A T4 tumor invades neighbor organs or structures or perforates the visceral peritoneum. Advanced T category is associated with decreased survival.3 Lymph node involvement, “N,” is also associated with decreased survival rates. N1 indicates metastasis to 1 to 3 lymph nodes, whereas N2 is defined as spreading to 4 or more lymph nodes.3 Distant metastasis, M1, will often imply that the disease is incurable and has very poor prognosis.
Tumor Morphology
Tumors referred to as CRC are almost exclusively adenocarcinomas, originating from the glandular epithelium of the colon and rectum. There are histologic subtypes of adenocarcinomas, such as mucinous adenocarcinoma (5%-15%) and signet ring cell tumors (1%). Typically, signet ring cell adenocarcinoma is associated with younger patients, more advanced stages, and poor outcome, compared with non-signet ring histology. Large population-based studies have shown that signet ring morphology is associated with lower survival rates in both colon and rectum.5 Mucinous adenocarcinoma is also associated with young age and lower survival rates, but only in rectum cancer, with no impact on survival in colon cancer.5
Histologic Grade
Histologic grade is defined in the TNM classification and describes the differentiation of the tumor cells. Well-differentiated tumors are graded G1 and moderately differentiated tumors, which are most common, are graded G2. Poorly and undifferentiated tumors are graded G3 and G4, respectively. Tumors with high histologic grade have a worse prognosis than G1 and G2 tumors.7
Carcinoembryonic Antigen
Preoperative elevated levels of carcinoembryonic antigen (CEA) are associated with a higher risk of recurrence of CRC. It is also included in most national surveillance programs after curative CRC surgery.8,9 In recent meta-analyses by Tan et al, where 20 studies were analyzed, they concluded that CEA has high specificity but insufficient sensitivity to detect CRC recurrence in isolation. Carcinoembryonic antigen is, however, useful as a surveillance test, based on serial measurements demonstrating temporal trends, in addition to radiological surveillance.10
Lymph Node Harvest
Several studies have identified the total number of examined lymph nodes in the resected specimen as a prognostic marker of recurrence. This is true for both node-positive and node-negative diseases. Goldstein et al showed that improved survival was associated with the number of recovered lymph nodes. The 5-year survival rate was 62% for those with less than 8 nodes, whereas those with more than 17 lymph nodes had a 76% 5-year survival.11 Sjo et al12 showed a similar positive association with improved survival with an increased number of examined lymph nodes.
It is accepted among colorectal surgeons that a D3 resection, ie, a resection including high tie of the feeding artery, draining veins, and resection of all adjacent lymph nodes, is the preferred method as this ensures the resection of a higher number of lymph nodes and a better oncological result.13,14 However, the details of a correct D3 resection are still debated, and several studies have shown that there exists a significant variance of anatomical appearance in the left and right colon. In a recent paper by Spasjeovic et al,15 they divided a predefined D3 area into 3 vertical compartments. In a postmortem study of 26 cadavers, they found that resection of the posterior vertical compartment had a potential of 5 to 6 additional lymph nodes. Similarly, they found that a postoperative computed tomographic (CT) scan can visualize the arterial stumps and that these stumps appear to be significantly longer than previously anticipated, implying that there is a significant improvement potential when surgical D3 dissection techniques are applied.16
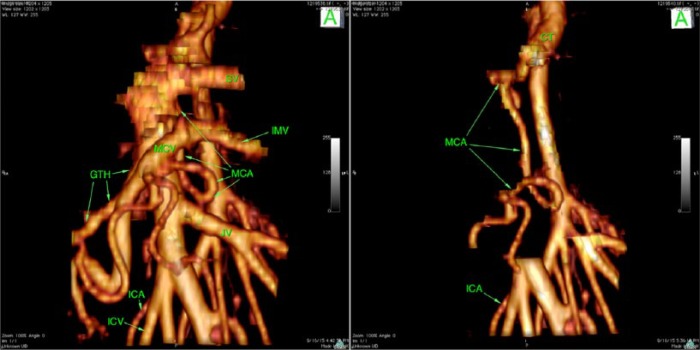
Awareness of anatomy is critical for performing safe surgery within the root of the mesentery. In a recent study by Nesgaard et al, they assessed the use of 3-dimensional reconstructed CT images. They found significant variations in the vascular anatomy in the mesenteric root but were able to identify 4 different crossing patterns of arteries and veins in the D3 area (Figure 1).17
Figure 1.
An example of the unique individualized anatomy in the D3 area. Arteria ileocolica (MCA) originates from the celiac trunk (CT), taking a serpiginous course, arching to the right; descends posterior to the portal vein (PV), splenomesenteric junction, and behind the superior mesenteric vein (SMV); and then loops 270° counterclockwise and joins the MCV in front of the SMV, bifurcating into right and left branches. A significant number of lymph nodes are located in the D3 area, making this a target area for increased lymph node harvest. Used with permission from Dr Dejan Ignatovic.
Tumor Invasion in Lymphatic and Venous Vessels
Tumor invasion in veins and lymphatic vessels plays a major role in hematologic and lymphatic tumor spread. However, the frequency of invasion varies widely between different series and may be caused by differences in sectioning of the tumor, number of examined tumor blocks, use of special staining, and interobserver variability. Furthermore, there are no standard guidelines for the pathologic examination of the tumor regarding lymphatic or venous tumor invasion.
There is a positive association between lymphatic invasion and depth of the tumor, poor differentiation, tumor budding, and lymph node metastases.18 However, there are diverging results related to whether tumor invasion in lymphatic vessels is associated with reduced survival. Liang et al18 showed an association with poor prognosis in univariate analysis, but not significant when adjusting for other risk factors. Venous invasion is associated with more advanced T category and tumor depth. It is an independent prognostic marker of distant metastases and reduced survival.18,19 Recent surveys have also argued that the grade extramural venous invasion should be used as a marker to tailor radiochemotherapy.20
The scientific works performed by Dukes and colleagues21,22 in the 1940s and 1950s, still, represent the cornerstone papers related to the metastatic spread of tumor cells through lymphatic vessels. They showed that the lymphatic spread of cancer cells from the rectum follows a distinct pathway and that survival is associated with the extent of lymphatic spread. They differentiated between A, B, C1, and C2 cases. The A and B cases represented no lymphatic spread, whereas C1 and C2 cases represented lymphatic spread along the arteries. In 1938, they showed for the first time that lymphatic spread follows an orderly and distinct pattern, which is important to have in mind when performing the dissection along the mesorectal fascia.22
Similarly, Dukes23 showed that venous spread was identified in 11% of all patients with resected rectal cancer. Interestingly, they showed that venous spread was more than twice as common in patients who had lymph node metastases. Of importance is that venous drainage of rectum follows 2 distinct pathways. In the lower third of the rectum, the hemorrhoidal veins empty into the iliac veins and after that to the vena cava inferior, the right heart, and the pulmonary circulation. In contrast, the upper two-thirds of the rectum drain directly to the inferior mesenteric vein, which then empties into the portal vein before it enters the liver. Some have argued that this difference in the venous return may affect the metastatic spreading pattern.24 The mesorectum is the part of the mesentery supporting the rectum and includes the perirectal fat, arteries, venous drainage, nerves, and lymphatic vessels. A layer of fibroareolar tissue, the mesorectal fascia, surrounds the mesorectum, and dissection along the surface of the fascia (ie, the holy plane) is the key to successful surgical treatment of rectal cancer. Staying in the loose, nonvascular tissue between the mesorectal and endopelvic fascia also minimizes blood loss and protects the surrounding neurovascular anatomy.25
Perineural Invasion
Perineural invasion is defined as direct cancer growth into the perineurium of autonomic nerves within the superior and inferior mesenteric nerve plexus.26,27 According to several recent trials, its underrecognized route of metastatic spread is an emerging pathologic feature in CRC.28 It has been postulated that this invasion facilitates movement of cancer cells from the primary site to distant metastatic sites through perineural routes leading to soft tissue tumor deposits.27 Perineural tumor invasion may explain the poor prognosis in patients who have right-sided colon cancer with spreading to the central lymph nodes around the superior mesenteric vessels due to their proximity to the superior mesenteric nerve plexus. Perineural invasion is reported as high as 33% in some surveys.27 Recently, Chablani et al found that perineural invasion was associated with a significantly worse prognosis in locally advanced rectal cancer; median disease-free survival was 13.5 months for those with perineural invasion compared with 39.8 months for those without perineural invasion (P < .0001). Chablani et al28 suggest that their data support further testing of the role of adjuvant chemotherapy in patients with perineural invasion.
Tumor Location
Tumor location in the right colon, left colon, or rectum represents an independent predictor for metastatic spread and thus disease-free survival. Recently, Augestad et al1 showed that left-sided colon cancers have a 70% increased risk of isolated liver metastases, compared with right colon and rectum cancers. Patients with rectum cancers have a more than 200% risk of local recurrence or isolated lung metastases, compared with right-sided colon cancers. Ding et al24 have shown similar patterns. It is shown that tumors from different portions of colon and rectum have different genetic features. For example, microsatellite instability (MSI), which is a good prognostic marker, is more common in tumors in the right colon than in tumors in the left colon and rectum. Hugen et al compared mucinous carcinoma, signet ring carcinoma, and adenocarcinoma and found that there are significant differences in anatomical location of the different cancer subtypes and that they are associated with a different spreading pattern. Moreover, they showed that rectal cancers more often spread to extra-abdominal sites.29 It is not known whether it is the genetic differences, histologic subtypes, or the difference in venous drainage/lymphatic return (ie, mechanical properties) that contributes most to the observed differences in metastatic spread.
Molecular Prognostic Factors
Microsatellite instability
There are 3 recognized molecular subtypes in sporadic CRC: MSI, chromosomal instability, and CpG island methylator phenotype.7,30,31 Microsatellite instability is caused by defect mismatch repair and is characterized by multiple mutations, mainly insertions and deletions, in repetitive sequences of DNA called microsatellites. Approximately, 15% of all sporadic CRC and all tumors caused by the Lynch syndrome display MSI. It has been demonstrated that MSI is an independent positive prognostic factor after curative resection of CRC, with a significantly increased disease-free survival, compared with tumors characterized as microsatellite stable (MSS).32 Microsatellite instability is, however, a negative predictor of response to 5-fluorouracil (FU) monotherapy. In a systematic review by Guastadisegni et al,33 they showed that MSS has a significant beneficial effect on 5-FU monotherapy compared with MSI.
KRAS
KRAS is a proto-oncogene and a member of the Ras-family. It has been recognized as one of the most commonly mutated oncogenes in cancer, and KRAS mutations are demonstrated in 30% to 40% of CRC in population-based surveys.34 The prognostic impact of KRAS mutation has been investigated in several studies, but the results are contradictory, and the prognostic value of KRAS is still uncertain.34 However, KRAS mutation is a recognized predictor of nonresponse of anti–epidermal growth factor receptor (EGFR) treatment in metastatic CRC. Treatment guidelines, therefore, recommend that this treatment is only given to patients with KRAS wild-type tumors (Figure 2).7
Figure 2.
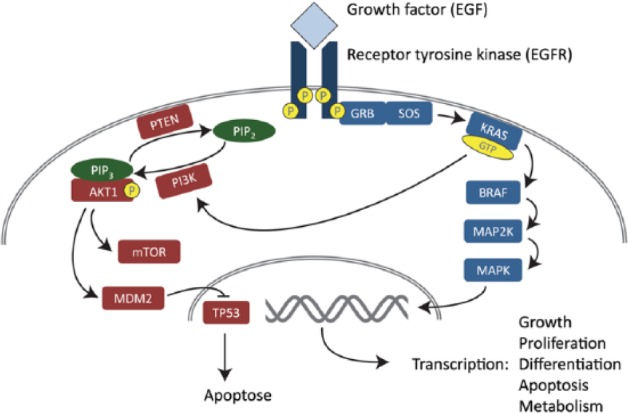
An outline of the RAS-RAF-MAPK and PI3K signaling pathways. Adapted with permission from Merok7 (No. 1745). EGF indicates epidermal growth factor; EGFR, epidermal growth factor receptor; GTP, guanosine triphosphate; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide-3 kinase.
BRAF
BRAF is a proto-oncogene and a member of the RAF kinase family.34,35 BRAF is activated by KRAS and regulates the mitogen-activated protein kinase cascade, including the extracellular signal–regulated kinase signaling pathway, which affects cell proliferation, cell-cycle arrest, terminal differentiation, and apoptosis (Figure 2). BRAF is mutated in 10% to 15% of CRC, the V600E substitution being the most common alteration. There are several studies supporting an adverse prognostic impact of BRAF mutation, especially in MSS tumors. Also, BRAF mutation is a probable negative predictive marker in anti-EGFR treatment and is therefore often analyzed before treatment decisions in metastatic CRC (Figure 2).
PIK3CA
PIK3CA is an oncogene, encoding the catalytic subunit of phosphoinositide-3 kinase (PI3K). Mutations in PIK3CA lead to constitutional activation and are observed in a range of different cancers (Figure 2).36 The mutation frequency is 10% to 15% in CRC, and the prognostic value has been evaluated in several studies. In recent meta-analyses by Mei et al,37 they found that PIK3CA mutation has a neutral effect on CRC overall survival and progression-free survival.
Circulating Tumor Cells
Circulating tumor cell was first observed in the blood of a man with metastatic cancer by Thomas Ashworth 150 years ago and is an acknowledged prognostic factor in CRC. The seed-soil principle, first postulated by Paget in 1889, still represents a major area of research and explores the molecular basis of circulating tumor cell (CTC) adherence in peripheral anatomical locations.38
Preceding this deposit and organ adherence is the physical process where molecules that originate from the primary tumor enter the circulation. Principally, these molecules are CTCs, circulating tumor DNA (ctDNA), microRNAs, and exosomes.39
Although much is unknown related to the mechanisms in which these molecules enter the circulation, there is increasing evidence that mechanical and physical models need to be used to understand how these molecules travel within the bloodstream and through the body. Weiss et al40 merged the seed-soil principle with a mechanical model for distant adherence of CTC and calculated the metastatic efficiency index (MEI). Scott et al38 published, in 2014, a paper in which new and more modern theories related to the MEI were forwarded. However, despite decades of research, there is still a considerable lack of knowledge related to the dynamics of CTC in the capillary bed of distant organs.
The ability to analyze ctDNA in the blood (ie, liquid biopsy) of people with cancer will facilitate the monitoring of therapy responses and patterns of early disease recurrence.41 Liquid biopsies may have numerous clinical applications.42 First, a liquid biopsy may be used for screening purposes and early detection of cancer. There are several commercial kits on the market. In 2014, the Food and Drug Administration approved Cologuard (stool testing), and in 2016, Epi proColon (blood sample). However, given the current challenges of identifying CTC in patients with low tumor burden, colonoscopy still remains the preferred method of screening. Second, liquid biopsies may be used for CRC surveillance to detect recurrent cancer after curative CRC surgery. Liquid biopsies have been found to be more sensitive in detecting CRC recurrence compared with CEA.42
The Consensus Molecular Subtypes of Colorectal Cancer
The CRC Subtyping Consortium published in Nature in 2015 a new framework for CRC molecular subtyping.43 The objective of the consortium was to assess core subtype patterns among existing gene expression–based subtype algorithms. The consortium also aimed to characterize the key biological features of the core subtypes, including disease-free and overall survival. The contortion aimed to establish a framework that would facilitate the division of molecular subtypes in the clinic. The consortium identified 4 consensus molecular subtypes (CMS 1-4) with distinguishing features:
CMS1 (MSI immune, 14% of all CRC), hypermutated, microsatellite unstable, and strong immune activation. CMS1 tumors were identified more frequently among women with right-sided lesions and had usually a higher histopathologic grade. CMS1 tumors have a very poor prognosis after recurrent cancer disease.
CMS2 (canonical, 37% of all CRC), epithelial with marked WNT and MYC signaling activation. CMS2 tumors are mainly left sided and have superior survival rates after cancer recurrence.
CMS3 (metabolic, 13% of all CRC), epithelial and have evident metabolic dysregulation.
CMS4 (mesenchymal, 23% of all CRC), prominent transforming growth factor activation, stromal invasion, and angiogenesis. CMS4 tumors tend to be diagnosed in a more advanced stage (stages III and IV) and result in worse overall survival and disease-free survival. The poor prognosis is significant, even when adjusting for histopathologic features, MSI status, and presence of BRAF/KRAF mutations.
A fifth subtype is classified as samples with mixed features (13%) possibly representing transition phenotype or intratumoral heterogeneity. According to the Consortium, the CMS groups are the most robust classification system currently available for CRR, with clear biological interpretability and the basis for future clinical stratification and subtype-based targeted interventions.43
The Future of Tailored CRC Treatment
Tailored neoadjuvant radiochemotherapy
Numerous of trials have been performed to assess the impact of pre- and postoperative radiation and chemotherapy for treatment of rectal cancer. In a Cochrane review from 2012, evaluations of preoperative chemoradiation for locally advanced rectal cancers in 6 randomized controlled trials were assessed. They found that there was no difference in overall survival between radiotherapy and chemoradiotherapy. However, chemoradiotherapy was associated with less local pelvic recurrence. It is shown that preoperative chemoradiation causes the tumor to shrink, which again is advantageous at surgery.44 Moreover, a Cochrane review by Petersen et al assessed postoperative chemotherapy among patients who had a curative resection for rectal cancer. They found that postoperative chemotherapy was beneficial, both regarding overall survival and disease-free survival after assessing 21 randomized controlled trials.45
Neoadjuvant radiochemotherapy is a standardized preoperative treatment for selected patients with rectal cancer, and 15% to 27% of the patients have a complete response with no residual tumor macroscopically or histologically.46 Maas et al46 show that patients with a complete response after radiochemotherapy have significantly better long-term outcomes compared with those with residual disease and conclude that complete response indicates a favorable biological tumor profile. Interestingly, patients with complete response have a favorable outcome also without surgery. However, we do not yet have the means to identify patients who will respond to the neoadjuvant radiochemotherapy, left alone the complete responders. Maas et al argue that tumors should be genetically profiled to identify the patients who will respond, but for now, we do not know what genetic alterations to look for. Furthermore, as discussed by Perez et al,47 several challenges still exist in gene profiling of CRC tumors, including intratumoral genetic heterogeneity. Perez concludes that the discovery of a clinically useful gene expression is unlikely shortly and argues that instead of looking at individual gene combinations in the search for gene signatures, an alternative strategy could be the search for deranged genetic pathways that may predict complete response.
However, in the area of personalized medicine, systemic chemotherapy involving infusion of 5-FU and leucovorin is defined as the cornerstone treatment for patients with CRC.
Tailored anti-EGFR and PIK3CA treatment
Approximately 15% to 20% of all patients with CRC have mutations in the PI3K pathway, making it one of the most frequently altered pathways in CRC (Figure 2).37 Mutations usually lead to constitutional activation. The prognostic role of this pathway is still debated, but it may be a target area in the future of tailored CRC treatment.
Of significant interest is that aspirin (ie, acetylsalicylic acid) has been shown to have a potential anticancer effect, targeting the PI3K pathway. Aspirin is a nonsteroidal anti-inflammatory drug that inhibits cyclooxygenases 1 and 2. Most colorectal adenomas and CRCs overexpress the cyclooxygenase-2 (COX-2) enzyme. Cyclooxygenase-2 interacts with the PI3K pathways in different ways, and inhibition of COX-2 results in inhibition of PI3K/AKT activity.48 Interestingly, a recent population-based survey by Baines et al49 showed an improved survival in patients using aspirin regularly. Aspirin downregulates PI3K signaling activity through COX-2 inhibition, leading to the hypothesis that the effect of aspirin varies according to PIK3CA mutational status. Baines et al linked data from the Norwegian Cancer Registry with the Norwegian Prescription Registry. More than 23 000 patients were included, of whom approximately 6000 used aspirin on a regular basis. They found that regular aspirin use after curative treatment reduced the hazard of having recurrent cancer (hazard ratio: 0.85). They conclude that regular aspirin use after curative treatment is independently associated with improved disease-free survival.48,49 However, rigorous designed randomized controlled trials are needed before we can make definitive conclusions. Recently, the ALASCCA trial (A randomized double-blinded placebo controlled study with ASA treatment in colorectal cancer patients with mutations in the PI3K signaling pathway) was initiated, which is a multicenter randomized controlled trial aiming to measure the impact of aspirin among those surgically cured for CRC (https://clinicaltrials.gov/ct2/show/NCT02647099?term=colorectal+cancer+ASA&rank=5).
Biological agents have been discovered that enhance the effect of cytotoxic therapy. Bevacizumab is a humanized monoclonal antibody (mAB) that targets vascular endothelial growth factor, a central regulator of angiogenesis. Cetuximab and panitumumab are monoclonal antibodies directed against the EGFR. Cetuximab, panitumumab, and bevacizumab have been approved and are in current use for the treatment of CRC in the United States. Many other mAbs targeting other pathways are currently being tested in clinical trials.50,51
Tailored immunotherapy
Immunotherapy has been postulated to be an emerging therapy with a high future potential. The immune system plays a major role in the development of CRC, ie, the development of the precancerous polyp and the development of the colorectal tumor, in the surgical treatment and postsurgical treatment. This has led to new innovative therapies, such as cancer vaccines and T-cell–stimulating therapies.52,53 Future potential cancer vaccines may be divided into autologous vaccines, peptide vaccines, dendritic cell vaccines, and viral antigen vaccines. Autologous vaccines use cells directly from the patient’s own cancer cell, an approach that guarantees that the vaccine will contain all tumor-specific antigens. Peptide vaccines attempt to target more specific tumor cells by identifying peptides that are unique to the specific tumor cell. Dendritic cells play a fundamental role in the immune system activation cascade and represent an area for tailored immunotherapy. Finally, viral vaccines may play a major role in the future, as it is hypothesized that viruses play a major role in the development of cancers.52 Furthermore, there are several other areas where stimulation of the immune system may inhibit tumor growth, including cytokines, toll-like receptor agonist, adoptive cell transfer, mAB-based therapy, and checkpoint inhibition (Table 1).52,53 Especially, immune checkpoint inhibition may have a huge potential, either as monotherapy or used together with chemotherapy. Immunotherapy with checkpoint inhibition has shown considerable clinical benefit especially in mismatch repair–deficient CRC. Immune checkpoint blockade via monoclonal antibodies leads to preferential activation of cancer-specific T cells and revival of tumor immunity. Current clinical trials of checkpoint inhibition have shown very promising results in the subset of patients with CRC.53
Table 1.
Tailored treatment of CRC—future perspectives.
| Individualized factors | Preoperative phase | Operative phase | Postoperative phase |
|---|---|---|---|
| Molecular/genetic factors | MSI vs MSS KRAS mutation BRAF mutation: BRAFV600E poor prognosis PIK3CA mutation: aspirin treatment and anti-EGFR treatment at metastatic CRC |
||
| Radiochemotherapy | Complete responders after rectal cancer radiochemotherapy | MSI vs 5-FU monotherapy | |
| Surgical technique | 3D reconstruction of D3 area to optimize surgical technique Preoperative MRI |
D3 dissection to improve lymph node harvest | |
| Identification of high-risk patients | Preoperative MDT evaluation: sex, age, TNM stage, CEA level, tumor location, and hereditary factors/Lynch syndrome | Pathology report: tumor morphology, histologic grade, CEA level, lymph node harvest, lymphatic invasion, venous invasion, perineural invasion | |
| Future perspectives | Improved identification of complete responders Radiation to activate the immune system |
Measurement of circulating tumor cells Improved surgical technique |
Risk-adopted postoperative surveillance programs Improved genetic profiling |
| Future perspectives of immunotherapy | Vaccination: whole tumor cell vaccines, peptide vaccines, viral vector vaccines, dendritic cell vaccines T-cell–stimulating therapy/checkpoint therapy |
Adoptive cell transfer therapy |
Abbreviations: 3D, 3-dimensional; CEA, carcinoembryonic antigen; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; FU, fluorouracil; MRI, magnetic resonance imaging; MSI, microsatellite instability; MSS, microsatellite stable.
Several agents augment host immunity against tumor antigens. Of special interests is that conventional chemotherapies may have some effect through the immune system. It is shown that oxaliplatin triggers cell death that is immunogenic. It is believed that the antitumor activity of oxaliplatin may also be related to its immune modulatory effect, and not only as a cytotoxic drug. It is believed that several cytostatic agents may have this immune modulatory effect, mainly by increasing the number of T cells.50,53 However, these potentially tailored therapies are not fully developed, and rigorous research is needed before routine clinical use.
Tailored surgical technique
Total mesorectal excision is the surgical criterion standard in rectal cancer surgery. This technique, where surgical dissection is performed along the “holy plane,” ie, the mesorectal fascia that surrounds the rectum, is associated with a significantly lower local recurrence rate and increased disease-free survival.54,55 A high tie (D3 resection) of the inferior mesenteric artery is recommended, as this will ensure that a high number of lymph nodes are removed with the surgical specimen.21,22,25,56 There is no consensus regarding the separate ligation of veins. Moynihan and coworkers argued that the importance of venous spread carries implications for surgical technique in the treatment of rectal cancer and provides a rational basis for the early ligation of the superior hemorrhoidal vein.57,58 This is, however, still debated, and there is no consensus on exactly how the colonic venous drainage should be ligated.
Recently, it is argued that colon surgery should follow similar surgical principles as for rectal cancer surgery, ie, complete mesocolic excision (CME) following Toldts plane with D3 dissection of lymph nodes.59 However, there exist limited evidence related to survival after CME. In 2008, Hohenberger et al59 published a paper arguing for an increased focus on standardized surgical techniques for colon cancer. In a recent paper by Merkel et al,60 they showed that 5-year cancer-related survival rate increased from 61% to 80%. This survival improvement corresponds with the nationwide implementation of CME. Similarly, recently published papers have shown promising results related to the new innovative techniques of D3 resection of the right hemicolon.16,17 In our opinion, a tailored and individualized preoperative planning of surgery is needed, especially due to the significant anatomical variance in the D3 area.
Tailored postoperative surveillance
It is well known that approximately 40% of all patients surgically cured for CRC will have recurrent cancer disease within 5 years after the surgery. Of those, roughly 80% of the recurrences appear within 2 years after the initial surgery.3 This high recurrence rate is used as an argument to enroll most patients in national postoperative surveillance programs. The aim of these surveillance programs is to detect recurrences early, offer metastatic salvage surgery, monitor the effects of monitoring programs, and monitor the social consequences of cancer survivorship.61,62 Previously, it was argued that these surveillance programs improve overall survival by more than 10%.63 However, recent results from the FACS (Follow-up After Colorectal Surgery) randomized controlled trial have shown that intensive surveillance after curative CRC treatment does not increase the number of patients being offered curative metastases surgery. Similarly, in recent meta-analyses, it is shown that intensive surveillance does not improve survival.64 Furthermore, in studies by Augestad and colleagues,65,66 it is shown that surveillance programs adhere to a considerable cost, which questions these surveillance programs’ cost-effectiveness. Recent studies have shown that individualized risk prediction for patients with CRC is feasible, and these approaches may play a major role in the future of risk-adopted postoperative surveillance programs.8,67
Conclusions
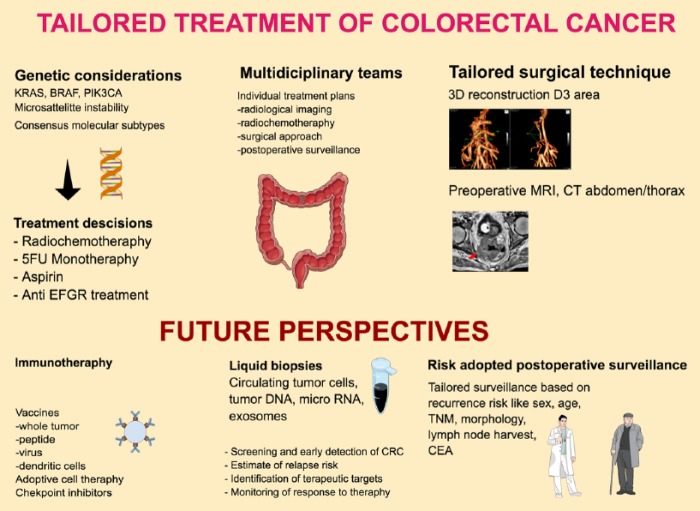
There are several areas where treatment and surveillance of patients with CRC may be individualized. In such an individualized approach, all prognostic markers (sex, age, histologic subtypes, TNM stage, CEA level, genetic factors, radiological images, and anatomical factors, among others) should be identified and discussed in preoperative multidisciplinary team meetings, resulting in individualized treatment plans (Figure 3). These plans can include plans for preoperative radiochemotherapy, surgical treatment, and postoperative surveillance. First, all tumors should be analyzed for known prognostic and predictive genetic markers, such as MSI and mutations in KRAS and BRAF. This will guide the postoperative treatment and surveillance.
Figure 3.
The concepts of tailored CRC treatment. 3D indicates 3-dimensional; CEA, carcinoembryonic antigen; CRC, colorectal cancer; CT, computed tomography; EGFR, epidermal growth factor receptor; FU, fluorouracil; MRI, magnetic resonance imaging.
Second, it is important that surgery is also individualized based on the considerable variation in anatomy (Figure 1). We argue that a 3-dimensional CT reconstruction of the regional vessels is of importance in an individualized surgical approach to ensure safe surgery and to optimize dissection in the D3 area.
Third, in the postoperative period, an individualized risk-adopted surveillance program should be developed and implemented.61,62
In conclusion, individualized CRC treatment based on genetic profiles and anatomical variance will gain increased acceptance in the following years.68 It is easy to imagine a scenario where preoperative radiology, genetic profiling, and liquid biopsies will be used to make individualized plans for surgical treatment, radiochemotherapy, and postoperative surveillance. Finally, immunotherapy may represent an interesting tailored treatment option in the future.
Footnotes
Peer Review:Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1169 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: KMA, MM, and DI contributed to idea generation and conceptualization of the manuscript. MM contributed with expertise on molecular mechanisms of CRC spread, whereas DI contributed with expertise on D3 mesocolic surgery. KMA wrote the first draft of the manuscript. All authors contributed to writing of the manuscript and approved the final manuscript version.
References
- 1. Augestad KM, Bakaki PM, Rose J, et al. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol. 2015;39:734–744. [DOI] [PubMed] [Google Scholar]
- 2. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dørum LM, Guren MG. Annual report Norwegian cancer registry 2014. The Norwegian Cancer Registry. October 2015:1–114. https://www.kreftregisteret.no/en/. [Google Scholar]
- 4. Storli KE, Søndenaa K, Bukholm IRK, et al. Overall survival after resection for colon cancer in a national cohort study was adversely affected by TNM stage, lymph node ratio, gender, and old age. Int J Colorectal Dis. 2011;26:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyngstrom JR, Hu C-Y, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawai K, Ishihara S, Yamaguchi H, et al. Nomogram prediction of metachronous colorectal neoplasms in patients with colorectal cancer. Ann Surg. 2015;261:926–932. [DOI] [PubMed] [Google Scholar]
- 7. Merok MA. Genetic and clinical prognostic markers for colorectal cancer (ed Nesbakken A.) [PhD thesis]. Oslo: Faculty of Medicine, University of Oslo; 2014. https://www.duo.uio.no/handle/10852/40418. [Google Scholar]
- 8. Rose J, Augestad K, Cooper GS. Colorectal cancer surveillance: what’s new and what’s next? World J Gastroenterol. 2014;20:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Augestad K, Rose J, Crawshaw B, Cooper GS, Delaney CP. Do the benefits outweigh the side effects of colorectal cancer surveillance? a systematic review. World J Gastrointest Oncol. 2014;6:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15–24. [DOI] [PubMed] [Google Scholar]
- 11. Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–189. [DOI] [PubMed] [Google Scholar]
- 12. Sjo OH, Merok MA, Svindland A, Nesbakken A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum. 2012;55:307–315. [DOI] [PubMed] [Google Scholar]
- 13. Kanemitsu Y, Komori K, Kimura K, Kato T. D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum. 2013;56:815–824. [DOI] [PubMed] [Google Scholar]
- 14. Lee SD, Lim S-B. D3 lymphadenectomy using a medial to lateral approach for curable right-sided colon cancer. Int J Colorectal Dis. 2008;24:295–300. [DOI] [PubMed] [Google Scholar]
- 15. Spasojevic M, Stimec BV, Dyrbekk APH, et al. Lymph node distribution in the D3 area of the right mesocolon: implications for an anatomically correct cancer resection. A postmortem study. Dis Colon Rectum. 2013;56:1381–1387. [DOI] [PubMed] [Google Scholar]
- 16. Spasojevic M, Stimec BV, Gronvold LB, Nesgaard J-M, Edwin B, Ignjatovic D. The anatomical and surgical consequences of right colectomy for cancer. Dis Colon Rectum. 2011;54:1503–1509. [DOI] [PubMed] [Google Scholar]
- 17. Nesgaard JM, Stimec BV, Bakka AO, Edwin B, Ignjatovic D; The RCC Study Group. Navigating the mesentery: a comparative pre- and per-operative visualization of the vascular anatomy. Colorectal Dis. 2015;17:810–818. [DOI] [PubMed] [Google Scholar]
- 18. Liang P, Nakada I, Hong J-W, et al. Prognostic significance of immunohistochemically detected blood and lymphatic vessel invasion in colorectal carcinoma: its impact on prognosis. Ann Surg Oncol. 2007;14:470–477. [DOI] [PubMed] [Google Scholar]
- 19. Washington MK. Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med. 2008;132:1600–1607. [DOI] [PubMed] [Google Scholar]
- 20. Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2013;110:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958;12:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabriel WB, Dukes CE, Bussey HJ. Lymphatic spread in cancer of the rectum. Br J Surg. 1942;23:395–413. [Google Scholar]
- 23. Dukes CE. The surgical pathology of rectal cancer. J Clin Pathol. 1949;2:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding P, Liska D, Tang P, et al. Pulmonary recurrence predominates after combined modality therapy for rectal cancer: an original retrospective study. Ann Surg. 2012;256:111–116. [DOI] [PubMed] [Google Scholar]
- 25. Augestad KM, Crawshaw B, Delaney CP. Chapter 30. Cancer of the rectum: operative management. In: Fazio VW, Kiran R, Delaney CP, eds. Current Therapy in Colon and Rectal Surgery. Amsterdam, Netherlands: Elsevier; 2016:1–6. [Google Scholar]
- 26. Yang Y, Huang X, Sun J, et al. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19:1113–1122. [DOI] [PubMed] [Google Scholar]
- 27. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. [DOI] [PubMed] [Google Scholar]
- 28. Chablani P, Nguyen P, Pan X, et al. Perineural invasion predicts for distant metastasis in locally advanced rectal cancer treated with neoadjuvant chemoradiation and surgery [published online ahead of print December 22, 2015]. Am J Clin Oncol. doi: 10.1097/COC.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hugen N, van de Velde CJ, de Wilt H, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2013;25:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Söreide K, Slewa A, Stokkeland PJ, et al. Microsatellite instability and DNA ploidy in colorectal cancer: potential implications for patients undergoing systematic surveillance after resection. Cancer. 2008;115:271–282. [DOI] [PubMed] [Google Scholar]
- 31. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. [DOI] [PubMed] [Google Scholar]
- 32. Merok MA, Ahlquist T, Royrvik EC, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788–2798. [DOI] [PubMed] [Google Scholar]
- 34. Foltran L, De Maglio G, Pella N, et al. Prognostic role of KRAS, NRAS, BRAF and PIK3CA mutations in advanced colorectal cancer. Future Oncol. 2015;11:629–640. [DOI] [PubMed] [Google Scholar]
- 35. Søreide JA, Janssen E, Söiland H, Körner H, Baak J. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. [DOI] [PubMed] [Google Scholar]
- 36. Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2016;2:1565–1573. [DOI] [PubMed] [Google Scholar]
- 37. Mei Z, Duan C, Li C, Cui L, Ogino S. Prognostic role of tumor PIK3CA mutation in colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:1836–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott JG, Fletcher AG, Maini PK, Anderson ARA, Gerlee P. A filter-flow perspective of haematogenous metastasis offers a non-genetic paradigm for personalised cancer therapy. Eur J Cancer. 2014;50:3068–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–491. [DOI] [PubMed] [Google Scholar]
- 40. Weiss LL, Grundmann EE, Torhorst JJ, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. [DOI] [PubMed] [Google Scholar]
- 41. Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu J, Strickler JH. Clinical applications of liquid biopsies in gastrointestinal oncology. J Gastrointest Oncol. 2016;7:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dienstmann R, Wang X, de Reyniès A, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCarthy K, Pearson K, Fulton R, Hewitt J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:CD008368. [DOI] [PubMed] [Google Scholar]
- 45. Petersen SHS, Harling HH, Kirkeby LTL, Wille-Jørgensen PP, Mocellin SS. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2011;3:CD004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 47. Perez RO, Habr-Gama A, São Julião GP, et al. Should we give up the search for a clinically useful gene signature for the prediction of response of rectal cancer to neoadjuvant chemoradiation? Dis Colon Rectum. 2016;59:895–897. [DOI] [PubMed] [Google Scholar]
- 48. Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bains SJ, Mahic M, Myklebust TÅ, et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. J Clin Oncol. 2016;34:2501–2508. [DOI] [PubMed] [Google Scholar]
- 50. Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol. 2015;6:208–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. [DOI] [PubMed] [Google Scholar]
- 52. Lynch D, Murphy A. The emerging role of immunotherapy in colorectal cancer. Ann Transl Med. 2016;4:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh PP, Sharma PK, Krishnan G, Lockhart AC. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep. 2015;3:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. [DOI] [PubMed] [Google Scholar]
- 55. Wibe AA, Møller BB, Norstein JJ, et al. A national strategic change in treatment policy for rectal cancer—implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45:857–866. [DOI] [PubMed] [Google Scholar]
- 56. Augestad KM, Lindsetmo RO, Reynolds H, et al. International trends in surgical treatment of rectal cancer. Am J Surg. 2011;201:353–358. [DOI] [PubMed] [Google Scholar]
- 57. Lange MM, Buunen M, van de Velde CJH, Lange JF. Level of arterial ligation in rectal cancer surgery: low tie preferred over high tie. A review. Dis Colon Rectum. 2008;51:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moynihan BG. The surgical treatment of cancer of the sigmoid flexure and rectum. Surg Gynecol Obstet 1908;463. [Google Scholar]
- 59. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis. 2009;11:354–364. [DOI] [PubMed] [Google Scholar]
- 60. Merkel S, Weber K, Matzel KE, Agaimy A, Göhl J, Hohenberger W. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br J Surg. 2016;103:1220–1229. [DOI] [PubMed] [Google Scholar]
- 61. Jeffery M, Hickey BE, Hider PN, See AM. Follow-up Strategies for Patients Treated for Non-Metastatic Colorectal Cancer. Chichester, UK: John Wiley & Sons; 2016. [Google Scholar]
- 62. Mokhles S, Macbeth F, Farewell V, et al. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. [DOI] [PubMed] [Google Scholar]
- 64. Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. J Am Med Assoc. 2014;311:263–270. [DOI] [PubMed] [Google Scholar]
- 65. Augestad KM, Norum J, Dehof S, et al. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open. 2013;3:e002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Augestad KM, Norum J, Rose J, Lindsetmo RO. A prospective analysis of false positive events in a National Colon Cancer Surveillance Program. BMC Health Serv Res. 2014;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rose J, Augestad KM, Kong CY, et al. A simulation model of colorectal cancer surveillance and recurrence. BMC Med Inform Decis Mak. 2014;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kalady MF. Lessons learned from the quest for gene signatures that predict treatment response in rectal cancer. Dis Colon Rectum. 2016;59:898–900. [DOI] [PubMed] [Google Scholar]