Abstract
In exonization events, Ds1 may provide donor and/or acceptor sites for splicing after inserting into genes and be incorporated into new transcripts with new exon(s). In this study, the protein variants of Ds1 exonization yielding additional functional profile(s) were studied. Unlike Ds exonization, which creates new profiles mostly by incorporating flanking intron sequences with the Ds message, Ds1 exonization additionally creates new profiles through the presence or absence of Ds1 messages. The number of unique functional profiles harboring Ds1 messages is 1.3-fold more than that of functional profiles without Ds1 messages. The highly similar 11 protein isoforms at a single insertion site also contribute to proteome complexity enrichment by exclusively creating new profiles. Particularly, Ds1 exonization produces 459 unique profiles, of which 129 cannot be built by Ds. We thus conclude that Ds and Ds1 are independent but synergistic in their capacity to enrich proteome complexity through exonization.
Keywords: Ds1 transposon, exonization, alternative splicing, nonsense-mediated decay pathway
Introduction
Evolution and speciation are believed to be driven in large part by the insertion of transposable elements (TEs) within eukaryotic genes.1 The TEs can integrate into a gene with either a forward or reverse pattern according to the transcription directionality of the inserted gene and the transposase gene. When inserting into the exons of genes, TEs may disrupt and cause the loss of function of genes into which they are inserted. However, the insertion of TEs within the intronic sequences of a given gene can also have the effect of altering pre–messenger RNA through alternative splicing (AS) and/or exonization.2 In such instances, the AS that results from TE insertion may cause interference with the normal splicing of the inserted gene’s transcribed region, whereas the exonization results in a cryptic splice site of the inserted TE to generate a new exon of the inserted gene. Any additional variant due to AS or exonization may subsequently evolve to form a protein with new functions. Moreover, the operation of natural selection may even serve to enhance the novel splice sites and, in turn, to raise the production level of the new variant if it is advantageous.3 Alternative splicing is commonly seen in higher eukaryotes, but its role in expanding the functions of the plant proteome is limited.4 In contrast, exonization can potentially introduce a portion or portions of a TE into the resulting transcripts, thereby altering the reading frames so as to enhance the complexity of proteomes, as was found, for example, in our previous study involving the insertion of a mini Ds transposon into the modified tobacco marker gene epsps.5
The Ac/Ds system includes the first TEs recognized in the scientific literature, having been identified by Barbara McClintock 60 years ago. Ds transposons of the nonautonomous (transposase defective) variety consist of 11 bp terminal inverted repeats as well as approximately 250 bp of both ends (ie, terminal regions) of their full form transposon, Activator (Ac).6 There are 3 different types of Ds elements, namely, Ds, Ds1, and Ds2. Ds1 has 13 bp at the 5′ terminal and 26 bp at the 3′ terminal in common with Ac, whereas the internal region of Ds1 is not homologous to Ac.7 Ds1 can be mobilized not only by Ac but also by another TE, Uq, which does not trans-activate Ds elements of the Ds family.8
Ds and Ds1 are identical for the first 19 bp containing 2 discontinuous but in-framed premature termination codons (PTCs; Figure 1). In addition, Ds and Ds1 are both biased toward providing splice donor and/or acceptor sites located close to their terminal regions.5,9 Ds provides only donors (1 forward and 4 reverse insertion patterns), whereas Ds1 provides 3 donors and 2 acceptors associating with 11 possible exonizing patterns (all in reverse insertion patterns).10,11 These different features between Ds and Ds1 imply possibly independent evolutionary impacts of Ds and Ds1 induced through exonization. To investigate their roles from an evolutionary perspective, we have simulated all putative Ds-exonized as well as Ds1-exonized transcripts in the rice genome.10,11 The exonized transcripts were translated into proteins and characterized as C-terminal variants (ie, those for which the C-terminus of the reference protein was replaced by the output peptides) and interior variants (ie, those for which additional peptides were inserted in the middle of the original transcript even as the same original termination codon was used). We also performed a functional profile analysis based on the PROSITE database12 and revealed the possibility of proteome enrichment by Ds exonization.13
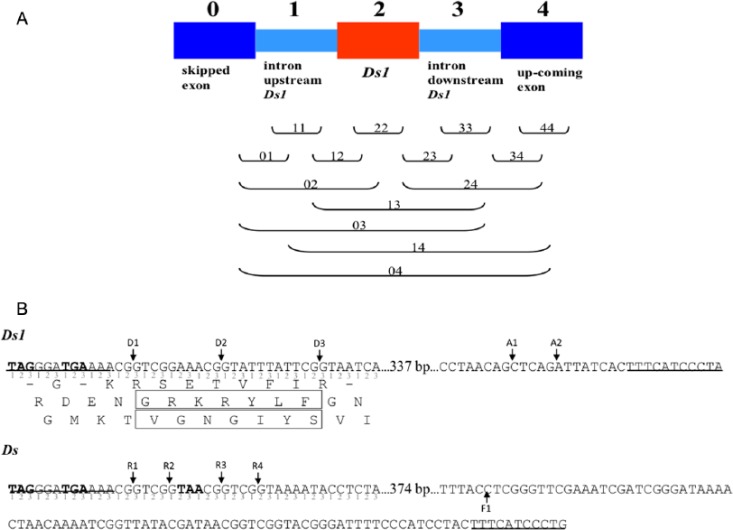
Figure 1.
(A) Classification of the profiles built by Ds1 and its flanking sequences. (B) Ds1 and Ds sequences yielding splice acceptor (A) or donor (D) junctions (arrows) as well as premature termination codons in exonized transcripts (bold). The translated products of Ds1 are also shown, of which gains/losses of 7 amino acids (boxes) yielded using D3/D1 were important to compose functional profiles. The termini repeat sequences of each transposable element are underlined. Note that Ds could provide 5 donors, R1, R2, R3, R4, and F1, but no acceptor. The donor, F1, is used for exonization by the opposite insertion pattern.
In this study, we investigated the behavior of Ds1 in exonization and compared it with that of Ds. A protocol similar to that used by Chien et al,13 with some modifications (see “Materials and Methods” section), was applied to the Ds1-exonized protein variants. We found that the Ds1-exonized messages with no more than 59 nucleotides were actively involved in creating diverse functional profiles. In particular, protein variants exonized from a single Ds1 insertion site with 2-amino-acid differences corresponded to 18 different functional profiles. Although Ds1 and Ds can build functional profiles per intron with similar efficiencies, Ds1 exonization produces 459 unique profiles, of which 129 are not produced by Ds. We thus conclude that Ds and Ds1 are independent but synergistic in their capacity to enrich proteome complexity through exonization.
Materials and Methods
The Ds1-exonized transcripts of rice were constructed previously.11 In this study, open reading frame (ORF) analysis was conducted for all of these exonized transcripts beginning at the original start codon and ending at the first in-frame stop codon. The transcripts were categorized as type I, type II, type III, or type IV transcripts depending on, respectively, whether the in-frame stop codon was located at the conserved region of the original splice junction, at the intron inserted by Ds1, at the Ds1 transposon itself, or at any exon occurring after the Ds1 insertion. Furthermore, in the event that the ORF analysis revealed no in-frame stop codon, the corresponding transcript was categorized as a type V transcript, and the incomplete transcript (ie, incomplete in that it lacked a stop codon) was output directly. In addition, the transcripts were further categorized as belonging to 1 of 2 subtypes: if the termination codon of the transcript was the same as that of the reference transcript (ie, the transcript without the Ds1 insertion), it was categorized as an interior transcript; otherwise, it was categorized as a C-terminal transcript.
If a given transcript contained a termination codon that was located more than 55 nucleotides upstream from the last exon/exon junction, it was considered a potential target for the nonsense-mediated decay (NMD) pathway14,15 and was therefore omitted from isoform prediction. The proteins of these transcripts not targeted by the NMD pathway were called non-NMD protein variants and were further translated to protein sequences. The original protein sequences and protein sequences from type III, IV, and V non-NMD variants were subject to protein profile analysis, in which we scanned the sequences to search for domains (profiles) previously reported in the PROSITE database (version 20.83).12 The PROSITE database contains a total of 2442 entries that describe the various protein domains, families, and functional sites, in addition to the various amino acid patterns, profiles, and signatures contained within them. Instead of using all the entries in PROSITE, we only scanned for the 1308 patterned ones to consistently identify the newly developed profiles using the same standard.
The functional profiles of each protein variant were compared with the ones of its reference protein. Only those variants yielding additional functional profile(s) were collected. As described in the text above, the new functional profiles in the protein isoforms were classified into subclasses named with 2 digits from “0” to “4” to indicate the start and the end of the amino acids from which the profiles originated, where “0” to “4” denoted the skipped exon, the flanking intron upstream from Ds1, Ds1 itself, the flanking intron downstream from Ds1, and the upcoming exon, respectively (Figure 1A). Following the same logic, the profile names shown in the text combined the information of interior (I) or C-terminal (C) type as well as the Ds1 portion used. For example, a class I22-D1 profile indicates a “22” profile observed in an interior functional variant (I) when Ds1 provided the first donor (D1). Further analyses of the resulting protein variants in different types were conducted using R (version 2.15.1).16
Results and Discussion
New functional profiles are introduced by Ds1 alone or together with its flanking exons and introns
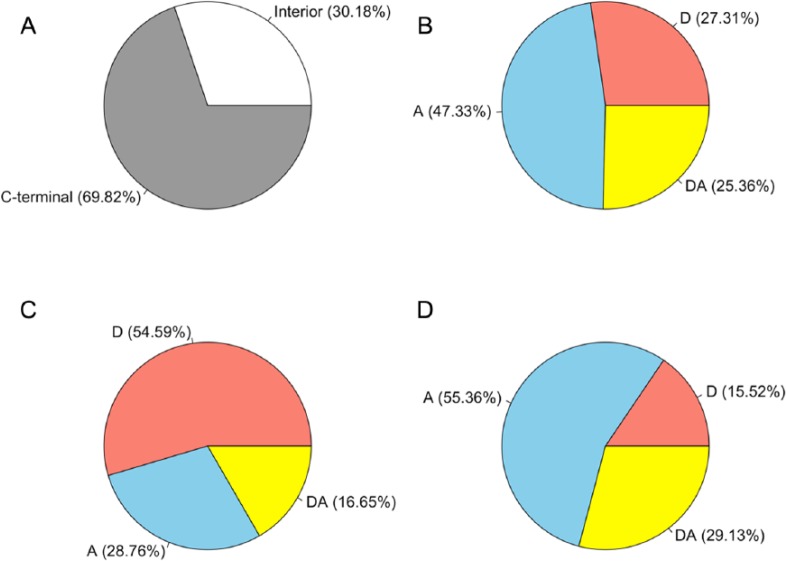
Functional profile analyses were performed on the previously simulated Ds1-exonized non-NMD protein variants in rice11 according to the patterned-profile database in PROSITE.12 From a total of 38 427 898 non-NMD variants, only 14 258 780 (4 303 236 interior and 9 955 544 C-terminal) variants yielding additional functional profile(s) were collected and termed as functional variants (Table 1 and Figure 2). There were 47.33% (6 748 622 of 14 258 780), 27.31% (3 893 991 of 14 258 780), and 25.36% (3 616 167 of 14 258 780) of the functional variants using an acceptor (A) alone, a donor (D) alone, or both a donor and an acceptor (DA) of Ds1, respectively (Table 1 and Figure 2). Most of the interior variants (54.59%) originated from Ds1 providing donors only, whereas most of the C-terminal variants (55.36%) originated from Ds1 providing acceptors.
Table 1.
Number of variants, profiles, and profiles per variant yielded by each donor, acceptor, and donor-acceptor combination.
| Number of variants |
Number of profiles |
Number of profiles per variant |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ds1 | Interior | C-terminal | Interior + C-terminal | Ds1 | Interior | C-terminal | Interior + C-terminal | Ds1 | Interior | C-terminal | Avg. |
| D1 | 834 649 | 459 547 | 1 294 196 | D1 | 1 649 507 | 1 604 133 | 3 253 640 | D1 | 1.9763 | 3.4907 | 2.5140 |
| D2 | 538 473 | 578 306 | 1 116 779 | D2 | 1 259 036 | 2 234 207 | 3 493 243 | D2 | 2.3382 | 3.8634 | 3.1280 |
| D3 | 976 104 | 506 912 | 1 483 016 | D3 | 2 066 945 | 1 821 524 | 3 888 469 | D3 | 2.1175 | 3.5934 | 2.6220 |
| Sub-total | 2 349 226 | 1 544 765 | 3 893 991 | Sub-total | 4 975 488 | 5 659 864 | 10 635 352 | Avg. | 2.1440 | 3.6492 | 2.7547 |
| D1A1 | 140 406 | 500 362 | 640 768 | D1A1 | 386 510 | 1 652 797 | 2 039 307 | D1A1 | 2.7528 | 3.3032 | 3.1826 |
| D1A2 | 88 020 | 429 108 | 517 128 | D1A2 | 213 532 | 1 315 830 | 1 529 362 | D1A2 | 2.4259 | 3.0664 | 2.9574 |
| D2A1 | 95 605 | 485 683 | 581 288 | D2A1 | 288 212 | 1 729 509 | 2 017 721 | D2A1 | 3.0146 | 3.5610 | 3.4711 |
| D2A2 | 139 554 | 443 916 | 583 470 | D2A2 | 793 056 | 1 613 625 | 2 406 681 | D2A2 | 5.6828 | 3.6350 | 4.1248 |
| D3A1 | 156 369 | 554 037 | 710 406 | D3A1 | 460 560 | 1 870 458 | 2 331 018 | D3A1 | 2.9453 | 3.3761 | 3.2812 |
| D3A2 | 96 485 | 486 622 | 583 107 | D3A2 | 250 906 | 1 503 768 | 1 754 674 | D3A2 | 2.6005 | 3.0902 | 3.0092 |
| Sub-total | 716 439 | 2 899 728 | 3 616 167 | Sub-total | 2 392 776 | 9 685 987 | 12 078 763 | Avg. | 3.2370 | 3.3387 | 3.3377 |
| A1 | 653 001 | 2 911 994 | 3 564 995 | A1 | 92 | 6 951 881 | 6 951 973 | A1 | 0.0001 | 2.3873 | 1.9501 |
| A2 | 584 570 | 2 599 057 | 3 183 627 | A2 | 49 | 6 280 165 | 6 280 214 | A2 | 0.0001 | 2.4163 | 1.9727 |
| Sub-total | 1 237 571 | 5 511 051 | 6 748 622 | Sub-total | 141 | 13 232 046 | 13 232 187 | Avg. | 0.0001 | 2.4018 | 1.9614 |
| Total | 4 303 236 | 9 955 544 | 14 258 780 | Total | 7 368 405 | 28 577 897 | 35 946 302 | Avg. | 1.7123 | 2.8706 | 2.5210 |
Figure 2.
The proportions of functional variants: (A) all variants, (B) all variants, (C) interior variants, and (D) C-terminal variants. A indicates acceptor; D, donor; DA, donor and acceptor.
The additional functional profiles yielded by the variants were further classified into subclasses named with 2 digits from “0” to “4” to indicate the start and the end of the amino acids from which the profiles originated, where “0” to “4” denoted the skipped exon, the flanking intron upstream from Ds1, Ds1 itself, the flanking intron downstream from Ds1, and the upcoming exon, respectively (Figure 1A). For example, a class “02” profile indicates that the functional profile in question was made from amino acids combining the messages (sequences) of the skipped exon, flanking upstream intron, and Ds1; a “22” profile indicates a profile made from the Ds1 message alone; and a “04” profile indicates a functional profile made from combining the messages all the way from the skipped exon to the upcoming exon. The “04” profiles were expected to be rare because 95% of the patterned profiles presented in PROSITE are composed of less than 30 amino acids. Indeed, “04” profiles accounted for only about 0.4% of the total profiles (Supplementary Table S1A and S1C). Following the same logic, a class I22-D1 profile is a “22” profile observed in an interior functional variant (I) when Ds1 provided the first donor (D1). Supplementary Table S1C and S1D presents the numbers of unique profiles in all classes of interior and C-terminal variants, respectively. Some classes not existing by definition are labeled “N” in Supplementary Table S1A and S1B. For example, exonization caused using donors alone (D) cannot yield profiles starting/ending with “3” (the intron downstream from Ds1).
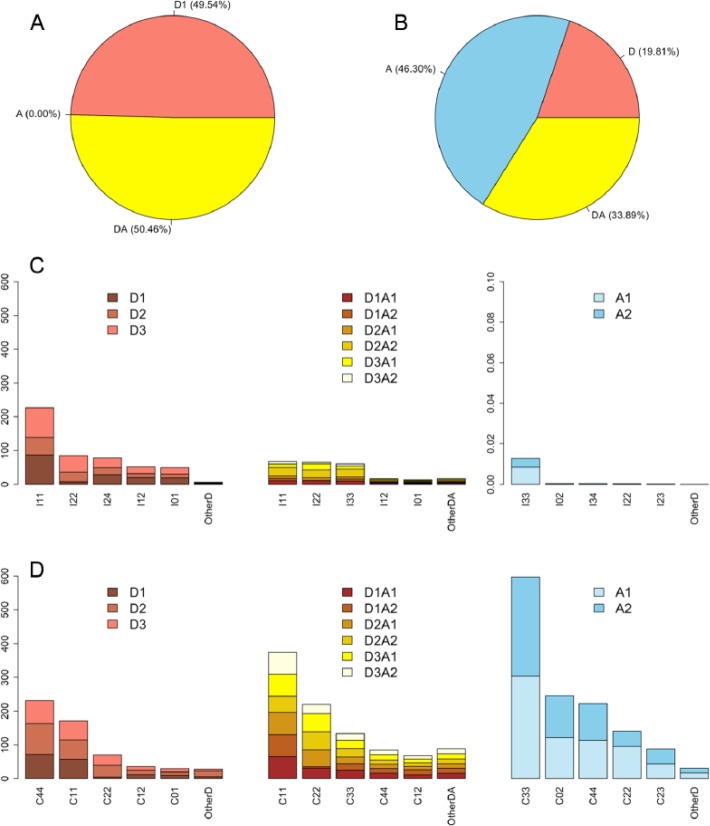
About 68% (4 975 488 out of 7 368 405) of the interior profiles (ie, additional profiles from interior functional variants) originated from Ds1 providing donors only (Table 1 and Figure 3A). The classes I11-D1 and I11-D3 yielded the highest number of new profiles (870 463) composed of 59 unique ones (Supplementary Table S1A and Figure 3C). The number of D2 interior profiles was generally lower than the numbers of D1 and D3 interior profiles due to fact that the D2 variants share the same reading frame with the stop codon, TAG, in the upstream Ds1 (Figure 1B). For C-terminal protein isoforms, 46.3% of the profiles originated from Ds1 providing acceptors only (Table 1 and Figure 3B). The classes C33-A1 and C33-A2 created the most additional profiles composed of 113 and 120 unique ones, respectively (Supplementary Table S1B and SID and Figure 3D). A C-terminal isoform was constructed using a reading frame different from that of the reference protein, and therefore, a greater diversity of C-terminal profiles than interior profiles were expected. In fact, among the 1308 profiles defined in the PROSITE database, 459 unique profiles were observed in all isoforms in this study, where 334 and 399 unique profiles appeared only in interior variants and only in C-terminal variants, respectively.
Figure 3.
The proportions and numbers of functional profiles: (A) interior profiles, (B) C-terminal profile, (C) numbers of interior profile (in thousands), and (D) numbers of C-terminal profiles (in thousands). A indicates acceptor; D, donor; DA, donor and acceptor.
On average, 1 variant included 2.52 new profiles (Table 1). The numbers and types of new functional profiles from D-variants (meaning the functional variants using a donor alone), A-variants (meaning the functional variants using an acceptor alone), and DA-variants (meaning the functional variants using both DA) were very different from each other (Figure 3). Most of functional profiles from A-variants resulted from C-terminal variants, for about 1.96 profiles per variant, but DA-variants yielded an average of 4.1 profiles per variant (Table 1). Interestingly, the classes yielding the most additional profiles were not necessarily the classes yielding the most unique profiles. For example, the profile number of class I12-D1 (204 859) was 4.3-fold less than that of I11-D1 (870 463), but more unique profiles were yielded by I12-D1 (111) than by I11-D1 (69). This implies the contribution of incorporated Ds1 messages, even those consisting of merely 14 bp, for yielding variants with new functions.
Exonized messages specifically from Ds1 contributed to new functional profiles
The functional profiles from classes “01,” “11,” “33,” “34,” and “44” only using the intron/exon sequences of the affected transcripts are Ds1 independent, whereas the other classes are Ds1 dependent. The number of Ds1-independent profiles (23 618 314) was about 1.9-fold higher than the number of Ds1-dependent ones (12 327 988). Because only a maximum of 59 bp from a Ds1 message would be incorporated into the resulting protein isoforms, the major contribution of a TE to exonization was expected to be the incorporation of the message of TE-inserted intron of the affected transcripts rather than the message itself.11 However, the Ds1-independent and Ds1-dependent profiles were composed of 314 and 398 unique profiles, respectively, with 253 overlapping profiles (Supplementary Table 2). This implies that the exonized Ds1 message is important for building functional profiles for selective advantage, either via the Ds1 message alone or together with its flanking intron/exon. There were 1 506 257 (20.44% of all the interior profiles) and 4 302 547 (25.58% of all the C-terminal profiles) class “22” interior and C-terminal profiles, respectively, built using the Ds1 message alone. These abundant class “22” profiles were composed of only 6 unique profiles, PS00004, PS00005, PS00006, PS00007, PS00008, and PS00009 (the underlined profiles were also yielded by Ds alone). The remaining 139 unique Ds1-dependent profiles were therefore built using the message of Ds1 together with its flanking intron/exon, and that number is still 2.3-fold (=139/61) (Supplementary Table 2) more than the number of unique Ds1-independent profiles.
Although Ds1 may yield 11 exonized transcript isoforms at a single insertion site, the translated protein products might be similar to each other. For example, isoforms yielded by D2A1 and D3A2 differ from each other by a mere 2 amino acids (Figure 4). This feature seems to underestimate the contribution of Ds1 exonization to proteome complexity. However, these small differences in isoforms from a single insertion site surprisingly contributed various new profiles, which was further illustrated using the two particular examples of (1) comparing isoforms using either D3 or D1 and (2) comparing isoforms using one of D1A1, D2A1, and D3A2.
Figure 4.
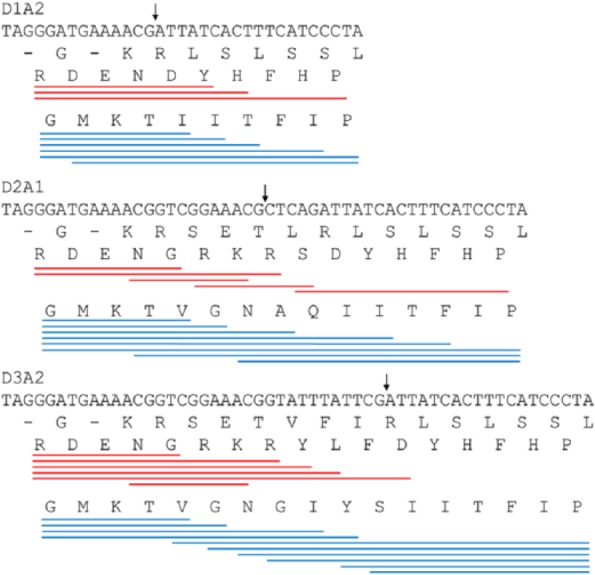
Distinct donor/acceptor combinations (ie, D1A2, D2A1, and D3A2) resulting in proteins that differ from other proteins by only a few amino acids.
The translated protein isoforms of D3 and D1 at the same insertion site differed by only 7 amino acids (either as “VGNGIYS” or “GRKRYLF” according to the reading frames) because the splice junction of D3 is located 21 bp downstream from that of D1 (Figure 1B). The 7-amino-acid sequences were responsible for 10 more unique profiles in class I12-D3 than in class I12-D1 (Figure 2). However, the lack of these 7-amino-acid sequences meant that class I14-D3 yielded only 21 unique profiles, less than the 78 yielded by class I14-D1. These results indicate that both the presence and absence of a part of the Ds1 message would act positively for building unique profiles in exonization events. Table 2 shows the IDs of the profiles yielded by gaining or losing 7 amino acids. The presence or absence of the 7 amino acids caused by alternatives of the D1 or D3 sites of Ds1 provided 133 unique profiles, of which 81 and 27 were exclusively yielded by D1 and D3, respectively. It is notable that the total number of profiles composing a class may not be equal to the number of unique profiles of that class shown in Figures 2 and 3 because messages other than these 7 amino acids can also contribute to creating functional profiles. Similar results were observed when comparing profiles from D1A1 and D3A1 isoforms or those from D1A2 and D3A2 isoforms.
Table 2.
Unique functional profiles yielded by gaining or losing 7 amino acids (either as “VGNGIYS” or “GRKRYLF”), which would be exonized using D1 and D3 donors because the splice junction of D3 is located downstream from D1 by 21 bp.
| Class | Gain or loss of Ds1 messages for translated amino acids | Profile ID |
|---|---|---|
| I02-D3 | GainVGNGIYS | PS00098; PS00186; PS00371; PS00447; PS01067 |
| I02-D3 | GainGRKRYLF | PS00636; PS00743; PS00761 |
| I12-D3 | GainVGNGIYS | PS00098; PS00186; PS00371; PS00420; PS00551; PS00595; PS00878; PS01067; PS01143 |
| I12-D3 | GainGRKRYLF | PS00027; PS00041; PS00636; PS01143 |
| I22-D3 | GainGRKRYLF | PS00009 |
| I04-D1 | LossVGNGIYS | PS00012; PS00027; PS00028; PS00029; PS00041; PS00059; PS00079; PS00086; PS00098; PS00189; PS00211; PS00212; PS00216; PS00251; PS00285; PS00310; PS00356; PS00358; PS00371; PS00389; PS00445; PS00527; PS00583; PS00589; PS00592; PS00615; PS00636; PS00652; PS00666; PS00678; PS00770; PS00778; PS00878; PS00909; PS00957; PS01008; PS01067;PS01145; PS01249; PS01353 |
| I04-D1 | LossGRKRYLF | PS00007; PS00029; PS00041; PS00079; PS00136; PS00189; PS00299; PS00451; PS00464; PS00595; PS00652; PS00698; PS00743; PS01202 |
| I04-D3 | GainVGNGIYS | PS00052; PS00292; PS00634; PS01094; PS01109; PS01171 |
| I04-D3 | GainGRKRYLF | PS01117 |
| I14-D1 | LossVGNGIYS | PS00012; PS00027; PS00028; PS00029; PS00053; PS00062; PS00079; PS00086; PS00095; PS00098; PS00128; PS00133; PS00146; PS00163; PS00186; PS00189; PS00194; PS00211; PS00212; PS00251; PS00262; PS00285; PS00299; PS00316; PS00338; PS00358; PS00371; PS00389; PS00392; PS00445; PS00551; PS00589; PS00592; PS00615; PS00636; PS00657; PS00672; PS00678; PS00818; PS00889; PS00914; PS01067; PS01103; PS01186; PS01275 |
| I14-D1 | LossGRKRYLF | PS00007; PS00022; PS00024; PS00028; PS00029; PS00063; PS00079; PS00107; PS00133; PS00194; PS00216; PS00232; PS00236; PS00280; PS00296; PS00362; PS00410; PS00422; PS00451; PS00464; PS00595; PS00678; PS01159; PS01186; PS60014 |
| I14-D3 | GainVGNGIYS | PS00218; PS00559; PS00605; PS01094; PS01109 |
| I14-D3 | GainGRKRYLF | PS00605; PS00634; PS01117 |
| I24-D1 | LossVGNGIYS | PS00165; PS00187; PS00237; PS00304; PS00671; PS00778; PS00915; PS01319 |
| I24-D1 | LossGRKRYLF | PS00018; PS00213 |
| I24-D3 | GainVGNGIYS | PS00062; PS00079; PS00107; PS00170; PS00211; PS00259; PS00290; PS00380; PS00588; PS00589; PS00598; PS00606; PS00636; PS01032 |
| I24-D3 | GainGRKRYLF | PS00070; PS00214; PS00674; PS01238 |
| C02-D3 | GainVGNGIYS | PS00098; PS00186; PS00189; PS00551 |
| C02-D3 | GainGRKRYLF | PS00583; PS00636; PS00761 |
| C12-D3 | GainVGNGIYS | PS00073; PS00186; PS00189; PS00371; PS00447 |
| C12-D3 | GainGRKRYLF | PS00041; PS00098; PS00636; PS00761 |
| C22-D3 | GainGRKRYLF | PS00009 |
| C04-D1 | LossVGNGIYS | PS00028; PS00029; PS00041; PS00061; PS00079; PS00211; PS00389; PS00551 |
| C04-D1 | LossGRKRYLF | PS00007; PS00028; PS00029; PS00041; PS00159; PS00189; PS00464; PS00583; PS01176; PS01249 |
| C04-D3 | GainVGNGIYS | PS00107; PS01047; PS01094 |
| C04-D3 | GainGRKRYLF | PS00527; PS01117; PS01143 |
| C14-D1 | LossVGNGIYS | PS00012; PS00022; PS00028; PS00029; PS00061; PS00073; PS00079; PS00086; PS00132; PS00133; PS00163; PS00189; PS00211; PS00285; PS00371; PS00392; PS00447; PS00527; PS00605; PS00678; PS00878; PS00889 |
| C14-D1 | LossGRKRYLF | PS00007; PS00021; PS00022; PS00028; PS00029; PS00063; PS00079; PS00223; PS00270; PS00296; PS00527; PS00652; PS00678; PS00761; PS01186 |
| C14-D3 | GainVGNGIYS | PS00214; PS00217; PS00622; PS01047 |
| C14-D3 | GainGRKRYLF | PS00217 |
| C24-D1 | LossVGNGIYS | PS00217; PS00615 |
| C24-D1 | LossGRKRYLF | PS00018; PS00027; PS00213 |
| C24-D3 | GainVGNGIYS | PS00092; PS00107; PS00205 |
| C24-D3 | GainGRKRYLF | PS00012; PS00216; PS00276 |
| C44-D1 | LossVGNGIYS | PS00435 |
The second example mentioned above consisted of comparing the translated protein isoforms of D1A2, D2A1, and D3A2 differing by only 2 to 7 additional amino acids (Figure 4). Table 3 shows the profiles that were built in protein isoforms by 1 or 2 (but not all) of the D1A2, D2A1, and D3A2 isoforms from a single Ds1 insertion site. For example, 13, 5, and 6 profiles were yielded only by D1A2, D2A1, and D3A2, respectively (bold-faced profiles in Table 3). D1A2 provided the lowest number of Ds1 messages, but a specific amino acid sequence, GMKTIITFIP, from D1A2 exonization exclusively contributed 7 profiles, PS00445, PS00622, PS00634, PS00740, PS00838, PS01241, and PS01359, which were not observed in D2A1 and D3A2 isoforms (Figure 4 and Table 3). Although PS00189, PS00371, PS01067, and PS00041 were present in all the D1A2, D2A1, and D3A2 isoforms, they originated from different Ds1 insertion sites and, consequently, were built by independent translated Ds1 messages. For example, PS00189 was matched by the translated Ds1 messages “RDENDY,” “NAQIITFIP,” and “GMKTVGNGIY” from D1A2, D2A1, and D3A2 patterns, respectively, due to its pattern being relatively broadly defined. Taken together, all the functional profiles shown in Tables 2 and 3 contributed about one-third (48 of 145) of the Ds1-dependent exclusive profiles (Supplementary Table 2) using merely 59 bp.
Table 3.
Unique translated Ds1 messages for the functional profiles of exonized protein isoforms yielded by joining specific donor and acceptor sites of D1A2, D2A1, and D3A2.
| Unique translated Ds1 messages for the functional profiles | Interior | C-terminal |
|---|---|---|
| D1A2 | ||
| RDENDY | PS00007 | PS00007; PS00189 |
| RDENDYH | — | PS01173 |
| RDENDYHFHP | PS00028; PS00223 | PS00028 |
| GMKTI | — | PS00371 |
| GMKTII | PS00079; PS00251 | PS00079; PS00636 |
| GMKTIIT | — | PS01067 |
| GMKTIITFI | PS00356 | PS00098; PS00356 |
| GMKTIITFIP | PS00041; PS00107; PS00189; PS00445; PS00622; PS00634; PS00740; PS00838; PS01241; PS01359 | PS00041; PS00189; PS00223; PS00622; PS00634; PS00716; PS00838; PS01047 |
| MKTIITFIP | — | PS01319 |
| D2A1 | ||
| RDENG | — | PS00761 |
| RDENGRKR | PS00041 | — |
| NGRK | PS00009 | PS00009 |
| RKRS | PS00004 | PS00004 |
| SDYHFHP | PS00214 | PS00214 |
| GMKTV | — | PS00371 |
| GMKTVG | PS00186; PS00589; PS00878 | PS00186; PS00420; PS00589; PS01067 |
| GMKTVGNA | PS00012 | PS00012 |
| GMKTVGNAQII | — | PS00373 |
| GMKTVGNAQIITF | PS00362; PS01047 | — |
| GMKTVGNAQIITFIP | PS00041; PS00716; PS01047 | PS00716; PS01047 |
| TVGNAQIITFIP | PS00223 | — |
| NAQIITFIP | PS00189 | — |
| D3A2 | ||
| RDENG | — | PS00761 |
| RDENGRKR | PS00041 | — |
| RDENGRKRY | PS00636 | PS00636 |
| RDENGRKRYL | — | PS00260 |
| RDENGRKRYLFD | — | PS00073 |
| NGRK | PS00009 | PS00009 |
| GMKTV | — | PS00371 |
| GMKTVG | PS00186; PS00589; PS00878 | PS00186; PS00420; PS00589; PS01067 |
| GMKTVGNGI | — | PS00098 |
| GMKTVGNGIY | — | PS00189 |
| VGNGIYSIITFIP | PS00107 | PS00107 |
| GNGIYSIITFIP | PS00189 | — |
| NGIYSIITFIP | PS00323 | PS00052; PS00323 |
| GIYSIITFIP | PS00079 | PS00079 |
| YSIITFIP | PS00027 | PS00027 |
| SIITFIP | PS00392 | PS00392 |
The resulting variants differ from others by only a few amino acids, which build unique profiles by 1 (bold) or 2 patterns only. Although PS00189, PS00371, PS01067, and PS00041 present in all three patterns, each profile was built by independent translated Ds1 message (see text). Profiles yielded by gaining or losing 7 amino acids (either as “VGNGIYS” or “GRKRYLF”), which are exonized using D1A2 and D3A2, were not shown.
Ds1 also differs from Ds by providing 2 acceptors for exonization. New interior profiles due to exonization only using Ds1 acceptors accounted for less than 1% of the interior profiles (Figure 3A), but new C-terminal profiles using Ds1 acceptors accounted for about 46% of the C-terminal profiles (blue bars in Figure 3). Similarly, either gain or loss of the Ds1 messages using Ds1 acceptors enriches proteome complexity through the creation of unique profiles. For example, the number of unique profiles in class C33-A2, 120, yielded by providing an extra 19 bp was a bit higher than that in C33-A1, 118 (Figure 3); however, the number of unique profiles in class C23-A2, 184, was less than that in C23-A1, 193. All these results suggest that Ds1 messages play a different role than Ds messages in building new functional protein isoforms for selective advantage.
Ds1 yields more exclusive profiles than Ds does
As shown in Figure 1B, the Ds and Ds1 transposons share a large degree of similarity in their sequences. However, the behaviors of these 2 TEs for exonization involved in splicing events are different: Ds provides only donors (1 forward and 4 reverse insertion patterns), whereas Ds1 provides both donors and acceptors.10,11 Only 1 splice donor site, D1 for Ds1 and R1 for Ds, appears in both TEs, and the same set of protein isoforms were yielded in both simulations using this particular loci (Figure 1B). However, the new profiles yielded from functional variants using sites other than R1 in Ds1 are considered to be Ds1-specific profiles.
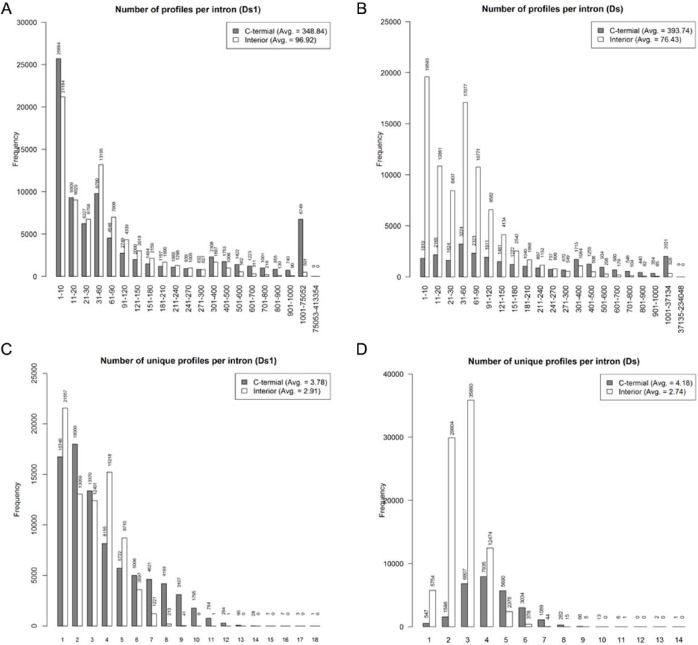
For interior variants, an intron may yield up to 75 052 (average = 96.92) new functional profiles via Ds1 insertion and subsequent exonization events (Figure 5A). For C-terminal variants, an intron may yield up to 413 354 (average = 348.84) new profiles (Figure 5A). The particularly high number of profiles that a given intron could produce in terms of C-terminal variants could result from the peptides generated as a result of a new reading frame being used to replace the reference protein’s C-terminus. For the unique profiles, the interior variants can yield up to 12 unique profiles (average = 2.91) via an intron, whereas the C-terminal can yield up to 18 (average = 3.78) unique profiles (Figure 5C).
Figure 5.
The distributions of (A and B) numbers and (C and D) unique numbers of profiles per intron in rice yielded by Ds1 and Ds exonization, respectively.
Through a comparison with those profiles obtained with Ds exonization in rice (Figure 5B and 5D), we found that Ds insertion yielded more profiles and unique profiles per intron than Ds1 insertion did via exonization. We reason that the insertion of the specific forward-pattern donor, F1, provided by Ds might possibly cause more distinct variants being exonized (Figure 1B); this forward splice donor is, however, absent in Ds1. The inflated number of variants by F1 insertion rationally brought on a higher number of (unique) profiles per intron, particularly in C-terminal variants. However, from a total number of 1308 patterned profiles in the PROSITE database, Ds1 and Ds built 459 and 365 unique profiles, respectively, with 330 identical ones (Supplementary Table 3), meaning that there are only 129 and 35 unique profiles exclusively built by Ds1 and Ds, respectively. This implies that although the termini of Ds and Ds1 are highly conserved, they yield independent sets of exonized protein isoforms and would thus be synergistic in contributing to the evolution of proteome complexity.
Conclusions
Ds and Ds1, which belong to the same TE family, share an identical 13 bp at 5′-terminal and 26 bp at 3′-terminal sequences. For exonization, the small difference in sequences between Ds1 and Ds may result in different PTCs, donor sites, and incorporated TE messages, which could, consequently, build independent sets of protein variants. As demonstrated in this study, this small difference in sequences makes Ds1 act unlike Ds in exonization. We have previously reported that Ds passively enriches proteome complexity in exonization by mainly adopting the messages of the flanking introns after Ds insertion sites.13 However, by offering acceptors that create new A-variants and DA-variants, Ds1 is more actively involved in exonization, either through its message alone or together with its flanking intron/exon, allowing the building of various new functional protein variants. We also demonstrated that, although a few of the 11 possible exonizing patterns from that Ds1 inserting into a single site are very similar to each other, even differences of only a few amino acids are enough to result in a wide spectrum of new profiles among these isoforms. All these features suggest that the evolutionary impacts of Ds and Ds1 due to exonization are distinct in various respects. We thus conclude that Ds and Ds1 exonizations are independent and synergistic in their effects on evolutionary proteome complexity enrichment. Incorporating further molecular analysis, for example, determining the changes in the priority of exonization sites under various stresses, would provide more information about the evolutionary impact of TE exonization.
Supplementary Material
Footnotes
Peer Review:Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 621 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by Ministry of Science and Technology in Taiwan (grant no. MOST 105-2321-B-002-037).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: YC and LDL conceived and designed the experiments. LH and LDL analyzed the data. YC wrote the first draft of the manuscript. YC, LH, and LDL contributed to the writing of the manuscript. YC, LH, and LDL Agree with manuscript results and conclusions. YC, LH, and LDL jointly developed the structure and arguments for the paper. YC and LDL made critical revisions and approved final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Sela N, Kim E, Ast G. The role of transposable elements in the evolution of non-mammalian vertebrates and invertebrates. Genome Biol. 2010;11:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmitz J, Brosius J. Exonization of transposed elements: a challenge and opportunity for evolution. Biochimie. 2011;93:1928–1934. [DOI] [PubMed] [Google Scholar]
- 4. Severing E, van Dijk A, Stiekema W, van Ham R. Comparative analysis indicates that alternative splicing in plants has a limited role in functional expansion of the proteome. BMC Genomics. 2009;10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang K-C, Yang H-C, Li K-T, Liu L, Charng Y-C. Ds transposon is biased towards providing splice donor sites for exonization in transgenic tobacco. Plant Mol Biol. 2012;79:509–519. [DOI] [PubMed] [Google Scholar]
- 6. Haring MA, Rommens CMT, Nijkamp HJJ, Hille J. The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol Biol. 1991;16:449–461. [DOI] [PubMed] [Google Scholar]
- 7. Kunze R, Weil CF. The hAT and CACTA superfamilies of plant transposons. In: Craig N, Craigie R, Gellert M, Lambowitz A. eds. Mobile DNA II. Washington, DC: ASM Press; 2002:565–610. [Google Scholar]
- 8. Pisabarro AG, Martin WF, Peterson PA, Saedler H, Gierl A. Molecular analysis of the Ubiquitous (Uq) transposable element system of Zea mays. Mol Gen Genet. 1991;230:201–208. [DOI] [PubMed] [Google Scholar]
- 9. Wessler SR. The maize transposable Ds1 element is alternatively spliced from exon sequences. Mol Cell Biol. 1992;11:6192–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu LD, Charng Y-C. Genome-wide survey of Ds exonization to enrich transcriptomes and proteomes in plants. Evol Bioinform Online. 2012;8:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charng Y-C, Liu LD. The extent of Ds1 transposon to enrich transcriptomes and proteomes by exonization. Bot Stud. 2013;54:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hulo N, Bairoch A, Bulliard V, et al. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chien TY, Liu L-YD, Charng Y-C. Analysis of new functional profiles of protein isoforms yielded by Ds exonization in rice. Evol Bioinform Online. 2013;9:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang Y-F, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. [DOI] [PubMed] [Google Scholar]
- 15. Hori K, Watanabe Y. Context analysis of termination codons in mRNA that are recognized by plant NMD. Plant Cell Physiol. 2007;48:1072–1078. [DOI] [PubMed] [Google Scholar]
- 16. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.