Abstract
Introduction:
Cancer cachexia is one of the most frequent effects of malignancy, is often associated with poor prognosis, and may account for up to 20% of cancer deaths. The aim of our study was to evaluate the relationship of cancer cachexia and serum levels of resistin and leptin in patients with advanced non–small cell lung cancer.
Methods:
A total of 67 chemotherapy-naïve patients with advanced-stage non–small cell cancer and a control group containing 20 healthy individuals without a known chronic disease were enrolled in this study. All individuals in the control group were age and sex matched. Demographic, anthropometric, laboratory data and serum levels of adipokines were measured for 2 groups. Progression-free survival and overall survival were estimated using the Kaplan-Meier method. Survival among various factors was calculated using the log-rank test.
Results:
Patients presented significantly higher serum resistin (P = .0001) and lower serum leptin levels (P = .025) than the control group. Lower serum levels of leptin were correlated with overall survival (P = .011).
Conclusions:
Serum leptin and resistin levels play key role as proinflammatory cytokines in lung cancer and cancer cachexia; however, their use as diagnostic or prognostic markers is not possible yet, and further large-scale studies are required to confirm our findings.
Keywords: cancer cachexia, lung cancer, leptin, resistin
Introduction
Currently, cancer is the second leading cause of death following cardiac diseases. Despite recent developments in tumor biology and genetics, an effective treatment method for a great deal of advanced cancer types has not yet been developed. Cancer treatment and improvement of the quality of life or sustained well-being during palliative treatment constitute the basis of treatment strategies. In this context, the most important approach is to provide nutritional support for these patients.
Although cachexia is used in the meaning of fasting or severe disease-related weight loss in the literature, it, indeed, defines the conditions with a body mass index (BMI) <20 kg/m2. Recently, it is defined as >5% weight loss accompanied by hypercatabolic state within the past 6 months during the course of life-threatening diseases such as cancer.1 One of the most frequent problems in advanced-stage cancers is cachexia. Lung cancer and gastrointestinal system (GIS) cancers are the 2 most common tumors causing cancer cachexia.2 Cachexia has major effects on mortality and by itself is a cause of death in at least 20% of the patients.3 Cachexia index is a novel index for patients with lung cancer to assess the survival and quality of life.4
The pathogenesis of cachexia in patients with cancer has not been exactly clarified, yet. Apart from the hypercatabolic process, it is thought to be related to tumor itself and some factors produced by the body. Inflammatory process and associated secretion of cytokines, such as tumor necrosis factor-α (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6), and IFN-γ, are thought to be responsible for this hypercatabolic process.5 Furthermore, food intake and energy balance are regulated by some mediators affecting arcuate nucleus in the hypothalamus, and leptin and resistin are among these mediators.
In recent years, the importance of adipose tissue is emphasized in several studies related to cancer cachexia.6 The major function of adipose tissue is to provide energy deposition. Adipose tissue also secretes bioactive peptides called adipokines. Adiponectin, leptin, and resistin are among the well-known adipokines. When leptin binds the receptors, feeding behavior is regulated, metabolism rate is arranged, sympathetic nervous system is activated, angiogenesis is stimulated, thermogenesis increases, and ultimately cellular growth and proliferation develop.7 Resistin is a polypeptide rich in cysteine.8 It is termed as resistin as the protein causes insulin resistance (IR). The relation between resistin and inflammation and the stimulating effects of nuclear transcription factors demonstrate that these adipokine levels may be partially effective in cancer development or its course. The potential role of resistin and leptin in non–small cell cancer (NSCLC) and their influence on cancer progression and cachexia syndrome are not entirely explained. Increasing recent evidence suggests the involvement of resistin in a variety of numerous inflammatory and autoimmune processes, such as atherosclerosis, steatosis, rheumatic diseases, inflammatory bowel diseases, asthma, and cancer.9 In vitro studies suggest that resistin is capable of inducing endothelial cell proliferation and promoting angiogenesis by upregulating vascular endothelial growth factor expression.9 Previous studies have demonstrated a link between many cancer types and resistin; however, there is still a limited number of data on this subject.10
In this study, we aimed to investigate the role of serum leptin and resistin levels in the pathogenesis of cancer cachexia to evaluate whether these peptides are effective in predicting cachexia and to investigate their effects on the quality of life of the patients.
Materials and Methods
Study population
A total of 67 chemotherapy-naïve patients who were admitted to Medical Oncology Department for the first time and were pathologically diagnosed as NSCLC were evaluated. A written informed consent was obtained from each participant. The study protocol was approved by the Medical Ethics Committee of Pamukkale University Medical Faculty (no: 02/date: February 24, 2009). The study was conducted in accordance with the principles of the Declaration of Helsinki.
The patients at advanced stage (stage IIIB and stage IV) whose performance status (PS) was 0, 1, 2 according to the World Health Organization classification were included in the study. The patients whose PS was 3 and worse on admission, those with cranial metastasis or suspicion of cranial metastasis, patients aged 80 years and above, those at early stage (I, II, and IIIA), those who were unwilling to give a written informed consent, and those who refused to answer the questionnaire were excluded from the study.
The control group consisted of a total of 20 healthy individuals without a known chronic disease. All individuals in the control group were age and sex matched.
Age, sex, and anthropometric measurements of the patient and the control groups, type of tumor, treatment, and pretreatment performances of the patients were recorded. The presence of weight loss at the time of diagnosis was questioned. Weight loss at the time of diagnosis was defined as a weight loss of more than 10% within the past 6 months. Cachexia was defined as a BMI ⩽20.
Biochemical analysis
Blood samples were taken before treatment in the patient group, and blood samples were taken between 08:00 and 09:00 am following an 8- to 12-hour fasting in the control group. A complete blood count was measured using the CELL-DYN 3700 Systems and CELL-DYN Sapphire device. Albumin, C-reactive protein (CRP), lactate dehydrogenase, ferritin, glucose, insulin, and cortisol were measured with Roche/Hitachi Cobas c Systems, e 601 Module device. For the analysis of IR, the homeostasis model assessment (HOMA-IR) tool which provides practical examination of β-cell function and IR using fasting glucose and insulin levels was used. It was calculated as fasting insulin value (µIU/mL) × fasting glucose level (mg/dL)/405. In healthy individuals, HOMA value is lower than 2.7, whereas values above 2.7 indicate IR.11
Furthermore, 6 mL of venous blood samples was taken from both the patient and control groups, for leptin and resistin analysis, were transferred to vacuumed tubes, and were centrifuged at 15 000 rpm for 10 minutes. Serum was, then, separated and stored in a deep freezer at −70°C. It was measured by enzyme-linked immunosorbent assay (ELISA) (Digital and Analog System, DAS, Palombara Sabina, Italy). The ELISAs were performed by the Human Resistin and Human Leptin ELISA kits obtained from Merck Millipore Corporation. The cut-off values of leptin and resistin were calculated using an automatic program. The cut-off values for leptin and resistin were 5.5 and 4.9 ng/mL, respectively. The values at and below this value were evaluated as low, and the values above this value were evaluated as high.
Anthropometric measurements
Height, weight, waist circumference, hip circumference, arm circumference, and triceps thickness were measured before treatment in the patient group and on admission in the control group. Height and weight measurements were performed on an empty stomach in height-scale and calibrated weight-scale device by a single investigator. The BMI was calculated according to the following formula: weight (kg)/height2 (m2). A BMI value between 20 and 24.9 kg/m2 was defined as normal, whereas BMI values above 25 kg/m2 were defined as overweight and obese.12 For the waist circumference, the narrowest diameter between the costal arch and anterior posterior (superior) iliac spinous process was measured by tape and recorded. For the hip circumference, the widest diameter through gluteus maximus at the back and symphysis pubis on the front was measured and recorded. The waist/hip ratio was calculated (normal value is <0.95 cm in men and <0.80 cm in women).12 The arm circumference was measured at the midpoint between olecranon and acromion. Values below 18 cm in women and below 20 cm in men were accepted as pathological values.13 For the evaluation of subcutaneous fat tissue, triceps thickness was measured at the midpoint of the distance between olecranon and acromion in the unused arm 3 times using a special caliper device, and the mean value was obtained. This value below 10 mm in men and 13 mm in women was accepted as nutritional deficiency.13
Statistical analysis
Statistical analysis was performed using SPSS version 16.0, for Windows software (SPSS Inc., Chicago, IL, USA). The χ2 square and Mann-Whitney U tests were used to compare characteristics of the patient and control groups. The Spearman and Pearson correlation tests were used for correlation analysis. The Kaplan-Meier plot was used for overall survival (OS) and progression-free survival (PFS) and time-survival curves. Logistic regression analysis was conducted to analyze factors affecting survival and progression. P < .05 was considered statistically significant with a confidence interval (CI) of 95%.
Results
The patient and control groups were compared in terms of age, sex, anthropometric measurements, biochemical values, and serum leptin and resistin values (Table 1).
Table 1.
A comparison of the patient and the control groups (mean ± SD).
| Patient (n = 67) | Control (n = 20) | P value | |
|---|---|---|---|
| Age, y | 62.9 ± 8.7 | 63.1 ± 6.2 | .988 |
| Age (below 55/above 55) | 18/49 | 2/18 | .169 |
| Sex (female/male) | 5/62 | 4/16 | .202 |
| Weight, kg | 70.07 ± 12.8 | 80.6 ± 10.8 | .002* |
| Weight loss (yes/no) | 42/25 | 0/20 | .042* |
| Smoking (yes/no) | 54/13 | 0/20 | .027* |
| BMI, kg/m2 | 25.4 ± 4.9 | 27.2 ± 2.9 | .078 |
| Waist circumference, cm | 89.4 ± 11.6 | 98.2 ± 7.6 | .0001* |
| Hip circumference, cm | 95.5 ± 7.8 | 104.2 ± 5.4 | .0001* |
| Triceps thickness, mm | 1.9 ± 0.8 | 3.6 ± 0.5 | .0001* |
| Arm circumference, cm | 26.4 ± 3.7 | 32.2 ± 2.2 | .0001* |
| Hemoglobin, g/dL | 12.8 ± 1.7 | 14.5 ± 1.0 | .0001* |
| Albumin, g/dL | 4.1 ± 0.4 | 4.4 ± 0.3 | .014* |
| CRP, mg/dL | 5.7 ± 6.1 | 0.3 ± 0.1 | .0001* |
| LDH, IU/L | 411.9 ± 108.1 | 194.1 ± 7.1 | .015* |
| Leptin, ng/mL | 8.5 ± 1.6 | 12.8 ± 2.4 | .025* |
| Resistin, ng/mL | 6.3 ± 1.9 | 4.4 ± 0.5 | .0001* |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; LDH, lactate dehydrogenase; n, number of patients; SD, standard deviation.
Results of Mann-Whitney U test.
P < .05 indicates statistical significance.
We described a correlation between BMI and serum leptin levels with Pearson correlation test (P < .01). However, there was no correlation between serum resistin levels and BMI (P = .18).
The increase in resistin levels was found to be statistically significant in the patient group (P = .0001). Further analysis among the patient group indicated that leptin levels were significantly lower compared with controls (P = .025). The frequency distribution of leptin and resistin in the patient and control groups is shown in Table 2.
Table 2.
Frequency distribution of leptin and resistin in the patient and control groups.
| Patient | Control | |||
|---|---|---|---|---|
| Leptin | Low (⩽5.5 ng/mL) | 41 | 7 | χ2 = 4.357 P = .037* |
| High (>5.5 ng/mL) | 26 | 13 | ||
| Resistin | Low (⩽4.9 ng/mL) | 16 | 15 | χ2 = 17.53 P = .0001* |
| High (>4.9 ng/mL) | 51 | 5 |
Results of χ2 test.
P < .05 indicates statistical significance.
Of the 67 patients included in the study, 42 patients (62.7%) had weight loss at the time of diagnosis. The characteristics of the patients with and without weight loss at the time of diagnosis were compared (Table 3).
Table 3.
Characteristics of patients with and without weight loss at the time of diagnosis (mean ± SD).
| Patients without weight loss (n = 25) | Patients with weight loss (n = 42) | P value | |
|---|---|---|---|
| Sex (female/male) | 0/25 | 5/37 | .073 |
| Age, y | 60.8 ± 1.4 | 64.2 ± 1.4 | .139 |
| Histologic type (A/S/O) | 6/18/1 | 9/26/7 | .299 |
| Smoking (present) | 19 | 35 | .539 |
| Albumin, g/dL | 4.2 ± 0.0 | 4.0 ± 0.0 | .027* |
| Leptin, ng/mL | 10.7 ± 2.5 | 7.2 ± 2.1 | .044* |
| Resistin, ng/mL | 5.6 ± 0.2 | 6.7 ± 0.3 | .054 |
| Patient status (exitus/alive) | 15/10 | 25/17 | .969 |
Abbreviations: A, adenocarcinoma; CT, chemotherapy; O, other; S, squamous cell carcinoma.
Results of Mann-Whitney test.
P < .05 indicates statistical significance.
Although resistin levels were higher in patients who had weight loss at the time of diagnosis, it did not reach statistical significance (P = .054). In addition, leptin levels were lower in patients who had weight loss at the time of diagnosis, indicating statistical significance (P = .044). Of the 42 patients who had weight loss at the time of diagnosis, progression was seen in 23 patients (54.8%) and exitus was seen in 25 patients (59.5%).
Leptin levels were 5.3-fold higher in obese patients (P = .002) and 5-fold higher in patients with IR (P = .02). Other remarkable findings are demonstrated in Table 4.
Table 4.
Patients’ clinical features and high serum leptin/resistin level relations.
| High leptin levels (No. of patients) | P value | ||
|---|---|---|---|
| Sex (female/male) | 20 | 5 | .006* |
| Cachexia (±) | 24 | 1 | .04* |
| Comorbidity (±) | 29 | 11 | .034* |
| Smoking (±) | 9 | 16 | .007* |
| Anemia (±) | 21 | 4 | .004* |
| High resistin levels (No. of patients) | P value | ||
| Sex (female/male) | 48 | 2 | .08 |
| CRP, mg/dL | 46 | 4 | .09 |
Abbreviation: CRP, C-reactive protein.
Results of the Fisher exact test.
P < .05 indicates statistical significance.
Of the 67 patients, progression developed in 33 patients (49.25%). The median PFS rate was 50.7% with a median PFS of 60 weeks (95% CI: 49.2-70.7). A total of 50% of the patients had progression within a year. The median OS rate was 40.3%, and median OS was 40 weeks (95% CI: 10.5-69.4). One-year and 2-year survivals in patients were 45% and 19%, respectively. Only low levels of leptin was found to be a statistically significant factor which affected OS (6.4-fold, P = .011) (Figure 1).
Figure 1.
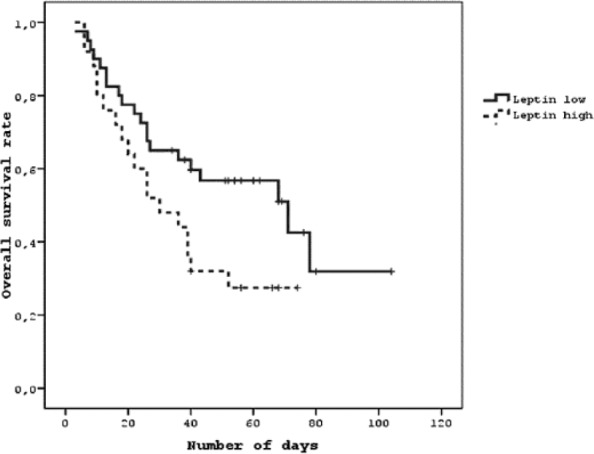
Kaplan-Meier overall survival curve of serum leptin levels.
Discussion
In this study, we investigated the correlation between leptin and resistin levels and nutritional status and disease progression in patients with advanced-stage NSCLC. We believe that these findings would make a contribution to the literature by assessment of adipokine levels, presentation of nutritional status, and assessment of the length of life.
More than 90% of the patients with lung cancer define complaints at the time of admission. In a previous study, it was reported that only 6% of the patients with lung cancer were without complaints, 27% had complaints related to primary tumor, 27% had nonspecific systemic complaints, such as loss of appetite and weight loss, and 32% had complaints suggesting metastasis.14 In this study, according to the medical history and nutritional status, 92.5% of the patients had complaints at the time of admission. The most common complaint was weight loss, followed by sputum production. However, in the previous studies, the most common complaint was dyspnea, followed by hemoptysis.14,15
In our study, BMI, waist circumference, hip circumference, arm circumference, triceps thickness, hemoglobin, and albumin values in the patient group were found to be lower, compared with the control group, due to the catabolic process, as expected in patients with cancer. We found that others were significant, except BMI.
Although there is no widely adopted definition of cancer cachexia at the present time, the main symptoms are weight loss and anorexia. However, there is a consensus for the definition of weight loss and the terminology used in the studies, namely, weight change within the past 6 months.16 In this study, the presence of weight loss of more than 10% within the past 6 months was defined as weight loss. The patients with weight loss constituted 62.7% of all patients in our study.
Furthermore, waist circumference, hip circumference, waist/hip ratio, triceps thickness, arm circumference, BMI, and albumin and hemoglobin levels were lower in patients who had weight loss at the time of diagnosis. Consistent with our study results, a previous study found a correlation between weight loss and low albumin and hemoglobin levels in patients with NSCLC.17
Leptin is produced more in subcutaneous fat tissue, compared with the visceral fat tissue, and leptin levels have best positive correlation with BMI and body fat ratio.18 In this study, leptin level was found to be lower in patients with cachexia. Leptin level was lower in patients with low BMI and other anthropometric measurements. In our study, leptin levels were lower in the patient group and in patients with weight loss at the time of diagnosis and higher in patients with BMI >25.
In the previous report presented by Wolf et al,19 it is found that plasma leptin levels were gender dependent, and significantly lower levels were found in cachectic women than among cachectic men. However, their sample size was very small with only 6 cachectic men and 12 cachectic women. Our study sample is also small. In our noncachectic patient group, there are 25 men and no woman. Our cachectic patient group consists of 5 women and 37 men. Further large-scale studies are required to confirm gender differences.
Although the correlation between leptin levels and cancer and cancer cachexia differs according to cancer types, it has not been clearly established, yet. Leptin levels were found to be lower in patients with colon20,21 and gastric cancers,21 whereas they were higher in those with breast22 and ovarian cancers.23 In a previous study including 76 patients with advanced-stage NSCLC and 30 healthy controls, BMI and serum leptin levels were found to be significantly lower in the patient group,24 consistent with our results. As acute-phase response, including anorexia, hypercatabolism, and some cytokines, plays role in the pathogenesis of cancer and cancer cachexia, we can suggest that increased leptin levels can also have a role in the pathogenesis. It has been explained by leptin OB gene overexpression by proinflammatory cytokine, TNF-α, and IL-1 which are involved in the pathophysiology of cancer cachexia. However, although acute-phase reactants (CRP, ferritin, platelets) increased in the aforementioned study, as in our study, leptin levels did not increase in contrast to this hypothesis. These results demonstrate that although cytokines such as TNF-α, IL-6, and IL-2 were unable to be evaluated in this study, leptin production seems not to be induced with inflammatory process, and leptin levels strongly show a correlation with adipose tissues. Albumin, BMI, and other anthropometric measurements, which are the indicators of nutritional status and loss of adipose tissue, have a positive correlation with leptin, which can be considered as evidence. Increased acute-phase reactants can be correlated with low leptin levels and poor nutritional status. Therefore, leptin can be regarded as a marker of nutritional status, rather than an acute-phase reactant. This hypothesis has been supported in previous studies related to chronic obstructive lung disease and chronic heart failure, which also have a course with cachexia.25,26
In a previous study including 20 NSCLC patients with weight loss and 13 healthy controls, leptin values were found to be significantly lower in the patient group. In the aforementioned study, weight loss, IL-6, and CRP were not associated with leptin values.27 Adipokine productions were normal and might be included in the pathogenesis both by its role in abnormal fat metabolism and by its role in systemic inflammatory response. In another study, leptin level was found to be significantly lower in cachexic patients with GIS cancer, and low leptin levels were correlated with both loss of adipose tissue and increased inflammatory cytokines.28
In a previous study including 101 patients with advanced-stage NSCLC (76 patients without weight loss and 25 patients with weight loss) and 51 healthy controls, leptin levels at the time of admission were found to be significantly lower in the patient group and in patients with weight loss.29 When leptin levels were remeasured at the end of the third and sixth cycles of chemotherapy, these levels were found to increase significantly. Furthermore, leptin levels were found to be higher during relapses. However, leptin levels in patients who were responsive to chemotherapy were not found to be different from the other group. In addition, there was no correlation between leptin levels at the time of diagnosis and OS and time to disease progression. Although a significant correlation was not found with the time to disease progression, the individuals with weight loss and poor performance were prone to poor survival.
In another study conducted in patients with NSCLC, leptin levels were found to be significantly higher during the early period and significantly lower in advanced stage.30,31 As leptin has proliferative and antiapoptotic effects, the correlation between leptin or its receptors and colorectal carcinoma,32 breast cancer,33 and melanoma34 has been demonstrated in many in vivo studies and in vitro cancer cell cultures.31 In addition, leptin receptor expressions in breast, ovary, bladder, endometrium, and hepatocellular cancer cells have been in the in vivo setting.31,35 In these studies, increased leptin levels were attributed to its proliferative properties. Furthermore, it has been suggested that leptin secretion secondary to weight loss during early period might have increased as a compensatory response. Although the number of studies on NSCLC is limited in the literature, increased expression and polymorphism of leptin gene were associated with lung cancer risk in a molecular study.36
Moreover, it has been suggested that resistin has a mediator role in the regulation of metabolism, particularly glucose homeostasis, and it has a regulator role in adipogenesis and a modulator role in inflammation process.37 Cell population producing and expressing resistin in human beings are peripheral blood mononuclear cells, macrophages, and bone marrow cells.37 In vitro macrophage stimulation and administration of exogenous endotoxin result in increased resistin production. As a result, it was found that higher resistin levels were present in sepsis and other inflammatory diseases.38,39 It is thought that the role of resistin in this process is that it plays a role in systemic inflammatory response through a potent reciprocal interaction with cytokines such as TNF-α, IL-6, and IL-10 which play a role in the regulation of systemic inflammatory response, angiogenesis, cellular proliferation, differentiation, and migration.39
There are limited number of studies investigating the relationship between resistin and cancer.40 In this study, resistin level was found to be higher in the patient group and male patients. Although resistin level was higher in patients with weight loss at the time of diagnosis, it did not reach statistical significance. In a previous study including 41 patients with breast cancer and 43 healthy controls, resistin levels were found to be significantly higher in the patient group.41 Similar to this study, resistin levels were found to be significantly higher in the NSCLC patient group.29 Therefore, we concluded that increased resistin levels not only originated from adipose tissue, but it could be also related to monocyte activation during the inflammatory process. In this study, a correlation between resistin and age, sex, BMI, stage of the disease, OS, and time to disease progression was unable to be identified. In addition, in the aforementioned study, increased resistin levels were correlated with poor performance, and the resistin levels in patients with weight loss at the time of diagnosis were significantly higher.29 In this study, we can attribute high resistin levels in patients with weight loss at the time of diagnosis without any significance to the small sample size. High resistin values in patients with weight loss at the time of diagnosis demonstrate that resistin can play a role in hypercatabolic process. In this study, median PFS time was shorter in patients with high resistin levels; however, no effect on progression and survival was shown.
In conclusion, leptin and resistin measurements were done only at the time of diagnosis. The median PFS was shorter in patients with high leptin values; however, it did not reach statistical significance. Although median OS was higher in patients with low leptin levels, it was significant. The leptin and resistin ELISA kits which we applied in our study contain only 96 plates. Our sample was a total of 87 individuals (67 patients and 20 controls), and we spared the rest 9 plates as misapplications. The small sample size and lack of measurements at the end of the chemotherapy might have led to these results. Our study results demonstrates that resistin levels were found to be higher in patients with high CRP levels, which is an important proinflammatory cytokine in cancer and cancer cachexia; however, its use as a diagnostic or prognostic marker is not yet possible, and further large-scale studies are required to confirm our findings.
Footnotes
Peer Review:Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1436 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration Of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors reviewed and approved of the final manuscript.
References
- 1. Consul N, Guo X, Coker C, et al. Monitoring metastasis and cachexia in a patient with breast cancer: a case study. Clin Med Insights Oncol. 2016;10:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fortunati N, Manti R, Birocco N, et al. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep. 2007;18:1521–1527. [PubMed] [Google Scholar]
- 3. Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. [DOI] [PubMed] [Google Scholar]
- 4. Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia index in advanced non-small-cell lung cancer patients. Clin Med Insights Oncol. 2015;9:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson G, Salle A, Lorimier G, et al. Cancer cachexia: measured and predicted resting energy expenditures for nutritional needs evaluation. Nutrition. 2008;24:443–450. [DOI] [PubMed] [Google Scholar]
- 6. Ryden M, Arner P. Fat loss in cachexia—is there a role for adipocyte lipolysis? Clin Nutr. 2007;26:1–6. [DOI] [PubMed] [Google Scholar]
- 7. Kralisch S, Bluher M, Paschke R. Adipokines and adipocyte targets in the future management of obesity and the metabolic syndrome. Mini Rev Med Chem. 2007;7:39–45. [DOI] [PubMed] [Google Scholar]
- 8. Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. [DOI] [PubMed] [Google Scholar]
- 9. Ntikoudi E, Kiagia M, Boura P, Syrigos KN. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev. 2014;40:22–30. [DOI] [PubMed] [Google Scholar]
- 10. Hou WK. Adipocytokines and breast cancer risk. Chin Med J. 2007;120:1592–1596. [PubMed] [Google Scholar]
- 11. Lebovitz HE. Insulin resistance and the insulin resistance syndrome. Clinician’s manual on insulin resistance. Science. 2002;9:1–15. [Google Scholar]
- 12. World Health Organization (WHO). Obesity: preventing and managing the global epidemic. Report of a WHO consultation, WHO Technical Report Series no. 894. Published 2000. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 13. Davies M. Nutritional screening and assessment in cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:64–73. [DOI] [PubMed] [Google Scholar]
- 14. Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123:97–104. [DOI] [PubMed] [Google Scholar]
- 15. Scangliotti GV. Symptoms, signs and staging of lung cancer. Eur Respir Monogr. 2001;17:86–119. [Google Scholar]
- 16. Lasheen W, Walsh D. The cancer anorexia-cachexia syndrome: myth or reality? Support Care Cancer. 2009;9:772–776. [DOI] [PubMed] [Google Scholar]
- 17. Bozzetti F, Mariani L. Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. J Parenter Enteral Nutr. 2009;33:361–367. [DOI] [PubMed] [Google Scholar]
- 18. Emral R. Adiponectin and other cytokines. J Med Sci. 2006;26:409–420. [Google Scholar]
- 19. Wolf I, Sadetzki S, Kanety H. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. 2006;106:966–973. [DOI] [PubMed] [Google Scholar]
- 20. Kumor A, Daniel P, Pietruczuk M, Małecka-Panas E. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275–281. [DOI] [PubMed] [Google Scholar]
- 21. Bolukbas FF, Kilic H, Bolukbas C. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. [DOI] [PubMed] [Google Scholar]
- 23. Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129:353–357. [DOI] [PubMed] [Google Scholar]
- 24. Aleman MR, Santolaria F, Batista N, et al. Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine. 2002;19:21–26. [DOI] [PubMed] [Google Scholar]
- 25. Takabatake N, Nakamura H, Abe S, et al. Circulating leptin in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1215–1219. [DOI] [PubMed] [Google Scholar]
- 26. McEntegart MB, Awede B, Petrie MC, Sattar N, Dunn FG, MacFarlane NG, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829–835. [DOI] [PubMed] [Google Scholar]
- 27. Nigel B, Jamieson, Duncan JF, et al. Adiponectin and the systemic inflammatory response in weight-losing patients with non-small cell lung cancer. Cytokine. 2004;27:90–92. [DOI] [PubMed] [Google Scholar]
- 28. Dülger H, Alici S, Sekeroğlu MR, et al. Serum levels of leptin and proinflammatory cytokines in patients with gastrointestinal cancer. Int J Clin Pract. 2004;58:545–549. [DOI] [PubMed] [Google Scholar]
- 29. Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer. 2008;61:391–397. [DOI] [PubMed] [Google Scholar]
- 30. Agapios T, Theodoros NS, Antonopoulos G, et al. Elevated serum leptin levels: a risk factor for non-small-cell lung cancer? Oncology. 2009;76:19–25. [DOI] [PubMed] [Google Scholar]
- 31. Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–244. [DOI] [PubMed] [Google Scholar]
- 32. Chia VM, Newcomb PA, Lampe JW, et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2697–2703. [DOI] [PubMed] [Google Scholar]
- 33. Liu CL, Chang YC, Cheng SP, et al. The roles of serum leptin concentration and polymorphism in leptin receptor gene at codon 109 in breast cancer. Oncology. 2007;72:75–81. [DOI] [PubMed] [Google Scholar]
- 34. Gogas H, Trakatelli M, Dessypris N, et al. Melanoma risk in association with serum leptin levels and lifestyle parameters: a case control study. Ann Oncol. 2008;19:384–389. [DOI] [PubMed] [Google Scholar]
- 35. Wang SN, Chuang SC, Yeh YT, et al. Potential prognostic value of leptin receptor in hepatocellular carcinoma. J Clin Pathol. 2006;59:1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ribeiro R, Araujo AP, Coelho A, et al. A functional polymorphism in the promoter region of leptin gene increases susceptibility for non-small cell lung cancer. Eur J Cancer. 2006;42:1188–1193. [DOI] [PubMed] [Google Scholar]
- 37. McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol. 2006;17:170–175. [DOI] [PubMed] [Google Scholar]
- 38. Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin-implications for inflammatory bowel disease. Mol Nutr Food Res. 2008;8:855–866. [DOI] [PubMed] [Google Scholar]
- 39. Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3:29–34. [PubMed] [Google Scholar]
- 40. Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. [DOI] [PubMed] [Google Scholar]
- 41. Kang J, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007;22:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


