Abstract
With advances in sequencing technologies, there has been an increase in the discovery of viruses that do not group with any currently described virus families. The newly described taxon Negevirus encompasses a group of viruses displaying an insect-specific phenotype which have been isolated from multiple host species on numerous continents. Using a broad-spectrum virus screening assay based on the detection of double-stranded RNA and next-generation sequencing, we have detected a novel species of negevirus, from Anopheles, Culex, and Aedes mosquitoes collected in 4 geographically separate regions of Australia. Bioinformatic analysis of the virus, tentatively named Castlerea virus, revealed that it is genetically distinct from previously described negeviruses but clusters in the newly proposed Nelorpivirus clade within this taxon. Analysis of virions confirmed the presence of 2 proteins of 24 and 40 kDa which support previous bioinformatic predictions of negevirus structural proteins.
Keywords: Virus discovery, negevirus, mosquito, insect-specific virus
Introduction
Recently, a vast number of insect-only viruses (ISVs) have been isolated from multiple mosquito species globally.1–3 Making up a large percentage of these viruses are insect-specific flaviviruses (ISFs) that have predominately been found within Aedes and Culex species mosquitoes. However, isolations of Palm Creek virus from Coquillettidia sp. and Nakiwogo virus from Mansonia sp. suggest that ISFs exhibit a broad mosquito host range.2–4 Interestingly, multiple other ISVs have been isolated from other virus families, including Bunyaviridae, Reoviridae, Togaviridae, Mesoniviridae, and most recently from a newly described taxon, Negevirus.5–9
The taxon Negevirus was first proposed by Vasilakis and colleagues who described 6 viruses that were distantly related to plant-infecting viruses of the genus Cilevirus.10 Viruses in the Negevirus taxon are enveloped, positive-sense single-stranded RNA viruses ((+) ssRNA) with a 9- to 10-kb genome that is polyadenylated at the 3′ end and encodes 3 open reading frames (ORFs) flanked by noncoding intergenic regions (IGRs).10,11 Open reading frame 1 is approximately 7 kb and codes for the RNA-replication machinery domains: ribosomal RNA (rRNA) methyltransferase (MTase), viral MTase, helicase, and RNA-dependent RNA polymerase (RdRP). Open reading frames 2 and 3 are approximately 1.5 kb and 600 base pairs (bp), respectively, and it has been hypothesised that they encode structural proteins, although little physical evidence has been provided to support these potential functions to date.6,10,12 Open reading frame 3 encodes a small protein which contains a domain with similarity to the p24 and p23 membrane proteins found in the plant virus genera Cilevirus, Higrevirus, and Blunervirus.12–14 More recently, with the use of sequence profile-based domain prediction programs, such as PSI-BLAST and HHblits, ORF2 has been reported to contain a small glycoprotein domain indicating that it may function as a spike protein.13,14
To date, 12 viruses have been described which are thought to represent individual species of negeviruses. These viruses have been isolated from multiple mosquito genera, as well as Lutzomyia sandflies from Europe, Africa, and the United States, with numerous isolates of some virus species being isolated from several different geographic locations and from multiple host species.6,10-12,15
Recent bioinformatic analyses undertaken by Kallies et al16 revealed that negeviruses can be classified into 2 distinct clades within the taxon. The Nelorpivirus clade consists of viruses that cluster with Negev (NEGV), Loreto (LORV), and Piura (PIUV) viruses, whereas the Sandewavirus clade includes the more divergent Santana (SANV), Dezidougou (DEZV), Wallerfield (WALV), and related viruses.6,11,16
In virus culture using the dicer-2–deficient C6/36 and C7/10 (Aedus albopictus) cell lines, these viruses can cause extensive cytopathic effect (CPE) from 24 hours post infection (hpi), characterised by cell rounding and detachment of the monolayer.10,15,17,18 Additional analysis using vertebrate cell culture (Vero, African green monkey; BHK, baby hamster kidney; and HEK 293, human adrenal ganglion/neuron-derived cell line) and intracerebral inoculation of newborn mice has indicated that negeviruses likely exhibit an insect-specific phenotype.10
Little is known about the mode of transmission of negeviruses in nature. Isolation of Okushiri virus (OKV) from mosquito larvae suggests the potential for vertical transmission of some negeviruses,13 whereas horizontal transmission via blood meal has been demonstrated to be possible, but fairly inefficient, for the prototype strain of NEGV (EO-329).10 Although attempts to culture these viruses in vertebrate cell lines have thus far been unsuccessful, the isolation of some negeviruses from multiple host species and genera indicates the potential for horizontal transmission via a cryptic vertebrate host or a host range that includes plants.6,10,12
Further isolations of negeviruses will provide crucial information about the evolution and persistence of these viruses and interactions that occur within the mosquito virome, such as the potential to interfere with the transmission of secondary infections with pathogenic vertebrate-infecting viruses, as has been shown for the ISFs.19,20 Herein, we describe a putative species of negevirus, tentatively named Castlerea virus (CsV), which represents the first isolation of a negevirus from Australian mosquitoes.
Results
Isolation of prototype CsV from Brisbane, Australia
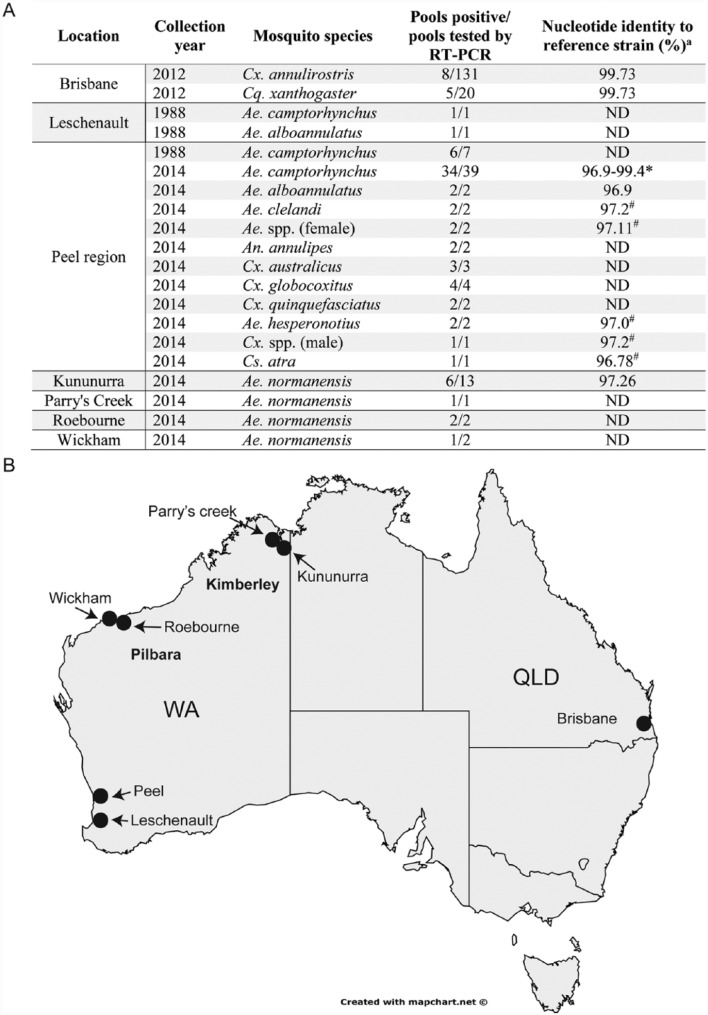
A total of 200 pools of mosquitoes (4302 mosquitoes) from Culex, Mansonia, and Aedes genera collected in Brisbane during 2012 were screened for the presence of (+) ssRNA or double-stranded RNA (dsRNA) viruses by fixed-cell enzyme-linked immunosorbent assay (ELISA) using Monoclonal Antibodies against Viral RNA Intermediates in Cell culture (MAVRIC) which detects long dsRNA molecules (>30 bp) produced during viral replication21 (Table 1). From the 24 pools identified as positive by this assay, a single pool of Culex annulirostris mosquitoes (B185775), which also caused substantial CPE in cell culture 24 hpi, was selected for Illumina sequencing. Initial BLAST-X analysis of elucidated contigs showed that the sequence shared a high (75% across 2282 amino acids) amino acid sequence identity with Ngewotan virus (NWTV), a negevirus isolated from Culex vishnui mosquitoes collected in Indonesia.10 This virus was tentatively named Castlerea virus after Castlerea street in Tingalpa where the mosquitoes from pool B185775 were collected. Based on the prototype sequence, a CsV-specific reverse transcription polymerase chain reaction (RT-PCR) using primers to the rRNA MTase domain of ORF1 was designed to perform retrospective analysis of the cohort of 24 MAVRIC-positive pools collected in 2012. This analysis yielded an additional 12 isolates from Cx annulirostris and Cq xanthogaster mosquitoes (Figure 1A, Table 1).
Table 1.
Analysis of MAVRIC-positive pools from Brisbane mosquito cohort, 2012.
| Pool ID | Mosquito species | Number per pool | RT-PCR |
||
|---|---|---|---|---|---|
| CsV | Alphamesonivirus 1 | LNV | |||
| B185774 | Cx annulirostris | 25 | + | − | + |
| B185775 | Cx annulirostris | 25 | +a | − | + |
| B185776 | Cx annulirostris | 25 | + | + | + |
| B185792 | Ae vigilax | 11 | − | + | + |
| B185798 | Ae vigilax | 11 | + | − | + |
| B185805 | Cx annulirostris | 25 | + | − | + |
| B185814 | Ae vigilax | 15 | + | − | + |
| B185816 | Cx annulirostris | 25 | + | − | + |
| B185827 | Cx annulirostris | 25 | + | − | + |
| B185829 | Cq xanthogaster | 23 | + | − | + |
| B185860 | Cq xanthogaster | 15 | + | + | + |
| B185869 | Cq xanthogaster | 18 | − | + | + |
| B185883 | Cx annulirostris | 10 | − | − | + |
| B185884 | Cq xanthogaster | 2 | + | − | + |
| B185885 | Cq xanthogaster | 9 | − | − | + |
| B185896 | Cq xanthogaster | 20 | − | + | + |
| B185926 | Cx annulirostris | 30 | − | − | ND |
| B185931 | Cq xanthogaster | 8 | − | + | + |
| B185945 | Cq xanthogaster | 35 | − | + | + |
| B185953 | Cq xanthogaster | 27 | − | + | + |
| B185959 | Cq xanthogaster | 22 | + | − | + |
| B185962 | Cx sitiens | 29 | − | + | + |
| B185967 | Cq xanthogaster | 25 | + | + | + |
| B185970 | Cx bitaeniorhynchus | 30 | − | − | − |
Abbreviations: Ae, Aedes; Cq, Coquillettidia; Cx, Culex; CsV, Castlerea virus; LNV: Liao ringing virus; MAVRIC: Monoclonal Antibodies against Viral RNA Intermediates in Cell culture; RT-PCR, reverse transcription polymerase chain reaction.
Figure 1.
Isolations of Castlerea virus (CsV) in Australia. (A) Summary of CsV isolations from Queensland and Western Australia. aNucleotide similarity across 700 base pairs unless otherwise stated. *Four isolates of CsV from Ae camptorhynchus collected in 2014 were sequenced; one sample was sequenced as a representative for all other mosquito species. Isolates depicted with (#) represent sequences obtained by next-generation sequencing. Ae indicates Aedes; An, Anopheles; Cq, Coquillettidia; Cs, Culiseta; Cx, Culex; ND, sequencing not performed. (B) Map of Australia depicting the approximate locations from which CsV isolates were found (black dots).
MAVRIC positive mosquito pools from the cohort of mosquitoes collected in Brisbane in 2012 were further assessed by RT-PCR of extracted RNA from the virus cultures using a panel of specific primers generated to viruses that are frequently present in Australian mosquito populations1,22,23 These analyses confirmed the presence of Liao ning virus (LNV) and Alphamesonivirus 1 in many of the mosquito pools and co-isolation of LNV in all CsV-positive pools (Table 1).
CsV is present in mosquito populations of a large geographic range and in archival mosquito pools
MAVRIC positive virus cultures obtained from an additional 10 mosquito pools collected from the Peel region of Western Australia (WA) in 2014, at a distance of over 4000 km from where the prototype was isolated (Figure 1B), were analysed by Illumina sequencing. This resulted in 10 additional negevirus sequences from a range of host species, including a single male Culex mosquito (Table 2). All sequences shared 97.42% to 97.72% nucleotide identity with the prototype CsV strain (B185775) over 9020 bp (ORFs 1-3) (Table 2). Further analysis of extracted RNA from cultures of 50 mosquito homogenates from these cohorts using RT-PCR yielded a further 45 CsV-positive pools. Additional retrospective analysis of 7 archival pools collected from the Peel region in 1988 yielded 6 isolates from Aedes camptorhynchus, and pools collected from Leschenault in the same year yielded 1 isolate from Ae camptorhynchus and one isolate from Ae alboannulatus, representing the oldest isolates of this virus in Australia (Table 2). Finally, 13 pools of Ae normanensis collected in Kununurra and 1 from Parry’s Creek in the Kimberley region during 2014 yielded 6 and 1 isolate of CsV, respectively, whereas 3 isolates in total were obtained from 2 pools of Ae normanensis from Roebourne and 1 pool collected in Wickham from the Pilbara region in 2014 (Figure 1).
Table 2.
Details of isolates with full genome sequence available.
| Pool ID | Mosquito species | Sex | Pool size | Location | Nucleotide identity to CsV prototype (%)a | Genbank accession no. |
|---|---|---|---|---|---|---|
| B185775 | Cx annulirostris | Female | 25 | Tingalpa | 100 | KX886280 |
| DC60042 | Ae camptorhynchus | Female | 20 | Peel region | 97.74 | KX903294 |
| DC59219 | Ae camptorhynchus | Female | 18 | Peel region | 97.42 | KX903303 |
| DC59240 | Ae camptorhynchus | Female | 20 | Peel region | 97.50 | KX903302 |
| DC59669 | Ae camptorhynchus | Female | 20 | Peel region | 97.48 | KX903300 |
| DC59991 | Ae hesperonotius | Female | 2 | Peel region | 97.59 | KX903295 |
| DC59899 | Ae hesperonotius | Female | 2 | Peel region | 97.72 | KX903298 |
| DC59932 | Ae clelandi | Female | 10 | Peel region | 97.53 | KX903296 |
| DC59801 | Ae spp. | Female | 21 | Peel region | 97.57 | KX903299 |
| DC59341 | Culiseta atra | Female | 2 | Peel region | 97.53 | KX903301 |
| DC59916 | Cx spp (male) | Male | 1 | Peel region | 97.61 | KX903297 |
Abbreviations: Ae, Aedes; Cx, Culex; CsV, Castlerea virus; ORF, open reading frame.
Identity across entire ORF1-3 sequence (9020 bp).
Genome organisation and nonstructural protein analysis of CsV
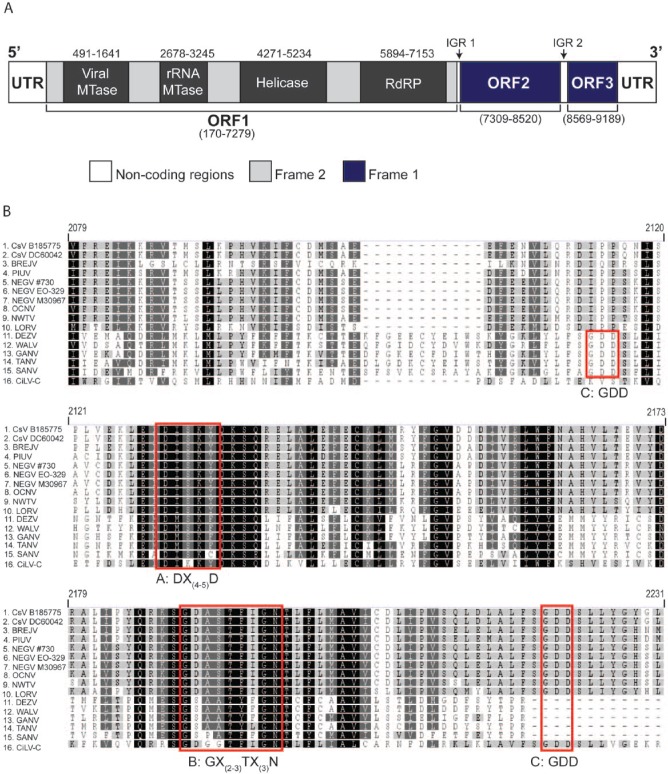
Annotation of the CsV genome showed that it follows the conventional genome organisation of viruses in the Negevirus taxon, encoding 3 ORFs that are separated by 2 intergenic regions (IGRs) and flanked by 5′ and 3′ untranslated regions (UTRs). The 3′ UTR also displays a polyadenylated tail (Figure 2A). Castlerea virus ORF1 is 7110 bp long and encodes a 2369-amino-acid polyprotein containing the 4 conserved domains typical of negeviruses: a viral MTase domain (aa 108-491), rRNA MTase domain (aa 837-1025), helicase core domain (aa 1368-1688) and RNA-dependent RNA polymerase domain (aa 1909-2328) (Figure 2A). The CsV RdRP domain (RdRP_2 family pfam: PF00978) follows the canonical A-B-C pattern of motifs consistent with its closest relatives NEGV and NWTV (Figure 2B).6,12,16 ORFs 2 and 3 are encoded one frameshift down from ORF1 and are 1212 and 621 bp long (Figure 2A).
Figure 2.
Organisation of the Castlerea virus (CsV) genome. (A) A schematic of the CsV genome organisation. Regions shaded in dark blue are translated in the first frame, and light grey in the second frame and white represent regions of noncoding sequence. Intergenic regions (IGR) 1 and 2 are depicted with arrows. The black boxes represent the various replication machinery motifs within ORF1 with nucleotide positions depicted above each motif. (B) Amino acid sequence alignment of the RNA-dependent RNA polymerase (RdRP) of various negeviruses and Citrus Leprosis virus C (CiLV-C). Red boxes indicate the position of motifs – A: DX(4-5)D, B: GX(2-3)TX(3)N, and C: GDD. IGR indicates intergenic region; MTase, methyltransferase; ORF, open reading frame; UTR, untranslated region,
CsV groups with the Nelorpivirus clade of negeviruses
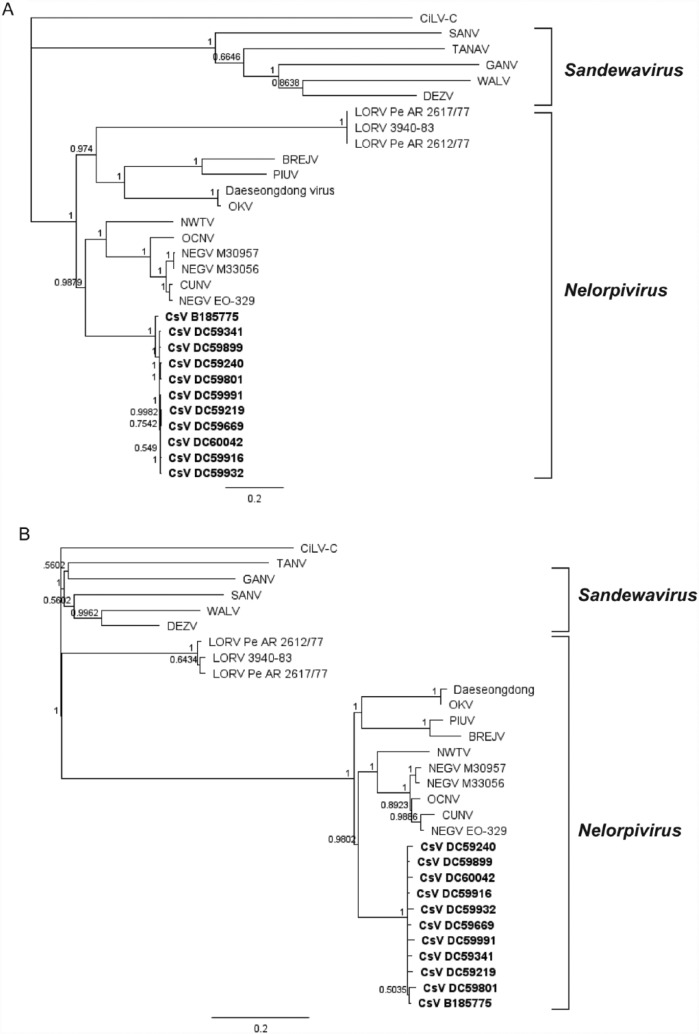
To determine the relationship of CsV to other negeviruses, phylogenetic analysis was performed using the ORF1 sequences (nucleotide 170-7279) of the 11 isolates of CsV for which ORF1-3 sequence was available. This analysis showed that CsV groups in the Nelorpivirus clade of negeviruses with its closest relatives NWTV and NEGV (Figure 3A). This phylogenetic position is consistent with the A-B-C motif pattern observed for the RdRP of CsV and conserved within this clade (Figure 2B). Additional analysis of the amino acid sequence of ORF3 (CsV aa 15-204) confirmed this relationship (Figure 3B). A comparison of nucleotide and amino acid identity across ORF1 showed CsV shared 67.9% nucleotide and 74.9% amino acid identity with NWTV and 68.3% nucleotide and 73.1% amino acid identity with NEGV. This high sequence divergence strongly suggests that CsV is a new species of negevirus (Table 3).
Figure 3.
Bayesian phylogenies of Castlerea virus (CsV) prototype strain B185775 and WA isolates with all published negevirus sequences over (A) ORF1 nucleotide sequence (CsV nucleotides 431-7279) and (B) amino acid sequence of the membrane protein encoded by ORF3 (CsV aa 15-204). Horizontal branch lengths represent posterior probabilities. Both trees have been rooted using the outgroup CiLV-C. Accession numbers are as follows: CiLV-C (NC_008169), SANV (JQ675606), TANV (NC_024071), GANV (KF588036), WALV (KF042857), DEZV (JQ675604), LORV Pe AR 2617/77 (JQ675612), LORV 3940-83 (JQ675610), LORV Pe AR 2612/77 (JQ675611), BREJV (KM350512), PIUV (JQ675607), Daeseongdong (KU095841), OKV (AB972669), NWTV (JQ686833), OCNV (HF913429), NEGEV M30957 (JQ675608), NEGEV M33056 (JQ675609), NEGEV EO-329 (JQ675605), CUNV (AB935183). CiLV-C indicates Citrus Leprosis virus C; ORF, open reading frame; WA, Western Australia.
Table 3.
Amino acid/nucleotide identity table.
| CsV B185775 | LORV 3940-83 | PIUV P60 | NWTV | NEGEV EO-329 | OKV | OCNV | BREJV | SANV | |
|---|---|---|---|---|---|---|---|---|---|
| CsV B185775 | 100/100 | 42.8 | 59.8 | 74.9 | 73.1 | 63.9 | 73.0 | 60.5 | 19.0 |
| LORV 3940-83 | 50.3 | 100/100 | 40.9 | 42.9 | 42.5 | 42.8 | 42.5 | 40.3 | 18.6 |
| PIUV P60 | 59.3 | 47.0 | 100/100 | 60.9 | 60.0 | 59.8 | 60.1 | 80.4 | 18.7 |
| NWTV | 67.9 | 48.2 | 58.5 | 100/100 | 79.2 | 74.9 | 79.1 | 60.1 | 19.3 |
| NEGEV EO-329 | 68.3 | 49.0 | 58.6 | 70.1 | 100/100 | 73.1 | 96.6 | 58.9 | 19.4 |
| OKV | 61.6 | 47.9 | 59.1 | 60.3 | 61.0 | 100/100 | 62.7 | 60.1 | 18.8 |
| OCNV | 67.9 | 48.8 | 58.2 | 69.9 | 86.8 | 60.6 | 100/100 | 58.9 | 19.3 |
| BREJV | 59.2 | 47.5 | 68.9 | 57.5 | 57.4 | 58.9 | 57.5 | 100/100 | 18.7 |
| SANV | 40.0 | 33.1 | 35.1 | 36.7 | 36.7 | 36.0 | 37.0 | 35.4 | 100/100 |
Bold text indicates amino acid identity. Nonbold text indicates nucleotide identity.
Morphological characterisation of CsV
Morphological analysis of CsV virions was undertaken using transmission electron microscopy (TEM) to examine a gradient-purified preparation of CsV isolate DC59801. This analysis revealed elliptical particles, with a diameter of approximately 50 nm, consistent with the morphology described for other negeviruses (Figure 4).10,12 Each particle displayed a single, short projection, which has also been reported for the Sandewavirus Tanay virus (TANV) (Figure 4).12
Figure 4.
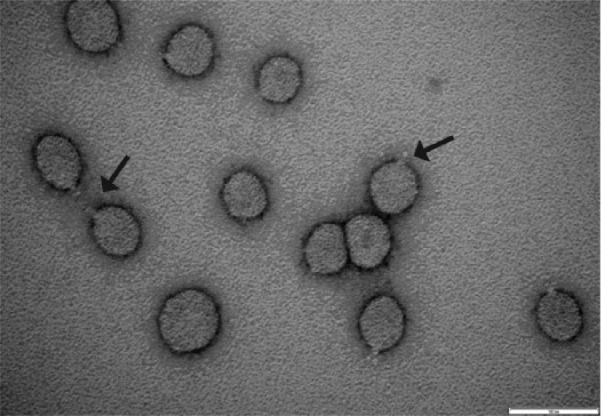
Morphology of Castlerea virus (CsV). Transmission electron micrograph of negatively stained potassium tartrate gradient–purified CsV virions. Arrows indicate presumed glycoprotein projections.
Analysis of the structural proteins of CsV
Additional bioinformatics analyses of the second and third ORFs were performed to further characterise the structural proteins of CsV. ORF2 is predicted to encode a 403-amino-acid protein with an expected molecular weight of 46 kDa (Figure 5A). This protein contains a putative glycoprotein domain (pfam: PF16506.2) between amino acids 56 and 106. ORF 2 also encodes an N-terminal signal peptide and a transmembrane domain at the C-terminus followed by a predicted cytoplasmic tail (Figure 5A). A disulphide bridge is predicted to form between cysteines at positions 300 and 386. ORF 3 encodes a 206-amino-acid protein with a predicted molecular weight of 22 kDa. HMMER predicts a putative virion membrane protein domain (pfam: PF16504.2) between amino acids 60 and 180 (Figure 5B). This protein contains 3 transmembrane domains at amino acids 64 to 81, 129 to 150, and 171 to 189 and 2 predicted cytoplasmic regions at amino acids 1 to 63 and 151 to 170. N-glycosylation sites were predicted at amino acids 83 and 127 for the ORF2 protein and amino acid 201 for the ORF3 protein using NetNGlyc 1.0 Server (Figure 5A and B).24
Figure 5.
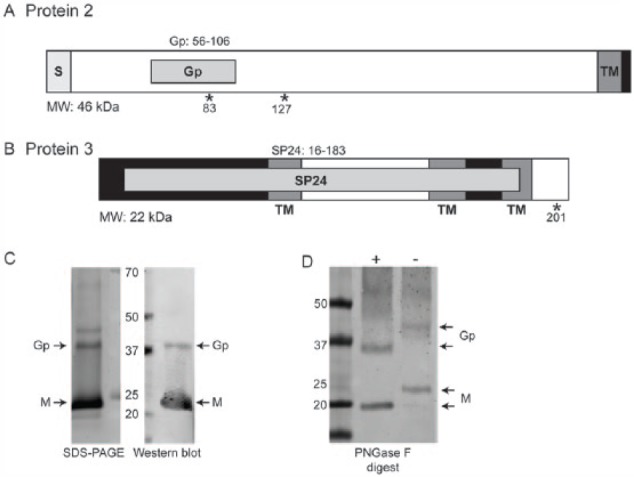
Bioinformatic and physical analyses of Castlerea virus (CsV) structural proteins. (A) ORF2 of CsV encodes a putative 46 kDa glycoprotein. This protein contains an N-terminal signal peptide (S) and a C-terminal transmembrane domain (TM) followed by a short predicted cytoplasmic domain (black). A small glycoprotein domain is predicted between amino acids 56 and 106. (B) ORF3 encodes a putative virion membrane protein with a predicted molecular weight of 23 kDa. This protein contains 3 transmembrane (TM) regions and 2 predicted cytoplasmic domains (black). * depicts predicted N-glycosylated sites. (C) Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis performed on purified CsV virions using anti-CsV mouse immune serum shows 2 abundant proteins at apparent molecular weights (MWs) of 40 and 24 kDa corresponding to the glycoprotein (Gp) and membrane protein (M), respectively. (D) Western blot analysis using anti-CsV immune serum samples to probe CsV-infected lysate with (+) and without (−) PNGase F digestion.
To further analyse the major structural proteins of CsV, purified virions were assessed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot. Two proteins with apparent molecular masses of 40 and 24 kDa, respectively, were resolved (Figure 5C), presumably the ORF2 (glycoprotein) and ORF3 (membrane) proteins. Confirmation of the identity of the 24-kDa protein as that expressed by ORF3 was provided through the detection of 2 ORF3-specific peptides by mass spectrometry (Figure S1). Mouse immune serum samples generated against purified CsV virions bound only the 40- and 24-kDa proteins in western blot (Figure 5C). Furthermore, this antiserum efficiently neutralised CsV in a microneutralisation assay up to a dilution of 1/80, whereas the control serum had no detectable neutralising activity, adding strength to the hypothesis that the proteins expressed from ORFs 2 and 3 likely form the virion (Figure 5C). PNGase F digestion confirmed that the proteins produced by both ORFs were glycosylated as indicated by increased mobility of the proteins through the gel after deglycosylation (Figure 5D).
CsV growth in RNA interference–deficient and RNA interference–competent cell lines
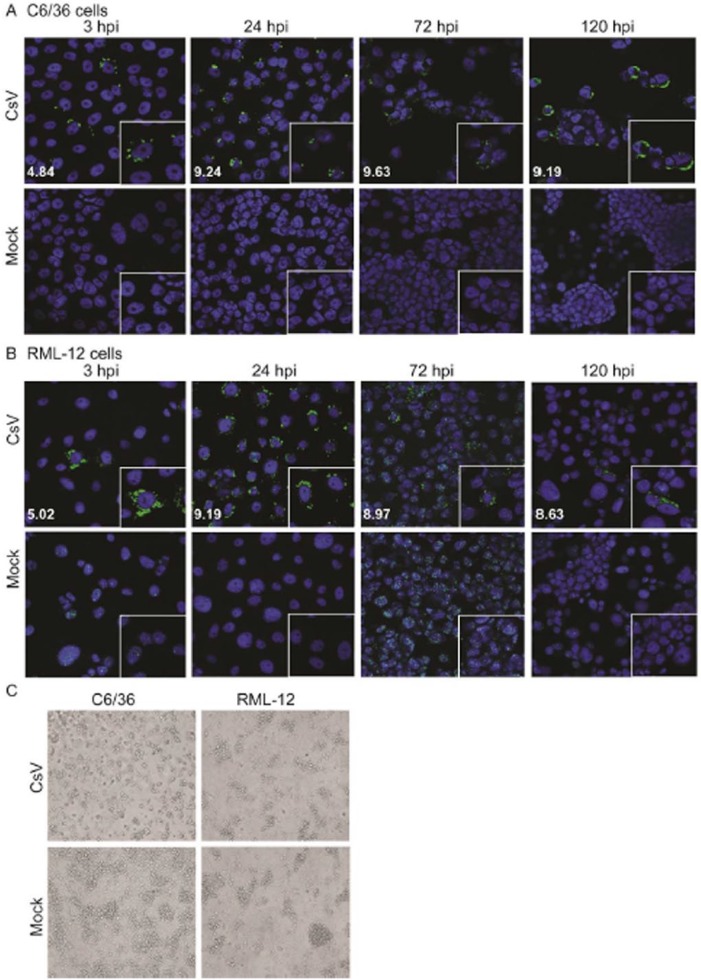
Negeviruses are commonly reported to cause extensive CPE and grow to high titres in cell culture. However, most of these observations have been in the RNA interference (RNAi)-deficient C6/36 and C7/10 cells.6,10,17,18 To further characterise the replication of CsV in cell culture, we inoculated CsV into 2 Ae albopictus–derived cell lines – the RNAi-deficient C6/36 and RNAi-competent RML-12 cells.18 Viral replication was assessed by dsRNA immunolabelling using MAVRIC and quantification of viral particles in the cell supernatant. A perinuclear immunostaining pattern was observed in infected cells consistent with replication patterns observed for other (+) ssRNA viruses1,21 (Figure 6A and B). DsRNA staining could be detected in both cell lines at 3 hpi, and perinuclear staining was detected through to 120 hpi in C6/36 cells. In contrast, although a strong perinuclear signal was detected at 24 hpi in RML-12 cells, this signal became less clear at 72 and 120 hpi. Some dsRNA staining was observed in the nucleus of mock-infected cells as described previously.21 Both cell lines produced high titres of virus, with peak titres being detected in C6/36 cells at 72 hpi (109.63/mL) and at 24 hpi for RML-12 cells (109.19/mL) (Figure 6A and B). No cytophathic effect was noted in RML-12 cells after 72 hpi, whereas cell rounding and monolayer clearance were observed for infected C6/36 cells (Figure 6C).
Figure 6.
Analysis of Castlerea virus (CsV) replication in C6/36 and RML-12 cells. Immunofluorescent staining (green fluorescence) of double-stranded RNA (dsRNA) replicative intermediates produced by CsV infection in (A) C6/36 cells and (B) RML-12 cells. Main images were taken at ×40 magnification, whereas inset shows ×63 magnification. The log of average titre per millilitre harvested at each of these time points is shown as in white text in each panel. (C) Comparison of cytopathic effect production by CsV in C6/36 and RML-12 cell lines at 72 hours post infection (hpi).
Discussion
Since the Negevirus taxon was first described, a number of these viruses have been isolated from multiple geographic locations, including Europe, United States, Indonesia, and Africa. However, the diversity of these viruses was yet to be investigated in Australian mosquitoes. Using a combination of a novel ELISA-based detection platform that targets the dsRNA intermediates produced during viral infection (MAVRIC) and Illumina sequencing, several isolates of a phylogenetically distinct negevirus tentatively named Castlerea virus were identified from Culex, Aedes, Anopheles, and Culiseta mosquitoes collected in Queensland and WA.21 Phylogenetic analysis of the prototype CsV isolate (B185775) from Brisbane indicated that this sequence was related to, but distinct from, its closest relative NWTV, a negevirus isolated from Cx vishnui mosquitoes in Indonesia, with approximately 70% nucleotide identity over ORF1, which contains highly conserved motifs, including the RdRP.10 Recent phylogenetic analysis performed by Kallies et al16 suggested that the Negevirus taxon can be separated into 2 clades – nelorpiviruses and sandewaviruses. Analyses from this study showed that characterised negeviruses in the Nelorpivirus clade share between 54.1% and 72.1% amino acid identity.16 In line with these criteria, we propose that CsV represents a new viral species within the Nelorpivirus clade of the Negevirus taxon.
There is limited information to suggest how these viruses persist in wild mosquito populations. Given the results of the oral exposure of Ae aegypti to NEGV, whereby mosquitoes became infected via a blood meal spiked with virus,10 horizontal transmission of negeviruses cannot be discounted. Recent detection of OKV in 3 pools of field collected Aedes larvae from Okushiri Island, Japan, suggests that this negevirus may be vertically transmitted to progeny.13 Interestingly, we have reported a single isolation of CsV from a male Culex mosquito. Such isolations in larvae and males provide evidence for vertical transmission of these viruses from parent mosquito to progeny as has been demonstrated for other insect-specific viruses, in particular the ISFs.4,25 However, unlike the restricted host range of many ISFs, retrospective analysis of mosquitoes from Brisbane and WA revealed that CsV can be isolated from multiple mosquito genera from geographically separated regions of Australia.18 These isolates share a high nucleotide similarity with the prototype isolate B185775 ranging from 96.78% to 99.73% nucleotide identity over ORF1, demonstrating a seemingly genetically stable genome across a wide geographic separation and between diverse mosquito genera. Similar patterns of negevirus distribution can also be observed for NEGV and PIUV, both of which have been isolated from multiple mosquito genera and geographic locations.6,10 In addition, a recently described negevirus sequence identified in Cx pipiens mosquitoes from South Korea, Daeseongdong virus 1, appears to be a strain of OKV, showing once again the isolation of the same virus from a different location and mosquito species.13,26 The wide diversity of mosquito host species and the high incidence of these viruses within mosquito populations might suggest a complex transmission cycle involving vertical transmission and potentially horizontal transmission via a cryptic host, such as mites, or the acquisition of viral infection during the mosquito larval stage through interaction with their environmental habitat.
Transmission electron microscopy analysis of a Western Australian isolate of CsV (DC59801) revealed a morphology unique to negeviruses – elliptical particles of approximately 50 nm diameter with a single, short projection.6,10,12 Analysis of purified virions by SDS-PAGE revealed 2 proteins with approximate molecular weights of 40 and 24 kDa consistent with the sizes predicted for the proteins encoded by ORF2 and ORF3, respectively. Based on the HMMER domain prediction software, the protein encoded by ORF3 is likely to be a membrane protein, whereas ORF2 encodes a protein containing a glycoprotein domain (Figure 5A and B). From these data, we speculate that the short projection observed on CsV virions comprises the glycoprotein encoded by ORF2.
As previous findings have demonstrated that negeviruses induce severe CPE and grow to high titres in RNAi-deficient C6/36 and C7/10 cells, further analysis of a pure CsV isolate was assessed in the RNAi-competent Ae albopictus RML-12 cell line.6,10,18 Comparative analysis showed that CsV titres were slightly reduced in RML-12 cells compared with C6/36 cells. Interestingly, the peak titre was detected for CsV in RML-12 cells at 24 hpi compared to 72 hpi in C6/36 cells; however, it is feasible that the peak may be at 48 hpi for both cell lines. Immunolabelling for the replicative dsRNA intermediate in CsV-infected cells revealed perinuclear staining in both cell lines at early time points (3 and 24 hpi). However, perinuclear staining in the RML-12 cell line became less obvious after 72 hpi and staining in CsV-infected cells became more reminiscent of that observed in the mock cells, perhaps indicating the effects of RNAi on the viral replication in this cell line. The perinuclear staining pattern observed in this study suggests that CsV is likely to replicate in a similar manner to other (+) ssRNA viruses by manipulating the host cell endoplasmic reticulum membranes.1,21,27,28
In conclusion, we report the first isolation of a phylogenetically distinct negevirus tentatively termed CsV from Australian mosquitoes. Although the full diversity of these viruses is currently unknown, the isolation of CsV prompts the inclusion of negevirus-specific screening tools in virus surveillance and discovery programs in mosquitoes in Australia. Negeviruses may contribute significantly to the virome of their hosts, and further studies to elucidate replication strategies, transmission mechanisms, and potential to interfere with the transmission of select medically significant arboviruses are required.
Methods
Mosquito collection and processing
Mosquitoes were collected from the suburb of Tingalpa, Brisbane (QLD, Australia), using CO2-baited Centers for Disease Control light traps (Model 512; John Hock Co., Gainesville, FL) and sorted according to species in pools of up to 35 mosquitoes. Samples were stored at −80°C until processing.36
The northern Western Australian mosquito collection sites have previously been described in detail.29–31 Briefly, adult mosquitoes were collected using methods already described and sorted according to species in pools of up to 25.32 Methods for collections from the Peel region in 2014 and Peel and Leschenault in 1988 have been summarised previously; these mosquitoes were sorted by species into pools of up to 20.33,34
Cell and virus culture
C6/36 (Ae albopictus) and RML-12 (A albopictus) cells were cultured at 28°C in RPMI 1640 medium supplemented with 5% foetal bovine serum (FBS), 50 U penicillin/mL, 50 mg streptomycin/mL, and 2 mM l-glutamine.
Stocks of the viral isolates were propagated by inoculating C6/36 cells and incubating at 28°C for 3 days. Stocks were titrated by serial 10-fold dilution onto monolayers of C6/36 cells in 96-well plates using 10 wells per dilution. After 3 days, the culture supernatant was removed and the cell monolayers fixed with acetone fixative buffer (20% acetone, 0.02% bovine serum albumin [BSA] in phosphate buffered saline [PBS]). Castlerea virus–infected wells were detected by ELISA using MAVRIC and previously described methods.21 Virus titres were determined as 50% tissue culture infective dose (TCID50) using the method of Reed and Muench.35
A pure stock of CsV B185775 was prepared by limit-diluting the mosquito pool homogenate with a neutralising antibody against LNV. After a 1-hour incubation at 28°C, the homogenate/antibody mixes were inoculated onto C6/36 cells and incubated for 3 days at 28°C. The CsV B185775 stock was confirmed to be free of LNV by RT-PCR.
Virus isolation and identification from mosquito homogenates
Collected mosquitoes from Brisbane were homogenised in 1.5 mL of culture medium (RPMI 1640) supplemented with 2% FBS using a TissueLyser II (Qiagen, Hilden, Germany) for 8 minutes at 30.0 Hz. Western Australian mosquitoes were ground in 2.5 mL M199 media with 2% FBS using sterile glass grinders (for 1988 samples) or glass beads in 5 mL tubes in the SPEX mixer mill. Homogenates were then centrifuged, filtered through a double 0.2 µm/0.8 µm filter, and stored at −80°C until use. Two hundred microlitres of filtered (or un-filtered for some cohorts) mosquito homogenates was inoculated onto semi-confluent C6/36 Ae albopictus cells and incubated at 28°C for 5 to 6 days. Supernatant was harvested and screening by fixed-cell ELISA using the MAVRIC system as described previously.21 RNA was extracted from 150 µL of culture supernatant of the ELISA-positive samples using the Macherey-Nagel NucleoSpin Viral RNA isolation kit, and RNA from 11 samples was submitted for Illumina sequencing.
Sequencing of viral isolates
Brisbane and WA CsV isolates were sequenced by the Roslin Institute (Glasgow, UK) and the Australian Genomics Research Facility, respectively, using the Illumina HiSeq2000 platform. The genome of the Brisbane isolate was assembled using Geneious 8.1.4 by mapping paired reads to the reference genome NWTV (JQ686833) with high sensitivity. The genome of one of the WA isolates was assembled using Trinity to map to NWTV after removing adapter sequences and trimming reads with Cutadapt and Trim Galore. Trimming was performed using a minimum quality score of 30 and removing 5 bp from the 5′’ end of each read. The remaining 9 genomes from WA were assembled with Geneious 8.1.4 using the consensus sequence obtained from a pairwise alignment of the 2 available CsV genomes as a reference.
Genome annotation
The 3 ORFs were predicted based on alignment to related negeviruses. Domains of the translated ORFs were predicted using the HMMER program (www.ebi.ac.uk/Tools/hmmer). Transmembrane domains and membrane topology were predicted using the Phobius server (http://phobius.binf.ku.dk/). Disulphide bonds were predicted using the DISULFIND Server.37 Molecular weights were predicted using the protein molecular weight prediction tool from the sequence manipulation suite at http://www.bioinformatics.org/sms/prot_mw.html.38
The 5′ and 3′ ends of the genome were confirmed using the GeneRacer kit (Life Technologies, California, USA) according to the manufacturer’s instructions using the gene-specific primers 5′Rev: TCAGCTCTGTGCATGGCGTCAGCAA, 5′Rev_nested: GCAGCGCGTGTGAGGCGTTGCAG, and 3′Fwd: GCACGATTCCTCGGTCTGGCCAT.
RT-PCR screening
Primers for RT-PCR screening were designed to the rRNA MTase region (2059-2851 nucleotides) of CsV ORF1 (CsV_Fwd: AGCCGTTATCAACTCTCTCG; CsV_Rev: CGGTGAGAAGTCGATGAG). Reverse transcription polymerase chain reaction was performed with the Superscript III One-Step RT-PCR System with Platinum Taq DNA polymerase (Invitrogen, California, USA) with the following conditions – RT: 45°C/30 minutes; PCR: 94°C/2 minutes, followed by 40 cycles of 94°C/30 seconds, 46°C/30 seconds, and 68°C/45 seconds and a final extension of 68°C/5 minutes.
Liao ringing virus was detected using primers designed to segment 10 (Sead_Seg10F 5′- GTTATTTTTTCTAAGTGACA-3′ and Sead_Seg10R 5′- GCTAAGATTGTAAACACGGTT-3′).22 Reverse transcription polymerase chain reaction was performed using the Superscript III One-Step RT-PCR System with Platinum Taq DNA polymerase (Invitrogen) under the conditions stated previously with the exception of annealing temperature: 45°C/30 seconds. Detection of Alphamesonivirus 1 was undertaken using pan-mesonivirus primers (Nido F1/R1) and was performed as per Warrilow et al.1
Sequence alignments and phylogenetic analysis
Nucleotide alignment of ORF1 of 11 CsV sequences (with full ORF1 available) and 18 published negevirus sequences and CiLV-C (NC_008169) was performed in Geneious (v8.1.4) using the MUSCLE algorithm with maximum 10 iterations.39 A phylogenetic tree based on this alignment (nucleotide positions 431-7279 for CsV) was constructed in Geneious using MrBayes v3.2.2 under the Bayesian Markov chain Monte Carlo (MCMC) model with a General Time Reversible substitution model, gamma distribution (5 discrete gamma categories), and invariant rates among sites as determined by jModelTest2 via the CIPRES gateway.40-43
Amino acid alignments were performed using the ClustalW alignment tool in Geneious (Cost matrix: BLOSUM, gap open cost: 10, gap extend cost: 0.1). A phylogenetic tree based on the alignment of protein 3 was constructed using MrBayes under the MCMC model with default parameters.43 Citrus Leprosis virus C p24 protein sequence (extracted and translated from NC_008170) was used as an outgroup.
Purification of CsV and TEM analysis
Supernatant containing CsV particles propagated in C6/36 cells was harvested 2 days post infection and clarified by centrifugation at 3000 rpm for 15 minutes at 4°C. Supernatant was added to a 40% PEG 8000 solution and left to stir overnight at 4°C before purification through a potassium tartrate gradient as per McLean et al.18 Visible bands in the gradient which contained purified virions were harvested and stored at 4°C for immediate TEM analysis. Samples were prepared for TEM on glow-discharged carbon/formvar-coated copper grids and negatively stained with 1% uranyl acetate. All imaging was performed on a JEOL 1011 transmission electron microscope.
PNGase F assay
CsV-infected C6/36 lysate was harvested in NP-40 lysis buffer and clarified by centrifugation at 10 000g for 10 minutes at 4°C. Lysate was resuspended in glycoprotein denaturing and reaction buffers (NEB) as per the manufacturer’s instructions, with or without the addition of 500 U PNGase F. Each preparation was incubated at 37°C for 1 hour, prior to the addition of LDS sample buffer (Life Technologies), separation on a 4% to 12% Bis-Tris SDS-PAGE gel (Life Technologies), and transfer to a nitrocellulose membrane. Castlerea virus proteins were detected by incubation with CsV-immune mouse serum followed by IRDye 800CW Goat anti-Mouse IgG (H+L) (LI-COR) and visualised on the Odyssey imaging system (LI-COR, Nebraska, USA).
Mass spectrometry analysis
Protein bands were excised after running through an SDS-PAGE gel and destained in 50% acetonitrile/50 mM ammonium acetate solution overnight at 37°C. Destained slices were then dried and digested overnight with 0.5 µg trypsin in 50 mM ammonium acetate with 10 mM dithiothreitol. Digested peptides were desalted and analysed using liquid chromatography-mass spectrometry (LC-MS)/MS as previously described.44 Proteins were identified using ProteinPilot software.
Generation of mouse antiserum to CsV
All animal procedures had received prior approval from the University of Queensland Animal Ethics Committee (AEC #SCMB/329/15/ARC) and where necessary were performed under ketamine:xylazine anaesthesia. Six-week-old BALB/c mice (Animal Resources Centre, Murdoch, WA, Australia) were immunised twice via the subcutaneous route with purified CsV, along with inulin-based adjuvant Advax (Vaxine Ltd, Adelaide, SA, Australia). Mice were kept on clean bedding and given food and water ad libitum. Immunised mice were bled via the tail vein at least 2 weeks following immunisation and the serum samples tested for evidence of seroconversion to CsV using a fixed-cell ELISA as previously described.45
Control antiserum to Casuarina virus (CASV – Alphamesonivirus 4) was prepared as described above with the use of purified CASV virions as the immunogen.
Assessment of CsV-induced CPE in RML-12 cells
C6/36 and RML-12 cells were cultured by methods previously described and seeded onto glass coverslips at a density of 1 × 105 per well. Monolayers were inoculated with CsV at a multiplicity of infection of 10 with CsV in biological replicates. After incubation at 28°C for 1 hour, the inoculum was removed and wells were washed 3 times with sterile PBS with fresh 2% FBS/RPMI media added for further incubation at 28°C. Supernatant was harvested and coverslips fixed at time points of 3, 24, 72 and 120 hpi and titrated on C6/36. Cytopathic effect was imaged at ×20 magnification at 72 hpi using a Nikon Eclipse TE200 microscope. Immunofluorescence assay was performed using MAVRIC as described previously.18 Infective viral titres from each time point were determined by TCID50 assay as described earlier and averaged across replicates.
Microneutralisation assay
Microneutralisation assay using anti-CsV immune serum samples was performed as per previously described methods with slight modification.46 Briefly, serial 2-fold dilutions of serum samples were made (1:20-1: 2560) and incubated with CsV for 1 hour at 28°C before an adsorption phase on C6/36 cells in a 96-well plate for 1 hour at 28 °C. Inoculum was then removed and replaced with 2% FBS/RPMI. Neutralisation effect was measured by CPE and MAVRIC ELISA. A negative control was performed in parallel using anti–serum samples generated against alphamesonivirus 4.1
Acknowledgments
The authors thank Peter Simmonds (Roslin Institute, University of Edinburgh. United Kingdom) for performing the Illumina sequencing of the prototype isolate of CsV. They are grateful to the following people for their excellent technical assistance: Marcus Mah, Waylon Wiseman (The University of Queensland), and the University of Queensland Semester 1, 2016 cohort of MICR 3002 students, tutors, and laboratory staff. The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. The Western Australia (WA) Department of Health funded the Arbovirus Surveillance Program and assisted with mosquito collections (from which the WA mosquito homogenates arose). They thank the Arbovirus Surveillance Lab members for technical support associated with mosquito collections and processing for virus isolation.
Footnotes
Peer Review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totalled 1655 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Australian Research Council (ARC DP120103994).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: BJM, CAO, JH-P, RAH, NDN, and BLS conceived and designed the experiments. CAO and BJM analysed and wrote the first draft of the manuscript. JH-P, RAH, AvdH, SH-M, AMGC, JJH, DW, CAJ, HB-O, NDN, and BLS contributed to the writing of the manuscript. BJM, CAO, JH-P, RAH, AvdH, SH-M, AMGC, JJH, DW, CAJ, HB-O, NDN, and BLS agreed with manuscript results and conclusions. CAO, BJM, and JH-P jointly developed the structure and arguments for the paper. JH-P made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Warrilow D, Watterson D, Hall RA, et al. A new species of mesonivirus from the Northern Territory, Australia. PLoS ONE. 2014;9:e91103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cook S, Moureau G, Harbach RE, et al. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J Gen Virol. 2009;90:2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobson-Peters J, Yam AWY, Lu JWF, et al. A new insect-specific flavivirus from Northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE. 2013;8:e56534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blitvich BJ, Firth AE. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses. 2015;7:1927–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marklewitz M, Handrick S, Grasse W, et al. Gouléako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J Virol. 2011;85:9227–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carapeta S, do Bem B, McGuinness J, et al. Negeviruses found in multiple species of mosquitoes from southern Portugal: isolation, genetic diversity, and replication in insect cell culture. Virology. 2015;483:318–328. [DOI] [PubMed] [Google Scholar]
- 7. Nasar F, Palacios G, Gorchakov RV, et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A. 2012;109:14622–14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Attoui H, Mohd Jaafar F, Belhouchet M, et al. Expansion of family Reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus). Virology. 2005;343:212–223. [DOI] [PubMed] [Google Scholar]
- 9. Zirkel F, Roth H, Kurth A, Drosten C, Ziebuhr J, Junglen S. Identification and characterization of genetically divergent members of the newly established family Mesoniviridae. J Virol. 2013;87:6346–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasilakis N, Forrester NL, Palacios G, et al. Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. J Virol. 2013;87:2475–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auguste AJ, Carrington CV, Forrester NL, et al. Characterization of a novel negevirus and a novel bunyavirus isolated from Culex (Culex) declarator mosquitoes in Trinidad. J Gen Virol. 2014;95:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nabeshima T, Inoue S, Okamoto K, et al. Tanay virus, a new species of virus isolated from mosquitoes in the Philippines. J Gen Virol. 2014;95:1390–1395. [DOI] [PubMed] [Google Scholar]
- 13. Kawakami K, Kurnia YW, Fujita R, et al. Characterization of a novel negevirus isolated from Aedes larvae collected in a subarctic region of Japan. Arch Virol. 2016;161:801–809. [DOI] [PubMed] [Google Scholar]
- 14. Kuchibhatla DB, Sherman WA, Chung BYW, et al. Powerful sequence similarity search methods and in-depth manual analyses can identify remote homologs in many apparently ‘orphan’ viral proteins. J Virol. 2014;88:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira DD, Cook S, Lopes A, et al. Characterization of an insect-specific flavivirus (OCFVPT) co-isolated from Ochlerotatus caspius collected in southern Portugal along with a putative new negev-like virus. Virus Genes. 2013;47:532–545. [DOI] [PubMed] [Google Scholar]
- 16. Kallies R, Kopp A, Zirkel F, et al. Genetic characterization of goutanap virus, a novel virus related to negeviruses, cileviruses and higreviruses. Viruses. 2014;6:4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, Myles KM. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 2012;8:e1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLean BJ, Hobson-Peters J, Webb CE, et al. A novel insect-specific flavivirus replicates only in Aedes-derived cells and persists at high prevalence in wild Aedes vigilax populations in Sydney, Australia. Virology. 2015;486:272–283. [DOI] [PubMed] [Google Scholar]
- 19. Hall-Mendelin S, McLean BJ, Bielefeldt-Ohmann H, Hobson-Peters J, Hall RA, van den Hurk AF. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit Vector. 2016;9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Brien CA, Hobson-Peters J, Yam AWY, et al. Viral RNA intermediates as targets for detection and discovery of novel and emerging mosquito-borne viruses. PLoS Negl Trop Dis. 2015;9:e0003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coffey LL, Page BL, Greninger AL, et al. Enhanced arbovirus surveillance with deep sequencing: identification of novel rhabdoviruses and bunyaviruses in Australian mosquitoes. Virology. 2014;448:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall RA, Bielefeldt-Ohmann H, McLean BJ, et al. Commensal Viruses of Mosquitoes: Host Restriction, Transmission, and Interaction with Arboviral Pathogens. Evolutionary Bioinformatics. 2016;Suppl. 2: 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Paper presented at: Pacific Symposium on Biocomputing; 2002, Hawaii, 3–7 January, 2002. [PubMed] [Google Scholar]
- 25. Bolling BG, Eisen L, Moore CG, Blair CD. Insect-specific flaviviruses from Culex mosquitoes in Colorado, with evidence of vertical transmission. Am J Trop Med Hyg. 2011;85:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hang J, Klein TA, Kim H-C, et al. Genome sequences of five arboviruses in field-captured mosquitoes in a unique rural environment of South Korea. Genome Announc. 2016;4:e01644–e01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Angelini MM, Neuman BW, Buchmeier MJ. Untangling membrane rearrangement in the Nidovirales. DNA Cell Biol. 2014;33:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quan P-L, Williams DT, Johansen CA, et al. Genetic characterization of K13965, a strain of Oak Vale virus from Western Australia. Virus Res. 2011;160:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison JJ, Warrilow D, McLean BJ, et al. A new orbivirus isolated from mosquitoes in North-Western Australia shows antigenic and genetic similarity to Corriparta Virus but does not replicate in vertebrate cells. Viruses. 2016;8:E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansen CA, Susai V, Hall RA, et al. Genetic and phenotypic differences between isolates of Murray Valley encephalitis virus in Western Australia, 1972–2003. Virus Genes. 2007;35:147–154. [DOI] [PubMed] [Google Scholar]
- 32. Broom AK, Wright AE, MacKenzie JS, Lindsay MD, Robinson D. Isolation of Murray Valley encephalitis and Ross River viruses from Aedes normanensis (Diptera: culicidae) in Western Australia. J Med Entomol. 1989;26:100–103. [DOI] [PubMed] [Google Scholar]
- 33. Johansen C, Broom A, Lindsay M, et al. Arbovirus and vector surveillance in Western Australia, 2004/05 to 2007/08. Paper presented at: Arbovirus Research in Australia; 2009;Coffs Harbour, Australia. [Google Scholar]
- 34. Lindsay MD, Broom AK, Wright AE, Johansen CA, Mackenzie JS. Ross River virus isolations from mosquitoes in arid regions of Western Australia: implication of vertical transmission as a means of persistence of the virus. Am J Trop Med Hyg. 1993;49:686–696. [DOI] [PubMed] [Google Scholar]
- 35. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 36. Jansen CC, Prow NA, Webb CE, et al. Arboviruses isolated from mosquitoes collected from urban and peri-urban areas of eastern Australia. J Am Mosq Control Assoc. 2009;25:272–278. [DOI] [PubMed] [Google Scholar]
- 37. Ceroni A, Passerini A, Vullo A, Frasconi P. DISULFIND: a disulfide bonding state and cysteine connectivity prediction server. Nucleic Acids Res. 2006; 34:W177–W181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques. 2000;28: 1102–1104. [DOI] [PubMed] [Google Scholar]
- 39. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller M, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE); November 14, 2010; New Orleans, FL. [Google Scholar]
- 41. Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Meth. 2012;9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. [DOI] [PubMed] [Google Scholar]
- 43. Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. [DOI] [PubMed] [Google Scholar]
- 44. Bailey UM, Jamaluddin MF, Schulz BL. Analysis of congenital disorder of glycosylation-Id in a yeast model system shows diverse site-specific under-glycosylation of glycoproteins. J Proteome Res. 2012;11:5376–5383. [DOI] [PubMed] [Google Scholar]
- 45. Clark DC, Lobigs M, Lee E, et al. In situ reactions of monoclonal antibodies with a viable mutant of Murray Valley encephalitis virus reveal an absence of dimeric NS1 protein. J Gen Virol. 2007;88:1175–1183. [DOI] [PubMed] [Google Scholar]
- 46. Hall RA, Kay BH, Burgess GW. An enzyme immunoassay to detect Australian flaviviruses and identify the encephalitic subgroup using monoclonal antibodies. Immunol Cell Biol. 1987;65:103–110. [DOI] [PubMed] [Google Scholar]