Abstract
Background
By the time clinical symptoms of Alzheimer’s disease (AD) manifest in patients there is already substantial tau pathology in the brain. Recent evidence also suggests that tau pathology can become self-propagating, further accelerating disease progression. Over the last decade several groups have tested the efficacy of protein-based anti-tau immunotherapeutics in various animal models of tauopathy. Here we report on the immunological and therapeutic potency of the first anti-tau DNA vaccine based on the MultiTEP platform, AV-1980D, in THY-Tau22 transgenic mice.
Methods
Starting at 3 months of age, m,ice were immunized intramuscularly with AV-1980D vaccine targeting a tau B cell epitope spanning aa2–18 followed by electroporation (EP). Humoral and cellular immune responses in vaccinated animals were analyzed by ELISA and ELISpot, respectively. Neuropathological changes in the brains of experimental and control mice were then analyzed by biochemical (WB and ELISA) and immunohistochemical (IHC) methods at 9 months of age.
Results
EP-mediated AV-1980D vaccinations of THY-Tau22 mice induced activation of Th cells specific to the MultiTEP vaccine platform and triggered robust humoral immunity response specific to tau. Importantly, no activation of potentially harmful autoreactive Th cell responses specific to endogenous tau species was detected. The maximum titers of anti-tau antibodies were reached after two immunizations and remained slightly lower, but steady during five subsequent monthly immunizations. Vaccinations with AV-1980D followed by EP significantly reduced total tau and pS199 and AT180 phosphorylated tau levels in the brains extracts of vaccinated mice, but produced on subtle non-significant effects on other phosphorylated tau species.
Conclusions
These data demonstrate that MultiTEP-based DNA epitope vaccination targeting the N-terminus of tau is highly immunogenic and therapeutically potent in the THY-Tau22 mouse model of tauopathy and indicate that EP-mediated DNA immunization is an attractive alternative to protein-based adjuvanted vaccines for inducing high concentrations of anti-tau antibodies.
Keywords: Tau immunotherapy, Alzheimer’s diseases, DNA epitope vaccine, anti-tau antibody, transgenic mice
1. Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disease and the most common cause of age-related dementia[1], with symptoms that manifest in cognitive, memory, and functional impairments[2]. Neuropathological features of AD include deposition of the amyloid-β (Aβ) fragment of amyloid precursor protein (APP) in senile plaques, accumulation of neurofibrillary tangles (NFT) composed of hyperphosphorylated tau protein, and death of neurons[3–7]. Although Aβ may be the primary initiator of AD pathogenesis, it is clear that pathological tau also plays a critical role in AD[8]. Importantly, by the time clinical signs of AD appear there is already substantial tau pathology in the brain[9, 10], which may also become self-propagating[11–14].
Anti-tau immunotherapy using protein-based adjuvanted vaccines targeting full-length tau [15] as well as various B cell tau epitopes (C-terminus[16] and phosphorylated epitopes[17–22]) have been tested in several mouse models. Importantly, some of these vaccines showed some degree of efficacy in preventing tau-like pathology in rodent models and two vaccines have recently advanced into Phase 1 clinical trials[16, 23]. Previously we showed that our EP-mediated DNA vaccination strategy based on the MultiTEP platform [24–27] provides an attractive alternative to the adjuvanted-protein vaccination approach for inducing of strong anti-Aβ antibody response in mice, rabbits, and monkeys. Based on these promising data we are currently conducting pre-clinical safety and toxicology studies in preparation for an Investigational New Drug (IND) application. In this paper, we have taken a parallel approach to develop a MultiTEP-based DNA vaccine targeting the N-terminus of tau, AV-1980D. To the best of our knowledge, this is the first examination of a DNA tau vaccine. We chose to target amino acids 2–18 of the tau N-terminus as a B cell epitope based on data showing that this region, comprising phosphatase-activating domain (PAD), (i) plays an important role in activation of a signaling cascade that leads to inhibition of anterograde fast axonal transport (FAT)[28–31]; (ii) is normally hidden in microtubule bound tau conformations but becomes highly exposed during tau aggregation[28, 29]; and (iii) plays an important role in polymerization of tau, and truncation or phosphorylation of this region may have a neuroprotective role[30, 32]. In this report, THY-Tau22 mice immunized with AV-1980D generated very high titers of anti-tau antibodies that recognized tangles in human AD brain tissue and reduced the accumulation of total tau in the brains of vaccinated mice.
2. Materials and methods
2.1. Mice
In this study we used female heterozygous THY-Tau22 mice maintained on a C57Bl6/J background[33]. THY-Tau22 mice are a well-established model of Tauopathy that express human 4 repeat tau with two frontotemporal dementia-associated point mutations (G272V and P301S) under control of the neuronal driven promoter Thy1.2[33]. Mice were anesthetized with 4% isoflurane (Vedco, Inc., St. Joseph, MO) and maintained in 3–3.5% isoflurane during all injections. All animals were housed in a temperature and light-cycle controlled facility, and their care was under the guidelines of the National Institutes of Health and an approved IACUC protocol at University of California, Irvine.
2.2. Antigen
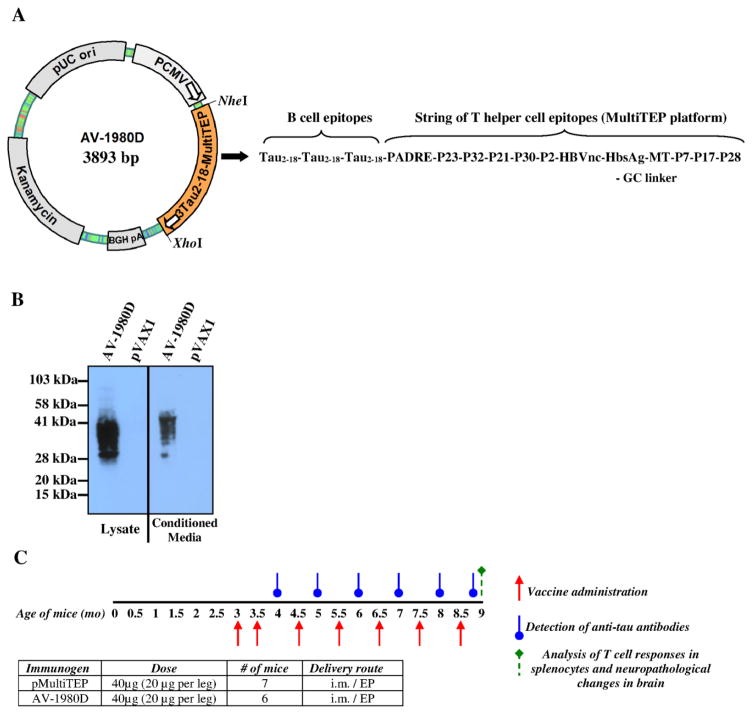
AV-1980D codes for a protein consisting of the Ig κ-chain signal sequence, three copies of the tau2-18 B cell epitope (sequence: AEPRQEFEVMEDHAGTY) linked to the MultiTEP platform, consisting of a string of twelve foreign Th epitopes [one synthetic peptide (PADRE), 8 epitopes from Tetanus Toxin (TT) (P2, P21, P23, P30, P32, P7, P17 and P28), 2 epitopes from hepatitis B virus (HBsAg, HBVnc) and one epitope from influenza matrix protein (MT)]. A polynucleotide encoding three copies of tau2-18 epitope separated by GS linkers was synthesized by GenScript (Piscataway, NJ) and subcloned in frame with the MultiTEP minigene (amplified by PCR from AV-1959D construct[26]) into the pVAX1 vector (Invitrogen, Carlsbad, CA)[24], that was designed to be coherent with current Food and Drug Administration (FDA) guidelines, using NheI/BamHI/BglII/XhoI restriction sites. DNA sequencing was performed to confirm that the generated plasmids contained the correct sequences. A map of AV-1980D is presented in Fig. 1A. Plasmids were prepared and purified by Aldevron (Fargo, ND). Gene expression was analyzed in transfected CHO cells and the protein was detected by western blot (WB) using anti-tau 1C9 monoclonal antibody (generated at The Institute for Molecular Medicine, Huntington Beach, CA, Fig. 1B).
Figure 1. Schematic representations of DNA vaccine construct; analyses of its expression and experimental design in THY-Tau22 transgenic mice.
(A) Strategy for cloning the gene encoding 3Tau2-18-MultiTEP (AV-1980D) into the pVAX1 vector and schematic representation of AV-1980 construct encoding 3 copies of Tau2-18 fused to MultiTEP, one universal synthetic Th epitope, PADRE and eleven foreign promiscuous Th epitopes from infectious agents. (B) Intracellular and secreted AV-1980 protein from transfected CHO cells detected by WB, visualized by staining with anti-tau Mab, 1C9. (C) Design of experimental protocol in Tg mice vaccinated with AV-1980D and pMultiTEP (control group).
2.3. Immunizations
Female, three month old THY-Tau22 mice (n=6) were injected into both tibialis anterior muscles with 40μg (20μg per leg) of AV-1980D vaccine. Immediately after DNA administration, the needle electrode made of two parallel rows of four 5-mm needles 0.3mm in diameter (1.5 × 4-mm gap) was inserted in such a way that the i.m. injection site was located between the two needle rows. The EP pulses were applied (“high amplitude, short duration” (450/0.05) two pulses and “low amplitude, long duration” (110/10) eight pulses) using the AgilPulse™ device from BTX Harvard Apparatus (Holliston, MA) [27]. Control group of THY-Tau22 mice (n=7) were injected with a pMultiTEP plasmid that lacked the Tau B cell epitope. Mice were vaccinated twice with 2-week interval then received monthly immunizations (total 7 immunizations). On day 12 after each immunization (starting from second one) blood was collected for analysis of anti-tau antibodies. Mice were terminated at 9 months of age for further neuropathological analysis. These experimental details and timeline are further illustrated in Fig. 1C.
2.4. Detection of IFN-γ producing splenocytes
Analysis of IFN-γ producing T helper (Th) cells was performed in splenocyte cultures from immunized mice by ELISpot assay (BD Biosciences, San Jose, CA), as previously described[26, 34–36]. Cultures of splenocytes were re-stimulated in vitro with 10μg/ml of individual peptides representing epitopes from the MultiTEP platform, tau2-18 or irrelevant peptides (GenScript, Piscataway, NJ) for 20 hours. The numbers of SFC per 106 splenocyte were counted.
2.5. Detection of tau-specific antibodies and isotyping
The concentrations of anti-tau antibodies in mouse sera were determined by ELISA as previously described[37]. Synthetic tau2-18 peptide (GenScript, Piscataway, NJ) was used for coating ELISA plates. Anti-tau antibody concentrations were calculated using a calibration curve generated with 1C9 mAb. HRP-conjugated anti-IgG1, IgG2ab, IgG2b and IgM specific antibodies (Bethyl Laboratories, Inc., Montgomery, TX) were used to characterize the isotype profiles of anti-tau antibodies. The isotypes of antibodies were detected in individual sera of mice at a dilution of 1:800. To analyze humoral responses against T helper epitopes plates were coated with individual peptides from MultiTEP platform, PADRE, P2, P21, P23, P30, P32, HBsAg, HBVnc, MT, P7, P17, P28 (GenScript, Piscataway, NJ). Sera from individual mice were used at dilution 1:200, 1:1000 and 1:5000 and antibody titers were measured as previously described [26, 34–36].
2.6. Detection of tau tangles in human brain tissues by IHC and Confocal Microscopy
Sera from mice immunized with AV-1980D and pMultiTEP were screened for the ability to bind to tau tangles using 50 μm brain sections of paraformaldehyde-fixed cortical tissues from severe AD case (received from Brain Bank and Tissue Repository, MIND, UC Irvine) using immunohistochemistry as described previously[26, 34–36]. Sections were imaged using an Olympus FX1200 confocal microscope, with identical laser and detection settings across a given immunolabel and DAPI nuclear counterstain was pseudocolored red.
2.7. Preparation of brain homogenates from AD cases and western blot analysis
Preparation of brain homogenates from AD cases and Western blot (WB) analysis were performed as previously described[37]. Briefly, 0.2g of brain tissue from four different AD cases were homogenized in 0.4ml TBS buffer with Halt™ Protease and Phosphatase Inhibitor Cocktail (100X, Thermo Scientific, CA), then centrifuged at 6400xg for 15 minutes at +4°C. Supernatants were collected and applied to electrophoresis on NuPAGE 4–12% Bis-Tris gel in MES buffer under reducing conditions (Invitrogen, CA) and electrotransferred onto nitrocellulose membrane (GE Healthcare, NJ). Tau were visualized by incubating with sera from mice immunized with AV-1980D and pMultiTEP followed by HRP-conjugated anti-mouse IgG (Santa Cruz Biotechnology, CA). Anti-Tau (TNT-1; EMD Millipore, CA) monoclonal antibody was used as positive control.
2.8. Tissue preparation, Immunohistochemistry, Confocal Microscopy and Quantitative Analysis
Following perfusion, one hemisphere from each mouse was postfixed in 4% paraformaldehyde for 48 hours then stored in PBS + 0.05% sodium azide. Fixed half-brains were placed in 30% sucrose for at least 48 hours before being cut in the coronal plane (40 μm sections) using a freezing sliding microtome. Brain sections were rinsed in PBS before blocking in PBS+0.05% Triton-X with 5% donkey or goat serum for one hour. The following primary antibodies were used: 1C9 (IMM, Huntington Beach, CA; 1:500) and HT7 (ThermoFisher; Waltham, MA 1:1000) against human Tau, conformational tau epitope MC-1 (generously provided by Peter Davies; 1:1000), and phospho-tau (pS199, Abcam, Cambridge, United Kingdom, 1:1000). Sections were then incubated in primary antibodies at 4°C overnight. The next day, sections were washed three times with PBS and placed in appropriate Alexa fluor-conjugated secondary antibody solutions at room temperature for one hour. Sections were rinsed three additional times, mounted onto slides and coverslipped using Fluoromount-G with DAPI. For confocal microscopy, immunofluorescent staining was performed on equivalent brain sections and imaged on the Olympus FX1200 confocal microscope. Tau protein was visualized using Z-stack images taken through the entire depth of the section at 1 μm intervals. Z-stacks were compressed into a single stacked image and quantified. For quantification of 1C9, HT7 and pS199 staining, four randomly selected square sub-areas were selected in hippocampus CA1 and quantified by optical density using ImageJ. Quantification of MC-1 labeling was performed by manual counting of MC-1 positive pyramidal neurons in hippocampus CA1 by a researcher blinded to treatment.
2.9. Biochemical Analyses
Right hemispheres, previously frozen on dry ice and stored at −80°C, were crushed on dry ice using mortar and pestle, then homogenized in solution of T-PER (Thermo Scientific, Waltham, MA) and phosphatase and protease inhibitor mixtures (Thermo Scientific, Waltham, MA and Roche, San Francisco, CA) and processed as previously described[38, 39]. Soluble and insoluble SDS-PAGE Western blot was performed following standard protocols as previously described[39]. Primary antibodies used for Western blot analysis included the following: 1C9 (IMM, Huntington Beach, CA), AT180 (pT231), anti-tau (pS404), anti-tau (pS199), anti-tau (pS396) (all from Abcam, Cambridge, United Kingdom), HT7, AT-270 (pT181), AT8 (pS202/T205), anti-tau (pS214), AT100 (pT212/S214), anti-tau (pT212), anti-tau (pS396/S404) (all from ThermoFisher Scientific, Waltham, MA), anti-tau (pS422; WuXi AppTec, San Diego, CA). All blot membranes with soluble samples were also labeled with anti-GAPDH antibodies (ThermoFisher Scientific, Waltham, MA) as loading control. All antibodies were used at dilution 1:1000.
Concentrations of human total and phosphorylated tau in samples (soluble and insoluble brain extracts) were determined by Tau (total) Human ELISA kit, Tau [pS199] Human ELISA Kit, Tau [pT181] Human ELISA Kit, and Tau [pT231] Human ELISA Kit (all from ThermoFisher Scientific, Waltham, MA), according to the manufacturer’s instructions.
2.10. Statistical analysis
Statistical parameters (mean, standard deviation (SD), standard errors (SE), significant difference, etc.) were calculated using the Prism 6 software (GraphPad Software, Inc., La Jolla, CA). Statistically significant differences were examined using a two-tailed t-test (a P value of less than 0.05 was considered significant).
3. Results
3.1. Immunogenecity of AV-1980D vaccine in THY-Tau22 mice
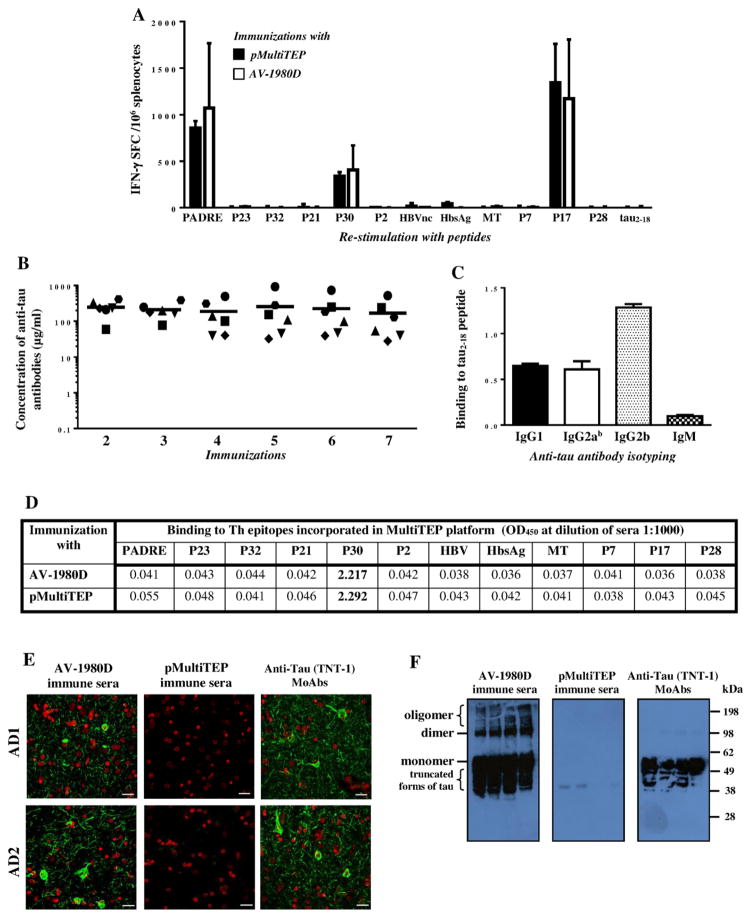
We generated a DNA vaccine, AV-1980D, composed of three copies of N-terminal B cell epitope from human tau protein (aa2-18) fused to the MultiTEP platform generated previously[24,26]. Immunizations of THY-Tau22 mice with AV-1980D followed by EP induced strong T cell immune responses (INF-γ secreting Th cells) specific to three different Th epitopes (PADRE, P30 and P17) incorporated into the MultiTEP platform of the AV-1980D vaccine, but not to the tau B cell epitope. Control mice immunized with pMultiTEP plasmid generated cellular immune responses specific to the same three epitopes, PADRE, P30 and P17 (Fig. 2A).
Figure 2. Cellular and humoral immune responses in THY-Tau22 mice vaccinated with AV-1980D.
(A) Numbers of IFN-γ producing T-cells were calculated by ELISPOT in splenocyte cultures obtained from vaccinated animals. Bars represent average ± SD (n=6 for pMultiTEP immunized group and n=7 for AV-1980D vaccinated group). (B) Concentration of anti-tau antibodies was detected in sera by ELISA. Lines indicate the mean values of antibody concentration (n=6). (C) Isotypes of anti-tau antibodies were analyzed by ELISA. Sera collected after two immunizations were used at dilution 1:800. (D) Binding to T helper epitopes incorporated in MultiTEP platform was detected in sera by ELISA. Sera collected after two immunizations were used at dilution 1:1000. (E) AV-1980D immune sera, but not pMultiTEP immune sera bound to the 50 μm brain sections of cortical tissues from two AD cases. Sera were used at dilution 1:1000. TNT-1 monoclonal antibody was used as a positive control. (F) AV-1980D immune sera, but not pMultiTEP immune sera bound to different forms of tau in soluble extracts from four AD cases detected by Western blot. Sera were used at dilution 1:1000. TNT-1 monoclonal antibody was used as a positive control.
THY-Tau22 mice generated strong anti-Tau humoral immune responses after two immunizations with AV-1980D (246.5 ± 119.8 μg/ml), then maintained the same antibody titers during five subsequent monthly immunizations (Fig. 2B). We measured the production of IgG1, IgG2ab, IgG2b, and IgM isotypes of anti-tau antibodies to characterize the type of humoral immune responses. The levels of IgG1, IgG2ab, and IgG2b immune responses were robust and stable, whereas the level of IgM was low (Fig. 2C). More specifically, EP-mediated i.m. delivery of AV-1980D induced strong IgG2b and equal amounts of IgG1 and IgG2ab antibodies specific to tau (Fig. 2C). The subclass of IgG that is induced against B cell epitope/s (Tau2-18) after immunization can be used as an indirect measure of the relative contributions of Th2 cytokines vs Th1 cytokines [40]. Therefore, these data indicate that i.m. delivery of AV-1980D vaccine followed by EP induced a mostly Th1 type of immune response, similar to that previously reported for the AV-1959D vaccine targeting pathological Aβ[27].
Next, we analyzed antibodies that might be generated against the Th epitopes incorporated in MultiTEP platform and showed that in these mice MultiTEP induces antibodies specific to P30 epitope only (Fig. 2D).
To determine whether the resulting AV-1980D induced anti-tau antibodies could recognize pathological forms of human tau, we next analyzed the binding of immune and control sera to neurofibrillary tangle (NFT) pathology within brain sections from two AD cases. Adjacent sections have also been stained with a commercial anti-tau antibody recognizing N-terminal region of tau, TNT-1. As expected, sera from mice immunized with AV-1980D, but not pMultiTEP plasmid, as well as commercial antibody TNT-1 specific to Tau N-terminus bound to both neuritic threads (NTs) and NFTs in human brain tissue (Fig. 2E). To show the binding of sera with various forms of Tau we performed WB of AD brain extracts and processed the membranes with sera and anti-Tau antibody, TNT-1. As shown in this new WB image, sera from vaccinated mice recognized monomeric and oligomeric forms of Tau, whereas TNT-1 antibody recognized only monomeric Tau. No binding was seen with control sera from mice immunized with pMultiTEP (Fig. 2F).
3.2. Changes of tau pathology in THY-Tau22 mice after immunization with AV-1980D
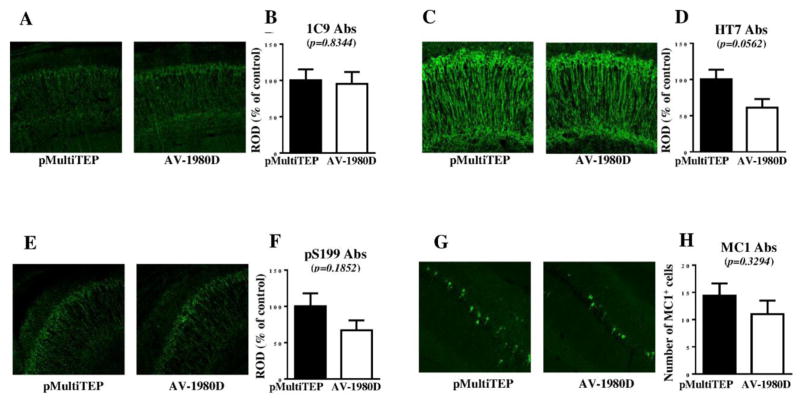
As a preclinical test of the efficacy of AV-1980D vaccination, we studied neurological changes in brains of nine month old THY-Tau22 mice vaccinated with AV-1980D. First, we investigated the impact of immunization on tau pathology in transgenic mice by performing immunohistochemistry (Fig. 3). Staining of brain tissue showed some reduction in total tau (1C9, p=0.8344 and HT7, p=0.0562; Fig. 3A–D), as well as phosphorylated tau (pS199, p=0.1852: Fig. 3G, H) following vaccination with AV-1980D, although these changes failed to reach significance. Similarly, we also observed a non-significant trend toward decreased MC1-positive cells in brain sections from AV-1980D vaccinated mice compared with pMultiTEP injected mice (p=0.3294, Fig. 3E, F).
Figure 3. Immunohistochemical changes measured in mice after DNA vaccination.
Level of total tau protein (A–D, G, H) and several phosphorylated tau species (E, F) in brain sections were analyzed by Confocal microscopy. Representative images of hippocampal CA1 region from vaccinated and non-vaccinated mice are presented (A, C, E, G). Error bars represent average ± SEM (n=6 for pMultiTEP immunized group and n=7 for AV-1980D vaccinated group).
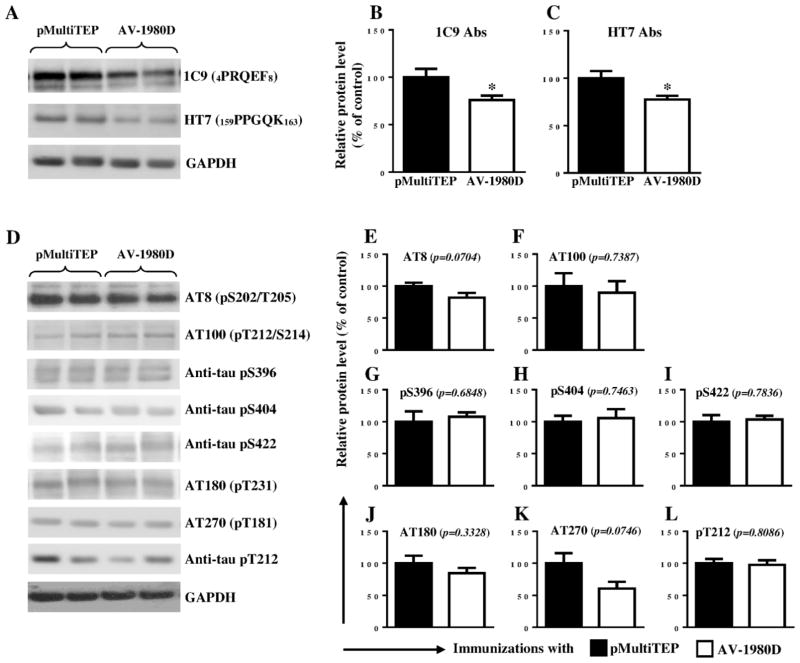
To further investigate tau aggregation, biochemical analyses were performed with soluble and insoluble protein fractions using two different techniques, WB and ELISA. WB analysis was carried out using antibodies raised against total and/or phosphorylated forms of tau. We observed that vaccination slightly, but significantly reduced the levels of total tau in soluble fractions of brain extracts from THY-Tau22 mice (1C9, p=0.0436 and HT7, p=0.0297; Fig. 4A–C). There were no obvious differences in the level of soluble phosphorylated tau epitopes AT100 (p=0.7387), pS396 (p=0.6848), pS404 (p=0.7463), pS422 (p=0.7836), AT180 (p=0.3328), and pT212 (p=0.8086) in vaccinated mice compared with controls (Fig. 4D and F, G, H, I, J, L, respectively). We did, however, observe a trend toward reduction in two soluble phosphorylated tau species, AT8 (p=0.0704) and AT270 (p=0.0746), in AV-1980D vaccinated mice, examining tau epitopes (Fig. 4D, E, K). Although, levels of these two soluble phosphorylated tau epitopes decreased in brains of vaccinated animals compared with that of control mice, these changes were not significant mainly due to variability in the extent of tau pathology between individual animals.
Figure 4. Effect of DNA vaccination on tau proteins in THY-Tau22 mice.
Level of total tau protein (A–C) and several phosphorylated tau species (D–L) in brain soluble extractions were analyzed by WB. Error bars represent average ± SEM. (*p<0.05, n=6 for pMultiTEP immunized group and n=7 for AV-1980D vaccinated group). In graphs A and D Lines 1 and 2 represent brain samples collected from mice immunized with pMultiTEP, and Lines 3 and 4 represent samples collected from mice immunized with AV-1980D.
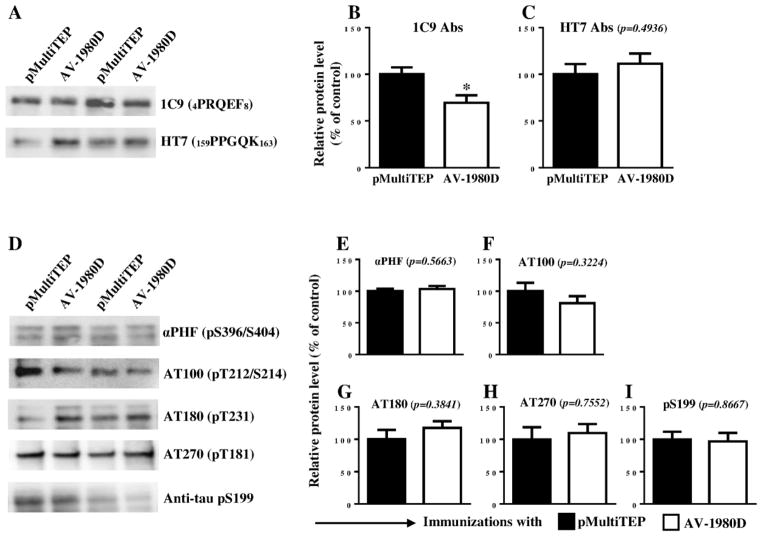
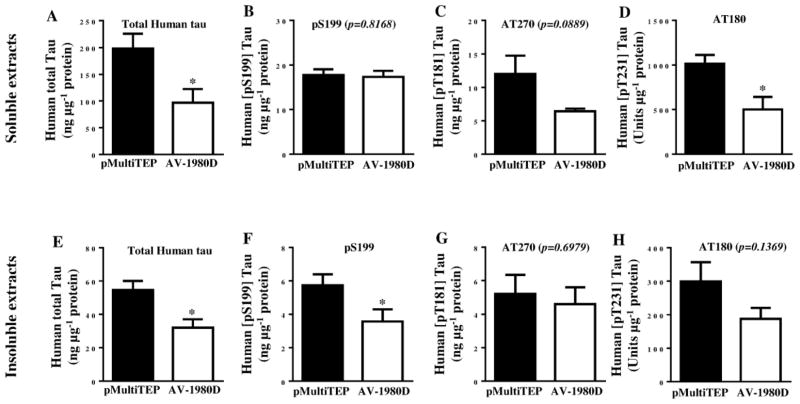
Next, we compared the level of total and phosphorylated tau in insoluble extracts from experimental and control brain homogenates by WB. Vaccination significantly reduced the level of total tau detected with 1C9 mAbs (p=0.0196; Fig. 5A, B), but not with HT7 mAbs (p=0.4936; Fig. 5A, C). No changes were detected in levels of phosphorylated insoluble tau after probing with αPHF-1 (p=0.5663), AT180 (p=0.3841), AT270 (p=0.7552) and pS199 (p=0.8667) antibodies (Fig. 5D and E, G, H, I, respectively). However, a subtle trend towards a reduction in levels of AT100 positive tau was observed in insoluble fractions of brain homogenates from AV-1980D vaccinated mice (p=0.3224; Fig 5D, F). To get more precise data we further analyzed the same brain homogenates by a more quantitative human tau-specific ELISA. As shown in Fig. 6A, E, both soluble and insoluble total human tau was significantly reduced in brain homogenates of AV-1980D vaccinated mice compared with control mice. Importantly, we detected significant decrease in soluble AT180 positive phospho-tau (Fig. 6D) as well as in insoluble pS199 positive phospho-tau (Fig. 6F). ELISA data showed also trends toward the reduction of soluble and insoluble AT270 (Fig. 6C, G), as well as insoluble AT180 positive phospho-tau.
Figure 5. Effect of DNA vaccination on tau proteins in THY-Tau22 mice.
Level of total tau protein (A–C) and several phosphorylated tau species (D–I) in brain insoluble extractions were analyzed by WB. Error bars represent average ± SEM (n=6 for pMultiTEP immunized group and n=7 for AV-1980D vaccinated group). In graphs A and D Lines 1 and 3 represent brain samples collected from mice immunized with pMultiTEP, and Lines 2 and 4 represent samples collected from mice immunized with AV-1980D.
Figure 6. Effect of DNA vaccination on tau proteins in THY-Tau22 mice.
Level of human total tau protein (A, E) and several phosphorylated tau species (B–D and F–H) in brain soluble and insoluble extractions were analyzed by ELISA. Error bars represent average ± SEM (n=6 for pMultiTEP immunized group and n=7 for AV-1980D vaccinated group).
Discussion
Immunotherapeutic approaches to treat tauopathies are promising strategy for AD[41]. Prior studies have shown that both active and passive tau-based immunotherapies clear some pathological forms of tau[42]. There is, however, still a risk that active vaccination against a full-length brain-derived self-protein could potentially induce encephalitis, which was observed in clinical trials with Aβ vaccination[43]. In fact, previously, Rosenmann et al. reported that immunization of C57BL/6 mice with full-length recombinant tau with CFA/pertussis toxin (PT), chosen as a proinflammatory adjuvant to analyze specifically safety issues, produced neurological deficits, NFT-like changes, gliosis, and inflammatory infiltration[15]. To reduce the risk of an adverse T cell-mediated immune response to tau immunotherapy, it is critical to design a vaccine that specificially targets the immunogenic B cell epitope of tau. During the last decade several anti-tau vaccines targeting different non-modified or phosphorylated epitopes, have been generated and evaluated in preclinical studies[42]. All these vaccines have shown varying degrees of efficacy, and did not induce adverse events in mice. However, subsequently, one group showed that harmful effects associated with vaccinations using phosphorylated tau as an epitope may still persist. In this study, E257T/P301S-tau Tg mice and wild-type mice were repetitively immunized with a mixture of three phospho-tau peptides (Tau195–213[p202/205], Tau207–220[p212/214], Tau224–238[p231]), formulated again in CFA/PT adjuvant[44]. This approach unfortunately led to the development of a paralytic disease accompanied by significant neurological disability in vaccinated mice[44]. In contrast, similar effects with antigens composed of other phospho-tau epitopes have not been observed[42].
In this study, we decided for the first time to develop and test a DNA vaccine targeting pathological non-phosphorylated tau. It is known that DNA immunization provides an attractive alternative to adjuvanted peptide/recombinant protein approach for inducing both cellular and humoral immune responses. More specifically, rapid accumulation of pre-clinical data demonstrating the potency of DNA vaccines in animal species ranging from mice to non-human primates has led to numerous Phase 1–3 human clinical trials[45, 46]. However, in clinical trials, DNA vaccines are generally immunogenic when they are delivered by devices such as electroporation systems or jet injectors[47, 48]. Therefore, in this study we focused on generation of an immunogenic and potentially safe DNA vaccine targeting a non-phospho-epitope located in N-terminal region of tau (tau2-18) and coupled this approach with electroporation. This N-terminal region of tau is normally folded and hidden in the native protein structure, yet becomes exposed due to the different biochemical modifications of pathological tau[29, 49]. Tau2-18 [termed as phosphatase-activating domain (PAD)] also plays an important role in the activation of a signaling cascade that leads to anterograde FAT inhibition, resulting in early synaptic dysfunction typical for AD and tauopathies[30]. In fact, it was reported that phosphorylation of Y18 in human tau can prevent the inhibitory effect of tau filaments on anterograde FAT, suggesting that physical blocking or binding of PAD can provide protection against the neurotoxic effects of pathogenic tau[30]. Lastly, it was reported that removal of tau2-18 decreased the polymerization of tau molecule, suggesting this region may serve as a seed for tau aggregation and thereby represents a promising therapeutic target[32]. Importantly, immunohistochemical and biochemical analyses of human postmortem brain tissues demonstrated that exposure of PAD in Tau is an early pathogenic event in AD and is closely associated in time with AT8 immunoreactivity, an early marker of pathological tau [28, 50]. Recently, it was demonstrated that induced pluripotent stem cell (iPSC) cortical neurons from AD patients secrete N-terminal tau fragments that negatively impact the neurons by inducing their hyperactivity and may elevate Aβ production in AD brain [51]. It was suggested that neutralization of these species can potentially slow the clinical progression into dementia. Dai et al reported the ability of monoclonal antibodies specific to Tau6-18 to reduce hyperphosphorylated Tau and improve reference memory in the Morris water maze task in 3xTg-AD mice [52].
We previously developed the MultiTEP vaccine platform that consists of a string of one synthetic and eleven non-self, pathogen-derived T helper (Th) epitopes[24–27], to which we can attach different B cell self-epitopes from neuronal proteins involved in AD pathogenesis. Using this approach we recently reported that a MultiTEP anti-Aβ DNA vaccine induced strong immune responses in mice, rabbits and monkeys[24–27]. Therefore, given these data and the universality of the MultiTEP approach, to generate strong anti-tau antibody responses without induction of possibly harmful autoreactive Th cells, we fused tau2-18 epitope to our universal MultiTEP platform (AV-1980D; Fig. 1A).
To test the efficacy of AV-1980D vaccine delivered in vivo by AgilePulseTM electroporation device we utilized the well-established THY-Tau22 transgenic model of tauopathy. THY-Tau22 mice overexpress human 4-repeat tau mutated at sites G272V and P301S under the control of Thy1.2 promoter and have previously been extensively characterized[33]. As these mice age they develop significant NFT-like pathology within the hippocampus, cortex, and amygdala that is accompanied by considerable gliosis and progressive cognitive impairments [21].
Repeated immunizations of young THY-Tau22 mice with AV-1980D induced strong T cell responses specific to the three epitopes from MultiTEP, but not to tau2-18 (Fig. 2A), thus avoiding the risk of unfavorable T cell-mediated autoimmune responses specific to tau. Importantly, DNA immunizations of THY-Tau22 mice induced very strong IgG-type (equal amount IgG1 and IgG2ab, higher level of IgG2b) anti-tau antibody production (Fig. 2B, C). Thus, i.m. delivery of DNA vaccine followed by EP induced strong cellular and humoral immune responses of Th1 type. Previously we showed that immunization with MultiTEP-based Aβ-epitope vaccine did not induce antibody responses to any of epitopes incorporated in MultiTEP in rabbits (unpublished data) and non-human primates [26]. The absence of antibody response to the carrier is extremely important, because antibodies specific to MultiTEP may more rapidly clear the vaccine from the body and decrease the immunogenicity of the vaccines in humans after booster injections. Thus, we measured the antibodies generated to each epitope incorporated into MultiTEP platform and showed that in THY-Tau22 mice AV-1980D vaccine induced antibodies specific to P30 only.
We also observed therapeutic efficacy of AV-1980D vaccination towards the reduction of non-phosphorylated tau. Recently, Yanamandra et. el. showed that monoclonal antibodies specific to tau25–30 that are capable of inhibiting tau propagation from cell to cell, may reduce tau pathology and improve cognitive functions in PS19 mice after intracerebroventricular (ICV) infusions or frequent high concentration in vivo injections[13, 53]. Another group tested the Mab specific to oligomeric tau conformational epitope in JNPL3 mouse models of tauopathy and showed a single intravenous (IV) injection of Mab cleared oligomeric tau, rescued the locomotor phenotype, and reversed the memory deficits associated with oligomeric tau pathology[54].
The goal of our current study was to examine whether we could generate an effective DNA vaccine that reduces tau in a stringent model of AD-like tau neuropathology. In that respect, immunization with AV-1980D led to significant decreases in the levels of total tau in soluble (Fig. 4A–C and 6A) and insoluble (Fig. 5A–C and 6E) fractions of brain extracts from vaccinated mice. In addition, we observed significant reduction in soluble AT180 phospho-tau (Fig. 6D) and insoluble pS199 phospho-tau (Fig. 6F). DNA vaccination also showed some trends toward reduction of soluble AT270 and insoluble AT180 phosphorylated tau species (Fig. 6C, H). Trend towards the reduction of total tau and pS199 phospho-tau and MC1 positive tau was seen by immunohisochemistry (Fig. 3). THY-Tau22 mice carry two pathogenic mutations in human tau, exhibit strong transgene expression, and develop very aggressive Tau pathology. This may at least partially explain why the efficacy of the DNA vaccine, AV-1980D is not strong.
These results are consistent with findings from other groups[17, 18] reporting that antibodies generated by peptide vaccination were found to cross the BBB, possibly due to impaired BBB in tau/Tg mice, bind phosphorylated tau, and reduce pathology without any adverse effects.
Therefore, we concluded that this initial study of tau DNA vaccination based on the MultiTEP platform suggests that this approach could potentially be used to induce strong immune responses in a broad population of vaccinated subjects with high MHC class II genes polymorphisms. Importantly, using tau B cell epitope we demonstrated that this immunogenic vaccine platform is truly universal and can be fused with virtually any B cell epitope to target the varying pathogenic molecules involved in AD or other neurodegenerative disorders.
Highlights.
THY-Tau22 immunized with AV-1980D followed by EP induced strong T cell immune responses specific to three Th epitopes incorporated into the MultiTEP platform of the AV-1980D vaccine, but not to the B cell epitope of tau.
THY-Tau22 mice immunized with AV-1980D followed by EP generated very high titers of anti-tau antibodies that recognized various forms of tau in the brain sections form AD cases.
Vaccinations of THY-Tau22 mice with AV-1980D followed by EP significantly reduced total Tau, as well as pS199 and AT180 phosphorylated tau levels in the brains extracts, but only in some extent reduced levels of other phosphorylated tau species.
Acknowledgments
This work was supported by funding from NIH (R01-NS050895, R01-AG020241, U01-AG048310, P50-AG016573, and RF1-AG048099).
Footnotes
Conflict of interest
MGA is a co-founder of Neuroimmune that licensed MultiTEP vaccine platform technology from the Institute for Molecular Medicine. The remaining author(s) declare no financial and commercial conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dementia, Fact Sheet. 2016 http://www.who.int/mediacentre/factsheets/fs362/en/.April.
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: a report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–4. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med. 1994;45:435–46. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerson JE, Kayed R. Formation and propagation of tau oligomeric seeds. Front Neurol. 2013;4:93. doi: 10.3389/fneur.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavaguera F, Lavenir I, Falcon B, Frank S, Goedert M, Tolnay M. “Prion-like” templated misfolding in tauopathies. Brain Pathol. 2013;23:342–9. doi: 10.1111/bpa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–14. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006;63:1459–67. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 16.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther. 2014;6:44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–66. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi M, Ittner A, Ke YD, Gotz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–85. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goni F, Herline K, Peyser D, Wong K, Ji Y, Sun Y, et al. Immunomodulation targeting of both Abeta and tau pathological conformers ameliorates Alzheimer’s disease pathology in TgSwDI and 3xTg mouse models. J Neuroinflammation. 2013;10:150. doi: 10.1186/1742-2094-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, Lopez-Deber MP, Reis P, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, et al. Refinement of a DNA based Alzheimer’s disease epitope vaccine in rabbits. Hum Vaccin Immunother. 2013;9:1002–10. doi: 10.4161/hv.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, et al. Epitope-based DNA vaccine for Alzheimer’s disease: Translational study in macaques. Alzheimers Dement. 2014;10:284–95. doi: 10.1016/j.jalz.2013.04.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Cribbs DH, et al. The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 2014;10:271–83. doi: 10.1016/j.jalz.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davtyan H, Hovakimyan A, Zagorski K, Davtyan A, Petrushina I, Agdashian D, et al. BTX AgilePulse(TM) system is an effective electroporation device for intramuscular and intradermal delivery of DNA vaccine. Curr Gene Ther. 2014;14:190–9. doi: 10.2174/1566523214666140522121427. [DOI] [PubMed] [Google Scholar]
- 28.Ward SM, Himmelstein DS, Lancia JK, Binder LI. Tau oligomers and tau toxicity in neurodegenerative disease. Biochem Soc Trans. 2012;40:667–71. doi: 10.1042/BST20120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–86. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, et al. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33:826.e15–30. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derisbourg M, Leghay C, Chiappetta G, Fernandez-Gomez FJ, Laurent C, Demeyer D, et al. Role of the Tau N-terminal region in microtubule stabilization revealed by new endogenous truncated forms. Sci Rep. 2015;5:9659. doi: 10.1038/srep09659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamblin TC, Berry RW, Binder LI. Tau polymerization: role of the amino terminus. Biochemistry. 2003;42:2252–7. doi: 10.1021/bi0272510. [DOI] [PubMed] [Google Scholar]
- 33.Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, et al. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–14. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, et al. Alzheimer’s Disease Peptide Epitope Vaccine Reduces Insoluble But Not Soluble/Oligomeric A{beta} Species in Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2007;27:12721–31. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, et al. Immunogenicity, Efficacy, Safety, and Mechanism of Action of Epitope Vaccine (Lu AF20513) for Alzheimer’s Disease: Prelude to a Clinical Trial. J Neurosci. 2013;33:4923–34. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davtyan H, Zagorski K, Rajapaksha H, Hovakimyan A, Davtyan A, Petrushina I, et al. Alzheimer’s disease Advax(CpG)-adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci Rep. 2016;6:28912. doi: 10.1038/srep28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–9. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc Natl Acad Sci U S A. 2016;113:E1316–25. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunology. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 41.Agadjanyan MG, Petrovsky N, Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: Active vaccination strategies to prevent and reverse Alzheimer’s disease. Alzheimers Dement. 2015;11:1246–59. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder SK, Joly-Amado A, Gordon MN, Morgan D. Tau-Directed Immunotherapy: A Promising Strategy for Treating Alzheimer’s Disease and Other Tauopathies. J Neuroimmune Pharmacol. 2016;11:9–25. doi: 10.1007/s11481-015-9637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orgogozo JM, Gilman S, Dartigues JM, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 44.Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, Nousiopoulou E, Kassis I, Abramsky O, et al. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp Neurol. 2013;248:451–6. doi: 10.1016/j.expneurol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 45.MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 46.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham BS, Enama ME, Nason MC, Gordon IJ, Peel SA, Ledgerwood JE, et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PLoS One. 2013;8:e59340. doi: 10.1371/journal.pone.0059340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, et al. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004;24:7895–902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, et al. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31:9858–68. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bright J, Hussain S, Dang V, Wright S, Cooper B, Byun T, et al. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015;36:693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Dai CL, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, et al. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J Neural Transm (Vienna) 2015;122:607–17. doi: 10.1007/s00702-014-1315-y. [DOI] [PubMed] [Google Scholar]
- 53.Yanamandra K, Jiang H, Mahan TE, Maloney SE, Wozniak DF, Diamond MI, et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015;2:278–88. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Gerson JE, Singh G, et al. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014;34:4260–72. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]