SUMMARY
Death of cochlear hair cells, which do not regenerate, is a cause of hearing loss in a high percentage of the population. Currently, no approach exists to obtain large numbers of cochlear hair cells. Here, using a small-molecule approach, we show significant expansion (>2,000-fold) of cochlear supporting cells expressing and maintaining Lgr5, an epithelial stem cell marker, in response to stimulation of Wnt signaling by a GSK3β inhibitor and transcriptional activation by a histone deacetylase inhibitor. The Lgr5-expressing cells differentiate into hair cells in high yield. From a single mouse cochlea, we obtained over 11,500 hair cells, compared to less than 200 in the absence of induction. The newly generated hair cells have bundles and molecular machinery for transduction, synapse formation, and specialized hair cell activity. Targeting supporting cells capable of proliferation and cochlear hair cell replacement could lead to the discovery of hearing loss treatments.
In Brief
Generation of hair cells after damage to the cochlea is a potential treatment for deafness. McLean et al. demonstrate that Lgr5+ supporting cells dissociated from the cochlear sensory epithelium form organoids and differentiate into hair cells in high yield after treatment with a combination of growth factors and drugs.

INTRODUCTION
Hearing impairment is a major health challenge estimated by the World Health Organization to affect over 5% of the world’s population (360 million people, including 32 million children). The sensory hair cells that detect sound and transmit their signal to the brain via the auditory nerve are susceptible to damage. After loss, the hair cells are never replaced (Cox et al., 2014; Fujioka et al., 2015), and thus, the number of cells, which is low (15,000 per ear in humans and 3,000 in mice) at the start of post-natal life, only decreases with age, and the absence of cell replacement leads to a high prevalence of acquired forms of deafness. Indeed, hair cell and auditory nerve damage, typically caused by noise exposure, ototoxic drugs, viral or bacterial infections, and aging, accounts for more than 80% of all cases of hearing loss (Davis, 1983).
Lgr5, an epithelial cell protein first discovered as a marker for intestinal stem cells and then shown to be critical for their function (Barker et al., 2007; Koo and Clevers, 2014), was recently shown to be expressed in cochlear supporting cells that surround the hair cells (Chai et al., 2012; Shi et al., 2012). These Lgr5-expressing cells could be induced to undergo limited proliferation when stimulated by Wnt in the normally post-mitotic cochlear sensory epithelium (Shi et al., 2013). Indeed, consistent with a progenitor role, supporting cells that expressed Lgr5 gave rise to new Lgr5+ cells by propagation and to hair cells that were Lgr5−, whereas supporting cells that did not express this receptor did not give rise to hair cells (Bramhall et al., 2014; Shi et al., 2012). Consistent with its role in upstream regulation of the transcription factor Atoh1 (Shi et al., 2010), which is a master regulator of hair cell differentiation (Edge and Chen, 2008; Kelley, 2006), upregulation of Wnt also increased hair cell differentiation. This combination of the ability to divide in response to Wnt signaling and the potency to differentiate into hair cells suggested that Lgr5+ cells were acting as progenitor cells of the cochlear epithelium (Shi et al., 2012). Indeed, in the newborn cochlea, Lgr5+ cells showed the capacity to regenerate spontaneously after damage (Bramhall et al., 2014; Cox et al., 2014). These data supported a role for the Lgr5+ cells as cochlear progenitor cells, but spontaneous regeneration capacity was lost after the first postnatal week, and, indeed, no cell division or cell replacement occurs in the sensory epithelium of the adult cochlea (Bramhall et al., 2014; Cox et al., 2014; Fujioka et al., 2015; Shi et al., 2012). Supporting cell transdifferentiation can lead to some hair cell replacement (Mizutari et al., 2013), but regenerating a functional cochlea would require both stimulating these cells to divide and differentiating them to hair cells, an approach that would benefit greatly from the isolation of Lgr5+ progenitor cells to develop protocols for their expansion and differentiation to hair cells.
The limited ability of cochlear cells to regenerate is unusual compared to other epithelia, but, despite different capacities for regeneration, Lgr5+ cochlear supporting cells have several characteristics in common with Lgr5+ cells from the intestine. Activation of both the Wnt and Notch signaling pathways has been independently demonstrated to be required for the establishment of Lgr5+ cells in the cochlea during development (Shi et al., 2010, 2014; Yamamoto et al., 2011). Wnt signaling is required for hair cell differentiation (Shi et al., 2014), which is increased by concurrent inhibition of Notch (Bramhall et al., 2014; Kelley, 2006; Korrapati et al., 2013; Shi et al., 2010, 2014). This is strikingly similar to intestinal epithelia, where Wnt and Notch signaling are required for stem cell expansion, and lack of Notch signaling with active Wnt leads to differentiation to mature epithelial cell types (de Lau et al., 2011; Koo and Clevers, 2014; van Es et al., 2005; Yin et al., 2014). Some expansion of Lgr5+ cells from the cochlea could be achieved by propagation as cochlear spheres (Shi et al., 2012), but heterogeneous cell populations are obtained and the yield of hair cells upon differentiation is low. Changes in gene expression of progenitors results in a loss of sphere-forming capacity in the adult mouse cochlea (Oshima et al., 2007). Studies on progenitor cells have thus been limited by the small number of Lgr5+ cells, which comprise a subset of the few cells in the cochlear epithelium, and inefficient protocols for their expansion. Here, by employing a cocktail of drugs and growth factors to modulate multiple pathways, we demonstrate mechanisms to clonally expand Lgr5+ cells from both newborn and normally unresponsive, adult tissue and to efficiently differentiate these colonies into nearly pure populations of hair cells in high yield. We show, furthermore, that the same drug cocktail drives the generation of hair cells and supporting cells in both healthy and damaged neonatal organ of Corti.
RESULTS
Lgr5+ Cochlear Cell Expansion
Lgr5+ cells represent a subset of supporting cells within the cochlear epithelium (Figure 1A). Using an Lgr5-GFP mouse line, we tested the activation or inhibition of multiple pathways to expand single Lgr5+ supporting cells isolated from the neonatal cochlea in a Matrigel-based 3D culture system (Figure 1B). Initially, we aimed to adapt conditions we previously developed for culture of intestinal stem cells to the inner ear progenitor cells (Yin et al., 2014). We added the glycogen synthase kinase 3β (GSK3β) inhibitor CHIR99021 (CHIR or C) and the histone deacetylase (HDAC) inhibitor valproic acid (VPA or V) to the growth factor cocktail that was previously used for the culture of inner ear spheres, which includes epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or F), and insulin like growth factor 1 (IGF-1 or I) (Li et al., 2003). In parallel, we tested conditions used for the culture of intestinal stem cells, which includes EGF (E), R-Spondin1 (R), and Noggin (N) (Sato et al., 2009). The addition of CHIR and VPA to EFI (EGF, bFGF, IGF-1) significantly increased the total number and percentage of Lgr5-GFP cells in culture, which outperformed the conditions previously used to expand intestinal Lgr5-GFP cells (ENR) (Figure S1A). The addition of CV to previously used factors led to the formation of large GFP+ colonies (Figure S1B), consistent with our previous finding in the intestine (Yin et al., 2014).
Figure 1. Diagram of Cochlear Lgr5+ Cell Culture.
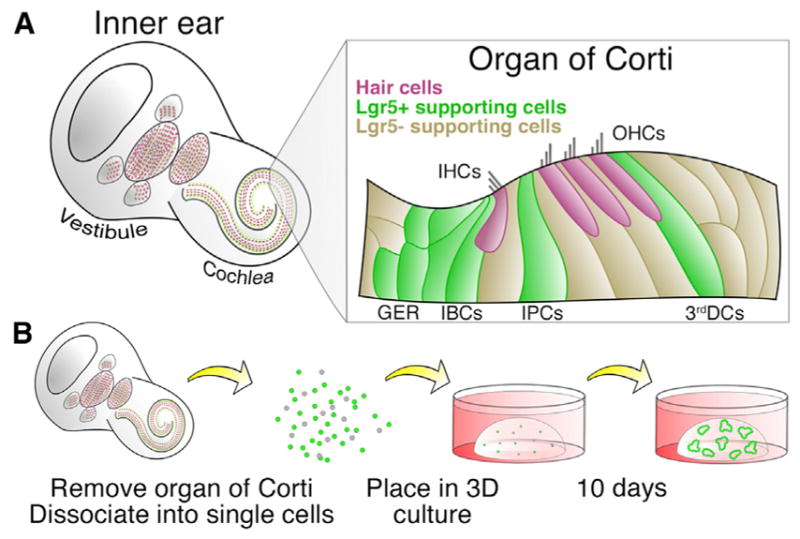
(A) Lgr5 is expressed in a subset of supporting cells surrounding the cochlear hair cells, including the greater epithelial ridge (GER), inner border cells (IBCs), inner pillar cells (IPCs), and third Deiters cells (3rd DCs). IHC, inner hair cell; OHC, outer hair cell.
(B) Diagram of cochlear epithelial cell isolation and culture as single cells in a 3D system for 10 days.
Without Wnt stimulation (EFI alone), Lgr5-GFP expression diminished, and, unlike the intestinal colonies, the number of Lgr5-GFP cells diminished after passage. Although the Lgr5-GFP cell numbers decreased after passage, further Wnt stimulation allowed Lgr5-GFP cells to be maintained in culture for extended periods of time (out to 45 days, the longest time point tested). We reasoned that other factors were needed to optimize the culture of Lgr5-GFP cells and thus performed screening to identify additional factors to enable the colonies’ prolonged culture and passaging. Addition of 2-phospho-L-ascorbic acid (pVc or P), a stable form of vitamin C, increased Lgr5+ cell expansion by an additional 2- to 3-fold (Figures S2A and 2B). Addition of a transforming growth factor β (TGF-β) receptor (Alk5) inhibitor, 616452 (or 6), also increased cell expansion (by 2- to 3-fold) and was required for the passage of colonies (Figures 2C, 2D, and S2C). Collectively, the addition of small molecules (CVP6), compared to growth factors alone, increased Lgr5+ cell numbers by >2,000-fold with high consistency (Figure 2B).
Figure 2. Characterization of Culture Conditions for Inner Ear Lgr5+ Cells.
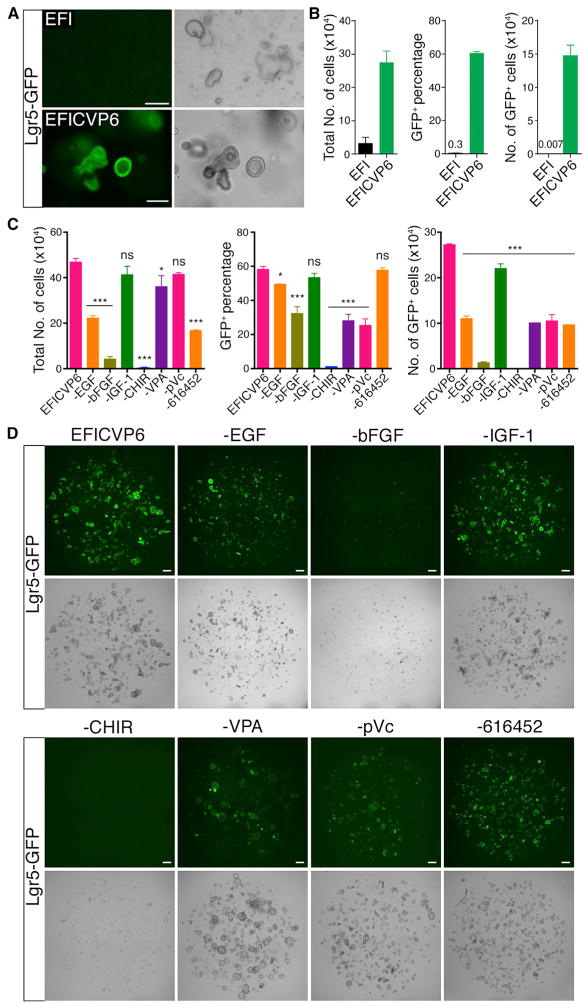
(A) GFP fluorescence and bright-field images of Lgr5-GFP colonies obtained from inner ear epithelial cells cultured for 10 days in the presence of EGF, bFGF, IGF-1 (EFI); EFI and CHIR, VPA, pVc, 616452 (EFICVP6). Scale bars, 200 μm.
(B) Number of live cells and percentage and number of Lgr5-GFP cells from inner ear epithelia cultured for 10 days. n = 3. Error bars represent mean ± SD.
(C) Number of live cells and number and percentage of Lgr5-GFP cells from inner ear epithelia cultured for 10 days. Lgr5+ cell number and percentage were highest in cultures containing EGF, bFGF, IGF-1, CHIR, VPA, pVc, and 616452 (EFICVP6) compared to cultures from which individual factors were removed. Each condition was compared to EFICVP6. n = 3. Error bars represent mean ± SD; ***p < 0.001; *p < 0.05; ns, not significant (p > 0.05).
(D) GFP fluorescence and bright-field images of cultures as shown in (C). Scale bars, 400 μm.
To examine the relative importance of individual factors in our culture system (without passaging), we separately removed each factor from the medium and quantified cell proliferation and Lgr5 expression of inner ear epithelial cells following 10 days of culture (Figures 2C and 2D). Removal of CHIR or bFGF had the greatest effect on Lgr5-GFP cell number and percentage, while removal of CHIR had the greatest effect on Lgr5 expression. Removing EGF or 616452 caused a significant reduction in Lgr5-GFP cell number, while removing VPA or pVc greatly reduced Lgr5 expression. The presence of IGF-1 had a marginal beneficial effect on Lgr5 cell number and percentage. The treatment with the combined agents (EFICVP6) yielded the highest number of total cells, Lgr5+ cells, and percentage of Lgr5+ cells following 10 days of culture. These results suggest that bFGF and CHIR were most critical to Lgr5+ cell culture, while the other factors promoted maximal Lgr5 cell growth and expression. Similar results were obtained by direct visualization of GFP expression and cell growth (Figure 2D).
We further examined the potential function of individual factors. The effects of CHIR in increasing Lgr5-GFP cell number and percentage could be partially replicated with Wnt3a in combination with R-spondin1 (Figures S2D–S2G), suggesting a role of CHIR in activating the Wnt pathway. Using an Atoh1-nGFP mouse line, we found that VPA suppressed spontaneous differentiation of supporting cells into hair cells (Figure S2H), which is consistent with the role of VPA in maintaining Notch activation in intestinal stem cells (Greenblatt et al., 2007; Yin et al., 2014).
Consistent with previous reports (Chai et al., 2012; Shi et al., 2012), Lgr5+ cells expressed the supporting cell marker Sox2, and a single optical slice revealed that Lgr5-GFP colonies cultured in EFICVP6 comprised pure populations of Sox2-expressing supporting cells with nuclear localization in the basal portion of the cell (Figure 3A). Exposure of EFICVP6 cultures to ethynyldeoxyuridine (EdU) revealed that Lgr5-GFP colonies were actively proliferating (Figure 3B). Tracking of single Lgr5-GFP cells over time revealed that Lgr5-GFP colonies were formed clonally (Figure 3C).
Figure 3. Lgr5+ Supporting Cells Actively Proliferate and Form Clonal Colonies.
(A) Lgr5+ colonies generated in EFICVP6 expressed the supporting cell marker Sox2 in nuclei located in the basal region of the cell. The dashed line indicates the border of a single cell with the apical surface (hollow arrowhead) facing the lumen. Image is a single optical slice. n = 4. Scale bar, 15 μm.
(B) EdU staining of an Lgr5-GFP colony. Image is a maximum projection of a z stack. n = 6. Scale bar, 15 μm.
(C) A single Lgr5+ cell tracked over 9 days while cultured in the presence of EFICVP6. n = 7. Scale bar, 15 μm. D, day.
Conversion of Lgr5+ Cells to Hair Cells
Although Notch inhibition and β-catenin expression were separately shown to promote hair cell differentiation in vitro from inner ear progenitor cells at a higher rate than removal of growth factors, the number of hair cells produced remained low due to the inability to expand progenitor cells and sufficiently convert them into hair cells (Jeon et al., 2011; Oshima et al., 2007; Shi et al., 2012, 2013). To test whether the expanded Lgr5+ cells were able to generate higher yields of hair cells after simultaneous Notch inhibition and Wnt activation, we treated Lgr5-GFP or Atoh1-nGFP cells, expanded by the above procedures, with LY411575, a γ-secretase inhibitor previously used to differentiate inner ear progenitor cells (Jeon et al., 2011; Mizutari et al., 2013), and CHIR, the GSK3β inhibitor. Following 10 days of differentiation, the expression of Lgr5 was diminished (Figure S3A), suggesting that they were differentiated cells. Atoh1-nGFP cells were rare during the expansion phase of the culture but increased in prevalence during the differentiation step of the protocol (Figure S3B). This suggests that the combination of LY411575 and CHIR induced the differentiation of the expanded Lgr5+ cells and transformed the colonies into high-purity populations of Atoh1-nGFP hair cells (Figure S3B).
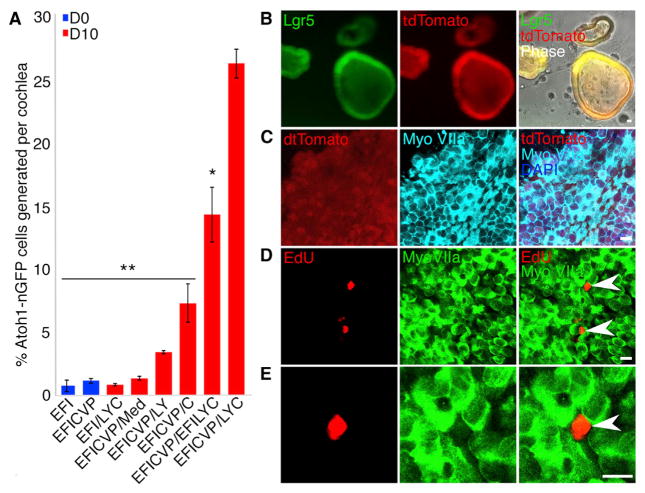
We quantified hair cell production using flow cytometry to count Atoh1-nGFP cells after expansion (day 0) and differentiation (day 10) phases of cultures originating from isolated epithelial cells from a single Atoh1-nGFP mouse cochlea (Figure 4A). We found that the addition of 616452 caused leakage of the enhancer-mediated Atoh1-nGFP (which was confirmed through co-staining with myosin VIIa during expansion). It was therefore removed from hair cell quantification assays using the Atoh1-nGFP reporter. Treatment with growth factors alone or with CHIR, VPA, and pVc produced few Atoh1-nGFP+ cells (0.72% with the growth factors and 1.108% in the presence of growth factors with drugs, p > 0.05) at the end of the 10-day expansion. Our results suggest that CHIR leads to greater differentiation than LY411575 (5.6-fold vs. 2.6-fold) when compared to removal of growth factors alone, and combining LY411575 and CHIR (LYC) further increases hair cell yield (Figure 4A). Expansion using EFICVP, followed by differentiation with LYC, produced the maximum number and purity of hair cells when multiple differentiation conditions were analyzed, suggesting that inclusion of growth factors in culture during differentiation reduces hair cell formation (Figure 4A). Expansion with EFICVP and differentiation with LYC resulted in ~26% of all cells in culture expressing Atoh1-nGFP, which corresponds to ~11,600 Atoh1-nGFP+ cells per cochlea compared to the 0.72% and average of 173 cells generated per cochlea when differentiating with LYC after expanding with EFI (p < 0.0001). Our optimal conditions generated a highly reproducible hair cell yield (26.3% ± 2.5%, n = 5 independent experiments; Figure 4A) that was 67-fold greater than previous methods using only growth factors to culture supporting cells (Oshima et al., 2007) and represented a 580-fold increase of viable hair cells during culture (20 Atoh1-nGFP cells).
Figure 4. Hair Cell Colonies Are Generated from Lgr5+ Colonies.
(A) Flow cytometry was performed for quantification of Atoh1+ cells in multiple expansion (blue bars) and differentiation (red bars) conditions in cultures originating from Atoh1-nGFP mice. Inner ear cell culture in growth factors (EFI) or growth factors with VPA and CHIR (EFICVP) did not change the percentage of Atoh1-nGFP cells after 10 days of expansion (shown as day 0 of the experiment; p > 0.05). A combination of LY411575 and CHIR (LYC) was the most effective for differentiation of Atoh1-nGFP cells from EFICVP-expanded cells and was therefore compared to each condition. n = 5. Error bars represent mean ± SD. **p < 0.0001; *p < 0.05; medium without growth factors or drugs (Med). D, day.
(B) Lgr5-Cre-tdTomato cells were cultured with EFICVP for 10 days. 4-Hydroxytamoxifen was added to the culture at day 0 of expansion. Expression of Lgr5-GFP and tdTomato is shown. Scale bar, 15 μm; n = 9.
(C) Immunocytochemical staining of Lgr5-Cre-tdTomato cells for myosin VIIa following 10 days of culture in LYC. Scale bar, 15 μm; n = 5.
(D) EdU and myosin VIIa cells following 10 days of differentiation. Scale bar, 15 μm; n = 5.
(E) High-power view of the EdU+ cell in (D). EdU was added at day 1.
The arrowheads in (D) and (E) refer to EdU-positive cells.
To determine whether Lgr5+ cells were the source of hair cells, we crossed Lgr5-Cre-ER mice with Rosa26-flox-tdTomato mice and followed tdTomato expression in any new cells. When 4-hy-droxytamoxifen was added to the culture at day 0 to activate the Lgr5-Cre, Lgr5+ cells formed colonies in the cocktail of growth factors and drugs, in which all cells within a colony were positive for tdTomato (Figure 4B). After differentiation with LY411575 and CHIR, hair cells (marked by hair cell-specific myosin VIIa (Weil et al., 1996)) were also tdTomato-positive (Figure 4C), indicating that the myosin VIIa+ cells were derived from Lgr5-expressing cells. Colonies that were tdTomato-negative did not produce myosin VIIa+ cells, indicating that Lgr5-negative cells do not generate hair cells, as also demonstrated previously (Shi et al., 2012). Cultures stained for EdU given 1 day after LY411575 and CHIR administration showed that cells expressing hair cell genes did not proliferate in the differentiation conditions (Figure 4D). These data thus suggest that hair cells did not proliferate during colony formation or differentiation.
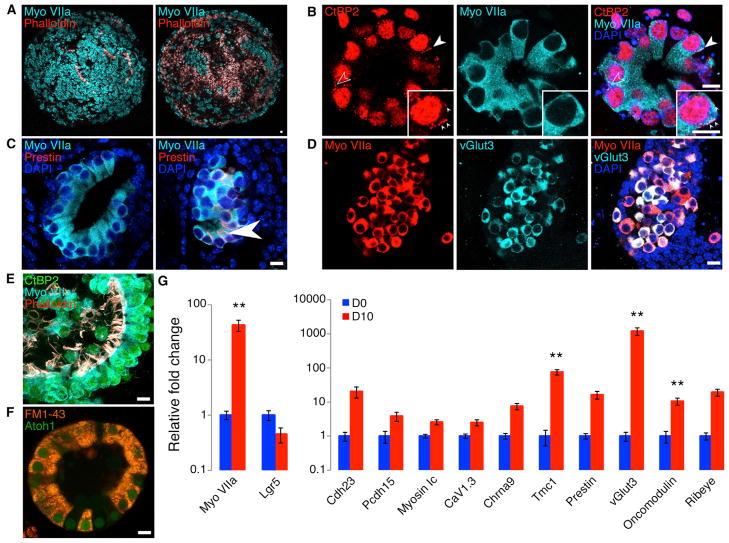
Further analyses showed that differentiating expanded supporting cells with LYC resulted in large colonies that were almost uniformly positive for myosin VIIa and contained actin-rich protrusions within the inner lumen (Figure 5A; Movie S1). Closer inspection of hair cell colonies revealed that myosin VIIa+ cells contained CtBP2+ ribbon synapse-like puncta in the basal region, where ribbon synapses are found in native hair cells (Figure 5B, arrowheads). Hair cell colonies were either negative (Figure 5C, left) or positive (Figure 5C, right, arrowhead) for prestin, a motor protein located in the membrane of outer hair cells, identifying a subset of the differentiated cells as outer hair cells (Dallos et al., 2008). Colonies of new hair cells also expressed vesicular glutamate transporter3 (vGlut3), an inner hair cell marker (Seal et al., 2008), which was only found in colonies that did not express prestin (Figure 5D). The staining also revealed that the actin-rich protrusions comprised several individual stereocilia on the cells’ apical surface (Figure 5E). The new colonies of hair cells rapidly accumulated the dye FM1-43, which enters hair cells through active transduction channels (Meyers et al., 2003) (Figure 5F).
Figure 5. Colonies of Inner Ear Progenitor Cells Generate Hair Cells In Vitro.
(A) The combination of CHIR and LY411575 (LYC) converted progenitor colonies into high-purity populations of myosin VIIa+ cells (left, surface view) with actin-rich protrusions projecting into the lumen (right, section through the colony). n = 11. Scale bar, 15 μm.
(B) Myosin VIIa+ cells had CtBP2+ puncta at the basal end of the cell near the membrane (arrowheads). n = 4. Scale bar, 15 μm.
(C) Myosin VIIa+/prestin− colonies, indicative of inner hair cells and myosin VIIa+/prestin+ (arrowhead) colonies, indicative of outer hair cells, were distinct. n = 4. Scale bar, 15 μm.
(D) Myosin VIIa+/vGlut3+ cells were also produced, indicative of inner hair cells. n = 5. Scale bar, 15 μm.
(E) Myosin VIIa+ cells had actin-rich bundles on the apical surface comprising several individual stereocilia. n = 11. Scale bar, 15 μm.
(F) Atoh1-nGFP colonies incorporated FM1-43 dye. Image is a single optical slice. n = 6. Scale bar, 15 μm.
(G) Key hair cell genes were compared by real-time qPCR at days 0 and 10 of differentiation. Myosin VIIa expression increased while Lgr5 expression decreased between days 0 and 10. Hair cell genes (CaV1.3, Ribeye, Chrna9, Tmc1, Pcdh15, Cdh23, myosin Ic, prestin, oncomodulin, and vGlut3), which include the markers measured by antibody staining (A–D), were strongly upregulated. n ≥5 independent samples per gene. Error bars represent mean ± SEM; p < 0.05 for all genes presented; **p < 0.005. D, day.
qPCR studies to determine gene expression profiles before and after differentiation using the optimal conditions for Atoh1-nGFP quantification (EFICVP/LYC) revealed that myosin VIIa was upregulated between day 0 and day 10 after expansion, while Lgr5 expression decreased (Figure 5G). The differentiated cells expressed the tip-link genes cadherin23 and protocad-herin15 (Hudspeth, 2008). The transduction adaptation component myosin Ic (Hudspeth, 2008), the synapse-associated calcium channel CaV1.3, and the ribbon synapse component ribeye were also upregulated (Khimich et al., 2005). The α9 acetylcholine receptor Chrna9 and the transduction channel component transmembrane channel 1 (Tmc1) (Pan et al., 2013a) both had increased expression. Prestin, the motor protein, and oncomodulin, a calcium modulator, both of which are found in outer hair cells, as well as the inner hair cell calcium modulator vesicular glutamate transporter 3, also showed increased expression. Our differentiation conditions thus generated inner and outer cochlear hair cell types that contained components of synaptic specializations, the transduction apparatus receptors, and ion channels of hair cells that were identified by both staining for specific markers and real-time qPCR.
Expansion and Hair Cell Differentiation of Lgr5-Expressing Cells from Adult Inner Ear Tissue
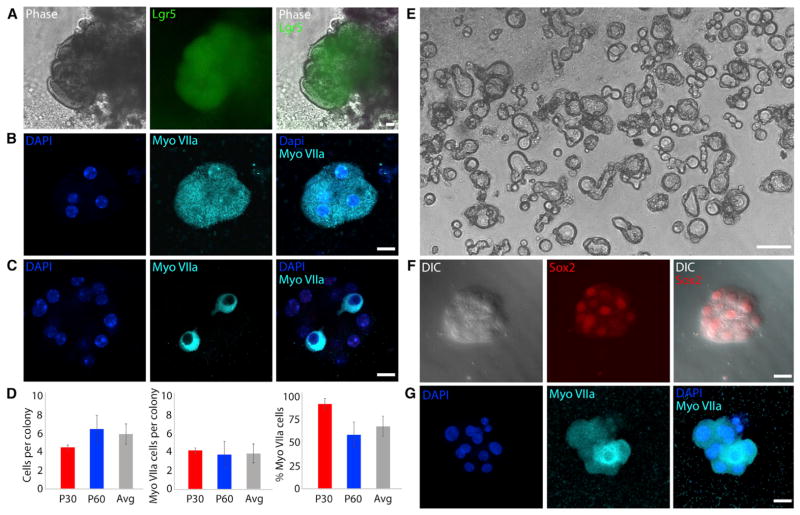
Previous studies have documented a decline in proliferative capacity and stem cell properties in the inner ear after the early postnatal period (Oshima et al., 2007; White et al., 2006). Since the drug combination applied here enhanced proliferation of neonatal cells compared to previous techniques, we next tested whether the compounds could be used to expand and differentiate otherwise quiescent adult cells into hair cells. Given the low numbers of Lgr5+ cells available from the adult mouse cochlea, we applied the cocktail of agents that we established for passaging neonatal cells, EFICVP6, to generate clonal colonies of adult cells positive for Lgr5 (Figure 6A). After the expansion, the cultures were treated with LY411575 and CHIR to differentiate the Lgr5+ cells. The colonies initiated expression of myosin VIIa only after differentiation (Figures 6B and 6C), indicating that adult Lgr5+ cells expanded and differentiated into cells that expressed hair cell markers. Myosin VIIa expression varied between colonies, with some colonies expressing the protein more robustly (Figures 6B and 6C). Cells isolated from mice at postnatal day 30 (4.67 ± 0.28 cells) and postnatal day 60 (6.75 ± 1.53 cells) formed similar sized colonies (average colony size across both ages of 6.18 ± 0.13 cells; Figure 6D). Colonies from postnatal day 30 (4.33 ± 0.28 cells) and postnatal day 60 (3.88 ± 1.48 cells) generated a similar number of myosin VIIa+ cells per colony (average across ages of 4.00 ± 1.06 myosin VIIa+ cells per colony; Figure 6D). The myosin VIIa+ cells were obtained in colonies generated from postnatal day 30 (93.3% ± 5.8% cells) and postnatal day 60 (59.6% ± 13.7% cells); (average across ages of 68.82% ± 0. 28% myosin VIIa cells; Figure 6D). No significant differences were seen across ages.
Figure 6. Expansion and Differentiation of Progenitor Cells from Adult Mouse, Rhesus Macaque, and Human Inner Ear.
(A) Adult mouse Lgr5-GFP cells formed colonies in EFICVP6. n = 4. Scale bar, 15 μm.
(B) Upon differentiation with LYC, subsets of colonies contained high purity populations of cells expressing hair cell gene, myosin VIIa (Myo VIIa). n = 4. Scale bar, 15 μm.
(C) Other colonies showed a smaller percentage of myosin VIIa+ cells. n = 4. Scale bar, 15 μm.
(D) Left: cells isolated from 30-day and 60-day old (p30 and p60) animals formed similar sized colonies (p > 0.05). Average (Avg) is also shown. Middle: colonies from 30- and 60-day-old animals generated a similar number of myosin VIIa+ cells per colony (p > 0.05). Average (Avg) is also shown. Right: myosin VIIa+ cells were represented in similar proportions in colonies from 30- and 60-day-old animals (p > 0.05). Average (Avg) is also shown. p30 n = 3; p60 n = 8. Error bars represent mean ± SD. P, postnatal day.
(E) Cells isolated from adult rhesus macaque inner ear epithelia generated clonal colonies in EFICVP6 for 7 days. n = 4. Scale bar, 50 μm.
(F) Cells isolated from human inner ear epithelia from a 40-year-old male generated clonal colonies that stained for Sox2 after a 12-day EFICVP6 treatment. n = 1. Scale bar, 15 μm. DIC, differential interference contrast.
(G) LYC treatment of human inner ear colonies generated populations of hair cell-like cells (Myo VIIa) with few myosin VIIa− cells (arrow). Colony size was 7.25 ± 1.74 cells. The number of myosin VIIa+ cells per colony was 5.25 ± 2.21. The proportion of myosin VIIa+ cells was 66.7% ± 18.0%. Scale bar, 15 μm.
We next tested whether the expansion and differentiation condition could be applied to non-human primates. Inner ear epithelial cells were isolated from adult rhesus macaques and cultured with EFICVP6. These preliminary results indicated that the cells formed clonal colonies (Figure 6E). However, differentiation to hair cells was not achieved due to repeated contamination likely caused by non-sterile conditions encountered during the temporal bone isolation.
We further tested the conditions using one sample of healthy human inner ear tissue isolated from a 40-year-old male patient undergoing a labyrinthectomy to access a tumor on the brain. The inner ear tissue was microdissected to remove bone, debris, and nerve tissue. The tissue was then treated identically to the mouse tissue to isolate single cells for culture. The single cells formed clonal colonies after 12 days under EFICVP6 conditions, although expansion was not as robust as that seen for neonatal cells (Figure 6F). The colonies stained for Sox2, a known marker of inner ear progenitor cells (Figure 6F). After 12 days of expansion, the cultures were treated with LY411575 and CHIR for 10 days to differentiate the colonies. The colonies stained positively for the hair cell marker myosin VIIa (Figure 6G), suggesting that sensory epithelium from adult human inner ear can also give rise to hair cell progenitors.
Hair Cell Generation In Situ in Cochlear Explants
The drugs that were critical for expansion and differentiation of Lgr5+ cells (HDAC inhibitors, GSK3β inhibitors, and γ-secretase inhibitors) have all been used clinically for other indications and could potentially be candidates for clinical development. To investigate their effects in a more clinically relevant tissue, we applied the drugs to cochlear explants. Supporting cells play a key role in cochlear function and homeostasis. Therefore, we treated intact and hair cell-damaged explants from postnatal day 2 mice with small molecules from the expansion conditions (CVP) rather than the differentiation conditions in an attempt to maintain a supporting cell population and permit spontaneous differentiation (Figure S4). We performed these cultures without growth factors in the presence of the surrounding tissue. These tests resulted in extensive proliferation of supporting cells and differentiation to hair cells. Whereas Lgr5-GFP was absent in a control cochlea in the region between the third Deiters cell and inner pillar cells (i.e., outer pillar cells, first and second Deiters cells), treatment with CVP for 3 days caused upregulation of Lgr5-GFP in all supporting cells (Figures 7A, 7C, and 7D). There was a highly significant (p < 0.001) ~2-fold increase in myosin VIIa+ inner and outer hair cells after 3 days of drug treatment (Figures 7B and 7D) as compared to control cochlea (Figure 7C). Addition of 616452 to VPA and CHIR did not increase the generation of hair cells. The new hair cells had morphology similar to the intact cochlea, with phalloidin+ stereociliary bundles and hair cells that were separated by intact supporting cells, suggesting that the treatment caused proliferation and subsequent differentiation (Figure 7E). Supporting cells had incorporated EdU, suggesting that they had divided (Figure 7F), and some of the hair cells, identified as “new” based on their expression of Sox2 (Bramhall et al., 2014; Kempfle et al., 2016), had transdifferentiated from supporting cells that had taken up EdU, similar to the division of supporting cells stimulated by Wnt signaling (Shi et al., 2013).
Figure 7. Increase in Hair Cell Numbers after Treatment of Cochlear Explants with GSK3β and HDAC Inhibitors.
(A) Cells between the third Deiters and inner border cells (arrowhead) had increased Lgr5-GFP expression in a cochlea treated with CHIR, VPA, and pVc (CVP). n = 9. Scale bar, 25 μm.
(B) Increased numbers of inner hair cells, outer hair cells, and total hair cells (IHCs, OHCs, and total HCs) were observed in treated as compared to control cochleae by myosin VIIa (Myo VIIa) expression. n = 4 each. Error bars represent mean ± SD; ***p < 0.001.
(C) Control cochleae had typical Lgr5-GFP expression, one row of inner hair cells, and three rows of outer hair cells. n = 3. Scale bar, 15 μm.
(D) A cochlear epithelium had an increased number of hair cell after CVP treatment. n = 4. Scale bar, 15 μm.
(E) Treated cochlear explant (left) had extra hair cells. The new hair cells possessed microvillar bundles in an orthogonal view (right). Supporting cells remained between new hair cells (arrowheads) as outlined by phalloidin staining. n = 5. Scale bars, 15 μm.
(F) Treated cochlear explant (top) showed supporting cells (Sox2+, arrowheads), hair cells (myosin VIIa+, asterisks), and EdU. Staining for EdU was visible in both supporting cells and hair cells (orthogonal view; bottom). n = 3. Scale bar, 25 μm.
(G) A cochlea damaged by gentamicin following a 3-day treatment with CVP had an increased number of Atoh1+ cells in the inner and outer hair cell regions. n = 3 each. Scale bar, 25 μm.
(H) Treatment with CVP increased hair cell numbers after gentamicin exposure. Dashed line represents hair cell counts in a healthy mouse cochlea. n = 3 each. Error bars represent mean ± SD. **p < 0.01.
(I) EdU incorporation into a gentamicin-treated cochlear explant (top) compared to a gentamicin and CVP-treated cochlear explant (bottom). EdU and CVP were added at 16 hr. n = 4. Scale bars, 15 μm.
Hair cell regeneration was achieved in cochlear explants treated with gentamicin, which causes hair cell death in the basal portion of the organ of Corti, where transduction channels are active in the neonate (Figures 7G–7I). Gentamicin caused extensive hair cell death in the base of the cochlea, but after 3 days of treatment with CVP, new Atoh1-nGFP hair cells appeared (Figures 7G and 7H). The number of hair cells was close to normal after treatment and 7-fold greater than that observed for control-treated cochlea (Figure 7H). Supporting cells were EdU+, indicating that supporting cell division was a part of the mechanism for hair cell replacement (Figure 7I). Thus, the treatment with CVP that expanded Lgr5+ cells from the cochlea after their isolation and placement into a 3D culture was also able to expand supporting cells in situ and force the generation of new hair cells.
DISCUSSION
Lgr5+ stem cells have been identified in epithelial cells of a number of tissues, including the intestine, colon, stomach, and liver (Barker et al., 2007; Koo and Clevers, 2014). Lgr5+ cells from these tissues can be induced to form organoids when cultured in the presence of Wnt pathway activators, including R-spondin 1, and contain a heterogeneous population of cells. Previously, we identified Lgr5 as a marker for progenitor cells in the newborn mouse cochlea (Chai et al., 2012; Shi et al., 2012, 2013). The cells that expressed Lgr5 were supporting cells that surround the hair cells of the cochlea. Similar to the Lgr5-expressing stem cells in the gut, these cells were Wnt responsive and could be stimulated to divide and differentiate to some extent by forced activation of Wnt signaling (Shi et al., 2013), even though the postnatal mammalian cochlea is normally quiescent. Further, although these previous studies showed Wnt stimulation could induce division in Lgr5+ cells, the limited potential for propagation and conversion to hair cells suggested that other pathways might be required to increase the stem cell capacity of Lgr5+ cells in the cochlea. Here, we show that Lgr5+ cells from the inner ear can also be extensively expanded with a GSK3β inhibitor to activate the Wnt signaling pathway combined with an HDAC inhibitor to activate Notch signaling. When provided with additional cues, specifically, 2-phospho-L-ascorbic acid, which was previously shown to facilitate induced pluripotent stem cell (iPSC) generation (Esteban et al., 2010), and the TGF-β inhibitor 616452, which regulates cell senescence (Hua and Thompson, 2001), neonatal cells could be passaged and clonal colonies of adult murine, primate, and human progenitor cells could be generated (Figures 6A, 6E, and 6F). Differentiating these cells by simultaneously activating Wnt and inhibiting Notch enabled conversion of progenitor cells into high purity populations of hair cells. Moreover, treatment of cochlear tissue with small molecules to simultaneously activate Wnt and Notch led to upregulation of Lgr5 in all supporting cells and increased numbers of Lgr5+ cells and hair cells (Figure 7). The increase in hair cell number was achieved even in cochlear tissue that had been depleted of hair cells by exposure to an aminoglycoside antibiotic. The effect of this drug combination on the cochlea suggests that small molecules activating Wnt and Notch could be useful as a therapeutic option to restore hair cells without loss of the supporting cells, which are important for cochlear homeostasis and mechanics.
In our previous work, we have shown that simultaneously providing Wnt and Notch signaling synergistically maintains self-renewal of Lgr5+ cells from the mouse small intestine, stomach, colon, and human small intestine. The expanded Lgr5+ cells could be used to generate mature intestinal epithelial cells, including Paneth cells, goblet cells, and enterocytes (Yin et al., 2014). Intestine, colon, stomach, and liver epithelia are actively renewed or activated upon injury, whereas the cochlear cells do not regenerate tissue spontaneously. However, like the stem cells from the intestine (de Lau et al., 2011; Koo and Clevers, 2014), the Lgr5-expressing cells in the cochlea show Wnt-dependent cell division (Shi et al., 2012). Wnt signaling and Notch inhibition also stimulate Atoh1-dependent differentiation to mature cells, similar to intestinal epithelium (Jacques et al., 2012; Shi et al., 2012, 2014; Yang et al., 2001). Lgr5 is a potentiator of the Wnt pathway through interaction with its ligand, R-spondin, and is uniquely driven by Wnt signals (de Lau et al., 2011; Koo and Clevers, 2014). R-spondin binding to Lgr5, along with Wnt binding to the Frizzled receptor, potentiates Wnt activity by as much as 100-fold (de Lau et al., 2011). The up-regulation of Lgr5 in organoids from the cochlea after treatment with the GSK3β inhibitor was consistent with a role of Wnt signaling upstream of Lgr5 (Barker et al., 2007). Expansion of Lgr5+ intestinal cells is dependent on Notch (van Es et al., 2005), which also expands progenitor cells in the cochlea (Jeon et al., 2011; Kelley, 2006; Pan et al., 2013b; Shi et al., 2010, 2014). In both the intestine and the cochlea, Notch plays a dual role, requiring cell-specific downregulation to establish the fate of supporting cells and hair cells in the patterning of the mature organ (Yamamoto et al., 2011). Wnts are released in response to injury, and this comprises one of the mechanisms through which stem cells may be activated in tissue repair (Liu et al., 2013; Whyte et al., 2012). Wnt signaling also stimulated the growth of Lgr5+ cells from the pancreas, which like the cochlea is a non-regenerative tissue (Huch et al., 2013). This study shows the generation of Lgr5+ organoids from non-endodermal epithelium. In the CNS, where a similar restricted role of stem cells has been noted, the stem cell compartments are Wnt dependent (Hochedlinger et al., 2005; Seib et al., 2013), and Wnt activity and responsiveness have been used to identify stem cell compartments (Denayer et al., 2008; Hochedlinger et al., 2005; Seib et al., 2013). These pathways provided a basis for the approach studied here for expanding Lgr5-expressing cells from the cochlea.
Although we have shown that inner ear cells could be isolated and cultured under neurosphere conditions and differentiated into hair cells and neural cells (Li et al., 2003; Martinez-Monedero et al., 2008; Oshima et al., 2007), the overall yield of hair cells was low. Since only a few thousand hair cells exist in the healthy adult mammalian cochlea and since the tissue is difficult to obtain and harvest from humans, a readily available source of bona fide hair cells would represent a major advance that would enable physiological studies as well as genetic screens using drugs, small interfering RNA (siRNA), or gene overexpression. Here, we demonstrated that Lgr5+ supporting cells from neonatal and adult cochlea responded to the protocols using Wnt stimulation and HDAC inhibition, giving rise to colonies with a high capacity for mature tissue cell differentiation. While the extent of expansion of Lgr5+ cells from adult mouse tissue was less than that from neonatal cells, the normally quiescent supporting cells from the adult mouse, rhesus, and human inner ear responded to the small-molecule cocktail used on neonatal tissue. However, additional molecules will likely be required to enhance the expansion of Lgr5+ cells from the adult cochlea. Importantly, hair cells produced from the protocols described here typify cochlear hair cells in both morphology and gene expression and possess key components necessary for proper function and communication with neurons. These hair cells possess stereociliary bundles that express transduction-associated genes (cadherin 23, protocadherin 15, myosin Ic, and transmembrane channel1), synaptic and neurotransmitter genes (CaV1.3, ribeye, α9-acetylcholine receptor, vesicular glutamate transporter 3, and oncomodulin), and other genes required for inner (vesicular glutamate transporter 3) and outer (prestin) hair cell function. We anticipate that Lgr5+ cells will need to be therapeutically targeted in situ to stimulate functional regeneration of hair cells, because cochlear mechanics rely on the precise anatomy of the organ and are unlikely to be recreated by disruptive cell transplantation approaches; thus, our identification of small molecules that can expand these cells is of particular significance. To date, difficulty in generating large numbers of primary hair cell progenitors has limited genetic and physiological studies for advancement of potential therapies. The small-molecule and growth factor cocktails used here enable the clonal expansion of Lgr5+ cells from mammalian cochlea and generation of sensory hair cells that will be useful for biological studies and will serve to generate a source of cells for both drug and genetic screens. Further, this work suggests that a small-molecule approach to activate Wnt and Notch could be a viable therapeutic route to restore hair cells.
EXPERIMENTAL PROCEDURES
Mouse Strains
Lgr5-EGFP-IRES-Cre-ER mice (The Jackson Laboratory, strain 8875) (Barker et al., 2007) were used to analyze the effects of small molecules on cochlear stem cell expansion. The same mice were crossed with Rosa26-td-Tomato reporter mice (The Jackson Laboratory, strain 7909) (Madisen et al., 2010) to create a mouse line that enabled lineage tracing of the cells that resulted from differentiated Lgr5-expressing cells. Atoh1-nGFP mice (Lumpkin et al., 2003) (provided by Dr. Jane Johnson) were used to identify differentiated hair cells.
Isolation of Stem Cells from the Inner Ear
All animal studies were conducted under an approved institutional protocol according to National Institutes of Health guidelines. For experiments with neonatal mice (postnatal days 1–3), the cochleae were dissected in Hank’s balanced salt solution (HBSS), and the organ of Corti (sensory epithelium) was separated from the stria vascularis (ion transport epithelium) and the modiolus (nerve tissue). The organs of Corti were then treated with Cell Recovery Solution (Corning) for 1 hr to separate cochlear epithelium from the underlying mesenchyme. Epithelia were then collected and treated with TrypLE (Life Technologies) for 15–20 min at 37°C. Single cells obtained by mechanical trituration were filtered (40 μm) and suspended in a Matrigel (Corning) dome for 3D culture. Laminin (0.015 mg/mL) was added to the Matrigel where indicated. For adult tissue, the stria vascularis was removed, but the epithelium was not removed from the underlying mesenchyme due to the limited amount of intact cochlea that could be extracted. Rhesus macaque tissue was acquired from the New England Primate Research Center. Temporal bones from adult rhesus macaques were dissected to obtain the inner ear and micro-dissected to remove bone, debris, and nerve tissue. The tissue was then processed and treated identically to the mouse cells to make a 3D culture of single cells. Human tissue was acquired after surgical removal in accordance with the policy of the Mass Eye and Ear Institutional Review Board for research use of discarded tissue. The tissue was then microdissected to isolate the sensory epithelium.
Expansion of Lgr5+ Cells
Cells were cultured in a 3D system and bathed in a serum free 1:1 mixture of DMEM and F12, supplemented with Glutamax (GIBCO), N2, B27 (Invitrogen), EGF (50 ng/mL; Chemicon), bFGF (50 ng/mL; Chemicon), IGF-1 (50 ng/mL; Chemicon), and small molecules, including CHIR99021 (3 μM), VPA (1 mM), pVc (100 μg/mL), and 616452 (2 μM) (Table S1). Media were changed every other day. 4-Hydroxytamoxifen (20 ng/mL) was added to cultures on day 0 for lineage tracing studies.
Differentiation of Lgr5+ Progenitor Cells
To differentiate stem cell colonies, the expansion media was removed and colonies remained in 3D culture. A serum-free 1:1 mixture of DMEM and F12, supplemented with Glutamax (GIBCO), N2, and B27, (Invitrogen) was added with various combinations of drugs or growth factors, as indicated in Figure 2. Small molecules were added to the culture to test their effect on differentiation. The optimal differentiation conditions included CHIR99021 (3 μM) and LY411575 (5 μM).
Quantification of Cells In Vitro
Lgr5+ cells were quantified after 10 days in culture in multiple conditions. Cell colonies were dissociated into single cells using TrypLE (GIBCO). Cell counting was performed with a hemocytometer. The cells were then stained with propidium iodide (PI) and analyzed using a flow cytometer for Lgr5-GFP expression. The number of GFP+ cells was calculated by multiplying the total number of cells by the percentage of GFP+ cells. Tukey’s post hoc test was used to analyze Lgr5-GFP expansion conditions.
To quantify hair cell production, cochlear epithelial cells were isolated as described above and seeded at one cochlea per well of a 24-well plate. This corresponded to a seeding density that was ~130,000 viable cells/mL, or 3,900 viable cells/well, which was determined using a hemocytometer and counting DAPI+ and DAPI− cells. More specifically, seeding at one cochlea per well corresponded to an Lgr5-GFP cell density of 23,750 cells/mL (710 cells/well) and an Atoh1-nGFP+ cell density of 740 cells/mL (20 cells/well).
Atoh1-nGFP+ cells were quantified at day 0 and day 10 of differentiation treatment to determine the number of Atoh1+ cells. Cell colonies were incubated in Cell Recovery Solution (Corning) to release the colonies from Matrigel and dissociated into single cells using TrypLE. The total number and percentage of GFP+ cells were quantified using fluorescence-activated cell sorting (FACS) of multiple culture conditions. ANOVA was used to compare means across conditions, and the two-tailed Student’s t test was used to compare each condition to the treatment with the highest yield.
Cochlear Explant Studies
Cochleae were isolated from 2-day-old Lgr5-GFP or Atoh1-nGFP mice and transferred to HBSS (Invitrogen). The organ of Corti was isolated from the otic capsule, and the nerve tissue and stria vascularis were removed. The basal hook portion of the organ of Corti was removed to allow for optimal plating of the organ. The organ of Corti was plated on a glass coverslip that had been coated with a 1:10 mixture of serum-free 1:1 DMEM/F12 and Matrigel to promote attachment to the coverslip. One cochlea from each animal served as a control, and the other was treated. Cochlear explants were cultured in a serum-free 1:1 mixture of DMEM and F12, supplemented with Glutamax, N2, and B27. For the treated cochlea, small molecules were added to this culture medium without growth factors present, while the control cochlea was bathed in culture media with DMSO at the same concentration (0.03%–0.13%) used in the treatments.
The number of cells expressing a hair cell protein within a 200-μm segment of the midbasal region of the cochlea was counted. The organs were treated with small molecules for 3 days. Lgr5-GFP cochlea and myosin VIIa staining were used for analysis of the undamaged cochlea.
To test hair cell generation after ablation, cochlear explants were prepared as described above using cochlear tissue isolated from Atoh1-nGFP mice. 1 hr after dissection, the organ of Corti explants were treated with 50 μM gentamicin (Sigma) for 16 hr to ablate hair cells as described previously (Bramhall et al., 2014). The organs were then treated for 3 days under control conditions that contained no growth factors or small molecules or with the small molecules used in our proliferation conditions. Atoh1-nGFP cells were counted in the basal portion of the cochlea using a 200-μm window.
Immunohistochemistry
Colonies or cochlear explants were fixed at room temperature in 4% paraformaldehyde/PBS for 15–20 min and then washed in PBS. Permeabilization of the cellular membrane (0.3% Triton X-100) was followed by blocking solution (15% heat inactivated donkey serum in PBS for 1 hr). Diluted primary antibody (0.1% Triton X-100 and 10% heat inactivated donkey serum in PBS) was applied for 4 hr at room temperature or overnight at 4°C. Primary antibody dilutions are listed in Table S2. Secondary antibodies (Alexa 488, Alexa Fluor 568, and Alexa Fluor 647 conjugated; Invitrogen) were used at 1:500 dilutions. Nuclei were visualized with DAPI (Vector Laboratories).
EdU studies were performed using the Click-iT EdU imaging kit (Thermo Fisher Scientific). For Lgr5+ cell proliferation studies, EdU (5 μM) was pulsed for 1.5 hr, and the cultures were washed and stained immediately after exposure. To test proliferation of hair cells, EdU was added 1 day after initial LYC exposure, and cultures were stained after 10 days. Explant cultures were exposed to EdU together with the expansion conditions for a total of 3 days.
For transduction assays, 5 μM FM1-43X (Invitrogen) was administered to cells for 30 s and then washed three times in 1× HBSS prior to fixation and further staining. Staining was visualized with confocal microscopy (TCD, Leica).
RNA Extraction and Real-Time qPCR
Colonies of expanded and differentiated cells were treated for 45–60 min with Cell Recovery Solution (Corning) to extract the cells from Matrigel and subsequently frozen in RLT buffer. RNA was extracted using the RNeasy Micro Kit (QIAGEN), and cDNA was generated using ImProm-II Reverse Transcription Kit (Promega). SYBR green real-time qPCR was performed in duplicates using SYBR Select Master Mix (Applied Biosystems) on a StepOne Real-Time PCR machine (Applied Biosystems). Specific primers were designed for all genes with mouse Gapdh as reference standard. A list of primers can be found in Table S3. The Student’s t test was used for analysis of gene expression, and at least five biologically distinct samples were analyzed for each condition.
Statistical Analysis
Results are reported as mean ± SD, where n represents the number of independent experiments conducted in the same manner. For quantification of cells in vitro, ANOVA and Tukey’s post hoc tests were used to compare means across conditions, while the two-tailed Student’s t test was used to compare each condition to the treatment with the highest yield. The Student’s t test was also used to compare hair cell counts from control and treated cochlear explants and to analyze gene expression levels at the beginning and end of in vitro differentiation. A p value < 0.05 was considered significant.
Supplementary Material
Highlights.
Lgr5+ cochlear supporting cells undergo clonal expansion after drug treatment
Colonies of Lgr5+ cells generate sensory hair cells in high yield
Hair cells can be generated from cells of the adult mouse and primate cochlea
Expansion of Lgr5+ cells and hair cells can be achieved in situ in the cochlea
Acknowledgments
We thank Lisa Ogawa and Marco Petrillo for contributions to this work. This work was supported by grants from the NIH (DE-013023, DC-007174, DC-013909, and RR-00168), a cooperative grant from the European Commission (FP7 Health 2013 Innovation), a Harvard-MIT IDEA2 award, the Shulsky Foundation, David H. Koch, and Robert Boucai.
Footnotes
Supplemental Information includes four figures, three tables, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.01.066.
AUTHOR CONTRIBUTIONS
W.J.M., X.Y., R.L., J.M.K., and A.S.B.E. designed research; W.J.M., X.Y., L.L., D.R.L., and D.M. performed research; W.J.M., X.Y., D.R.L., J.M.K., and A.S.B.E. analyzed data; and W.J.M., X.Y., D.R.L., J.M.K., and A.S.B.E. wrote the paper.
CONFLICTS OF INTEREST
J.M.K., R.S.L., X.Y., and W.J.M. hold equity in Frequency Therapeutics, a company that has an option to license IP generated by J.M.K., R.S.L., and X.Y. and that may benefit financially if the IP is licensed and further validated. W.J.M. is an employee of Frequency Therapeutics. The interests of J.M.K, R.S.L., and X.Y. were reviewed and are subject to a management plan overseen by their institutions in accordance with their conflict of interest policies.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2:311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Rubel EW, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141:816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AC. Hearing disorders in the population: first phase findings of the MRC National Study of Hearing. In: Lutman ME, Haggard MP, editors. Hearing Science and Hearing Disorders. Academic Press; 1983. pp. 35–60. [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Denayer T, Locker M, Borday C, Deroo T, Janssens S, Hecht A, van Roy F, Perron M, Vleminckx K. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells. 2008;26:2063–2074. doi: 10.1634/stemcells.2007-0900. [DOI] [PubMed] [Google Scholar]
- Edge AS, Chen ZY. Hair cell regeneration. Curr Opin Neurobiol. 2008;18:377–382. doi: 10.1016/j.conb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Okano H, Edge AS. Manipulating cell fate in the cochlea: a feasible therapy for hearing loss. Trends Neurosci. 2015;38:139–144. doi: 10.1016/j.tins.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Hua X, Thompson CB. Quiescent T cells: actively maintaining inactivity. Nat Immunol. 2001;2:1097–1098. doi: 10.1038/ni1201-1097. [DOI] [PubMed] [Google Scholar]
- Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron. 2008;59:530–545. doi: 10.1016/j.neuron.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, Dabdoub A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SJ, Fujioka M, Kim SC, Edge ASB. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J Neurosci. 2011;31:8351–8358. doi: 10.1523/JNEUROSCI.6366-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kempfle JS, Turban JL, Edge ASB. Sox2 in the differentiation of cochlear progenitor cells. Sci Rep. 2016;6:23293. doi: 10.1038/srep23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Koo BK, Clevers H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology. 2014;147:289–302. doi: 10.1053/j.gastro.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS ONE. 2013;8:e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA. Wnt signaling promotes Müller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2013;54:444–453. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68:669–684. doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge ASB. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013a;79:504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Chen J, Rottier RJ, Steel KP, Kiernan AE. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J Neurosci. 2013b;33:16146–16157. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build cryptvillus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin upregulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J Biol Chem. 2010;285:392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci USA. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge AS. β-Catenin is required for hair-cell differentiation in the cochlea. J Neurosci. 2014;34:6470–6479. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Weil D, Levy G, Sahly I, Levi-Acobas F, Blanchard S, El-Amraoui A, Crozet F, Philippe H, Abitbol M, Petit C. Human myosin VIIA responsible for the Usher 1B syndrome: a predicted membrane-associated motor protein expressed in developing sensory epithelia. Proc Natl Acad Sci USA. 1996;93:3232–3237. doi: 10.1073/pnas.93.8.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and transdifferentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol. 2012;4:a008078. doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353:367–379. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.