Abstract
Tobacco smoking remains one of the greatest public health problems facing the UK today. It varies significantly by ethnic group. This study aimed to determine whether ethnic differences in smoking behaviour are related to neighbourhood-level, own-group ethnic density across south and east London.
The association between ethnic density and individual smoking behaviour was assessed by multilevel logistic regression using the electronic health records of 688 397 general practitioner-registered patients. Restricted cubic splines were created to explore whether the effect of ethnic density on smoking behaviour was nonlinear.
Increasing own-group ethnic density was found to be associated with a significant reduction in the odds of being a current smoker in all ethnic groups, except for Black Caribbean women. The relationship between ethnic density and current smoking was found to be nonlinear, with the strength of association varying significantly by sex and ethnic group.
These novel findings point to a complex relationship between culture, neighbourhood-level experience of adversity or social support and smoking behaviour, and will allow us to target smoking cessation services differentially to individuals/groups living in relative ethnic isolation, who do not benefit from the potential cultural/social factors associated with reduced tobacco consumption.
Short abstract
The effect of ethnic density on smoking behaviour in London http://ow.ly/NQED308q9cO
Introduction
Tobacco smoking remains one of the greatest public health problems facing the UK today. Although smoking rates have decreased by over half since the 1970s, from 46% in 1974 to 16.9% in 2015, smoking is still a leading preventable cause of morbidity and mortality in the UK [1]. Smoking is a key risk factor for cardiovascular disease, respiratory disease, cancer and a range of other conditions. In 2014, 1.7 million hospital admissions and 78 000 deaths in Great Britain were attributable to smoking [2].
Patterns of smoking have been shown to differ significantly between men and women, by socioeconomic status and by ethnic group [3–6]. Smoking rates tend to be higher for men compared with women, with the difference most pronounced for South Asian groups [4]. In the UK, higher levels of deprivation have been associated with higher rates of smoking [6]. In 2016, smoking prevalence was found to be more than a third higher for people living in the most deprived decile of local authorities compared with those living in the most affluent decile of local authorities in England (20.4% versus 14.3%) [1].
A number of studies have linked ethnic minority health status to area-level ethnic density. This concept has been fruitfully explored in the field of mental health. In this case, the ethnic density hypothesis posits that members of ethnic minority groups may have better mental health when they live in areas with a higher density of people with the same ethnicity. A narrative review in 2012 suggested there was good evidence to support this proposition for the prevalence of psychotic disorders [7–10], with more recent studies suggesting a similar pattern for common mental disorders [11, 12]. In Utah in the USA, Hispanic immigrants were at greater risk of obesity the more isolated they were from their own ethnic group [13]. The explanatory mechanisms for these differences in prevalence of mental health problems and health risks are hypothesised to be related to the experience of reduced discrimination and enhanced social support in areas of higher own-group ethnic density, although it is challenging to demonstrate this empirically [14].
Whether the beneficial health effects of higher own-group ethnic density extend from disease prevalence and risk to health behaviours, such as smoking, remains to be explored [15]. Although the UK National Institute for Health and Clinical Excellence has highlighted the need to target smoking cessation services at ethnic minority groups [16], studies from across the UK indicate that smoking rates are already low among ethnic minority groups and thus the greatest need for smoking cessation services may be among the White majority population [3, 5, 17–20]. Acculturation towards the norms of the majority social group can change smoking behaviour among ethnic minority groups. When moving from a country with low smoking prevalence, acculturation tends to be associated with increased smoking behaviour, although this pattern may be offset by higher education levels among second-generation migrants, which are associated with reduced smoking rates [21, 22].
In addition to ethnic differences in the prevalence of current smoking, evidence also exists for ethnic differences in smoking intensity [23]. Ethnic differences in smoking intensity have been linked to genetic differences in cytochrome P450 (CYP2A6), which modulates nicotine metabolism and, ultimately, aspects of smoking behaviour [24–27].
Several studies have highlighted both the importance of developing culturally sensitive health promotion programmes and also a lack of evidence on how best to deliver these programmes to ethnic minority populations [28, 29]. Key considerations include lack of cultural acceptability, language differences, and lack of time and resources among healthcare practitioners [30].
The aims of this study are to determine whether ethnic differences in smoking behaviour are related to neighbourhood-level, own-group ethnic density across south and east London, and whether these effects vary by sex and age group after accounting for deprivation and geographical location. This study will 1) explore geographical variation in ethnic density in south and east London, and 2) assess the association between ethnic density and smoking prevalence and intensity.
Methods
This was a cross-sectional observational study using routinely collected primary care data of the association between ethnic density and smoking behaviour in ethnic groups in four inner-city boroughs of south and east London.
Data source
Routinely collected general practitioner (GP) health records for all patients registered in the boroughs of Hackney, Lambeth, Newham and Tower Hamlets were combined to conduct a cross-sectional study. The study population comprised all adults aged ≥18 years registered with 47 out of 48 practices in Lambeth in October 2013, and 140 out of 142 practices in Hackney, Newham and Tower Hamlets in June 2015. Patients were eligible for inclusion in the study if they were resident in the London boroughs of interest (i.e. Hackney, Lambeth, Newham and Tower Hamlets).
Individuals with an existing Read-coded diagnosis of chronic obstructive pulmonary disease (COPD) or lung cancer at the time of data capture were excluded from the analysis in order to identify a population of individuals free from established smoking-related respiratory disease (see supplementary material for code list) [31]. We excluded these individuals to capture a population suitable for targeting by smoking cessation services in primary care.
Individual-level variables
Individual data were anonymised prior to collection. Data collected included age, sex, self-reported ethnicity, smoking status, smoking intensity (cigarettes smoked per day), census-derived Lower Super Output Area (LSOA; an administrative area with an average population of 1614 individuals) based on postal code, Index of Multiple Deprivation (IMD) score and general practice with which registered. Individual self-reported ethnicity recorded at registration or consultation was reduced to 16 ethnic groups as defined in the 2011 UK Census [32]. The main analysis was restricted to the majority ethnic group of White British/Irish, and six ethnic minority groups of Other White, Indian, Pakistani, Bangladeshi, Black African and Black Caribbean as they represented the main ethnic minority groups resident across south and east London [32, 33]. South Asian and Black ethnic subgroups were considered separately throughout the analysis to account for known differences in migration history, geographic dispersion, cultural and religious influences on smoking, and established differences in smoking prevalence [4, 5, 34].
To identify current smokers, Read codes for tobacco consumption were reduced to two groups of current smokers and current nonsmokers (including ex-smokers and never-smokers). For the purpose of analysis, we summarised smoking intensity into low intensity (≤20 cigarettes per day) and high intensity (>20 cigarettes per day) (see supplementary material for code list).
Area-level variables
Area-level ethnic density for each ethnic group was defined as the percentage of people from that ethnic group living within each LSOA, based on the 2011 UK Census [35]. As a measure of socioeconomic deprivation we used the IMD score from 2010, with a score assigned to each patient based on home postcode [36].
Mapping
Mapping of ethnic densities was carried out to demonstrate their distribution in the geographical areas of investigation. To facilitate easier visualisation of this distribution, individuals were aggregated in the larger Middle Super Output Areas (an administrative area with an average population of 7787). The mapping was restricted to four ethnic groups: White, Bangladeshi, African and Caribbean, because of the low densities of the others [33]. Area-level ethnic density estimates were compiled from the current patient data, rather than relying on 2011 Census data, to ensure that they were up to date and as closely related as possible to the population under investigation [37].
Statistical analysis
All analyses were stratified by sex to account for established differences in smoking patterns between men and women.
1) A three-level logistic regression model, which nested patients within LSOAs within boroughs, was conducted separately for each ethnic group. A difference in ethnic density of 10% of the total population was set as the threshold interval above which an association with a change in the odds of being a current smoker was sought. A priori confounders included age, general practice, borough and IMD score.
2) To assess whether the relationship between change in ethnic density and smoking status differed by age group, we further stratified the analysis by those aged 18–35 years and those aged >35 years. We hypothesised that younger adults may be more acculturated to the majority ethnic group and thus show a different relationship between their smoking behaviour and own-group ethnic density, with the association between ethnic density and smoking behaviour being greater in older adults than in younger adults.
3) To explore whether the association between ethnic density and smoking behaviour was nonlinear, we repeated the analysis using restricted cubic splines which modelled the nonlinear change in the odds of being a current smoker for every 10% increase (10% of the total population) in own-group ethnic density [38]. A secondary analysis restricted to current smokers was conducted to examine the relationship between own-group ethnic density and smoking intensity, with high smoking intensity defined as smoking >20 cigarettes per day. All analyses were carried out using Stata version 14 (Statacorp, College Station, TX, USA).
Ethical approval
All data were anonymised and managed according to the UK NHS information governance requirements. Ethical approval was not required for this observational study as it relied solely on the use of Read-coded, nonidentifiable data with results published in aggregate form.
Results
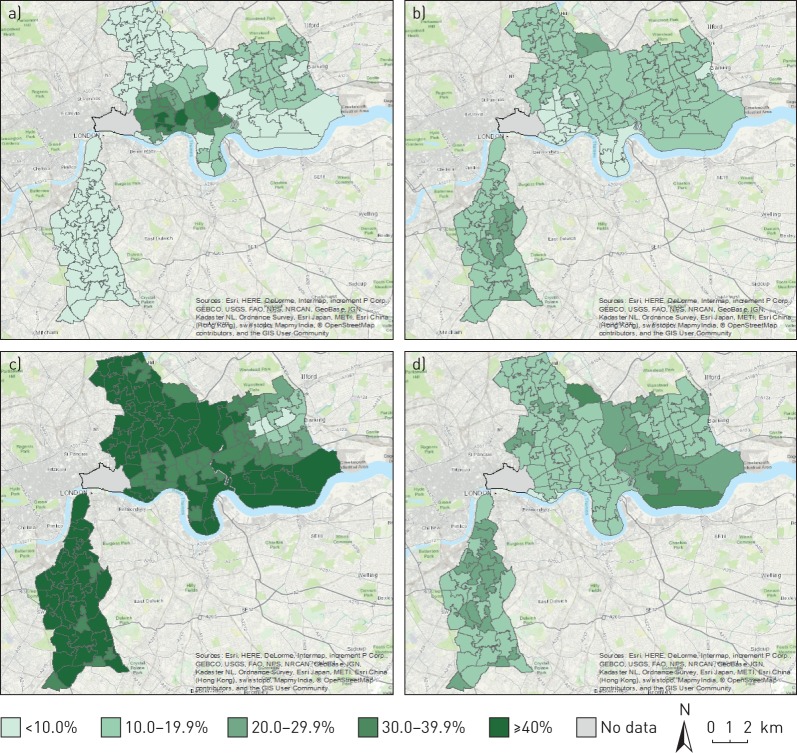
From a total GP-registered population of 1 000 388 adults ≥18 years across Lambeth and east London, 917 173 patients were resident within the study area of interest, and were free from COPD and lung cancer. From this population, 688 397 patients belonged to the pre-specified ethnic groups of interest (see supplementary material for population derivation flowchart). Figure 1 illustrates the wide geographical variation in neighbourhood-level ethnic density for Bangladeshi, Caribbean, White and African ethnic groups.
FIGURE 1.
Ethnic density distributions (%) by Middle Super Output Area mapped across south and east London for general practitioner-registered populations: a) Bangladeshi, b) Caribbean, c) White and d) African. © OpenStreetMap contributors. OpenStreetMap is open data, licensed under the Open Data Commons Open Database License by the OpenStreetMap Foundation.
Mean ethnic density was highest for the White British/Irish group and lowest for the Black Caribbean group (table 1). Large ethnic and sex differences in the proportion of current smokers and heavy smokers are apparent in the study population. The proportion of both current and heavy smokers is uniformly lower for women compared with men, with the difference most pronounced for South Asian groups, where the proportion of current smokers is up to up to six times higher for men compared with women.
TABLE 1.
Ethnic breakdown of sex, age, Index of Multiple Deprivation (IMD) score, ethnic density, current smokers and smoking intensity in south and east London
| White British/Irish | Other White | Indian | Pakistani | Bangladeshi | Black Caribbean | Black African | |
| Male | |||||||
| Patients n | 113 298 | 76 031 | 30 159 | 19 785 | 50 871 | 18 138 | 33 472 |
| Age years | 42.7±15.8 | 37.2±12.0 | 37.8±13.9 | 36.3±13.4 | 35.9±12.9 | 48.3±17.6 | 41.3±13.5 |
| IMD score | 36.6 (15.3) | 37.9 (14.5) | 38.8 (9.5) | 40.4 (9.6) | 43.8 (13.6) | 38.7 (12.9) | 41.5 (13.7) |
| Ethnic density % | 38.2 (20.0) | 15.4 (6.7) | 17.9 (42.3) | 13.0 (13.2) | 28.5 (30.7) | 8.6 (5.2) | 13.6 (10.2) |
| Current smokers % | 36.0 | 39.2 | 21.5 | 27.8 | 43.8 | 41.8 | 18.4 |
| High-intensity smokers# % | 12.1 | 7.6 | 2.4 | 3.7 | 4.0 | 3.6 | 2.8 |
| Female | |||||||
| Patients n | 115 654 | 90 531 | 23 801 | 12 673 | 42 513 | 24 039 | 37 432 |
| Age years | 42.3±17.7 | 36.0±12.8 | 39.6±15.6 | 38.2±14.5 | 36.9±14.5 | 48.3±17.7 | 40.8±14.0 |
| IMD score | 36.6 (15.2) | 38.1 (14.3) | 38.7 (9.7) | 40.1 (9.0) | 43.9 (13.5) | 38.8 (12.6) | 41.8 (13.5) |
| Ethnic density % | 38.4 (20.4) | 15.2 (6.6) | 15.1 (24.5) | 12.3 (13.4) | 28.9 (30.6) | 8.7 (5.9) | 13.7 (10.2) |
| Current smokers % | 31.8 | 31.4 | 5.0 | 5.3 | 7.2 | 24.2 | 6.2 |
| High-intensity smokers# % | 8.8 | 3.2 | 1.9 | 2.7 | 2.1 | 2.5 | 1.4 |
Data are presented as mean±sd or median (interquartile range; 75th–25th percentile), unless otherwise stated. #: high-intensity smoking >20 cigarettes per day.
Association between ethnic density and smoking status
The association between ethnic density and smoking status stratified by sex is presented in table 2. Each 10% increase in own-group ethnic density was associated with a 2–43% reduction in the odds of being a current smoker for all ethnic groups except for Black Caribbean women. For men, the largest association was found in the Black African group, for whom each 10% increase in own-group ethnic density was associated with an 18% reduction in the odds of being a current smoker (p<0.001). For women, the largest association was found in the Pakistani group, for whom each 10% increase in own-group ethnic density was associated with a 43% reduction in the odds of being a current smoker. For Black Caribbean women, no association between ethnic density and smoking status was evident (table 2).
TABLE 2.
Association (odds ratio (OR) adjusted for age, area deprivation and borough) between an increase of 10% in area ethnic density and the prevalence of current smoking by ethnic group
| Ethnic group | Male | Female | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| White British/Irish | 0.94 (0.92–0.97) | <0.001 | 0.96 (0.93–0.98) | <0.001 |
| Other White | 0.92 (0.87–0.97) | 0.003 | 0.93 (0.88–0.97) | 0.002 |
| Indian | 0.93 (0.90–0.96) | <0.001 | 0.62 (0.57–0.69) | <0.001 |
| Pakistani | 0.88 (0.83–0.93) | <0.001 | 0.57 (0.48–0.67) | <0.001 |
| Bangladeshi | 0.98 (0.95–1.00) | 0.056 | 0.92 (0.87–0.96) | <0.001 |
| Black African | 0.82 (0.77–0.86) | <0.001 | 0.80 (0.73–0.88) | <0.001 |
| Black Caribbean | 0.88 (0.79–0.99) | 0.034 | 0.97 (0.86–1.08) | 0.555 |
The relationship between ethnic density and smoking status stratified by sex and age group is presented in table 3. A significant reduction in odds of being a current smoker was evident in all male ethnic groups except for Black Caribbean after stratifying by age. The size of the reduction was comparable between those aged ≤35 years and those aged >35 years. Among women, the association between ethnic density and being a current smoker was larger for those aged ≤35 years compared with those aged >35 years in the Other White and Bangladeshi ethnic groups (table 3).
TABLE 3.
Association (odds ratio (OR) adjusted for age, area deprivation and borough) between 10% increase in area ethnic density and change in prevalence of current smoking stratified by age group
| Ethnic group | Age ≤35 years | Age >35 years | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Male | ||||
| White British/Irish | 0.96 (0.93–0.98) | 0.003 | 0.93 (0.91–0.95) | <0.001 |
| Other White | 0.90 (0.84–0.96) | 0.002 | 0.95 (0.89–1.01) | 0.110 |
| Indian | 0.93 (0.78–0.98) | 0.006 | 0.94 (0.89–0.98) | 0.003 |
| Pakistani | 0.87 (0.80–0.94) | <0.001 | 0.91 (0.84–0.99) | 0.028 |
| Bangladeshi | 0.97 (0.94–1.00) | 0.070 | 0.99 (0.96–1.02) | 0.548 |
| Black African | 0.77 (0.70–0.86) | <0.001 | 0.85 (0.80–0.91) | <0.001 |
| Black Caribbean | 0.95 (0.76–1.18) | 0.642 | 0.90 (0.78–1.02) | 0.104 |
| Female | ||||
| White British/Irish | 0.97 (0.94–1.00) | 0.042 | 0.94 (0.92–0.97) | <0.001 |
| Other White | 0.90 (0.85–0.96) | 0.001 | 0.96 (0.90–1.03) | 0.261 |
| Indian | 0.67 (0.60–0.78) | <0.001 | 0.57 (0.49–0.66) | <0.001 |
| Pakistani | 0.58 (0.47–0.71) | <0.001 | 0.56 (0.42–0.73) | <0.001 |
| Bangladeshi | 0.90 (0.85–0.95) | <0.001 | 0.94 (0.88–1.02) | 0.133 |
| Black African | 0.83 (0.73–0.94) | 0.001 | 0.77 (0.69–0.87) | <0.001 |
| Black Caribbean | 0.95 (0.79–1.14) | 0.580 | 0.98 (0.85–1.12) | 0.732 |
Ethnic density as a nonlinear effect
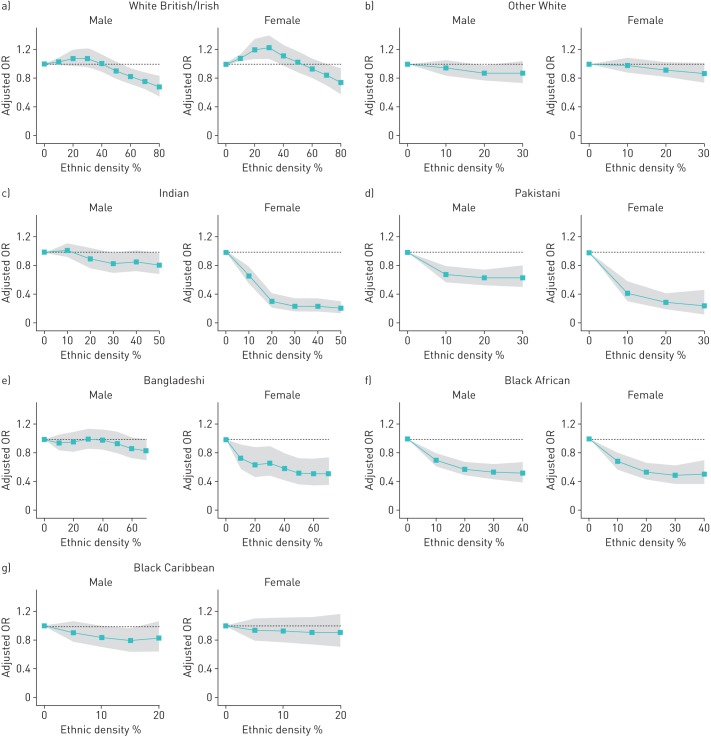
Restricted cubic splines were used to examine the nonlinear relationship between ethnic density and smoking status. Nonlinear relationships were apparent for all ethnic groups, for both men and women (figure 2). The shape of the relationship differed noticeably between men and women in all South Asian ethnic groups, but was comparable between men and women for White British/Irish and Black African and Black Caribbean groups.
FIGURE 2.
Relationship between own-group ethnic density (expressed in 10% increments of the whole population) and the adjusted odds ratio (OR; adjusted for age, general practice, borough and Index of Multiple Deprivation score) of being a current smoker, analysed by sex and ethnic group using restricted cubic splines: a) White British/Irish, b) Other White c) Indian, d) Pakistani, e) Bangladeshi, e) Black African and f) Black Caribbean. Shaded areas show the 95% confidence intervals.
Among White British/Irish men and women, the odds of being a current smoker increased until own-group ethnic density reached 30% of the total population and then decreased thereafter. For Bangladeshi, Indian and Pakistani women, the association between smoking status and ethnic density fell steeply with each 10% rise in ethnic density until ethnic density rose above 20% of the total population, at which point changes in ethnic density were not associated with changes in smoking status
Association between ethnic density and smoking intensity in current smokers
A secondary analysis examining the relationship between own-group ethnic density and the odds of being a high-intensity smoker (defined as smoking >20 cigarettes per day) was conducted for current smokers (table 4). Due to the small proportion of high-intensity smokers among women, the analysis of smoking intensity was restricted to men. There was no evidence of a relationship between own-group ethnic density and smoking intensity in six of the ethnic groups. The exception was Bangladeshi men, in whom a 10% increase in own-group ethnic density was associated with a 12% decrease in the odds of being a heavy smoker (odds ratio 0.88, 95% CI 0.82–0.95).
TABLE 4.
Association (odds ratio (OR) adjusted for age, area deprivation and borough) between 10% increase in area ethnic density and high-intensity smoking (males only)
| Ethnic group | Current smokers n | High-intensity smokers# % | OR (95% CI) | p-value |
| White British/Irish | 40 725 | 10.4 | 0.99 (0.96–1.04) | 0.799 |
| Other White | 29 783 | 6.2 | 0.90 (0.79–1.01) | 0.074 |
| Indian | 6481 | 2.1 | 0.84 (0.70–1.01) | 0.060 |
| Pakistani | 5504 | 3.3 | 1.08 (0.83–1.40) | 0.560 |
| Bangladeshi | 22 262 | 3.8 | 0.88 (0.82–0.95) | 0.001 |
| Black African | 6173 | 2.4 | 0.94 (0.68–1.03) | 0.706 |
| Black Caribbean | 7580 | 3.0 | 0.67 (0.41–1.10) | 0.115 |
#: high-intensity smoking >20 cigarettes per day.
After stratifying by age, the odds of being a high-intensity smoker were comparable between Bangladeshi males aged ≤35 years and Bangladeshi males aged >35 years (results in supplementary material). Restricted cubic splines did not provide any evidence of a nonlinear relationship between ethnic density and high-intensity smoking (results in supplementary material).
Discussion
This study highlights significant neighbourhood variation in ethnic density of key minority groups in south and east London, and identifies a highly significant relationship between area-level ethnic density and smoking behaviour in both men and women across most of these ethnic groups.
This study found strong evidence that higher own-group ethnic density is associated with a lower prevalence of current smoking across all ethnic and sex groups, with the exception of Black Caribbean women. This effect persisted after accounting for social deprivation, age and geographic location. The relationship was also found for the White British/Irish population, the ethnic majority population in the UK. The effect size was greatest in the Black African population for men and in the Pakistani population for women. The relationship between ethnic density and the odds of being a current smoker was nonlinear, with the shape of the pattern and strength of association varying significantly between sexes and ethnic groups.
The absence of association between ethnic density and smoking status among the Black Caribbean population may be due to a small sample size of this ethnic group and its greater geographic dispersal. In our study, as in others, the median ethnic density (8.6%) was lowest for the Black Caribbean population among the ethnic groups studied (table 1) [10].
With the exception of Bangladeshi men, the study found no evidence of a relationship between own-group ethnic density and high intensity of smoking behaviour. First, this may be because the analysis of smoking intensity was restricted to current smokers, few of whom self-reported as being high-intensity smokers. Low numbers may have resulted in low statistical power to detect a relationship between ethnic density and smoking intensity in our study population. This was particularly the case in ethnic minority groups where ≤4% were high-intensity smokers. Second, the use of cigarettes smoked per day as a measure of smoking intensity may be unreliable. Self-report of cigarettes smoked per day is prone to digit bias (rounding to multiples of 10 due to standard pack-sizes) and under-reporting [39]. Under-reporting may be particularly prevalent among high-intensity smokers due to social desirability bias.
We found no differences in the odds of being a current smoker between age groups. We hypothesised that younger adults may be more acculturated, and thus show a different relationship between their smoking behaviour and own-group ethnic density. It is possible that young people are more likely to smoke using methods such as cannabis, water pipes and electronic cigarettes, the latter being perceived as healthier alternatives to traditional cigarettes, and currently not well captured in the primary care record [40–42].
The lower prevalence of current and heavy smoking in South Asian groups compared with White and Black groups is likely to be determined by a combination of cultural, behavioural and genetic determinants. Studies have identified numerous genetic variants in nicotinic receptors, with a variation in frequencies between ethnic groups, which may reflect a differing propensity to make the transition from smoking to nicotine dependency [43, 44].
Sex differences in smoking behaviour are reflective of wide cultural disparities between men and women in different ethnic groups [45]. Women may under-report tobacco use to a greater extent than men due to social and cultural factors. This has been observed in British Bangladeshi and Pakistani populations, where smoking among men is a social activity, while smoking among women is associated with stigma and shame [46].
Strengths
Routine electronic health record databases provide up-to-date information on the current population makeup not available from national census records [47]. Furthermore, routinely recorded smoking data in a UK primary care database similar to our own (The Health Improvement Network database) has been validated against the Health Survey for England, confirming that GP-recorded data are of high enough quality to produce robust research findings [48].
The south and east London populations that are the subject of this study come from unselected and contiguous general practice lists of more than 1 million people that include 97% of the resident population in these areas [49]. The findings are relevant to other urban areas with high ethnic and social diversity.
Limitations
This study was unable to account for factors likely to influence smoking behaviour but not captured in the electronic patient record. These include education and employment status. These factors were to some extent represented in the IMD, which includes domains on employment and education [50].
Although self-reported religion and country of birth are captured in the primary record, these variables were not complete enough to be used without considerably reducing the number of complete cases available for analysis. These indicators would have allowed for better analysis of the role of migration and generational status in smoking behaviour.
We did not account for the influence of GP practice on the relationship between ethnic density and smoking behaviour. This was because patients in the same LSOAs do not all register with the same general practices. LSOAs could therefore not be used as a reliable indicator of general practice registration. GP interventions in smoking cessation may vary significantly between practices and this may lead to important differences in smoking behaviours between patient populations. Statistical methods such as cross-classified analysis would be well suited to exploring this relationship further [51, 52].
Recommendations
These novel findings of an association between higher own-group ethnic density and lower odds of current smoking point to a complex relationship between culture, neighbourhood-level experience of adversity or social support and smoking behaviour.
Recognising 1) that smoking prevalence is lower among ethnic minority groups and 2) that the odds of smoking are reduced further as ethnic density increases, findings from this study will allow us to consider whether smoking cessation services should be tailored differentially to individuals/groups living in relative ethnic isolation. It is possible that such individuals do not benefit from the potential cultural/social factors and support mechanisms characteristic of areas with high levels of own-group ethnic density, which may act to reduce tobacco consumption. At the same time, the influence of higher ethnic density on the likelihood of under-reporting smoking behaviour, particularly for women, should also be taken into consideration when designing these services.
Future work elucidating the relationship between ethnicity, ethnic density and smoking will benefit greatly from the linkage of primary care data with genetic and biological data, as in the UK Biobank and other similar studies. This will allow for studies that better characterise ethnic differences in propensity to smoke, as well as responses to smoking cessation strategies and medications, utilising a combination of genetic, biological and lifestyle information.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00130-2016_Supplement (718.8KB, pdf)
Acknowledgements
Thanks to Robert Walton (Centre for Primary Care and Public Health, Blizard Institute, London, UK) for his advice.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Support statement: The project was supported by a Curriers' Company Millennium Healthcare Bursary.
Conflict of interest: None declared.
References
- 1.Public Health England. Health Profiles – August 2016. www.gov.uk/government/statistics/2016-health-profiles Date last accessed: November 11, 2016.
- 2.Health & Social Care Information Centre. Statistics on Smoking. England, 2016. http://content.digital.nhs.uk/catalogue/PUB20781/stat-smok-eng-2016-rep.pdf Date last accessed: May 20, 2016.
- 3.Karlsen S, Millward D, Sandford A. Investigating ethnic differences in current cigarette smoking over time using the health surveys for England. Eur J Public Health 2012; 22: 254–256. [DOI] [PubMed] [Google Scholar]
- 4.Martin A, Badrick E, Mathur R, et al. Effect of ethnicity on the prevalence, severity, and management of COPD in general practice. Br J Gen Pract 2012; 62: e76–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilkes A, Ashworth M, Schofield P, et al. Does COPD risk vary by ethnicity? A retrospective cross-sectional study. Int J Chron Obstruct Pulmon Dis 2016; 11: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson CR, Hippisley-Cox J, Sheikh A. Trends in the epidemiology of smoking recorded in UK general practice. Br J Gen Pract 2010; 60: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield P, Ashworth M, Jones R. Ethnic isolation and psychosis: re-examining the ethnic density effect. Psychol Med 2011; 41: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 8.Das-Munshi J, Bécares L, Boydell JE, et al. Ethnic density as a buffer for psychotic experiences: findings from a national survey (EMPIRIC). Br J Psychiatry 2012; 201: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bécares L, Nazroo J, Stafford M. The buffering effects of ethnic density on experienced racism and health. Health Place 2009; 15: 670–678. [DOI] [PubMed] [Google Scholar]
- 10.Shaw RJ, Atkin K, Bécares L, et al. Impact of ethnic density on adult mental disorders: narrative review. Br J Psychiatry 2012; 201: 11–19. [DOI] [PubMed] [Google Scholar]
- 11.Schofield P, Das-Munshi J, Mathur R, et al. Does depression diagnosis and antidepressant prescribing vary by location? Analysis of ethnic density associations using a large primary-care dataset. Psychol Med 2016; 46: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das-Munshi J, Becares L, Dewey ME, et al. Understanding the effect of ethnic density on mental health: multi-level investigation of survey data from England. BMJ 2010; 341: c5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen M, Maloney TN. Latino residential isolation and the risk of obesity in Utah: the role of neighborhood socioeconomic, built-environmental, and subcultural context. J Immigr Minor Heal 2011; 13: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becares L, Shaw R, Nazroo J, et al. Ethnic density effects on physical morbidity, mortality, and health behaviors: a systematic review of the literature. Am J Public Health 2012; 102: e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dept of Health. Healthy Lives, Healthy People: A Tobacco Control Plan for England. 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213757/dh_124960.pdf Date last accessed: May 20, 2016.
- 16.National Institute for Health and Clinical Excellence. Stop smoking services: public health guideline. 2008. www.nice.org.uk/guidance/ph10/resources/stop-smoking-services-1996169822917 Date last accessed: June 16, 2016. [Google Scholar]
- 17.Mathur R, Hull SA, Boomla K, et al. Ethnic differences in primary care management of diabetes and cardiovascular disease in people with serious mental illness. Br J Gen Pract 2012; 62: e582–e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappuccio FP, Cook DG, Atkinson RW, et al. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart 1997; 78: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksen A, Tillin T, O'Connor L, et al. The impact of health behaviours on incident cardiovascular disease in Europeans and South Asians – a prospective analysis in the UK SABRE study. PLoS One 2015; 10: e0117364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyratzopoulos G, McElduff P, Heller RF, et al. Comparative levels and time trends in blood pressure, total cholesterol, body mass index and smoking among Caucasian and South-Asian participants of a UK primary-care based cardiovascular risk factor screening programme. BMC Public Health 2005; 5: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiss K, Lehnhardt J, Razum O. Factors associated with smoking in immigrants from non-western to western countries – what role does acculturation play? A systematic review. Tob Induc Dis 2015; 13: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An N, Cochran SD, Mays VM, et al. Influence of American acculturation on cigarette smoking behaviors among Asian American subpopulations in California. Nicotine Tob Res 2008; 10: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinidad DR, Pérez-stable EJ, Emery SL, et al. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res 2009; 11: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima M, Fukami T, Yamanaka H, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther 2006; 80: 282–297. [DOI] [PubMed] [Google Scholar]
- 25.Schoedel KA, Hoffmann EB, Rao Y, et al. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 2004; 14: 615–626. [DOI] [PubMed] [Google Scholar]
- 26.Park SL, Tiirikainen M, Patel Y, et al. Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risk of lung cancer and effect on their smoking intensity. Carcinogenesis 2016; 37: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derby KS, Cuthrell K, Caberto C, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev 2008; 17: 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu JJ, Davidson E, Bhopal R, et al. Adapting health promotion interventions for ethnic minority groups: a qualitative study. Health Promot Int 2016; 31: 325–334. [DOI] [PubMed] [Google Scholar]
- 29.Netto G, Bhopal R, Lederle N, et al. How can health promotion interventions be adapted for minority ethnic communities? Five principles for guiding the development of behavioural interventions. Health Promot Int 2010; 25: 248–257. [DOI] [PubMed] [Google Scholar]
- 30.White M, Bush J, Kai J, et al. Quitting smoking and experience of smoking cessation interventions among UK Bangladeshi and Pakistani adults: the views of community members and health professionals. J Epidemiol Community Health 2006; 60: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chisholm J. The Read clinical classification. BMJ 1990; 300: 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomis. Census 2011 KS201EW (Ethnic group): Nomis – Official Labour Market Statistics. www.nomisweb.co.uk/census/2011/ks201ew Date last accessed: September 14, 2016.
- 33.Tower Hamlets Council. Ethnicity in Tower Hamlets: Analysis of 2011 Census data. www.towerhamlets.gov.uk/Documents/Borough_statistics/Ward_profiles/Census-2011/RB-Census2011-Ethnicity-2013-01.pdf Date last accessed: April 12, 2016.
- 34.Bhopal R, Unwin N, White M, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ 1999; 319: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Office for National Statistics. Super Output Areas (SOAs). Census. 2011. www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/census/super-output-areas--soas-/index.html Date last accessed: November 23, 2016.
- 36.Dept for Communities and Local Government. The English Indices of Deprivation 2010. www.communities.gov.uk/documents/statistics/pdf/1871208.pdf Date last accessed: May 20, 2016.
- 37.Office for National Statistics. 2011 Census – Population and Household Estimates for England and Wales, March 2011. www.ons.gov.uk/ons/dcp171778_270487.pdf Date last accessed: August 11, 2016.
- 38.Harrell FE. Regression Modeling Strategies. With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer, 2001. [Google Scholar]
- 39.Jena PK, Kishore J, Jahnavi G. Correlates of digit bias in self-reporting of cigarette per day (CPD) frequency: results from Global Adult Tobacco Survey (GATS), India and its implications. Asian Pac J Cancer Prev 2013; 14: 3865–3869. [DOI] [PubMed] [Google Scholar]
- 40.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014; 129: 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jawad M, Wilson A, Lee JT, et al. Prevalence and predictors of water pipe and cigarette smoking among secondary school students in London. Nicotine Tob Res 2013; 15: 2069–2075. [DOI] [PubMed] [Google Scholar]
- 42.Lock K, Adams E, Pilkington P, et al. Evaluating social and behavioural impacts of English smoke-free legislation in different ethnic and age groups: implications for reducing smoking-related health inequalities. Tob Control 2010; 19: 391–397. [DOI] [PubMed] [Google Scholar]
- 43.Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 2008; 165: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Argos M, Tong L, Pierce BL, et al. Genome-wide association study of smoking behaviours among Bangladeshi adults. J Med Genet 2014; 51: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith M, Ramsay C, Mazure C. Understanding disparities in subpopulations of women who smoke. Curr Addict Reports 2014; 1: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bush J, White M, Kai J, et al. Understanding influences on smoking in Bangladeshi and Pakistani adults: Community based, qualitative study. Br Med J 2003; 326: 962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aspinall PJ, Mitton L. Smoking prevalence and the changing risk profiles in the UK ethnic and migrant minority populations: implications for stop smoking services. Public Health 2014; 128: 297–306. [DOI] [PubMed] [Google Scholar]
- 48.Marston L, Carpenter JR, Walters KR, et al. The validity of routinely recorded smoking status in UK primary care: a cross-sectional study. BMJ Open 2014; 4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mindell J, Biddulph JP, Hirani V, et al. Cohort profile: the Health Survey for England. Int J Epidemiol 2012; 41: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 50.Dept for Communities and Local Government. The English Indices of Deprivation 2010. www.gov.uk/government/uploads/system/uploads/attachment_data/file/6320/1870718.pdf Date last accessed: July 23, 2016.
- 51.Dunn EC, Richmond TK, Milliren CE, et al. Using cross-classified multilevel models to disentangle school and neighborhood effects: an example focusing on smoking behaviors among adolescents in the United States. Heal Place 2015; 31: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein H. Multilevel cross-classified models. Sociol Methods Res 1994; 22: 364–375. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00130-2016_Supplement (718.8KB, pdf)