Abstract
Background and aims
Resistin has been associated with atherosclerotic inflammation and cardiovascular complications. We and others have previously shown that PKC-epsilon (PKCε) is involved in resistin-induced smooth muscle cell (VSMC) dysfunction at a high pathological concentration. This study aimed to evaluate the role and potential pathways of resistin at a physiological concentration, in atherosclerosis-related inflammation.
Methods
Plasma from patients with atherosclerosis was analyzed for resistin concentration. Patients were divided into tertiles based on resistin levels and cytokines were compared between tertiles. Macrophages were then treated with resistin in the presence or absence of PKCε inhibitor and/or TLR4 blocking-antibody, and their inflammatory state was evaluated with ELISA, RT-PCR, immunocytochemistry, and Western blot.
Results
We observed significant associations between plasma resistin levels and TNF-α, IL-6, IL-12, MIP-1α, MIP-1β, and CD40L. Our in vitro analyses revealed that resistin activated PKCε via TLR4. This was followed by NF-kB activation and induction of a pro-inflammatory phenotype in macrophages, significantly upregulating CD40, downregulating CD206 and stimulating gene expression and secretion of the inflammatory cytokines, for which we found association in our plasma analysis. Resistin also induced persistent TRAM and CD40L upregulation up to 36 hours after resistin treatment. PKCε and TLR4 inhibitors suppressed gene expression to levels similar to control, especially when used in combination.
Conclusions
Resistin, at a physiological concentration, exacerbates the inflammatory response of macrophages. PKCε is a key upstream mediator in resistin-induced inflammation that may interact synergistically with TLR4 to promote NF-kB activation, while TRAM is an important signal. PKCε and TRAM may represent novel molecular targets for resistin-associated chronic atherosclerotic inflammation.
Keywords: resistin, macrophage, cytokine, atherosclerosis, protein kinase C epsilon, toll-like receptor 4
Introduction
Resistin, an adipokine with a cysteine-rich C terminus, was originally thought to link obesity and diabetes due to its ability to ‘resist’ insulin action in murine adipocytes.1 However, further investigations revealed key differences between mouse and human resistin. In humans, resistin is mainly produced by monocytes and macrophages, primary inflammatory mediators in atherosclerosis.2, 3 Unlike in mice, human resistin is a 12.5 kDa peptide whose gene resides on chromosome 19, and it has different promoter regions, suggesting different regulatory mechanisms and tissue distribution.4 Thus, the specific function and underlying mechanisms of resistin action in human pathology are still debated.
Clinical observations indicate that resistin has important roles in cardiovascular disease and atherosclerotic inflammation. Recently, the Multi-Ethnic Study of Atherosclerosis (MESA), with over 1900 subjects, found a strong association between higher resistin levels and incidence of cardiovascular disease (CVD) and heart failure.5 Resistin has also been associated with coronary artery disease and linked to increased risk of CVD for diabetic patients6 and cardiovascular events in patients with different comorbidities.7–10 These clinical studies support resistin roles in atherosclerosis and its associated complications. However, the mechanistic insights have not yet been elucidated.
Human plasma resistin level was found to be positively correlated with the levels of tumor necrosis factor-a receptor-2 (TNFa-R2) and interleukin 6 (IL-6) in non-diabetic individuals.11 Moreover, several in vitro studies have investigated resistin’s atherogenic properties. Human recombinant resistin has been shown to increase adhesion molecules expression in endothelial cells, promoting monocyte attachment.12, 13 We and others have demonstrated that resistin promotes vascular smooth muscle cell migration, proliferation, and dedifferentiation, and we reported that protein kinase C epsilon (PKCε) mediates such processes.14–16 Resistin can also induce IL-6 and TNF-α productions in peripheral blood mononuclear cells (PBMCs)17 and promote inflammation in macrophages.18 Tarkowski et al. have proposed that toll like receptor 4 (TLR4) may serve as a binding protein for resistin.19 However, the specific pathways triggered by resistin to promote its inflammatory properties in macrophages remain uncertain. Additionally, the levels of resistin being used in these studies ranged from 25 ng/ml to 100 μg/ml, which are vastly different from physiological (clinically relevant) and pathological levels reported in patients with atherosclerosis and cardiovascular disease.5, 8, 11 This study aimed to elucidate the role and mechanistic pathways of resistin in atherosclerosis-related inflammation at a clinically relevant concentration. Hence, using a highly translational approach, we first evaluated patients with atherosclerosis for resistin levels and the relationship of resistin with other inflammatory cytokines in our patient population. We then chose a resistin concentration consistent with our cohort findings and explored the molecular mechanisms triggering resistin-mediated inflammation in macrophages. We focused on the role of upstream modulators, specifically PKCε, its interaction with TLR4, and in TLR4-related downstream pathways in macrophages.
Materials and methods
Human plasma analysis
This study was approved by the Stanford University Investigational Review Board and the Palo Alto VA Research and Development Committee (IRB 23476), and it conforms to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from each patient included in the study. Plasma samples were collected from 99 patients undergoing carotid surgery interventions following an established protocol. Samples were stored at −80 °C and analyzed with a Luminex magnetic bead-based assay for circulating cytokine levels at the Stanford University Human Immune Monitoring Center.
Monocytes isolation and culture
PBMCs were collected from buffy coats of healthy control male donors (Stanford Blood Center), age >20 using the Ficoll-Paque density gradient method. Then, cluster of differentiation 14 (CD14)-positive monocytes, were isolated using negative magnetic sorting (Stemcell Technologies). Population purity was assessed with flow cytometry by conjugating a sample of sorted cells to CD14-FITC antibody (Invitrogen). Cells were differentiated to macrophages during a 7-day culture in RPMI media supplemented with 10% FBS and 100 ng/mL of macrophage colony stimulating factor (PeproTech). Effective macrophage differentiation was confirmed by immunocytochemistry as described below. Differentiated macrophages were then treated with resistin.
In vitro cell treatments
Human recombinant resistin (PeproTech) was used to treat cells. To choose a treatment concentration, we first evaluated resistin’s ability to polarize human macrophages by testing concentrations representative of our patient population (2, 5, and 10 ng/ml, Figure 2A). Based on results from these studies, we chose 10 ng/ml for further in vitro experiments. For all experiments, except for time-specific evaluations, cells were treated for 18 hours based on literature for macrophage polarization and phenotype-priming studies.20–22 The PKCε-specific inhibitor εV1–2 (from Dr. Mochly-Rosen Laboratory, Stanford University) and an anti-toll like receptor 4 (TLR4) antibody (Abcam)23 were used for inhibitor and receptor-blocking studies, respectably. εV1–2 was used at 1 μM concentration;24 anti-TLR4 at 0.5 μg/ml;23 with a 30 min pre-treatment prior to the addition of resistin. The TLR4 inhibitor TAK-242 (EMD Millipore) was also used for confirmatory studies at 2 μM concentration, with a 30 min pre-treatment time.
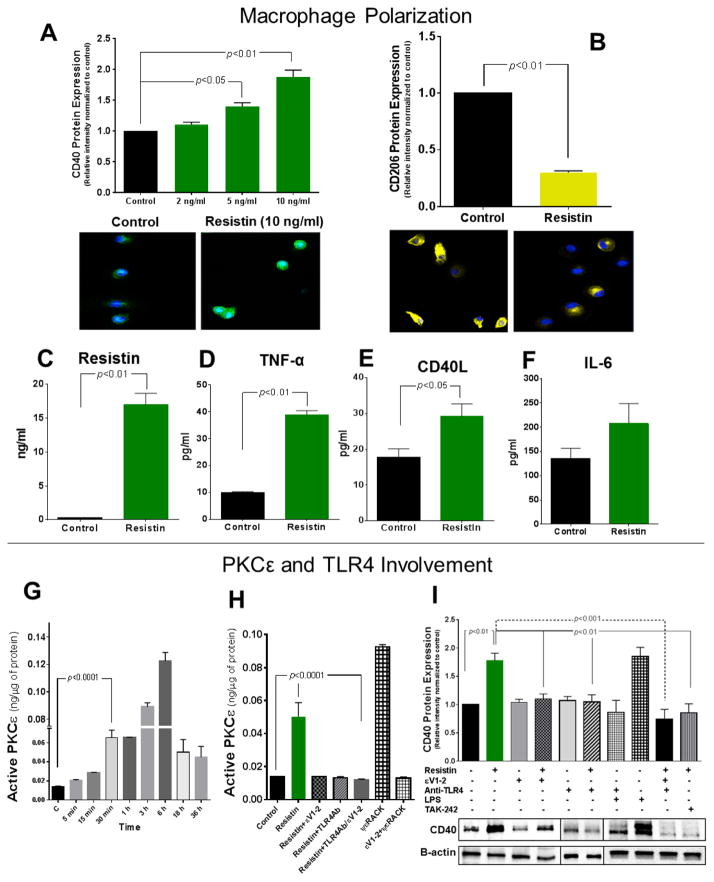
Fig. 2. Resistin induces a pro-inflammatory macrophage phenotype, activates PKCε via TLR4, and initiates a positive feedback loop in macrophages.
(A) Macrophages treated with resistin at various physiological levels, representative of our patient population, showed significant upregulation of CD40 expression after 18 hours of treatment, as demonstrated by Western blot (top panel) and immunofluorescence (bottom panel). (B) Resistin promoted decreased expression of CD206 at 10 ng/ml as analyzed by Western blot (top panel) and immunofluorescence (bottom panel). Resistin increased pro-inflammatory cytokine secretion of resistin (C), TNF-α (D), CD40L (E), and IL-6 (F), after 18 hours of 10 ng/ml treatment. (G) Specific PKCε activity was measured at various time points after 10 ng/ml of resistin treatment, and PKCε was significantly activated after 30 min of treatment. (H) Inhibition of TLR4 prevented PKCε activation (30 min time point). ψεRACK, a specific PKCε activator, was used as a positive control at 1 μM concentration. (I) PKCε inhibitor and TLR4-blocking prevent resistin-induced CD40 upregulation; LPS (100 ng/ml) was used as a positive control; and TAK-242, a TLR4 inhibitor, at 2 μM, was used to confirm TLR4 involvement in resistin-induced upregulation. Data are shown as mean + SEM of at least 4–12 independent experiments analyzed by one-way or two-way ANOVA.
Real-time polymerase chain reaction (RT-PCR)
Total RNA from macrophages was isolated using TRIzol according to standard protocols. SYBR green PCR master mix was used for real time PCR. Human primers are listed in Supplementary Table 1.25–29 RT-PCR was performed in a Mastercycler RT-PCR detection system (Eppendorf, Westbury, NY). The relative level of target (respective cytokine or key pathway modulators) gene in each group was normalized against internal housekeeping gene β-actin using the calculation formula of 2ΔCT(β-actin-target). The target gene levels in drug treated groups were further normalized against the control group.
Macrophage cytokine measurement
Conditioned media from macrophage cultures were analyzed using standard ELISA manufacturer’s protocols (Millipore, Invitrogen, and Thermo Scientific). Our analysis focused on macrophage pro-inflammatory cytokines that were found elevated in our patient population, were shown upregulated gene expression after resistin treatment, and have been proposed to play key pro-inflammatory roles in atherosclerosis. These included resistin, TNF-α, IL-6 and CD40L.
Fluorescent immunocytochemistry
Phenotypic signatures were evaluated with fluorescent immunocytochemistry. Briefly, cells were differentiated to macrophages as described above. After respective treatments, coverslips with macrophages were fixed, washed, and incubated with primary and fluorescence-conjugated secondary antibodies according to manufacturer’s recommendations and as previously described.30 Cells were visualized with laser scanning confocal microscope (ZEISS Confocal LSM-710) using DAPI staining as the focal reference point. Co-expression CD68 and CD11b was used as pan-macrophage marker, accounting for successful Macrophage differentiation. For the M1 subtype, we evaluated expression of cell surface markers CD40 and CD80; and for the M2 subtype, expression of CD163 and Mannose Receptor (CD206). At least 5 representative images (covering different areas of the coverslip) were recorded per treatment group. Mean fluorescence intensity (MFI) was measured using ImageJ and ZEN 2010 6.0 software packages, and relative MFI for each maker was compared among groups.
PKC activity
Total cell lysates were collected and the quantification of PKCε-specific activity was carried out with a PKC activity kit (Enzo Life sciences) according to the manufacturer’s instructions. Cell samples were assayed in the presence and absence of εV1–2 (1 μM) and the difference in values is used to calculate PKCε-specific activity. The assay was quantified with a spectrophotometric microplate reader (iMark, Bio-Rad) at a dual wavelength of 450/595 nm. Data is presented as amount of active PKCε ng/μg protein.
Western blot
Cell lysates (10 μg of protein) separated on a 4–20% polyacrylamide gel for electrophoresis were transferred onto nitrocellulose membranes using Bio-Rad Mini-Trans-Blot system. The membranes were blocked with 5% BSA in TBS with Tween-20 (TBST, 0.1% Tween-20) at room temperature. The blocked membrane was incubated with primary antibodies overnight at 4°C and with secondary antibody for 1 hour at RT. The immunoreactive bands were detected using a BIO-RAD chemiluminescence system, and the bands were captured and intensity quantified by with BIO-RAD ChemiDoc XRS+ camera and BIO-RAD Image Lab software respectively.
Statistical analysis
All in vitro experiments were performed independently at least four times in duplicate. Results are expressed as the mean +SEM. Statistical analyses were performed with THE GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) software package. Data sets were tested for parametric or non-parametric analysis, and the appropriate statistical test was selected. Dose-dependent data were compared by one-factor analysis of variance (ANOVA) followed by Dunnett’s test. Data comparing inhibitors effect, from PKCε inhibitor and TLR4-blocking experiments, on gene expression, protein expression, and resistin secretion, were analyzed using two-factor ANOVA followed by Tukey’s test. To compare differences between tertiles in cytokine levels and comorbidity distributions, categorical variables were analyzed with the Chi-square test and continuous variables with ANOVA, followed by Bonferroni correction for the multiple-comparisons test. Statistical significance was considered if the p-value was <0.05.
Results
Clinical characteristics and medical comorbidities in patients with varying levels of resistin
Arterial blood plasma from 99 male patients who underwent carotid interventions with a mean age of 69.3 years were analyzed. Medical comorbidities included hypertension (94%), diabetes (42%), CAD (49%), and obesity (42%), and most patients had a history of smoking (83%). Subjects were divided into tertiles (n=33 each) based on plasma resistin concentration (mean, range): low (2257.5 pg/ml, 1031.6 pg/ml – 3416.3 pg/ml), medium (4225.2 pg/ml, 3420.6 pg/ml – 4874.2 pg/ml), and high (7285.5 pg/ml, 4968.4 – 13266.5 pg/ml). The distribution of comorbidities, pre-operative symptoms, and medications was similar within each tertile (Table 1).
Table 1.
Distribution of comorbidities and risk factors in resistin tertiles.
| Variables | 1st tertile, n =33 (Low) | 2nd tertile, n=33 (Medium) | 3rd tertile, n=33 (High) | p-value |
|---|---|---|---|---|
| Age, average yrs (range) | 68 (54–84) | 70 (59–87) | 68 (55–91) | 0.24 |
| Procedure (CAS/CEA) | 16/17 | 22/11 | 18/15 | 0.32 |
| Risk factors and comorbidities | ||||
| History of smoking, n | 27 | 29 | 26 | 0.61 |
| Diabetes, n | 10 | 16 | 16 | 0.23 |
| Obesity, n | 9 | 16 | 17 | 0.09 |
| CAD, n | 18 | 12 | 19 | 0.18 |
| Hypertension, n | 29 | 31 | 33 | 0.20 |
| Preoperative symptoms | ||||
| Symptomatic, n | 16 | 18 | 13 | 0.46 |
| Prior stroke, n | 7 | 5 | 8 | 0.64 |
| Key medications | ||||
| Antiplatelets, n | 24 | 24 | 20 | 0.47 |
| Satins, n | 28 | 28 | 30 | 0.70 |
| Anticoagulants, n | 0 | 4 | 2 | 0.12 |
CAS, carotid artery stenting; CEA, carotid endarterectomy; CAD, coronary artery disease.
High resistin tertile exhibits higher levels of inflammatory cytokines/chemokines
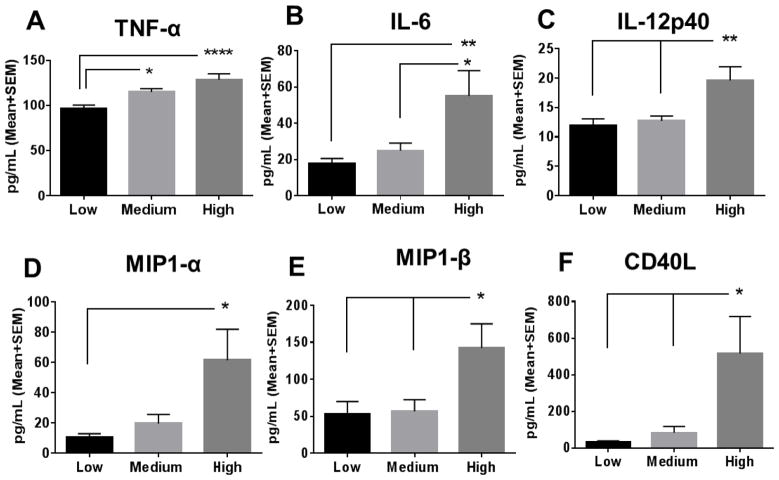
There was no significant difference in various growth factors (vascular endothelial growth factor A, macrophage colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor), neurotrophic factors (brain-derived neurotrophic factor and beta-nerve growth factor), or adhesion molecules (intercellular adhesion molecule 1 and vascular cell adhesion protein 1) between the tertiles. However, significantly higher levels of the pro-inflammatory cytokines/chemokines were observed in the high resistin tertile compared to the low tertile cohort including tumor necrosis factor alpha (TNF-α; p<0.001; Fig. 1A), interleukin-6 (IL-6; p <0.01; Fig. 1B), interleukin-12 subunit p40 (IL-12p40; p<0.01; Fig. 1C, macrophage inflammatory protein 1-alpha (MIP-1α; p<0.05; Fig. 1D), macrophage inflammatory protein 1-beta (MIP-1β; p<0.05; Fig. 1E), and soluble CD40 ligand (CD40L; p<0.05; Fig. 1F). Similarly, significantly higher levels of the aforementioned pro-inflammatory cytokines were observed in the high compared to the medium tertile: IL-6, p<0.05; IL-12p40, p<0.01; MIP-1β, p<0.05; and CD40L, p<0.05.
Fig. 1. Levels of inflammatory cytokines in patients with clinical atherosclerosis.
Subjects were divided in tertiles (n=33 each) based on resistin levels: low resistin (Low; mean=2257.5 pg/ml), medium resistin (Medium; mean =4225.2 pg/ml), and high resistin (High; mean=7285.5 pg/ml), and inflammatory cytokines were compared between tertiles. Significantly higher levels of (A) tumor necrosis factor alpha (TNF-α), (B) interleukin-6 (IL-6), (C) interleukin-12 subunit p40 (IL-12p40), (D) macrophage inflammatory protein 1-alpha (MIP-1α), (E) macrophage inflammatory protein 1-beta (MIP-1β), and (F) CD40 ligand (CD40L) were observed in the High tertile as compared to the Low tertile. Data are shown as mean + SEM, *p <0.05; **p<0.01; and ****p <0.0001.
Resistin promotes a pro-inflammatory phenotype in macrophages
We examined the direct effects of physiological levels of resistin on macrophage-mediated inflammation in vitro. To evaluate if resistin changes the relative polarization state of differentiated macrophages we used double markers to discern between the two end-of-the-spectrum macrophage states: M1 and M2. The M1 phenotype is typically associated with a pro-inflammatory state in macrophages, whereas the M2 phenotype is attributed to tissue repair activities. CD40 and CD80 have been observed to be upregulated in M1.31, 32 On the other hand, the mannose receptor (CD206) and CD163, a scavenger receptor, are generally attributed to the M2 phenotype.33, 34 First, macrophages were treated with resistin at a various resistin levels, representative of our patient population (2, 5, and 10 ng/ml) and examined for CD40 expression (Fig. 2A, top panel and Supplementary Fig. 1A). We observed that resistin dose dependently upregulated CD40 expression, and results at 10 ng/ml were verified with immunofluorescence (Fig. 2A, bottom panel). We therefore decided to use 10 ng/ml of resistin for our remaining experimental studies, which is within the high resistin tertile range (5–13 ng/ml) of our patient population. In addition, increased sections of key pro-inflammatory cytokines in macrophages including resistin itself (Fig. 2C), TNF-α (Fig. 2D), CD40L (Fig. 2E), and IL-6 (Fig. 2F) were observed following resistin treatment, while CD206 expression was downregulated as evidenced by Western blot results (Fig. 2B, top panel and Supplementary Fig. 1B) and immunofluorescence (Fig. 2B, bottom panel). However, not significant change in CD80 (Supplementary Fig. 1C) or CD163 (Supplementary Fig. 1D) was observed.
Resistin activates PKCe via TLR4 through a resistin-induced positive feedback loop
Resistin significantly increased PKCε activity as early as 30 min post treatment, and PKCε remained active throughout the time points investigated and peaking at 6 hours (Fig. 2G). As PKCε was significantly active at 30 min, we performed our inhibitory studies at this time point. We observed that blocking TLR4 or inhibiting PKCε with εV1–2 both prevented resistin-induced PKC activation (Fig. 2H). In addition, resistin-mediated CD40 upregulation was significantly inhibited by εV1–2 and by blocking TLR4 (Fig. 2I), reiterating the TLR4-PKCε connection in resistin-induced inflammation. Moreover, resistin treatment further increased resistin gene expression and protein secretion (Supplementary Fig. 2A and B), which was significantly higher than the treatment dose, suggesting a positive feedback loop. The increase in gene expression was time-dependent, peaking at 18 hours. Inhibition with εV1–2 and anti-TLR4 blocking mitigated gene upregulation and secretion of resistin (Supplementary Fig. 2A and B).
PKCe mediates resistin-induced upregulation of pro-inflammatory cytokine genes in macrophages
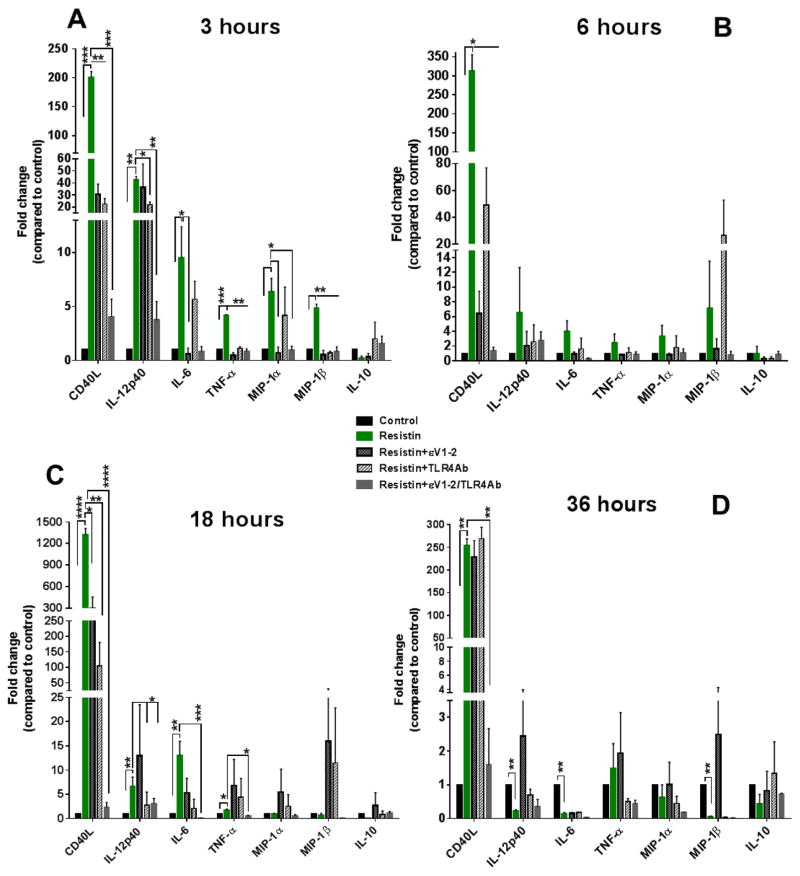
Resistin significantly increased gene expressions of CD40L, IL-12p40, IL-6, TNF-α, MIP-1α, MIP-1β after 3 hours of treatment at 10 ng/ml. The trend persisted at 6 hours and up to 18 hours for CD40L, IL-12p40, IL6, and TNF-1α (Fig. 3A–C). 36 hours after treatment, CD40L remained significantly elevated in the resistin-treated group (Fig. 3D). Our research group has shown that PKCε mediates resistin-induced vascular smooth muscle cell-migration,24 but its involvement in resistin-triggered macrophage activation had not been previously investigated. Thus, we used εV1–2, a PKCε specific inhibitor, to further study PKCε regulatory roles in macrophages. We observed that εV1–2 markedly inhibited resistin-induced cytokine gene upregulations (Fig. 3). In addition, we tested the involvement of TLR4, since it has been suggested as a receptor for resistin in mononuclear cells, 19 and we observed similar inhibitory trends as εV1–2 (Fig. 3). The relative suppression of resistin-induced upregulation of inflammatory cytokines varied for εV1–2 and TLR4-blocking, but it appeared to be more effective at the early time point of 3 hours. However, a combined inhibition of PKCε and TLR4 proved more effective, bringing cytokine/chemokine expression to levels similar to control.
Fig. 3. PKCe and TLR4 synergistically promote resistin-induced upregulation of pro-inflammatory cytokine genes in macrophages.
Resistin upregulated gene expression of inflammatory cytokines, which was examined after 3 hours (A), 6 hours (B), 18 hours (C), and 36 hours (D) of resistin treatment at 10 ng/ml. Inhibition of TRL4 or PKCε partially suppressed resistin-induced upregulation (A and C), but this was more effective when εV1–2/TLR4-blocking were used in combination. Resistin-treated cells were compared to control and to PKCε-inhibited/TLR4-blocked cells for the different cytokines. Data are shown as mean + SEM. *p<0.05; **p<0.01; ***p<0.001; and ****p<0.0001 by one-way or two-way ANOVA.
Resistin activates TLR4-mediated downstream pathways in macrophages
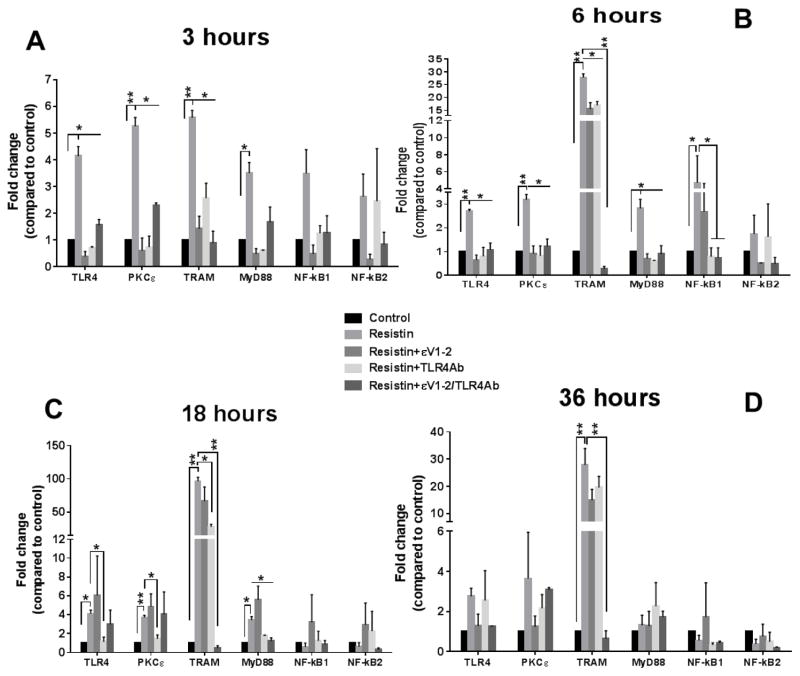
We observed significant increases in TLR4 and PKCε gene expressions at 3 hours (Fig. 4A), 6 hours (Fig. 4B), and 18 hours (Fig. 4C) following resistin treatment. Significant upregulations of TRIF-related adaptor molecule (TRAM) and myeloid differentiation primary response gene 88 (MYD88), well-known TLR4 downstream pathways, were also observed up to 18 hours; however, only TRAM upregulation was statistically significant after 36 hours of treatment (Fig. 4D). The PKCε inhibitor and TLR4 antibody suppressed resistin-induced gene upregulation of TLR4, PKCε, TRAM, and MYD88 at early time points, but they were less effective at later time points. Similarly to gene expression of cytokines, when both TLR4 was blocked and PKCε inhibited, gene expression returned to levels similar to control. The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) subunits showed a trend for upregulated expression in the early time points (3 and 6 hours), but only the NF-kB1 subunit was significant at 6 hours. In addition, we evaluated the protein expression of TLR4, PKCε, TRAM, and MYD88 after 18 hours of resistin treatment and observed similar trends (Supplementary Fig. 3). Finally, we tested NF-kB activation by evaluating IkBα phosphorylation in lysates from resistin-treated macrophages. Resistin induced increased IkBα phosphorylation, an indicator NF-kB activation (Supplementary Fig. 4A). When TLR4 or PKCε were inhibited in macrophages prior to resistin treatment, IkBα phosphorylation was prevented (Supplementary Fig. 4B), suggesting that TLR4 and PKCε mediate resistin-induced NF-kB activation.
Fig. 4. Resistin significantly upregulates TLR4 and PKCε gene expression as well as several downstream pro-inflammatory pathways in macrophages.
Resistin increased gene expression of TLR4, PKCε and key mediators of downstream inflammatory pathways after 3 hours (A), 6 hours (B), 18 hours (C), and 36 hours (D) of resistin treatment at 10 ng/ml. TRAM expression remained statistically significant after 36 hours of treatment (D). Inhibition of TRL4/PKCε prevented resistin-induced increased gene expression (A–D). Resistin-treated cells were compared to control and to PKCε-inhibited/TLR4-blocked cells for the different molecules/transcription factors. Data are shown as mean + SEM. *p<0.05 and **p<0.01 by one-way or two-way ANOVA.
Discussion
The specific roles of resistin in cardiovascular disease and atherosclerosis have long been debated. Although it has been suggested that resistin’s inflammatory properties promote its pathological consequences, the mechanisms and pathways remain uncertain. Furthermore, studies performed with super-pathological concentrations of resistin, which are far beyond the observed clinically relevant physiological ranges, have produced unclear results.18 To our knowledge, this is the first systematic study to evaluate the effects of resistin that are representative of a physiological level in patients with atherosclerosis. We validated the inflammatory property of resistin and identified several novel mechanistic interactions.
Reilly et al. were the first to propose that resistin serves as an atherosclerotic inflammatory marker after finding a positive association between plasma levels of resistin and TNF-α and IL-6 in the context of coronary atherosclerosis.13 Others have linked serum resistin to inflammatory activation in hypertension,35 metabolic syndrome,36 and rheumatoid arthritis.37 Comparing patients with similar comorbidities and demographics, we too observed significant associations between plasma resistin and TNF-α and IL-6. In addition, we identified several novel associations between resistin and IL-12, MIP-1α, MIP-1β, and CD40L, cytokines that are typically secreted by macrophages in their pro-inflammatory phenotype.38–40
Our observations in our patient cohort prompted a more detailed in vitro investigation into the role of resistin in the vasculature, its inflammatory stimuli, and the pathways involved. We utilized our clinical results to guide the selection of a clinically relevant resistin concentration for our in vitro studies and to select the markers and pathways to be investigated. Using a stepwise approach, we first evaluated macrophage phenotype changes, where we observed that resistin significantly induced CD40 upregulation and CD206 downregulation, suggesting pro-inflammatory transformation of macrophages.41 In addition, we also observed that resistin increased secretion of TNF-α, CD40L, and IL-6 and gene expression of CD40L, IL-12p40, IL-6, TNF-α, MIP-1α, and MIP-1β. These are well known, pro-inflammatory cytokines and markers, which further validate a pro-inflammatory macrophage transformation induced by resistin at high physiological levels. Although not significant, we observed a trend for downregulation for IL-10, an M2-related cytokine. These comprehensive evaluations prove, for the first time, that resistin has an independent inflammatory property that promotes macrophage pro-inflammatory polarization, inducing an inflammatory cascade of cytokines and chemokines. We also show for the first time that resistin induced further resistin expression and secretion in macrophages, possibly via a positive feedback loop. However, we cannot eliminate the possible contribution of other cytokines produced by resistin-treated macrophages to promote resistin expression and the overall inflammatory state of macrophages. Likewise, Shyu et al. have previously suggested that TNF-α induces resistin expression.42
Our previous studies have shown that PKCε mediates resistin-induced VSMC migration, proliferation, and dedifferentiation.16, 24 In this study, we observed that PKCε is a key mediator of resistin signaling for macrophage activation, suggesting that inhibiting PKCε has anti-inflammatory properties. Our study is in line with previous findings proposing that TLR4 participates in resistin-associated activity in macrophages.19 TLR4 signaling and interaction between CD40-CD40L have both been independently attributed to atherosclerotic plaque development and atherosclerosis-mediated inflammation.25, 43–45 Hoebe et al. have demonstrated that binding of lipopolysaccharides to TLR4 induced upregulation of co-stimulatory signals, including CD40 in macrophages via TRAM,46 and Lundberg et al. recently reported that TRAM signaling is crucial in TLR4-mediated atherosclerotic inflammation.47 Unique to this study, we observed persistent TRAM and CD40L upregulation up to 36 hours after resistin treatment, suggesting that TRAM-related downstream activation and CD40L expression mediate chronic cellular effects in macrophages. We suspect that the positive feedback mechanism triggered by resistin may contribute to continuous pro-inflammatory stimulation leading to the to100-fold increase in TRAM and up to 1400-fold increase in CD40L.
Studies have shown that PKCε is phosphorylated by TLR4 when signaling through MyD88 and TRAM.48, 49 Our results suggested that resistin may act through similar pathways to trigger pro-inflammatory properties in macrophages. First, we showed that it increased PKCε activity significantly as early as 30 min after treatment, and that this effect was prevented when TLR4 was blocked, indicating a sequential nature in PKCε activation. Second, we observed that resistin induced increased gene and protein expression of these downstream molecules (TRAM and MYD88) and that inhibition of PKCε/TLR4 blocks such effects. Finally, although resistin only moderately increased gene expression of NF-kB at early hours, it significantly triggered IkBα phosphorylation, an indicator of NF-kB activation, within an hour of treatment. IkBα holds NF-kB in the cytoplasm, but its phosphorylation releases NF-kB to translocate to the nucleus. NF-kB is a common transcription factor for TLR4-mediated inflammatory processes. Its activation indicates inflammatory transformation of macrophages. Overall, our findings suggest that resistin interacts with TLR4, activates PKCε within 30 min, and then elicits NF-kB activation to promote inflammation in macrophages, which further induces increased expression of pro-inflammatory cytokines and molecules.
There were a few unexpected observations in our study. We observed that TLR4 blocking and PKCε inhibition were more effective at early time points, and that each alone only partially blocked gene upregulations of TRAM and CD40L. When both TLR4 and PKCε were inhibited, expressions of TRAM and CD40L returned to control levels. These findings suggest that TLR4 and PKCε synergistically propagate resistin-mediated gene expression of inflammatory signals in macrophages. Finally, although we focused on inflammatory cascades in this study, we cannot ignore the possible connection between reactive oxygen species (ROS) production and inflammatory transformation of macrophages. We recently showed that resistin increased ROS production, NADPH oxidase (Nox) activity, and inflammatory cytokine secretion in VSMCs.16 We speculate that ROS production and Nox activity may also contribute to this pathway, and play important roles in the overall inflammatory cascades that are activated by resistin.
In conclusion, our study highlights the mechanistic insight in resistin-mediated atherosclerotic inflammation. Our findings, for the first time, suggest that PKCε is a key mediator for resistin-induced signaling and activation in macrophages. We also offer valid evidence that resistin at a physiological concentration has the potential to exacerbate the inflammatory response of macrophages, increasing cytokines and CD40/CD40L expression. Additionally, our findings allude to TRAM as an important downstream signaling molecule to induce NF-kB activation. Targeting resistin-mediated inflammation and its downstream signaling pathways may present a novel strategy in atherosclerosis prevention and management.
Supplementary Material
Highlights.
High plasma resistin levels are associated with high levels of inflammatory cytokines in patients with atherosclerosis.
Resistin induces a pro-inflammatory phenotype in macrophages via a TLR4 and PKCε.
Resistin stimulates NF-kB activity, significantly upregulating CD40, downregulating CD206 and stimulating gene/protein expression of inflammatory molecules and cytokines.
Resistin treatment further increased resistin gene expression and protein secretion, suggesting a positive feedback loop.
TRAM and CD40L are persistently upregulated up to 36 hours after resistin treatment.
Acknowledgments
Financial support
This work was supported by National Institutes of Health [grant number R01NS070308] and the United States Department of Veterans Affairs [grant number I01BX001398].
We thank Dr. Daria Mochly-Rosen (Stanford University) for providing the PKCε inhibitor and activator utilized in this investigation.
Abbreviations
- MΦ
macrophages
- PKCε
protein kinase C epsilon
- TLR4
toll-like receptor 4
- TRAM
TRIF-related adaptor molecule
- MyD88
myeloid differentiation primary response gene 88
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- CVD
cardiovascular disease
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1–5. doi: 10.1038/oby.2002.1. [DOI] [PubMed] [Google Scholar]
- 3.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 4.Yang R, Huang Q, Xu A, McLenithan JC, Eison JA, Shuldiner AR, Alkan S, Gong D. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 5.Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:101–108. doi: 10.1016/j.atherosclerosis.2014.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzaghi C, Bacci S, Salvemini L, Mendonca C, Palladino G, Fontana A, De Bonis C, Marucci A, Goheen E, Prudente S. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PloS One. 2013;8:e64729. doi: 10.1371/journal.pone.0064729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Koo BK, Cho SW, Kihara S, Funahashi T, Cho YM, Kim SY, Lee HK, Shimomura I, Park KS. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis. 2008;196:398–404. doi: 10.1016/j.atherosclerosis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Gencer B, Auer R, de Rekeneire N, Butler J, Kalogeropoulos A, Bauer DC, Kritchevsky SB, Miljkovic I, Vittinghoff E, Harris T. Association between resistin levels and cardiovascular disease events in older adults: the Health, Aging and Body composition study. Atherosclerosis. 2016;245:181–186. doi: 10.1016/j.atherosclerosis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weikert C, Westphal S, Berger K, Dierkes J, Mohlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 10.Watt KD, Fan C, Therneau T, Heimbach JK, Seaberg EC, Charlton MR. Serum adipokine and inflammatory markers before and after liver transplantation in recipients with major cardiovascular events. Liver Transpl. 2014;20:791–797. doi: 10.1002/lt.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 12.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine–endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 13.Hsu W, Chao Y, Tsai Y, Lien C, Chang C, Deng M, Ho L, Kwok CF, Juan C. Resistin induces monocyte–endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol. 2011;226:2181–2188. doi: 10.1002/jcp.22555. [DOI] [PubMed] [Google Scholar]
- 14.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 15.Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase Cε mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg. 2011;53:1044–1051. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghuraman G, Zuniga MC, Yuan H, Zhou W. PKCε mediates resistin-induced NADPH oxidase activation and inflammation leading to smooth muscle cell dysfunction and intimal hyperplasia. Atherosclerosis. 2016;253:29–37. doi: 10.1016/j.atherosclerosis.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 18.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 19.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjiu J, Chen J, Shun C, Lin S, Liao Y, Chu C, Tsai T, Chiu H, Dai Y, Inoue H. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 21.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, Van Zoelen MA, Nacken W, Foell D, Van der Poll T, Sorg C. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 24.Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase Cε mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg. 2011;53:1044–1051. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Bryan JL, DeLassus E, Chang LW, Liao W, Sandell LJ. CCAAT/enhancer-binding protein beta and NF-kappaB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1beta. J Biol Chem. 2010;285:33092–33103. doi: 10.1074/jbc.M110.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen H, Van Essen P, Koenen T, Joosten L, Netea M, Tack C, Stienstra R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153:5866–5874. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 30.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35-2094-10-35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281:51–61. doi: 10.1016/j.cellimm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos, Chris M, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Fang C, Lei J, Zhou SX, Zhang YL, Yuan GY, Wang JF. Association of higher resistin levels with inflammatory activation and endothelial dysfunction in patients with essential hypertension. Chin Med J (Engl) 2013;126:646–649. [PubMed] [Google Scholar]
- 36.Aquilante CL, Kosmiski LA, Knutsen SD, Zineh I. Relationship between plasma resistin concentrations, inflammatory chemokines, and components of the metabolic syndrome in adults. Metab Clin Exp. 2008;57:494–501. doi: 10.1016/j.metabol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Dessein PH, Norton GR, Woodiwiss AJ, Solomon A. Independent relationship between circulating resistin concentrations and endothelial activation in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1586–1588. doi: 10.1136/annrheumdis-2013-203587. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 40.Zuniga MC, White SL, Zhou W. Design and utilization of macrophage and vascular smooth muscle cell co-culture systems in atherosclerotic cardiovascular disease investigation. Vasc Med. 2014;19:394–406. doi: 10.1177/1358863X14550542. [DOI] [PubMed] [Google Scholar]
- 41.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2007;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 42.Shyu K, Chua S, Wang B, Kuan P. Mechanism of inhibitory effect of atorvastatin on resistin expression induced by tumor necrosis factor-α in macrophages. J Biomed Sci. 2009;16:1. doi: 10.1186/1423-0127-16-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 44.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 45.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 46.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 47.Lundberg AM, Ketelhuth DF, Johansson ME, Gerdes N, Liu S, Yamamoto M, Akira S, Hansson GK. Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc Res. 2013;99:364–373. doi: 10.1093/cvr/cvt033. [DOI] [PubMed] [Google Scholar]
- 48.Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold MyD88 acts to couple protein kinase Cε to Toll-like receptors. J Biol Chem. 2008;283:18591–18600. doi: 10.1074/jbc.M710330200. [DOI] [PubMed] [Google Scholar]
- 49.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O’Neill LA. Trif-related adapter molecule is phosphorylated by PKC{epsilon} during Toll-like receptor 4 signaling. Proc Natl Acad Sci USA. 2006;103:9196–9201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.