Abstract
Background
Although studies of oral immunotherapy (OIT) for food allergy have shown promise, treatment is frequently complicated by adverse reactions and, even when successful, has limited long-term efficacy as benefits usually diminish when treatment is discontinued.
Objective
We sought to examine whether the addition of omalizumab to milk OIT (MOIT) reduces treatment-related reactions and/or improves outcomes.
Methods
This was a double-blind placebo-controlled trial with subjects randomized to omalizumab or placebo. Open-label MOIT was initiated after 4 months of omalizumab/placebo with escalation to maintenance over 22–40 weeks, followed by daily maintenance dosing through month-28. At month-28, omalizumab was discontinued and subjects passing an oral food challenge (OFC) continued OIT for 8 weeks, after which OIT was discontinued with re-challenge at month-32 to assess sustained unresponsiveness (SU).
Results
Fifty-seven subjects (7–32 years) were randomized, with no significant baseline differences in age, milk-specific IgE, skin tests, or OFCs. At month-28, 24 (88.9%) omalizumab-treated subjects and 20 (71.4%) placebo-treated subjects passed the 10 gram “desensitization” OFC (p=0.18). At month-32, SU was demonstrated in 48.1% in the omalizumab group and 35.7% in the placebo group (p=0.42). Adverse reactions were markedly reduced during OIT escalation in omalizumab subjects for percent doses/subject provoking symptoms (2.1% versus 16.1%; p=0.0005), dose-related reactions requiring treatment (0.0% versus 3.8%, p=0.0008), and doses required to achieve maintenance (198 versus 225; p=0.008).
Conclusions
In this first randomized double-blinded placebo-controlled trial of omalizumab in combination with food OIT, we found significant improvements in measurements of safety, but not in outcomes of efficacy (desensitization and SU).
Trial Registration
OIT and XolairR (Omalizumab) in Cow’s Milk Allergy, NCT01157117, http://clinicaltrials.gov/show/NCT01157117
Keywords: Milk allergy, Oral immunotherapy, Omalizumab, Desensitization, Sustained unresponsiveness, Double-blind placebo-controlled trial, Dose-related adverse reactions
Introduction
Cow’s milk allergy (CMA) is the most common food allergy in young children, with an estimated prevalence of 2–3%1,2. While CMA is outgrown in most children, it persists in a subset into adulthood, often in a severe form3,4. The mainstay of treatment is avoidance, which is difficult given the ubiquitous nature of milk, leading to frequent and often severe reactions5,6. Oral immunotherapy (OIT) is under investigation for the treatment of persistent food allergy, with overall encouraging results. However, as with egg and peanut OIT, milk OIT (MOIT) has been associated with significant risks, with most subjects experiencing at least mild symptoms, some experiencing anaphylaxis, and 10–20% forced to discontinue due to adverse reactions7–11. In addition to the high rate of adverse reactions, implementation of OIT into clinical practice has been further constrained by the fact that the response to treatment is typically not sustained once therapy is discontinued10,12–16.
To mitigate the risks of OIT, and potentially enhance its efficacy, adjunctive treatment with omalizumab was investigated. Omalizumab is a recombinant humanized anti-IgE antibody, currently FDA-approved for treatment of allergic asthma and chronic urticaria, that has been shown to reduce adverse reactions with aeroallergen immunotherapy17,18, and suggested to have similar beneficial effects in small pilot studies of milk and peanut OIT19,20. In this study, subjects were randomized to receive omalizumab or placebo in combination with MOIT, with a primary endpoint of sustained unresponsiveness (SU), defined as persistence of desensitization after discontinuation of treatment. We hypothesized that the addition of omalizumab would result in significantly fewer adverse reactions, allow for more rapid dose escalation, and potentially enhance the development of SU by decreasing IgE-facilitated allergen presentation by IgE-bearing antigen-presenting cells 21–24 and facilitated uptake by IgE-bearing gut epithelial cells 25; thus reducing Th2 responses and increasing Th1 and T-regulatory cell responses.
METHODS
STUDY DESIGN
Subjects 7–35 years of age with a history of IgE-mediated CMA were recruited at 3 sites (Mount Sinai, Johns Hopkins, and Stanford). CMA was confirmed by (1) positive milk skin prick test (SPT, wheal ≥3mm greater than the negative control) or milk-specific IgE (>0.35 kUA/l) and (2) double-blind placebo-controlled OFC reactive to <2 grams of milk protein. Patients were excluded if there was history of life-threatening milk-induced anaphylaxis, i.e. cardiopulmonary failure, uncontrolled or severe persistent asthma, baseline FEV1 <80% predicted, or use of biologic therapy within one year. The study was approved by local IRBs and informed consent/assent was obtained on all subjects.
Subjects were randomized 1:1 to receive omalizumab or placebo. If the subject’s dose did not fall within the omalizumab package insert dosing chart, a formula (0.016mg/kg/IgE IU) provided by Genentech was utilized, and subjects having a weight-IgE combination that required a dose greater than 750mg were excluded. The first 16 months of the study consisted of blinded treatment with omalizumab/placebo injections every 2 or 4 weeks.
Open-label MOIT dosing was administered using nonfat dry powdered milk [Supplemental Table 1]. MOIT began two weeks after month 4 of omalizumab/placebo (i.e., 4 or 8 doses). All dose escalations were conducted under physician supervision. On the first day, dosing was initiated with 0.07mg of milk protein. In order to continue in the study, subjects were required to tolerate dose #6 (2.1mg milk protein) at dosing visit 1 and subsequently reach dose #8 (9mg) by visit 2. Daily home dosing was continued at the highest dose tolerated on the dose escalation day, after which subjects then returned every 2 weeks for dose escalation for a minimum of 22 weeks and a maximum of 40 weeks. Subjects were required to reach a minimum maintenance dose of 520mg milk protein (equivalent to 15mL of liquid milk). The goal maintenance dose was originally 3.3 grams, but was increased to 3.8 grams midway through the study when delivery was converted from pre-measured vials to a more economical bulk supply and a scoop. After 12 months of MOIT, treatment was unblinded, and omalizumab was continued for an additional 12 months in the active group, and injections were discontinued in placebo participants. At month 28, omalizumab was discontinued, and all subjects underwent a 10-gram OFC. Subjects passing this challenge received an additional 8 weeks of MOIT, then discontinued treatment for 8 weeks, followed by a final 10-gram OFC. Strict milk avoidance was maintained throughout the protocol, including the 8 weeks off therapy before the final OFC.
STUDY PROCEDURES
Double-Blind, Placebo-Controlled Milk Challenges
OFCs were conducted using placebo (corn starch) or milk powder, with cumulative doses of 2grams of milk protein for screening challenges and 10grams for post-treatment challenges. Each OFC was divided into 7 doses given at 15 minute intervals: 1%, 4%, 10%, 20%, 20%, and 25%; i.e. for the 2gram OFC, equivalent to 20, 80, 200, 400, 400, and 500 mg of milk protein; and for the 10gram OFC, equivalent to 100, 400, 1000, 2000, 2000, and 2500 mg of milk protein. Challenges were considered positive based upon clear objective symptoms (e.g. diffuse hives, vomiting, or wheezing), or marked subjective symptoms (e.g. severe persistent abdominal pain).
Endpoint titration skin prick testing
Endpoint titration SPTs were performed at baseline and months-28 and -32 using serial ten-fold dilutions of milk extract [Greer Lab; Lenoir, NC] utilizing the Greer Pick system. The starting concentration was the standard milk extract (1:20 wt/vol) with serial 10-fold dilutions (1:200, 1:2000, 1:20,000, 1:200,000 wt/vol).
Immunologic Assessments
In vitro immunologic assessments at baseline and months 4, 16, 22, 28, 30, and 32. Basophil activation was measured by CD63 up-regulation using flow cytometry, as previously described.26 Measurement of specific IgE, IgG, and IgG4 to milk, casein and β-lactoglobulin were performed utilizing ImmunCAPR (ThermoFisher Scientific, Inc)
Statistical Analysis
The primary endpoint was the comparison of subjects treated with omalizumab versus placebo in the development of SU, defined as the absence of dose-limiting symptoms in both the month-28 and -32 OFCs, i.e. SU measured after 8 weeks off of MOIT. Secondary endpoints included the percentage of subjects successfully desensitized (defined by the absence of dose-limiting symptoms in the month-28 OFC), change in OFC successfully consumed dose (SCD), incidence of MOIT-related adverse reactions and severe allergic reactions, time and number of doses to achieve maintenance, and changes in milk-specific IgE, IgG, IgG4, SPTs, and basophil activation.
Differences between groups in continuous variables were assessed using Wilcoxon rank-sum tests and changes from baseline within groups using signed-rank tests. Differences between groups in categorical data were examined using Fisher’s Exact test. Endpoint SPT titration was calculated as the sum of the wheals at each of the 5 dilutions, assessed using the Wilcoxon rank-sum test. Basophil activation and immunoglobulin levels were each evaluated in repeated-measurement models, with the baseline value, study visit, and treatment group as covariates. Hypothesis testing was performed using log 10 transformation for IgE, IgG, and IgG4, while summary statistics are reported on the observed scale. Exploratory logistic regression was used to identify baseline and month-16 clinical and immunologic variables that may predict month-28 and -32 OFC outcomes. No p-value adjustments were made for multiple comparisons, but a significance level of 0.01 was used for immunologic response. All analyses were performed with SAS software, v9.3 (SAS Institute).
RESULTS
Study Population
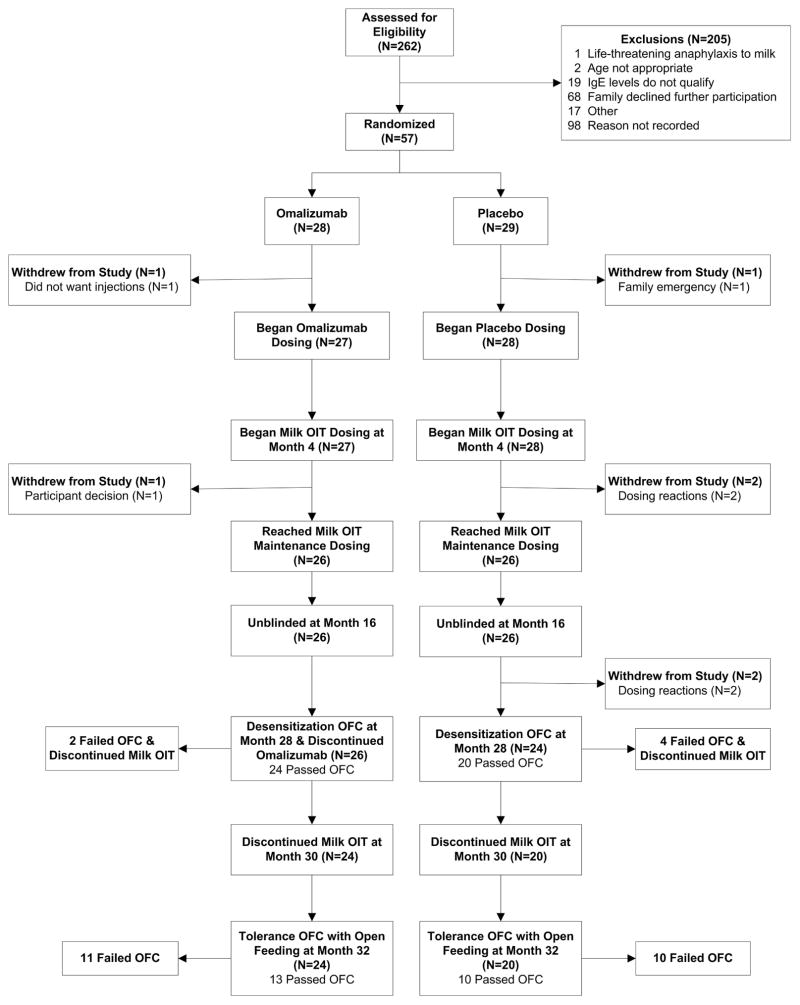
Fifty-seven subjects (7–32 years) were randomized between October, 2010 and April, 2012 (Mount Sinai-29, Johns Hopkins-23, and Stanford-5) [Figure 1]. There were no significant differences between the treatment groups for any baseline characteristic, including age (omalizumab versus placebo, median 11.7 versus 9.5 years), asthma, other food allergies, milk specific-IgE (median 42.1 versus 38.4 kUA/L), milk SPT (median wheal 8.8 versus 8.5mm), or milk OFC eliciting dose (median SCD: 20 versus 10mg) [Table 1].
Figure 1. Consort Diagram.
On the first day, dosing was initiated with 0.07mg of milk protein. Subjects were required to tolerate dose #6 (2.1mg) at dosing visit 1 and dose #8 (9mg) by visit 2. Daily home dosing was continued and subjects returned every 2 weeks for dose escalation for a minimum of 22 and a maximum of 40 weeks. Subjects were required to reach a minimum maintenance dose of 520mg milk protein (equivalent to 15mL of liquid milk).
Table 1.
Subject Demographics
| Characteristic | Omalizumab (n=28) | Placebo (n=29) |
|---|---|---|
| Male sex, no. (%) | 20 (71) | 20 (69) |
| Age (y), median (IQR) | 11.7(9.5–15.0) | 9.5 (8.0–13.2) |
| White race, no. (%) | 23 (82) | 26 (90) |
| Additional food allergies, no. (%) | 21 (75) | 19 (66) |
| Asthma, no. (%) | 20 (71) | 22 (76) |
| Allergic rhinitis, no. (%) | 21 (75) | 23 (79) |
| Atopic dermatitis, no. (%) | 12 (43) | 11 (38) |
| Baseline milk IgE (kUA/L), median (IQR) | 42.1 (9.4–83.0) | 38.4 (12.2–66.2) |
| Baseline milk skin prick test score (mm), median (IQR) | 8.8 (6.3–11.0) | 8.5 (6.5–11.0) |
| Baseline OFC Successfully Consumed Dose (mg), median (IQR) | 20 (0–100) | 10 (0–100) |
| Omalizumab dose (IU), median (IQR) | 300 (225–300) | --- |
Includes egg, peanut, tree nuts, sunflower seed, sesame seed, shellfish, fish, mustard, lamb, beef, pork, wheat.
All characteristics were not statistically significantly different between two treatment groups.
Clinical Outcomes
Significantly fewer MOIT doses were required to achieve maintenance in the omalizumab group (median: 198.0 versus 225; p=0.008), resulting in a shorter escalation phase (median: 25.9 versus 30.0 weeks; p=0.01). At month-28, 24 (88.9%) omalizumab-treated subjects and 20 (71.4%) placebo-treated subjects passed the 10 gram “desensitization” OFC (p=0.18) [Table 2]. At month-32 (16 weeks off omalizumab, 8 weeks off MOIT), SU was demonstrated in 13 (48.1%) in the omalizumab group and 10 (35.7%) in the placebo group (p=0.42). There were no significant differences in the median SCD for the month-28 or -32 OFCs (p=0.37 and 0.81, respectively) [Supplemental Table 2], nor in the degree of protection lost between months-28 and -32. For those losing protection, the median decrease in SCD between months-28 and -32 was 4,500mg for both groups. Clinically, this ranged from minimal loss of protection (e.g. mild reaction at the final dose), to nearly complete return of reactivity, with one omalizumab-treated subject requiring epinephrine at the first dose (100mg), after passing the month-28 OFC without symptoms.
Table 2.
Results of 28 and 32 month OFCs
| Treatment | |||||
|---|---|---|---|---|---|
| Omalizumab | Placebo | ||||
| N | % | N | % | P-value* | |
| Total Subjects with Omalizumab/Placebo Dosing | 27 | 100.0 | 28 | 100.0 | - |
| Month 28 10 gram Desensitization OFC | 24 | 88.9 | 20 | 71.4 | 0.18 |
| Success – Passed OFC | |||||
| Failure – Failed OFC | 2 | 7.4 | 4 | 14.3 | |
| Failure – Withdrew prior to OFC | 1 | 3.7 | 4 | 14.3 | |
| Month 32 10 gram Tolerance OFC** | 13 | 48.1 | 10 | 35.7 | 0.42 |
| Success – Passed OFC | |||||
| Failure – Failed OFC | 11 | 40.7 | 10 | 35.7 | |
| Failure – Did not complete OFC | 3 | 11.1 | 8 | 28.6 | |
P-value is for the comparison between treatment groups of the proportion of successes where both types of failures (failed OFC and withdrew prior to OFC/did not complete OFC) are combined for the analysis.
Re-analysis of the primary endpoint (Month 32 OFC) to include the 2 subjects who received no omalizumab/placebo treatment yielded the same P value.
Seven randomized subjects withdrew from the study [Figure 1]; 2 (7.4%) omalizumab vs 5 (17.8%) placebo subjects. Two subjects (one omalizumab/one placebo) withdrew prior to receiving any treatment (considered failures for the primary endpoint and excluded from other analyses); one omalizumab subject withdrew during escalation for family reasons; and 2 placebo subjects withdrew during escalation due to adverse reactions, one with frequent acute reactions and the other with abdominal pain and vomiting, diagnosed with eosinophilic esophagitis a month after discontinuing MOIT. Two additional placebo subjects withdrew during maintenance due to dosing symptoms, one with recurrent abdominal pain and one with intermittent acute reactions.
Safety Assessments
The overall percent of symptom-free doses during escalation was 91.5% among omalizumab subjects and 73.9% for placebo subjects (p<0.0001) [Supplemental Table 3]. The median percent of doses per subject with any symptoms was 2.1% and 16.1%, respectively (omalizumab versus placebo, p=0.0005), with significant differences occurring for all symptom categories except severe [Table 3]. Significant differences were also seen for dose-related reactions needing treatment (median: 0.0% versus 3.8% of doses per subject, p=0.0008) and severity grade of allergic reactions (mild: median 0.5% versus 7.9% doses per subject; p=0.0001; moderate: median: 0.0% versus 0.5% doses per subject; p=0.0005). Significant reductions in most safety parameters persisted into the maintenance phase for omalizumab-treated subjects [Table 4 and Supplemental Table 4].
Table 3.
Percent Doses per Subject with Dosing Symptoms during Escalation Period
| Treatment Group | |||||||
|---|---|---|---|---|---|---|---|
| Omalizumab (n=27) | Placebo (n=28) | ||||||
| Median | Lower Quartile | Upper Quartile | Median | Lower Quartile | Upper Quartile | P-value | |
| Total # Doses | 198.0 | 190.0 | 209.0 | 225.0 | 200.0 | 239.0 | 0.008 |
| Any Symptoms | 2.1 | 0.5 | 12.0 | 16.1 | 7.8 | 38.9 | 0.0005 |
| Any Symptoms Excluding Oral/Pharyngeal | 0.5 | 0.0 | 3.3 | 8.6 | 4.1 | 18.9 | 0.0001 |
| Duration >30 min. | 0.4 | 0.0 | 1.0 | 3.0 | 1.2 | 4.3 | 0.0001 |
| Treatment Used | 0.0 | 0.0 | 1.6 | 3.8 | 1.5 | 5.8 | 0.0008 |
| Oral/Pharyngeal Symptoms | 0.6 | 0.0 | 10.9 | 8.8 | 2.7 | 29.5 | 0.0025 |
| Skin Symptoms | 0.0 | 0.0 | 0.5 | 1.1 | 0.6 | 2.5 | 0.0004 |
| Respiratory Symptoms | 0.0 | 0.0 | 1.4 | 2.5 | 1.7 | 4.8 | <.0001 |
| GI Symptoms | 0.0 | 0.0 | 1.9 | 3.0 | 1.2 | 7.3 | 0.001 |
| Other Symptoms | 0.0 | 0.0 | 0.5 | 1.4 | 0.2 | 4.0 | 0.008 |
| Mild Symptoms | 0.5 | 0.0 | 3.3 | 7.9 | 3.5 | 18.1 | 0.0001 |
| Moderate Symptoms | 0.0 | 0.0 | 0.0 | 0.5 | 0.2 | 1.3 | 0.0005 |
| Severe Symptoms | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.35 |
| Treated with Epinephrine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.052 |
Table 4.
Percent Doses per Subject with Dosing Symptoms During Maintenance Period
| Treatment Group | |||||||
|---|---|---|---|---|---|---|---|
| Omalizumab (n=27) | Placebo (n=28) | ||||||
| Median | Lower Quartile | Upper Quartile | Median | Lower Quartile | Upper Quartile | P-value | |
| Total # Doses | 601.0 | 576.0 | 614.0 | 563.5 | 510.0 | 593.0 | 0.003 |
| Any Symptoms | 0.0 | 0.0 | 0.7 | 3.4 | 1.7 | 18.4 | <.0001 |
| Any Symptoms Excluding Oral/Pharyngeal | 0.0 | 0.0 | 0.3 | 2.3 | 1.2 | 12.5 | <.0001 |
| Duration >30 min. | 0.0 | 0.0 | 0.2 | 0.5 | 0.2 | 1.8 | <.0001 |
| Treatment Used | 0.0 | 0.0 | 0.2 | 1.2 | 0.2 | 4.0 | <.0001 |
| Oral/Pharyngeal Symptoms | 0.0 | 0.0 | 0.3 | 0.9 | 0.2 | 4.1 | 0.0005 |
| Skin Symptoms | 0.0 | 0.0 | 0.2 | 0.7 | 0.0 | 1.0 | 0.002 |
| Respiratory Symptoms | 0.0 | 0.0 | 0.0 | 0.9 | 0.2 | 2.0 | <.0001 |
| GI Symptoms | 0.0 | 0.0 | 0.2 | 0.4 | 0.0 | 1.3 | 0.007 |
| Other Symptoms | 0.0 | 0.0 | 0.2 | 0.5 | 0.0 | 3.0 | 0.0007 |
| Mild Symptoms | 0.0 | 0.0 | 0.3 | 2.3 | 1.2 | 12.3 | <.0001 |
| Moderate Symptoms | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.005 |
| Severe Symptoms | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.34 |
| Treated with Epinephrine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.09 |
In order to assess whether blinding had any effect on the reporting of dose-related reactions, we described reactions for maintenance doses administered during the blinded period and those administered during the unblinded period [Supplemental Tables 5 and 6]. As most reactions would be expected to occur during escalation, we excluded these when assessing a potential unblinding effect as escalation occurred entirely during the blinded phase. There continued to be significant reductions in most safety parameters for omalizumab-treated subjects during the blinded maintenance period as well as the unblinded maintenance period.
Reactions requiring epinephrine tended to be more common in the placebo group (P=0.052). When considering all subjects, 20 of 40,641 total doses led to reactions requiring epinephrine in 11 individuals, with 2 doses in 2 omalizumab-treated subjects and 18 doses in 9 placebo-treated subjects (including two subjects on 5 occasions and one on 2 occasions). Five reactions requiring epinephrine occurred during escalation visits and 15 with home doses.
Immunologic Responses
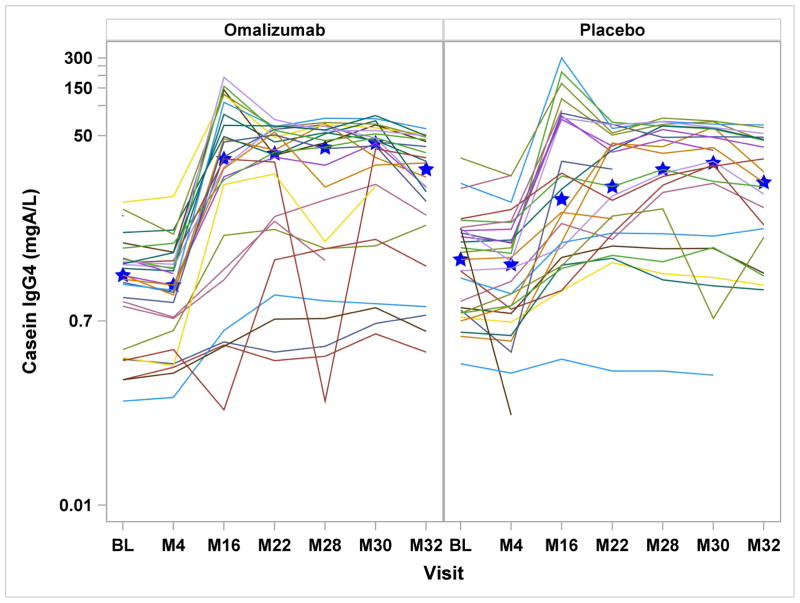
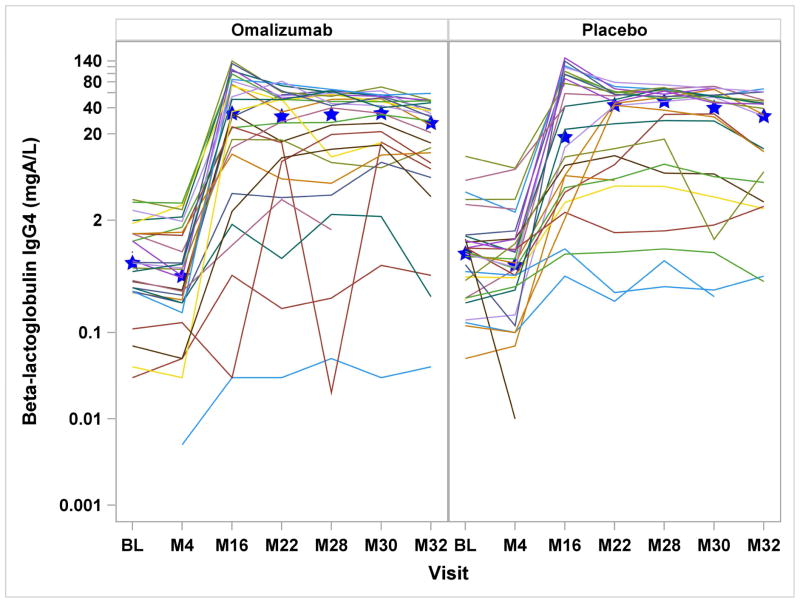
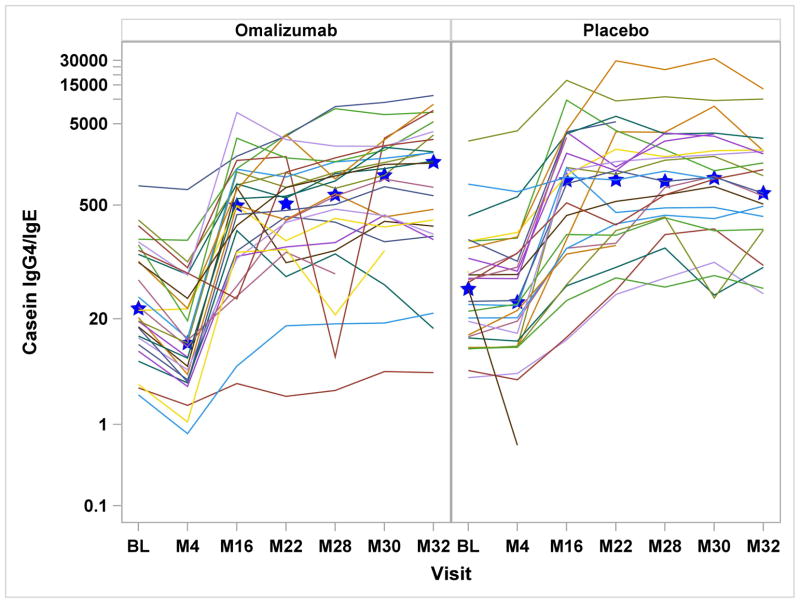
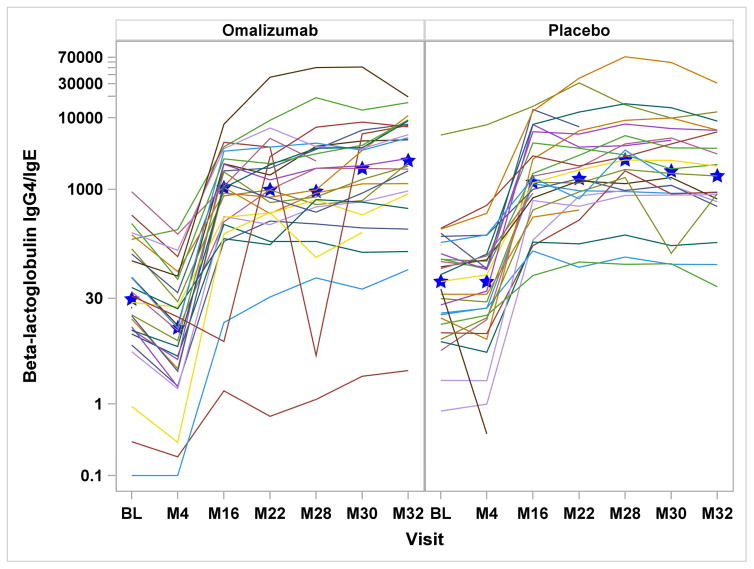
Serologic assessments included specific IgE, IgG, and IgG4 to milk, casein, and β-lactoglobulin. In the omalizumab group, milk- and casein-specific IgE were significantly increased at month-4 and reduced at month-32, while in the placebo group, all milk and casein IgE levels were significantly reduced after month-4 [Supplemental Figure 1a&b]. Comparing the treatment groups, there were significant differences in the change from baseline for milk-specific IgE through month-30, casein-specific IgE through month-28, and β-lactoglobulin-specific IgE through month-22. With regard to casein and β-lactoglobulin IgG4, IgG and IgG4/IgE ratios, both placebo and omalizumab-treated subjects had significant increases from baseline from month-16 onward, without any differences between the groups [Figure 2 and Supplemental Figure 1d&e].
Figure 2.
Figures 2a – 2d: Figures 2a&b: Casein and Beta-lactoglobulin IgG4 Levels: Casein and beta-lactoglobulin specific IgG4 levels were measured at baseline and months 4, 16, 22, 28, 30, and 32. Median values are represented by the blue stars. Significant increases from baseline were detected within both treatment groups from month 16 onward (all P<0.0001), with no differences seen between the two groups. Figures 2c&d: Casein and Beta-lactoglobulin IgG4/IgE Ratio: The ratio of casein- and β-lactoglobulin IgG4/IgE was calculated after IgG4 levels were converted from mgA/L to ng/mL and IgE level was converted from kUA/L to ng/mL with the formula (IgG4 × 1000) ÷ (IgE × 2.4). Significant increases from baseline were detected within both treatment groups from month 16 onward (all P<0.0001). The only significant difference between treatment groups was observed at month 4 with omalizumab subjects exhibiting decreased casein and beta-lactoglobulin IgG4/IgE ratio compared to placebo subjects.
For endpoint SPT titration, both groups exhibited significant decreases from baseline (P<0.0001 month-28 and -32), with no differences between the groups at any of the three time points at which it was assessed, or in the change from baseline [Supplemental Figure 2].
Assessment of basophil activation to milk stimulation in vitro revealed that the percent CD63+ cells was lower in the omalizumab group compared to placebo through month-28, at which point the the percent CD63+ cells increased to a level similar to the placebo group at month 32. Additional details regarding these measures are described in Supplemental Figure 3.
Relationship of Clinical or Immunologic Variables to Clinical Outcomes
Additional analyses were conducted to study possible relationships between baseline and post-treatment clinical and immunologic variables and treatment outcomes. When considering all subjects and adjusting for treatment, successful month-32 outcomes were associated with smaller baseline milk SPTs (P=0.012), smaller milk endpoint titration SPT (P=0.011), and lesser log10 milk-specific IgE (P=0.007, median milk-specific IgE 23.7 versus 56.8 kUA/L), log10casein-specific IgE (P=0.012) and percent milk-spcific IgE (p=0.003), and greater baseline casein-specific IgG4/IgE ratio (p=0.007) [Supplemental Table 7]. After 1 year of MOIT at month 16, smaller log10milk-specific IgE (p=0.005) and smaller log10casein-specific IgE (p=0.012) were associated with successful month-32 OFC outcomes [Supplemental Table 8]. Neither baseline nor month-16 IgG, IgG4, or basophil activation was associated with successful month-32 OFC outcomes, except lower baseline log10casein-specific IgG (p=0.037). In those with successful month-32 outcomes, milk- and casein-specific IgE levels were lower from month 16 onward for those on omalizumab but not placebo. Since the administration of omalizumab typically increases the apparent amount of serum IgE due to the formation of IgE complexes that clear more slowly27, the decrease in casein-specific IgE in omalizumab-treated subjects was unexpected and likely reflects the effect of omalizumab on decreasing IgE production in those achieving SU21. However, when considering all subjects with regard to successful versus unsuccessful month-32 OFCs, significant differences were detected at most time points (month-32 medians: milk-specific IgE 6.8 versus 29.8 kUA/L; casein-specific IgE 3.6 versus 17.8 kUA/L).
For safety outcomes, placebo subjects were analyzed separately, revealing that moderate/severe dosing symptoms or epinephrine use during dose escalation were associated with a baseline diagnosis of asthma (OR 8.0, 95% CI 1.2–55.3, P=0.035), and that the use of epinephrine during escalation was associated with increased baseline milk-specific IgE (OR 1.17, 95% CI 1.04–1.31, P=0.008).
DISCUSSION
Given that CMA is very prevalent and has the potential to result in severe and even fatal reactions, and that cow’s milk is ubiquitous in the food supply, safe and effective therapies for those who do not develop tolerance naturally is highly desirable, not unlike other common food allergens such as egg, peanut, tree nuts, etc. While results of previous food OIT studies generally have been encouraging, concerns regarding both safety and long-term efficacy may limit its application in clinical practice. Therefore, improved approaches, including the use of adjunctive therapies such as omalizumab, deserve investigation. In this first double-blind, placebo-controlled study of omalizumab in patients with severe, persistent milk allergy, we found that the addition of omalizumab to OIT led to marked improvements in safety, but no significant change in the rate of desensitization or SU.
Anti-IgE was first studied as a potential therapy for food allergy using a similar anti-IgE molecule, TNX-901 (Tanox Biosystems, Houston, TX, USA), in which a dose-related effect on peanut reactivity was clearly demonstrated28, as confirmed in two subsequent studies29,30. Omalizumab, as an adjunct to immunotherapy, was first studied with aeroallergen SCIT, demonstrating significant reductions in adverse reactions17,18. Adjunctive use of omalizumab with MOIT was first studied in a pilot study of 11 milk-allergic subjects in an open-label protocol19. MOIT was rapidly escalated over 7–11 weeks to 2000mg and among 2301 doses, 41 were associated with reactions, with 71% mild and 10% severe. A second small open-label pilot study used omalizumab with peanut OIT20, with 12/13 subjects successfully escalating to 4000mg in a median of 8 weeks with only 2% of doses precipitating adverse reactions.
In this study, we sought to expand on these pilot studies in a randomized trial studying both safety and efficacy. Subjects having weight-IgE combinations that required a dose greater than 750mg of omalizumab based on the algorithm provided in the package insert or the European dosing formula (0.016mg/kg/IgE IU) were not included in this study. Virtually all safety measures were markedly improved and the subject withdrawal rate and time needed to achieve maintenance dosing were decreased in the group on omalizumab compared to placebo. Significantly fewer MOIT doses were required to achieve maintenance in the omalizumab group resulting in a shorter escalation phase. However, there was no significant difference in clinical efficacy as determined by rates of desensitization or SU. In addition, no significant changes were detected in milk-component IgG or IgG4 responses between the two groups. A recent study of OIT in peanut-allergic subjects also demonstrated that OIT led to rapid suppression of basophil effector function, as well as decreases in dendritic cell activation and Th2 cytokine responses, during the initial phases of immunotherapy, but this suppression appeared to be temporary in most patients16.
Omalizumab therapy markedly reduces free IgE, which in turn leads to a loss of Fcε-receptors on mast cells, basophils and APCs.24 Earlier studies showed that increased high-affinity IgE receptor expression on APCs of allergic subjects [low or absent in normal individuals],31 increased antigen uptake and subsequent presentation by 100- to 1000-fold, i.e. antigen focusing or IgE-facilitated antigen presentation, in the induction of Th2 responses.32 In addition, the low-affinity IgE receptor (FcεRII/CD23) is upregulated on APCs of atopic individuals, and B cells bearing IgE were shown to fully activate allergen specific T cells via CD23-dependent allergen presentation using 100–1000-fold lower allergen concentrations.21 With natural low-dose allergen exposure or administration of low doses of allergen during initial immunotherapy, this IgE focusing (IgE-facilitated antigen presentation) will induce activation of T cells to produce Th2 cytokines that further promote IgE production by B cells and in turn leads to further increases of IgE in the mucosal tissue, perpetuating the cycle of T-cell activation and intensifying the inflammatory response.23 Therefore, it was hypothesized that decreasing free IgE with the consequent decrease in Fcε-receptors would lead to a loss of Th2 responses and a more sustained increase in Th1 and T-regulatory cell responses. Unfortunately this hypothesis was not supported by the clinical outcome of our study.
Strengths of this study include the sample size, placebo-controlled use of omalizumab, detailed immunologic assessments, and rigorous collection of data regarding adverse reactions. However, several potential limitations should also be noted. First, the omalizumab/placebo was unblinded after one year of OIT, which could limit the validity of adverse reaction comparisons during the maintenance phase of OIT. However, when observing adverse reactions from maintenance doses during the blinded period and adverse reactions from maintenance doses after unblinding, the placebo group continued to have similar, significantly higher reaction rates for most safety parameters compared to the omalizumab group. Also, since it is highly unlikely that this unblinding could have any effect on the primary outcome, the protocol was written to decrease the study burden for the placebo participants by limiting the need for frequent visits and unnecessary injections. Second, the OIT was open label throughout, leaving open the possibility that some participants could have become tolerant naturally over the course of the study. While possible, this would be highly unlikely in older children with high milk-specific IgE and very low baseline challenge thresholds. Third, the wide age range could include participants with different phenotypes, some more likely to respond to treatment or even gain tolerance naturally. Lastly, the 8 week period of avoidance may be too short to identify all participants who would eventually lose protection, although it is very unlikely that a longer period of avoidance would have changed the primary outcome.
While safety is of utmost importance in the development of new therapies, especially where the risk of anaphylaxis is a reality, not all patients treated with OIT experience major OIT-related adverse reactions, as noted in the majority of placebo-treated subjects in this study. Therefore, identifying clinical factors and/or biomarkers that distinguish high-risk subjects who might benefit most from adjunctive treatment is important. While no reliable biomarkers were identified, a history of asthma and higher baseline milk-IgE levels were associated with a higher risk of moderate/severe reactions or need for epinephrine as noted in other OIT trials10,12–16. For future studies and eventually clinical use, omalizumab should be considered as an adjunct to OIT in high-risk patients, although reliably identifying high-risk individuals with available pre-treatment biomarkers remains to be achieved.
The other major obstacle in bringing OIT to clinical practice is the loss of protection that is seen in most patients when treatment is discontinued13–15. While it would seem logical that protection could be maintained by simply keeping the food in the diet after treatment, this has not proven easy12, even for a food like milk that is available in so many forms. In this study, we found that milk- and casein-specific IgE (both at baseline and after one year of MOIT), baseline percent milk-specific IgE, and baseline casein-specific IgG4/IgE ratio predicted sustained unresponsiveness.
In conclusion, the addition of omalizumab to MOIT markedly improved safety with no significant effects on efficacy. With or without omalizumab, most subjects could be desensitized to a high dose (10g) of milk protein over a 24 month period, but half had increased reactivity after an 8 week period of avoidance. The prospects for the treatment of food allergy are on the horizon, but more research is needed to develop strategies to maintain sustained unresponsiveness.
Supplementary Material
Clinical Implications.
The use of omalizumab in conjunction with oral immunotherapy dramatically decreases the number of dose-related adverse reactions, markedly shifting the risk/benefit ratio of this procedure.
Acknowledgments
Supported by grant AI-44236 from NIAID and RR-026134 (Mount Sinai) and UL1 TR 001079 (Johns Hopkins) from NCATS, NIH; and a supplemental grant from the FARE (Food Allergy Research & Education).
Abbreviations
- APC
Antigen-presenting cell
- CMA
Cow’s milk allergy
- OFC
Oral food challenge
- OIT
Oral immunotherapy
- MOIT
Milk oral immunotherapy
- SCD
Successfully consumed dose
- SCIT
Subcutaneous immunotherapy
- SPT
Skin prick test
- SU
Sustained unresponsiveness
- Wt/vol
weight/volume
Footnotes
Omalizumab (XolairR) and omalizumab-placebo for this trial were kindly provided by Genentech, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert A. Wood, Johns Hopkins University School of Medicine.
Jennifer S. Kim, NorthShore University HealthSystem.
Robert Lindblad, The EMMES Corp.
Kari Nadeau, Stanford University School of Medicine.
Alice K. Henning, The EMMES Corp.
Peter Dawson, The EMMES Corp.
Marshall Plaut, NIAID.
Hugh A. Sampson, Icahn School of Medicine at Mount Sinai.
References
- 1.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. The Journal of allergy and clinical immunology. 2010;126:1105–18. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH. Epidemiology of food allergy. The Journal of allergy and clinical immunology. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Wood RA, Sicherer SH, Vickery BP, et al. The natural history of milk allergy in an observational cohort. The Journal of allergy and clinical immunology. 2013;131:805–12. doi: 10.1016/j.jaci.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. The Journal of allergy and clinical immunology. 2007;120:1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer DM, Perry TT, Atkins D, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130:e25–32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyano-Martinez T, Garcia-Ara C, Pedrosa M, Diaz-Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow’s milk proteins. The Journal of allergy and clinical immunology. 2009;123:883–8. doi: 10.1016/j.jaci.2008.12.1125. [DOI] [PubMed] [Google Scholar]
- 7.Skripak JM, Nash SD, Rowley H, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. The Journal of allergy and clinical immunology. 2008;122:1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo G, Barbi E, Berti I, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. The Journal of allergy and clinical immunology. 2008;121:343–7. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Meglio P, Giampietro PG, Gianni S, Galli E. Oral desensitization in children with immunoglobulin E-mediated cow’s milk allergy--follow-up at 4 yr and 8 months. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2008;19:412–9. doi: 10.1111/j.1399-3038.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 10.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. The Journal of allergy and clinical immunology. 2012;129:448–55. 55 e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–9. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 12.Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA. Long-term follow-up of oral immunotherapy for cow’s milk allergy. The Journal of allergy and clinical immunology. 2013;132:737–9. e6. doi: 10.1016/j.jaci.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. The New England journal of medicine. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) The Journal of allergy and clinical immunology. 2014;133:500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik M, Narisety SD, Guerrerio AL, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casale TB, Busse WW, Kline JN, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. The Journal of allergy and clinical immunology. 2006;117:134–40. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Massanari M, Nelson H, Casale T, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. The Journal of allergy and clinical immunology. 2010;125:383–9. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. The Journal of allergy and clinical immunology. 2011;127:1622–4. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. The Journal of allergy and clinical immunology. 2013;132:1368–74. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heijden FL, van Neerven RJ, Kapsenberg ML. Relationship between facilitated allergen presentation and the presence of allergen-specific IgE in serum of atopic patients. Clin Exp Immunol. 1995;99:289–93. doi: 10.1111/j.1365-2249.1995.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Neerven RJ, van Roomen CP, Thomas WR, de Boer M, Knol EF, Davis FM. Humanized anti-IgE mAb Hu-901 prevents the activation of allergen-specific T cells. Int Arch Allergy Immunol. 2001;124:400–2. doi: 10.1159/000053770. [DOI] [PubMed] [Google Scholar]
- 23.Wilcock LK, Francis JN, Durham SR. IgE-facilitated antigen presentation: role in allergy and the influence of allergen immunotherapy. Immunol Allergy Clin North Am. 2006;26:333–47. viii–ix. doi: 10.1016/j.iac.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. The Journal of allergy and clinical immunology. 2003;112:1147–54. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Berin MC. Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol. 2012;34:633–42. doi: 10.1007/s00281-012-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. The Journal of allergy and clinical immunology. 2013;131:180–6. e1–3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton RG, Marcotte GV, Saini SS. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab (Xolair) therapy. J Immunol Methods. 2005;303:81–91. doi: 10.1016/j.jim.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. The New England journal of medicine. 2003;348:986–93. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 29.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. The Journal of allergy and clinical immunology. 2012;130:1123–9. e2. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson HA, Leung DY, Burks AW, et al. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. The Journal of allergy and clinical immunology. 2011;127:1309–10. e1. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 31.Novak N, Kraft S, Bieber T. Unraveling the mission of FcepsilonRI on antigen-presenting cells. The Journal of allergy and clinical immunology. 2003;111:38–44. doi: 10.1067/mai.2003.2. [DOI] [PubMed] [Google Scholar]
- 32.Maurer D, Ebner C, Reininger B, et al. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.