Abstract
Gap junctions underlie electrical synaptic transmission between neurons. Generally perceived as simple intercellular channels, “electrical synapses” have demonstrated to be more functionally sophisticated and structurally complex than initially anticipated. Electrical synapses represent an assembly of multiple molecules, consisting of channels, adhesion complexes, scaffolds, regulatory machinery, and trafficking proteins, all required for their proper function and plasticity. Additionally, while electrical synapses are often viewed as strictly symmetric structures, emerging evidence has shown that some components forming electrical synapses can be differentially distributed at each side of the junction. We propose that the molecular complexity and asymmetric distribution of proteins at the electrical synapse provides rich potential for functional diversity.
Keywords: Electrical synapse, Gap junction, Connexin, Innexin, Synapse formation and plasticity
Introduction
Communication between neurons takes place at specialized cellular structures called synapses. At these junctions, transmission can be either chemically-mediated (chemical synapses) or made possible by intercellular channels that allow the passage of intracellular ions carrying electrical currents (electrical synapses). Together these synapses shape the fast form of signaling that is characteristic of neurons. Electrical synapses also serve as conduits for the diffusion of small messenger molecules and metabolites between coupled cells, creating a reticulum between neurons, with passage being restricted by charge and size (Fig. 1). These intercellular channels cluster into structures known as “gap junctions” (GJs) (Fig. 2A) (Goodenough and Paul, 2009), which are not restricted to neurons and are present in most multicellular organisms.
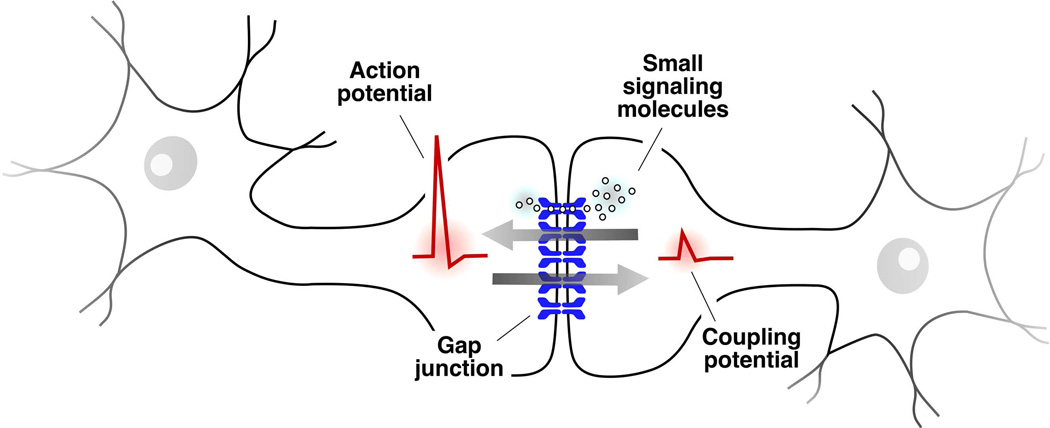
Figure 1. Gap junctions between neurons mediate electrical transmission.
The cartoon schematically depicts a canonical GJ between two, unspecified, neuronal processes (neuronal gap junction can between dendrites, somas, axons, or between axonal terminals and various cellular processes). GJs provide a pathway for the spread of electrical currents between neurons, a cellular type that characteristically rely on electrical signaling. Changes in the membrane potential of one of the cells evoke corresponding changes in the membrane potential of a second, coupled cell. Many electrical synapses are bi-directional, and therefore when depolarization propagates to a coupled cell (an action potential in this case but changes in membrane potential can also be subthreshold) the depolarization evoked in this cell (coupling potential) simultaneously propagates back to the cell on which the depolarization initially originated (arrows). In addition to supporting electrical signaling, electrical synapses have metabotropic functions providing a conduit for the exchange of small molecules such as metabolites and signaling molecules.
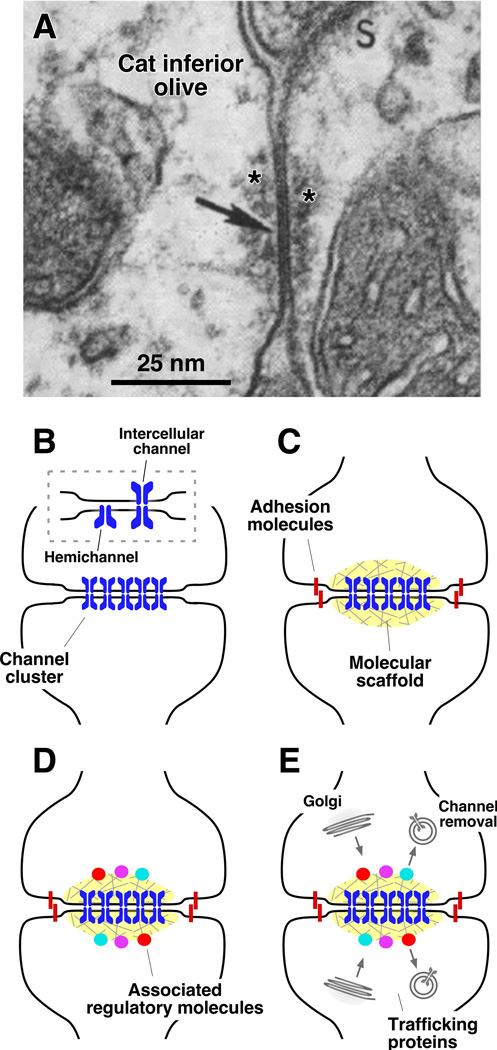
Figure 2. Electrical synapses are complex and contain many components.
Electrical synapses represent more than simple aggregates of intercellular channels. The cartoon aims to conceptualize the various components of an electrical synapse. A, Electron micrograph showing a neuronal GJ (arrow) between two dendritic profiles in the cat inferior olive, which were located in an extraglomerular position. Note the electron dense material (asterisks) that can be observed at each side of the GJ. We call this structure the Electrical Synaptic Density (ESD). Modified from Sotelo et al., 1974, with permission (Sotelo et al., 1974). B, Intercellular channels are formed by the apposition of two hemichannels, each contributed by one of the coupled cells. These intercellular channels cluster intro structures known as GJ plaques. C-E, A number of additional proteins have been identified at gap junctions. Most of these proteins are thought to be involved in the maintenance and regulation of intercellular communication. C, Adhesion and scaffolding proteins contribute to the clustering of channels. D, Channel forming proteins have been shown to interact with associated regulatory proteins. E, GJs have distinct pathways for synthesis, trafficking, removal and degradation.
As with chemical transmission, electrical transmission is a highly dynamic form of synaptic communication. Rather than simply behaving as passive channels, the functional state of electrical synapses in under the constant influence of various regulatory processes (Pereda et al., 2013; O’Brien, 2014). GJ conductance at electrical synapses was shown to be modified by modulatory neurotransmitters such as dopamine (Piccolino et al., 1984; Lasater and Dowling, 1985; Lasater et al., 1987; Kothmann et al., 2009), the activity of nearby glutamatergic synapses (Yang et al., 1990; Pereda and Faber, 1996; Landisman and Connors, 2005; Kothmann et al., 2012; Turecek et al., 2014; Wang et al., 2015b), as well changes in the intracellular concentration of Ca++ following intense cellular activity (Kohmann et al., 2016). Moreover, GJ channels can in some cases be sensitive to differences in the membrane potential of the coupled neurons, a property that can underlie rectification, the ability to offer differential resistance to the spread of electrical currents between the coupled cells (Oh et al., 1999; Palacios-Prado et al., 2014b). The existence of such complex functional properties suggests that electrical transmission should be supported by a similarly complex molecular machinery at the synaptic structure.
While the molecular complexity of chemical synapses has been long recognized, electrical synapses were generally perceived as structurally simple. This misperception probably originates in the inappropriately assigned conceptual simplicity of electrical transmission being based solely on the GJ channels, as well as on its functional stability, which allows high-fidelity transmission unlike the probabilistic nature of chemical synapses. However, growing evidence indicates that electrical synapses are made of more than just intercellular channels. Current views support the notion that GJs represent multi-molecular complexes at which intercellular channels are supported by a wide range of associated scaffolding and regulatory proteins (Hervé et al., 2004). Such structural complexity is emerging at electrical synapses as well (Burr et al., 2005; Alev et al., 2008; Lynn et al., 2012; Siu et al., 2016). Moreover, while chemical synapses are inherently structurally asymmetric, with a presynaptic site specialized in transmitter release and a postsynaptic one specialized for reception, electrical synapses are generally perceived to be structurally symmetric. Contrasting this perception, recent evidence indicates that electrical synapses might be asymmetric (Phelan et al., 2008; Rash et al., 2013) with some of its molecular components differentially distributed at each gap junction “hemi-plaque” (see below) suggesting that, as at chemical synapses, certain electrical synapses might have distinct cellular pre- and postsynaptic sites (this distinction is in mostly conceptual and arbitrary as electrical transmission is bi-directional).
Here we discuss the structural and functional complexity of electrical synapses. Rather than a complete review of the literature, this article focuses on evidence indicating that, rather than simple aggregates of intercellular of channels, electrical synapses represent true synaptic structures and that the complexity and asymmetry of their structural components are likely to contribute to their functional diversity.
What makes an electrical synapse?
As already mentioned, transfer of information at electrical synapses occur at cell junctions known as the “gap junction plaque” or simply “gap junction” that represent clusters of intercellular channels. Each of the intercellular channels at these structures is formed by the apposition of two “hemichannels” or “connexons”, each contributed by one of the coupled cells (Goodenough and Paul, 2009) (Fig. 2B). Hemichannels are composed of hexamers of proteins known as connexins (Cx) in chordates and recent evidence indicates they are octamers of innexins (Inx) in invertebrates; although whether all Inx-based hemichannels are octamers is not clear (Phelan, 2005; Goodenough and Paul, 2009; Oshima et al., 2016). Although Cxs and Inxs share similar membrane topology and form similar intercellular structures their sequences are unrelated (Phelan, 2005; Oshima et al., 2016). To date, mammalian genomes encode approximately twenty Cx-encoding genes whereas C. elegans and D. melanogaster genomes encode twenty five and eight Inx-encoding genes, respectively (Phelan and Starich, 2001; Söhl et al., 2005; Simonsen et al., 2014). Currently, at least five Cxs (Cx30.2, Cx36, Cx45, Cx50, Cx57) are definitively known to be expressed within mammalian neurons, while five others (Cx29, Cx30, Cx32, Cx43, Cx47) are expressed exclusively in glia (Nagy et al., 2004) – at least in mature circuits. Similarly, only a subset of Inxs are known to be expressed in invertebrate neurons (Simonsen et al., 2014). This diversity of Cx/Inx genes provides ample opportunity for complex GJ formation where each hemi-channel could be created from multiple Cx/Inxs (heteromeric) or hemi-channels created by different Cx/Inxs could couple between cells (heterotypic, discussed below). While some Cxs appear to be promiscuous in their interaction partners, others are quite restrictive in those with which they can form partners (White et al., 1994, 1995). In an animal the diversity of GJ makeup is further restricted due to spatially and temporally restricted patterns of Cx/Inx expression; although often these expression patterns overlap within a single neuron and/or coupled cells expressing multiple Cxs or Inxs genes (Connors and Long, 2004; Söhl et al., 2005; Simonsen et al., 2014).
The diversity of Cxs at electrical synapses support their communication functions, yet surprisingly only a small fraction of the channels (0.1 to 18%) are estimated to be functional and sufficient to support electrical transmission. This was observed in fish (Tuttle et al., 1986; Lin and Faber, 1988; Flores et al., 2012) and mammalian electrical synapses (Curti et al., 2012; Szoboszlay et al., 2016) as well as at GJs created from Cx36 in cell expression systems (Marandykina et al., 2013), and indicates that heterogeneous populations of channels can co-exist in a GJ plaque. Such a remarkable property is likely to result from the interaction of GJ channels with associated regulatory and scaffolding proteins (discussed below). Further, it suggests that Cx proteins can have multiple functions at GJ and in addition to conductive functions (electrical and metabolic coupling) they can also serve as adhesion molecules (Elias et al., 2007) providing perhaps mechanical stability to the intercellular junction (Agullo-Pascual and Delmar, 2012; Flores et al., 2012; Pereda, 2016).
While much emphasis has been put on the structure and function of the GJ channel, increasing evidence is leading us to look at electrical synapses in a new, more comprehensive, way. At chemical synapses, regulatory and functional properties of synaptic release and reception are supported by macromolecular structures known as the presynaptic Active Zone (Siksou et al., 2011) and the Postsynaptic Density (PSD), both recognizable for their characteristic electron dense profiles identifiable in electron microscope (EM) thin sections (Kennedy, 1997; Carroll et al., 2001). While often overlooked, EM of electrical synapses characteristically exhibit electron dense structures described as a “semi-dense cytoplasmic matrix” or “cytoplasmic semi-dense matrix” (Sotelo and Korn, 1978) (Fig. 2). This electron-dense matrix or “electrical synapse density” (ESD) likely represents the proteins associated with the GJ channels and thereby support the structure and function of the electrical synapse.
An ever growing number of proteins have been identified as being associated with the GJ channels at electrical synapses (Hervé et al., 2004). Most of these proteins are thought to be involved in the maintenance and regulation of intercellular coupling. Adhesion (E- and N-cadherins) (Meyer et al., 1992; Segretain and Falk, 2004) and scaffolding proteins (Duffy et al., 2002; Hervé et al., 2004) contribute to the clustering of channels (Fig. 2C) and channel forming proteins interact with regulatory proteins (Fig. 2D) (Li et al., 2004; Duffy et al., 2002; Hervé et al., 2004; Ciolofan et al., 2006; Lynn et al., 2012). Many of these proteins are shared with other membrane junctions such as tight and adherens junctions found in epithelia where the proteins are known to scaffold the junctional complexes to kinases, signaling molecules and cytoskeletal elements (Hervé et al., 2004). At neuronal GJs, Cx36 (Li et al., 2004) and some of its fish homologs (Flores et al., 2008) were shown to interact with the first PDZ domain of zonula occludens-1 (ZO-1). ZO-1 is a protein with scaffolding function that could serve to recruit signaling proteins into Cx36-based GJs, as suggested by its interaction with the accessory scaffolding proteins multi-PDZ domain protein 1 (MUPP1), AF-6, and cingulin (Lynn et al., 2012). Cx36 has also been shown to co-localize and interact with MUPP1, which interacts with both ZO-1 and CaMKII, thus serving as an anchor for this regulatory kinase protein (Lynn et al., 2012). Moreover, Cx36 was shown to directly interact with the alpha subunit of Ca++-calmodulin dependent kinase II (CaMKII), in a fashion that resembles the interaction of this kinase with the NR2B subunit of the NMDA receptor (Alev et al., 2008), and to interact with calmodulin (Burr et al., 2005; Siu et al., 2016). Cx36 also co-localizes and interacts with AF-6, a protein that is targeted by the Epac-Rap1 signaling pathway and that contributes to the regulation of other cell-cell junctions (Lynn et al., 2012). Finally, Cx36 was also reported to co-localize with cingulin, a protein that as a result of its interaction with alpha-actin links junctions to the cytoskeleton and is capable of mediating signaling via the RhoA system (Lynn et al., 2012). Although less is known, there is increasing evidence for the role of associated proteins in Inx-based electrical synapses (Chen et al., 2007; Norman and Maricq, 2007; Meng et al., 2016). Thus, rather than simple aggregates of channels, the current notion of the electrical synapse includes the participation of other proteins in gap junctional communication, forming multi-molecular complexes that has been termed the “nexus” in non-neuronal cells (Duffy et al., 2002) and we suggest are likely to contribute to the EM-observed ESD.
Within this complex of interacting proteins, GJs in non-neuronal tissue are known to be highly dynamic structures. Trafficking and turnover of recombinant GJ channels have been observed and well-characterized in cell expression systems (Laird, 1996, 2006; Gaietta et al., 2002; Lauf et al., 2002; Piehl et al., 2007). A wealth of evidence indicates that new connexins are trafficked in vesicles from the Golgi as undocked hemichannels (Fig. 2E). Hemichannels are then inserted into the plasma membrane at the periphery of existing GJ plaques rapidly docking with hemichannels inserted in the apposed membrane (Gaietta et al., 2002; Lauf et al., 2002). Hemichannels do not undock during removal; rather connexin removal occurs from the center of plaques as intact regions of the junction containing intercellular channels which are internalized into one or the other of the coupled cells (Laird, 1996, 2006; Gaietta et al., 2002; Lauf et al., 2002; Falk et al., 2016). The removed structures are then transported to lysosomes for their degradation (Laird, 1996; Lauf et al., 2002; Falk et al., 2016). Turnover of gap junction channels was observed at electrical synapses in vivo at auditory mixed synapses on the Mauthner cell (Flores et al., 2012) and the existence of this phenomenon at mammalian neuronal GJs is supported by the existence of internalized annular GJs at inhibitory interneurons in the rat striatum (Fukuda 2009). Similar turnover was observed for Cx36 in cell expression systems (Wang et al., 2015a). This dynamic turnover must require exo- and endocytosis machinery and is likely strictly regulated by multiple proteins. The trafficking and stabilization of AMPA receptors at glutamatergic chemical synapses is controlled, at least partially, through interactions of the AMPA receptor’s carboxy-terminus with several different cytosolic scaffolding proteins (Lüscher et al., 1999; Ehlers, 2000; Hanus and Ehlers, 2016). We expect that the regulation of GJ-channel insertion and removal via the molecular complexity found within the ESD will be a critical regulator of formation and function of the electrical synapse.
The molecular complexity and trafficking of GJ channels are also likely to participate in electrical synapse plasticity wherein the strength of communication can be modified by a number of pathways. In contrast to chemical synapses, less is known about proteins that play a functional role in regulation of electrical synapses. Trafficking of ionotropic receptors involves processes of membrane insertion and retrieval that maintains the strength of chemical synapses and underlies several forms of synaptic plasticity (Lüscher et al., 1999; Xia et al., 2000; Hanus and Ehlers, 2016). In analogy with chemical synapses, the existence of Cx turnover suggests that trafficking of GJ channels could potentially be regulated (increased or decreased) to produce modifications of synaptic strength, a possibility suggested by the observation that the strength of electrical synapses can be readily altered (minutes) by interfering with the insertion and removal of GJ channels (Flores et al., 2012). In addition to the distinct pathways for the synthesis, trafficking, and degradation of GJs (Musil and Goodenough, 1993; Falk et al., 1997; Diez et al., 1999; Evans et al., 1999) neuronal Cxs are subject to posttranslational modifications (Moreno and Lau, 2007). Most studies of mechanisms that regulate Cx36-mediated electrical synapses have converged on the involvement of two protein kinases, namely, cAMP-dependent protein kinase A (PKA) and the multifunctional CaMKII (Pereda et al., 1998; Urschel et al., 2006; Kothmann et al., 2007, 2009; Alev et al., 2008). Activation of CaMKII requires an increase in the intracellular concentration of Ca++, such as that which occurs during sustained activation of NMDA receptors, and its activation usually leads to enhancement of GJ conductance (Pereda et al., 1998; Kothmann et al., 2012; Turecek et al., 2014). PKA is usually recruited by the activation of G protein-coupled receptors via various neurotransmitters modulators (Urschel et al., 2006; Kothmann et al., 2007, 2009). Activation of PKA generally leads to a decrease in GJ conductance (Piccolino et al., 1984; Lasater and Dowling, 1985; McMahon et al., 1989; Mills and Massey, 1995; Zsiros and Maccaferri, 2008), but has also been reported to lead to its enhancement (Pereda et al., 1992, 1994; Cachope et al., 2007; Li et al., 2009, 2013). Biochemical analysis indicated that enhancement of GJ conductance is mediated by direct phosphorylation of Cx36, whereas reduction of GJ conductance is mediated via an indirect pathway that leads to the activation of protein phosphatase 2 (Kothmann et al., 2009). Cx36 (and its teleost homologs) contains multiple phosphorylation sites for both PKA and CaMKII (Urschel et al., 2006; Kothmann et al., 2007; Alev et al., 2008), and two of them (S110 and S293) are targeted by both kinases. The emerging molecular complexity at the electrical synapse thus represents a critical point of regulation where many scaffolds and effectors support the structure of the GJs but also serve as a platform for the precise regulation of electrical synapse function.
Are electrical synapses necessarily symmetric?
As a result of their structural arrangement, it has been assumed that electrical synapses are molecularly and structurally symmetric. However, data indicates that each side of an electrical synapse can be unique. In vivo GJ channels at electrical synapses can be formed by hemichannels that are molecularly different (Phelan et al., 2008; Rash et al., 2013; Palacios-Prado et al., 2014b). This channel asymmetry then may provide unique intracellular interactions allowing for asymmetry of associated proteins, a possibility that is consistent with the fact that the amount of associated electron dense area surrounding neuronal GJs (ESD) can be asymmetric in some contacts (Fig. 3A). Contacts between the lateral giant axon and the giant motor fiber in crayfish provide a clear example of structural asymmetry at electrical synapses, in which vesicles that are apparently tethered to the GJ are restricted to the presynaptic site (Fig. 3B,C) (Hanna et al., 1978). We discuss in the next sections known and potential sources of asymmetry at electrical synapses (Fig. 4).
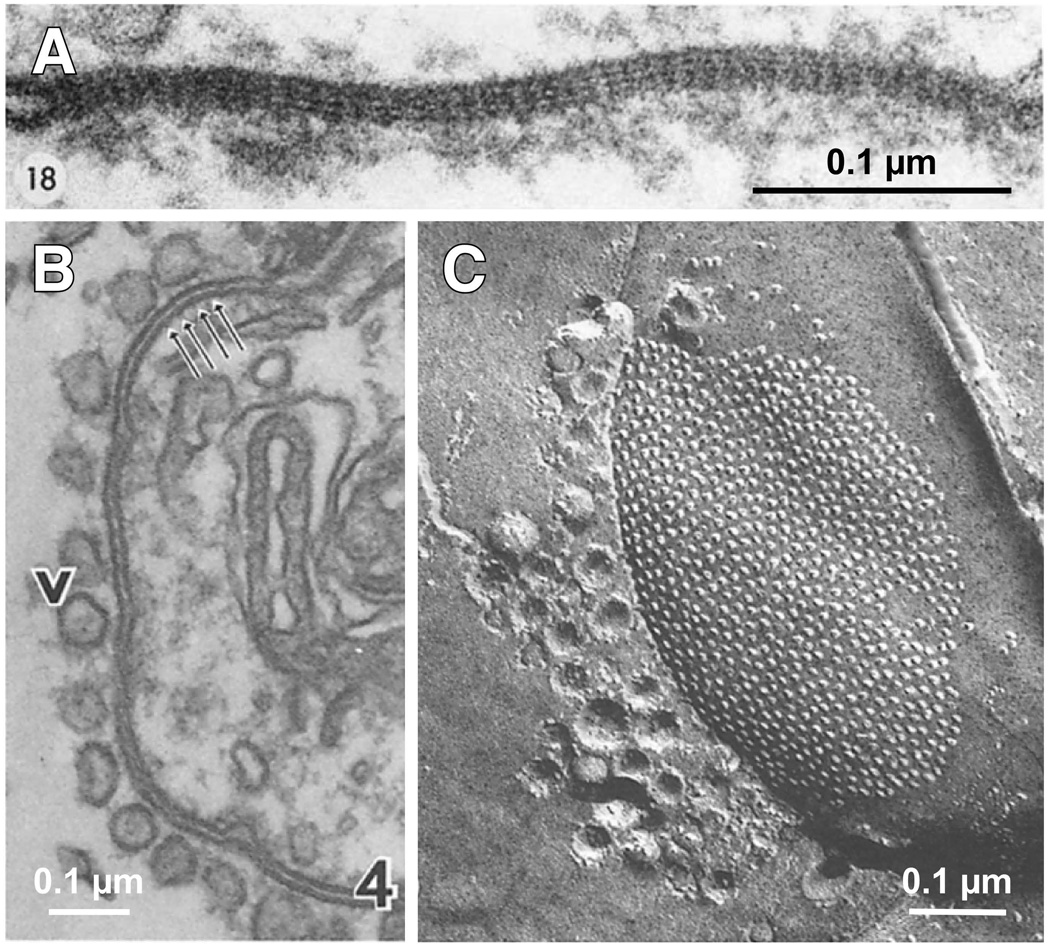
Figure 3. Structural asymmetry at electrical synapses.
Morphological asymmetries can be observed at both Cx-based and Inx-based electrical synapses. A, Electron micrograph of GJ between a Club Ending and the lateral dendrite of the Mauthner cell of a goldfish (Carassius auratus). Note the asymmetry of the ESD at each side of the junction. Modified from Brightman and Reese, 1969, with permission (Brightman and Reese, 1969). B, Electron micrograph of a GJ situated in the synaptic contact between the lateral giant axon and the giant motor fiber of a crayfish (Procambarus clarkii). The presynaptic side can be easily identified by the presence of vesicles (v), which seem to be connected to the junctional membrane. C, Freeze-fracture image (e-face) of a GJ in another crayfish electrical synapse showing the close arrangement of particles and vesicles, which are only present in the cytoplasm of the presynaptic lateral giant axon. Panels B and C modified from Hanna et al, 1978, with permission (Hanna et al., 1978). There are structural differences between vertebrate and invertebrate gap junctions including membrane separation (B) and inter-channel distance and regularity (C).
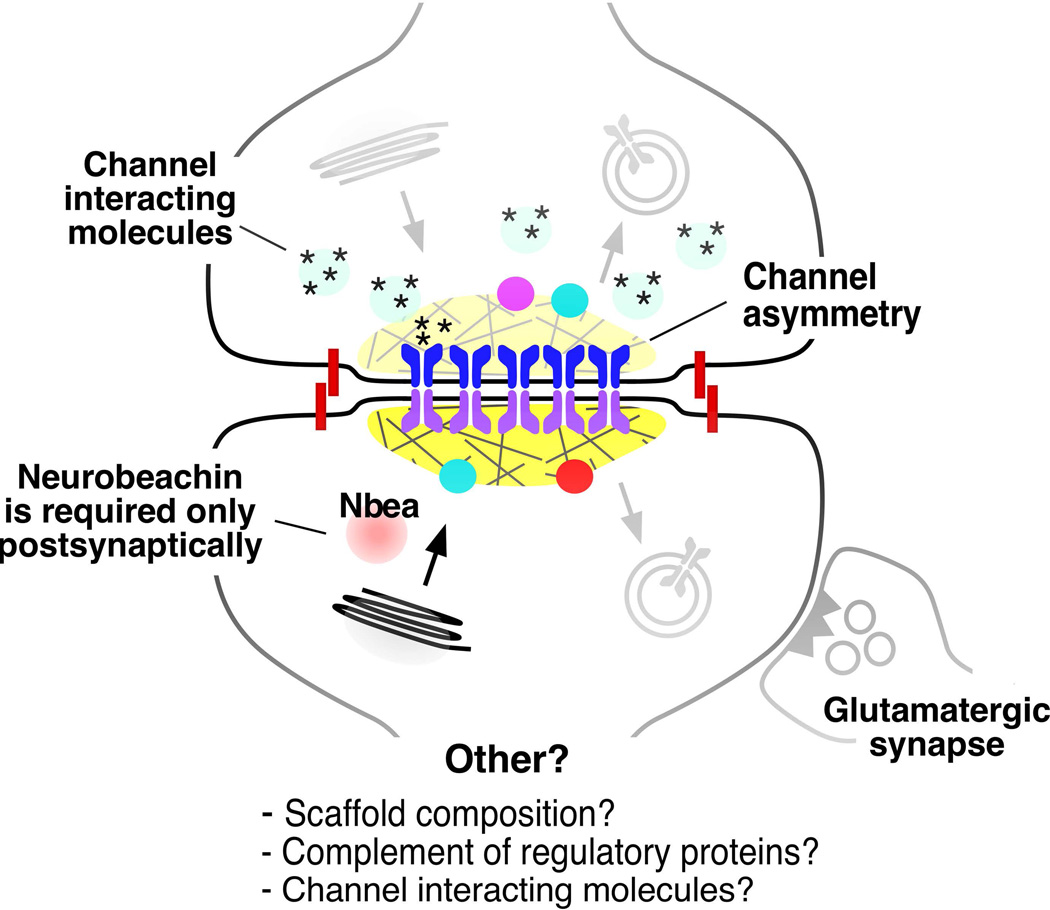
Figure 4. Potential sources of structural and functional asymmetry at vertebrate electrical synapses.
The cartoon illustrates known and possible forms of structural and functional asymmetry at an electrical synapse. Intercellular channels can be heterotypic, that is, each hemichannel is molecularly different (channel asymmetry). Both Cx-based and Inx-based electrical synapses have been shown to exhibit heterotypic channels, a configuration that is generally associated with electrical rectification (Palacios-Prado et al., 2014b). Scaffolding and trafficking proteins can be also differentially distributed; such is the case of Neurobeachin (Nbea) (Miller et al., 2015). Asymmetry can also be functional and could be mediated by the presence/absence of channel-interacting diffusible cytosolic factors at heterologous junctions, that is, between dissimilar cell types with different internal milieu (Palacios-Prado et al., 2013, 2014a). Functional interactions with glutamatergic synapses can occur asymmetrically, through one of the coupled cells (Pereda, 2014). These known asymmetries open the possibility that other components of an electrical synapse could also differentially distributed at each hemi-plaque, such as scaffold composition and the complement of regulatory molecules.
Asymmetry at the channel
The first evidence of molecular asymmetry of electrical synapses came from work in invertebrate systems. In the Drosophila giant fiber system (GFS) two Inx isoforms (Shaking-B(Neural+16) and ShakB(Lethal)) form heterotypic GJs with each Inx used only on one side of the synapse and being required for a fast escape response (Zhang and Wyman 1999, Phelan et al 2008). By expressing these channels in Cx-depleted Xenopus oocytes, the authors found that the heterotypic pairing of these Inxs is sufficient to produce asymmetric current flow, across the junction (Phelan et al., 2008). A different pair of Inxs (Inx7 and Inx6) forms a heterotypic electrical synapse between the APL neurons and DPN neurons of the mushroom body of flies – part of a circuit critical for associative olfactory learning and memory (Wu et al., 2011). Such molecular asymmetries at electrical synapses are also found in the C. elegans nervous system, for example the Inxs UNC-7S and UNC-9 form heterotypic GJs between command interneurons and motoneurons important for coordinated locomotion (Starich et al., 2009b). Molecular asymmetry at GJs is not restricted to invertebrates, as complex Cx arrangements are found in wide variety of non-neuronal cell types and organ systems throughout vertebrate lineages (de Boer and van der Heyden, 2005; Bukauskas et al., 2006; Scott et al., 2012; Xu and Nicholson, 2013; Irion et al., 2014). Within the vertebrate nervous system defining electrical synapse complexity has been challenging due to an incomplete understanding of the expression patterns and usage of Cxs. To date, the best evidence for Cx asymmetry at electrical synapses comes from ultrastructural data obtained with freeze-fracture immunolabeling (FRIL) that found that Cx35 and Cx34 were localized exclusively pre- and postsynaptically, respectively, at the Club ending synapses of the Mauthner cell in goldfish (Rash et al., 2013). The molecular asymmetry of GJ channels at M-cell synapses was found to be associated with functional asymmetry, with the rectified electrical synapse contributing to cooperativity amongst converging inputs (Rash et al., 2013). In zebrafish the gjd2a and gjd1a genes, encoding proteins similar to the goldfish Cx35 and Cx34, respectively, were recently found to be required for electrical synapse formation in the Mauthner cell network (Shah et al., 2015). Indeed, the genes encoding Cx35 and Cx34 are required exclusively pre-and postsynaptically, respectively, in the Mauthner network of zebrafish (ACM unpublished results). This represents the first molecularly defined asymmetry at electrical synapses in vertebrates. Such asymmetric configurations appear to be broadly distributed in the fish nervous system as further FRIL analysis identified the Cx35/Cx34 heterotypic pairings to be common throughout the brain and spinal cord (Rash et al., 2015). These examples of molecular asymmetry at electrical synapses exemplify two important points: 1) there are many different GJ forming proteins which could contribute to asymmetric complexity of electrical synapses, and 2) these heterotypic electrical synapses contribute to important functions in neural networks. It is very likely that electrical heterotypic junctions occur in all connectomes and are likely to contribute broadly to the development and mature function of various nervous systems.
Within the mammalian nervous system there is no definitive example of Cx asymmetry to date. However, despite the widespread distribution of Cx36 suggesting that most synapses are homotypic, there are several potential places at which heterotypic synapses may exist. For instance, horizontal cells in the retina make electrical synapses with one another and recent EM data suggests that at least a subset of these synapses are composed of Cx50 apposed to an as yet unidentified Cx in the adjacent neuron (Cha et al., 2012). Alternatively, these could represent unpaired Cxs, a phenomenon known in horizontal cells to be important for ephaptic (non-synaptic) inhibition of cone photoreceptors (Klaassen et al., 2011). Electrical synapses in the thalamic reticular nucleus (TRN) are functionally asymmetric and this rectification is modifiable and can be accentuated by activity (Haas et al., 2011). The molecular mechanisms underlying this shift are currently unknown, however the authors suggest that it might arise due to asymmetric regulation of hemichannels at each side of the synapse such as posttranslational modification of Cx (these synapses contain Cx36 which is regulated by CaMKII and PKA; see above), creating molecular asymmetry. Intriguingly, recent work found that in cx36(gjd2)−/− mutant mice, neurons of the TRN are still electrically coupled with more pronounced rectification across the GJs than in wildtype (Zolnik and Connors, 2016); while the remaining Cx proteins are yet to be identified, the authors suggest that Cx45 and/or Cx30.2 may be involved (Söhl et al., 2005; Kreuzberg et al., 2008). It is unclear if these GJs are present in wild type mice or if they represent a compensatory mechanism in cx36(gjd2)−/− mutants. Thus, while definite proof for molecular asymmetries at electrical synapses in mammals is still lacking, it seems unlikely that evolution would have given up on a strategy that provides neurons with an important tool for manipulating the flow of information amongst neurons, and therein a method for neural circuits to compute function. With the emerging tools to look at the localization and usage of Cxs throughout neural development and mature circuit function, GJ complexity in the nervous system is likely to be recognized as a rule as opposed to an exception. Moreover, molecular asymmetry may not be confined to the GJ-forming Cx and Inx proteins and asymmetries beyond the GJ channels are likely utilized.
Asymmetry beyond the channel
Electrical synapses often couple neurons of identical type together, promoting their function as ensembles that act in concert with one another (Connors and Long, 2004). In such cases, the neurons are generally expected to express the same genes/proteins, and thus such GJs are likely to be molecularly and functionally symmetric (although note that this need not be true, as with the asymmetry in the TRN discussed above (Haas et al 2011, Zolnik and Connors 2016)). Moreover, electrical synapses between the same neuronal type are often made between identical neuronal compartments, be it dendro-dendritic, axo-axonic, or cell-to-cell body. However, many electrical synapses are formed between neurons of different types and between different neuronal compartments, and such electrical synapses tend to be functionally asymmetric suggesting the possibility of additional sources of molecular asymmetry. For example, EM at rectifying crayfish junctions between the lateral giant axon and the giant motor fiber found remarkable differences of the pre- and postsynaptic content of electrical synapses. At these synapses 80nm vesicles were found localized across the length of the presynaptic GJ plaque apparently associated with the synapse (Fig. 4B,C). The associated vesicles were observed asymmetrically distributed (Peracchia, 1973, 1974; Hanna et al., 1978; Ohta et al., 2011), with more vesicles present in the presynaptic site, and this asymmetry was exaggerated by activity (Ohta et al., 2011). Mitochondria and smooth endoplasmic reticulum were present at both pre- and postsynaptic sites (Hanna et al., 1978). While such prominent asymmetries in structure were identified nearly 40 years ago, we still know little about how neurons use these differences for the function and formation of electrical synapses. However, such structural asymmetries of electrical synapses at the level of sub-cellular organelles suggest that very different machinery must be used to deliver and maintain the components of these GJs.
The initiation of electrical synapse formation and the subsequent trafficking and delivery of components of GJs is not well understood. The best model for the process comes from work in cultured cells showing that Cxs hexamerize into hemichannels within the ER/Golgi where they are loaded into vesicles for transport along microtubules (MTs) towards the sites of GJ formation (Segretain and Falk, 2004; Shaw et al., 2007). While neuronal Cx proteins are known to localize on vesicles within neurons (Flores et al., 2012), the molecular mechanisms of Cx delivery to electrical synapses in vivo remain unknown. However, at a basic cell biological level, axons and dendrites have very different cytoskeletal arrangements. MTs, themselves inherently polarized structures, are arranged with their plus ends distal in the axon whereas within dendrites MTs have mixed microtubule polarity (Baas et al 1989). These cytoskeletal differences underlie a very different MT-motor driven trafficking landscape with unique motors being used to transport synaptic molecules to each compartment (reviewed in (Hirokawa et al., 2010). Such differences in the cell biological underpinnings of the two compartments provide opportunity for neurons to make asymmetrical electrical synaptic structures. For example, the widespread heterotypic coupling of Cx35 and Cx34 at electrical synapses in fish occurs frequently between axonal and dendritic compartments (Rash et al., 2013, 2015; Shah et al., 2015) and ACM unpublished results). A simple model to explain the presynaptic use of Cx35 and postsynaptic use of Cx34 is that each is specifically transported to only the appropriate axonal and dendritic compartments, respectively, where they are incorporated into synapses. While the mechanisms of Cx trafficking in neurons have yet to be studied, a forward genetic screen in zebrafish identified the gene neurobeachin (nbea) as being required for electrical (and chemical) synapse formation (Miller et al., 2015). Nbea is an autism-associated gene that is localized to the trans-Golgi network where it is thought to regulate the trafficking of membrane-bound proteins (such as Cxs) in the secretory pathway (Wang et al., 2000; Nair et al., 2013). It was found that Nbea function was required exclusively postsynaptically for electrical synapse formation suggesting that Nbea controls the trafficking of Cxs into the dendritic compartment (Miller et al., 2015). While Nbea may be important in dendritic Cx trafficking, the mechanisms controlling presynaptic/axonal GJ trafficking in neurons are currently unknown.
What other asymmetries might GJs take advantage of in the nervous system? To date, there is no evidence that electrical synapses are structurally asymmetric beyond the Cx/Inx proteins themselves. But asymmetries can potentially originate on associated proteins that are part of the ESD. The best-known Cx-interacting scaffold is ZO-1 (discussed above), which is encoded by the tjp1 gene. Recently it was shown that tjp1b, a zebrafish ZO-1 homologue, is required for electrical synapse formation (Shah et al., 2015). Moreover, we have found that tjp1b is required exclusively postsynaptically for electrical synapse formation in zebrafish (ACM, unpublished). This suggests a model wherein the zebrafish electrical synapses are asymmetric in the makeup of their structural scaffolding molecules and, to our knowledge, represents the first evidence that molecular structural asymmetry of the GJ can extend beyond the gap. Moreover, it suggests that other related scaffolds (ZO-2, ZO-3, and MUPP1), effector molecules (AF6 and cingulin), and adhesion molecules (N-cadherin) (Ciolofan et al 2006, Li et al 2009, Lynn et. al. 2012), may also represent places where molecular asymmetry can arise at electrical synapses. Interestingly, while ZO-1 and cingulin seem obligatory and are always present, co-localization of Cx36 with CaMKII, MUPP-1 and AF-6 was reported to be variable and perhaps indicative of their functional state (Flores et al., 2010; Lynn et al., 2012), suggesting that molecular asymmetry may be dynamically created at each GJ hemiplaque. While currently there are only emerging hints of molecular asymmetry at electrical synapses beyond the gap, it is highly likely there are many complexities hiding in these important structures. Such asymmetry and complexity would provide broad opportunities for independent modification of the pre- and postsynaptic compartments, with implications for regulation akin to chemical synapses. Identifying the extent and composition of these asymmetries represents a critical new area in electrical synapse formation and function.
Asymmetries at neuronal GJs producing electrical rectification need not be restricted to proteins, and could arise from different concentrations of channel-interacting diffusible cytosolic factors between heterologous cell types with different intercellular milieu (Palacios-Prado et al., 2014b). Recent data supports the notion that Mg++ controls neuronal coupling via modulation of gating mechanisms of Cx36 GJs by directly interacting with a Mg++-sensitive domain located in the channel’s lumen (Palacios-Prado et al., 2013). Asymmetries in the concentration of Mg++ were shown to produce electrical rectification (Palacios-Prado et al., 2013, 2014a). Other intracellular diffusible cations such as H+, Ca++ and spermine were also shown to affect GJ coupling in a Cx-specific manner (White et al., 1990; Musa et al., 2004; Harris and Contreras, 2014) but their effect on asymmetry of electrical communication has not yet been reported. Finally, electrical synapses were shown to interact heterosynaptically with nearby glutamatergic synapses (reviewed in (Pereda, 2014). Activation of NMDA receptors located either at the post-synaptic density or extrasynaptically in the vicinity of the GJ were shown to lead to activation of CaMKII and enhancement of coupling (Pereda et al., 1998; Kothmann et al., 2012; Turecek et al., 2014). These interactions seem to occur within the postsynaptic cell, providing another source of asymmetry at electrical synapses. It is unknown if this interaction is mediated via the diffusion of molecules or is supported by a molecular structure that is common to both synapses. Interaction via activation of mGluR receptors was also reported, although it is unclear if its actions are restricted to only one of the cells (Landisman and Connors, 2005; Wang et al., 2015b). Known and potential forms of asymmetry at electrical synapses are summarized in Fig. 4.
Conclusions
Channels are the defining feature of the electrical synapse and GJs in general, but their function needs to be supported by a number of other proteins. A number of GJs associated proteins have been described (Hervé et al., 2004). The question is: are the neuronal GJs of the electrical synapse just intercellular channels with ancillary proteins or rather do they represent true synaptic structures? From our perspective, given their functional and molecular complexity, electrical synapses represent genuine synaptic structures whose function is supported by multiple interacting molecules, as is the case for chemical (Rizo and Xu, 2015; Hanus and Ehlers, 2016) and immunological synapses (Dustin and Groves, 2012). Differences of these synaptic components are likely to underlie functional diversity amongst electrical synapses across all nervous systems.
Another source of functional diversity can arise from asymmetries in the distribution of some of the synaptic components. Given their relevance, it was expected that asymmetry of channel composition to be the first evidence for asymmetry at an electrical synapse (Phelan et al., 2008). However, asymmetry was unexpectedly found at a scaffolding trafficking protein (Nbea)(Miller et al., 2015), suggesting that asymmetries can originate from other components of the synapse. Such structural asymmetry could represent a potential source of rich functional diversity at electrical synapses. Electrical synapses might be then more structurally complex and functionally diverse than anticipated.
One of the important remaining questions is the energetics of electrical transmission. While the role of mitochondria in chemical transmission has been established (Zenisek and Matthews, 2000; Vos et al., 2010; Jonas, 2014; Picard, 2015) little is known of the relationship of this organelle with electrical synapses, although mitochondria have been found in association with GJs in the heart (Forbes and Sperelakis, 1982; Li et al., 2016) and postsynaptically at electrical synapses in the crayfish (Hanna et al., 1978) [Also note the proximity of mitochondria to the GJ in Fig. 2A]. Originally thought as less energetically costly (Bennett, 1966), the sophisticated functional properties and molecular complexity of electrical synapses suggest this might not be the case. In other words, the synaptic complexity and sophistication required to maintain the remarkable stability of electrical transmission during long periods of time might be highly demanding from the energetic point of view. Thus, electrical synapses might represent highly sophisticated and molecularly complex structures.
Acknowledgments
This research was supported by National Institutes of Health grants DC03186, DC011099, NS055726, NS085772 and NS0552827 to A.E.P and NS085035 to A.C.M.
REFERENCES
- Agullo-Pascual E, Delmar M. The Noncanonical Functions of Cx43 in the Heart. J Membr Biol. 2012;245:477–482. doi: 10.1007/s00232-012-9466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MVL, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: Implications for atrioventricular conduction in the heart. Proc Natl Acad Sci. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr GS, Mitchell CK, Keflemariam YJ, Heidelberger R, O’Brien J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem Biophys Res Commun. 2005;335:1191–1198. doi: 10.1016/j.bbrc.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mackie K, Triller A, O’Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Cha J, Kim H-L, Pan F, Chun M-H, Massey SC, Kim I-B. Variety of horizontal cell gap junctions in the rabbit retina. Neurosci Lett. 2012;510:99–103. doi: 10.1016/j.neulet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liu Q, Ge Q, Xie J, Wang Z-W. UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol. 2007;17:1334–1339. doi: 10.1016/j.cub.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolofan C, Li X-B, Olson C, Kamasawa N, Gebhardt BR, Yasumura T, Morita M, Rash JE, Nagy JI. Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina. Neuroscience. 2006;140:433–451. doi: 10.1016/j.neuroscience.2006.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer TP, van der Heyden MAG. Xenopus connexins: how frogs bridge the gap. Differentiation. 2005;73:330–340. doi: 10.1111/j.1432-0436.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- Diez JA, Ahmad S, Evans WH. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur J Biochem. 1999;262:142–148. doi: 10.1046/j.1432-1327.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- Duffy HS, Delmar M, Spray DC. Formation of the gap junction nexus: binding partners for connexins. J Physiol. 2002;96:243–249. doi: 10.1016/s0928-4257(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Groves JT. Receptor signaling clusters in the immune synapse. Annu Rev Biophys. 2012;41:543–556. doi: 10.1146/annurev-biophys-042910-155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Elias LAB, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Evans WH, Ahmad S, Diez J, George CH, Kendall JM, Martin PE. Trafficking pathways leading to the formation of gap junctions. Novartis Found Symp. 1999;219 doi: 10.1002/9780470515587.ch4. 44–54–9. [DOI] [PubMed] [Google Scholar]
- Falk MM, Bell CL, Kells Andrews RM, Murray SA. Molecular mechanisms regulating formation, trafficking and processing of annular gap junctions. BMC Cell Biol. 2016;17(Suppl 1):22. doi: 10.1186/s12860-016-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–2716. doi: 10.1093/emboj/16.10.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Cachope R, Nannapaneni S, Ene S, Nairn AC, Pereda AE. Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35. J Neurosci. 2010;30:9488–9499. doi: 10.1523/JNEUROSCI.4466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Li X, Bennett MVL, Nagy JI, Pereda AE. Interaction between connexin35 and zonula occludens-1 and its potential role in the regulation of electrical synapses. Proc Natl Acad Sci U S A. 2008;105:12545–12550. doi: 10.1073/pnas.0804793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Nannapaneni S, Davidson KGV, Yasumura T, Bennett MVL, Rash JE, Pereda AE. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci U S A. 2012;109:E573–E582. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MS, Sperelakis N. Association between mitochondria and gap junctions in mammalian myocardial cells. Tissue Cell. 1982;14:25–37. doi: 10.1016/0040-8166(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Goodenough Da, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JS, Zavala B, Landisman CE. Activity-dependent long-term depression of electrical synapses. Science. 2011;334:389–393. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RB, Keeter JS, Pappas GD. The fine structure of a rectifying electrotonic synapse. J Cell Biol. 1978;79:764–773. doi: 10.1083/jcb.79.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol. 2016;39:8–16. doi: 10.1016/j.conb.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Harris AL, Contreras JE. Motifs in the permeation pathway of connexin channels mediate voltage and Ca (2+) sensing. Front Physiol. 2014;5:113. doi: 10.3389/fphys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé J-C, Bourmeyster N, Sarrouilhe D. Diversity in protein-protein interactions of connexins: emerging roles. Biochim Biophys Acta. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Irion U, Frohnhöfer HG, Krauss J, Çolak Champollion T, Maischein H-M, Geiger-Rudolph S, Weiler C, Nüsslein-Volhard C. Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. Elife. 2014;3:e05125. doi: 10.7554/eLife.05125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA. Contributions of Bcl-xL to acute and long term changes in bioenergetics during neuronal plasticity. Biochim Biophys Acta. 2014;1842:1168–1178. doi: 10.1016/j.bbadis.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HMM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic Transmission from Horizontal Cells to Cones Is Impaired by Loss of Connexin Hemichannels Wong ROL, ed. PLoS Biol. 2011;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmann D, Lüttjohann A, Seidenbecher T, Coulon P, Pape H-C. Short-term depression of gap junctional coupling in reticular thalamic neurons of absence epileptic rats. J Physiol. 2016;594:5695–5710. doi: 10.1113/JP271811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Trexler EB, Whitaker CM, Li W, Massey SC, O’Brien J. Nonsynaptic NMDA receptors mediate activity-dependent plasticity of gap junctional coupling in the AII amacrine cell network. J Neurosci. 2012;32:6747–6759. doi: 10.1523/JNEUROSCI.5087-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WWW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg MM, Deuchars J, Weiss E, Schober A, Sonntag S, Wellershaus K, Draguhn A, Willecke K. Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci. 2008;37:119–134. doi: 10.1016/j.mcn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Laird DW. The life cycle of a connexin: gap junction formation, removal, and degradation. J Bioenerg Biomembr. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Gonzalez A, Fischer C, Krebs EH. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Neurobiology. 1987;84:7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf U, Giepmans BNG, Lopez P, Braconnot S, Chen S-C, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chuang AZ, O’Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Jin D, Hata M, Takai S, Yamanishi K, Shen W, El-Darawish Y, Yamanishi H, Okamura H. Dysfunction of mitochondria and deformed gap junctions in the heart of IL-18-deficient mice. Am J Physiol Heart Circ Physiol. 2016;311:H313–H325. doi: 10.1152/ajpheart.00927.2015. [DOI] [PubMed] [Google Scholar]
- Li X, Lu S, Nagy JI. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Olson C, Lu S, Nagy JI. Association of connexin36 with zonula occludens-1 in HeLa cells, betaTC-3 cells, pancreas, and adrenal gland. Histochem Cell Biol. 2004;122:485–498. doi: 10.1007/s00418-004-0718-5. [DOI] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. J Neurosci. 1988;8:1302–1312. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lynn BD, Li X, Nagy JI. Under construction: building the macromolecular superstructure and signaling components of an electrical synapse. J Membr Biol. 2012;245:303–317. doi: 10.1007/s00232-012-9451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marandykina A, Palacios-Prado N, Rimkutė L, Skeberdis VA, Bukauskas FF. Regulation of connexin36 gap junction channels by n-alkanols and arachidonic acid. J Physiol. 2013;591:2087–2101. doi: 10.1113/jphysiol.2013.250910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989;86:7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Chen C, Yan D. Regulation of Gap Junction Dynamics by UNC-44/ankyrin and UNC-33/CRMP through VAB-8 in C. elegans Neurons Copenhaver GP, ed. PLOS Genet. 2016;12:e1005948. doi: 10.1371/journal.pgen.1005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992;119:179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Voelker LH, Shah AN, Moens CB. Neurobeachin is required postsynaptically for electrical and chemical synapse formation. Curr Biol. 2015;25:16–28. doi: 10.1016/j.cub.2014.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nair R, Lauks J, Jung S, Cooke NE, de Wit H, Brose N, Kilimann MW, Verhage M, Rhee J. Neurobeachin regulates neurotransmitter receptor trafficking to synapses. J Cell Biol. 2013;200:61–80. doi: 10.1083/jcb.201207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KR, Maricq AV. Innexin function: minding the gap junction. Curr Biol. 2007;17:R812–R814. doi: 10.1016/j.cub.2007.07.043. [DOI] [PubMed] [Google Scholar]
- O’Brien J. The ever-changing electrical synapse. Curr Opin Neurobiol. 2014;29C:64–72. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol. 1999;114:339–364. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Nishikawa K, Hiroaki Y, Fujiyoshi Y. Electron tomographic analysis of gap junctions in lateral giant fibers of crayfish. J Struct Biol. 2011;175:49–61. doi: 10.1016/j.jsb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Oshima A, et al. Atomic structure of the innexin-6 gap junction channel determined by cryo-EM. Nat Commun. 2016;7:13681. doi: 10.1038/ncomms13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Chapuis S, Panjkovich A, Fregeac J, Nagy JI, Bukauskas FF. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat Commun. 2014a;5:4667. doi: 10.1038/ncomms5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Hoge G, Marandykina A, Rimkute L, Chapuis S, Paulauskas N, Skeberdis VA, O’Brien J, Pereda A, Bennett MVL, Bukauskas FF. Intracellular Magnesium-Dependent Modulation of Gap Junction Channels Formed by Neuronal connexin36. J Neurosci. 2013;33:4741–4753. doi: 10.1523/JNEUROSCI.2825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Huetteroth W, Pereda AE. Hemichannel composition and electrical synaptic transmission: molecular diversity and its implications for electrical rectification. Front Cell Neurosci. 2014b:8. doi: 10.3389/fncel.2014.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Low resistance junctions in crayfish. I. Two arrays of globules in junctional membranes. J Cell Biol. 1973;57:66–76. doi: 10.1083/jcb.57.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Excitable membrane ultrastructure. I. Freeze fracture of crayfish axons. J Cell Biol. 1974;61:107–122. doi: 10.1083/jcb.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda a E, Bell TD, Chang BH, Czernik a J, Nairn a C, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci U S A. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci U S A. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci. 2014;15:250–263. doi: 10.1038/nrn3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE. The Variable Strength of Electrical Synapses. Neuron. 2016;90:912–914. doi: 10.1016/j.neuron.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Faber DS. Activity-dependent short-term enhancement of intercellular coupling. J Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Nairn AC, Wolszon LR, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosci. 1994;14:3704–3712. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phelan P, Goulding LA, Tam JL, Allen MJ, Dawber RJ, Davies JA, Bacon JP. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol. 2008;18:1955–1960. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- Picard M. Mitochondrial synapses: intracellular communication and signal integration. Trends Neurosci. 2015;38:468–474. doi: 10.1016/j.tins.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3’:5’-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl M, Lehmann C, Gumpert A, Denizot J-P, Segretain D, Falk MM. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–347. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, Flores CE, Yasumura T, O’Brien J, Lynn BD, Bukauskas FF, Nagy JI, Pereda AE. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–969. doi: 10.1016/j.neuron.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Kamasawa N, Vanderpool KG, Yasumura T, O’Brien J, Nannapaneni S, Pereda AE, Nagy JI. Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain. Neuroscience. 2015;285:166–193. doi: 10.1016/j.neuroscience.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Xu J. The Synaptic Vesicle Release Machinery. Annu Rev Biophys. 2015;44:339–367. doi: 10.1146/annurev-biophys-060414-034057. [DOI] [PubMed] [Google Scholar]
- Scott CA, Tattersall D, O’Toole EA, Kelsell DP. Connexins in epidermal homeostasis and skin disease. Biochim Biophys Acta. 2012;1818:1952–1961. doi: 10.1016/j.bbamem.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta - Biomembr. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Shah AN, Davey CF, Whitebirch AC, Miller AC, Moens CB. Rapid reverse genetic screening using CRISPR in zebrafish. Nat Methods. 2015;12:535–540. doi: 10.1038/nmeth.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan Y-N, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Triller A, Marty S. Ultrastructural organization of presynaptic terminals. Curr Opin Neurobiol. 2011;21:261–268. doi: 10.1016/j.conb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Simonsen KT, Moerman DG, Naus CC. Gap Junctions in C. elegans. Name Front Physiol. 2014;5:40. doi: 10.3389/fphys.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu RCF, Smirnova E, Brown CA, Zoidl C, Spray DC, Donaldson LW, Zoidl G. Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin. Front Mol Neurosci. 2016;9:120. doi: 10.3389/fnmol.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Korn H. Morphological correlates of electrical and other interactions through low-resistance pathways between neurons of the vertebrate central nervous system. Int Rev Cytol. 1978;55:67–107. doi: 10.1016/s0074-7696(08)61887-2. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Llinas R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 2009a;4:16. doi: 10.1186/1749-8104-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 2009b;4:16. doi: 10.1186/1749-8104-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoboszlay M, Lőrincz A, Lanore F, Vervaeke K, Silver RA, Nusser Z. Functional Properties of Dendritic Gap Junctions in Cerebellar Golgi Cells. Neuron. 2016 doi: 10.1016/j.neuron.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek J, Yuen GS, Han VZ, Zeng X-H, Bayer KU, Welsh JP. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron. 2014;81:1375–1388. doi: 10.1016/j.neuron.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle R, Masuko S, Nakajima Y. Freeze-fracture study of the large myelinated club ending synapse on the goldfish Mauthner cell: special reference to the quantitative analysis of gap junctions. J Comp Neurol. 1986;246:202–211. doi: 10.1002/cne.902460206. [DOI] [PubMed] [Google Scholar]
- Urschel S, Höher T, Schubert T, Alev C, Söhl G, Wörsdörfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lin Y-P, Mitchell CK, Ram S, O’Brien J. Two-color fluorescent analysis of connexin 36 turnover: relationship to functional plasticity. J Cell Sci. 2015a;128:3888–3897. doi: 10.1242/jcs.162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Herberg FW, Laue MM, Wullner C, Hu B, Petrasch-Parwez E, Kilimann MW. Neurobeachin: A protein kinase A-anchoring, beige/Chediak-higashi protein homolog implicated in neuronal membrane traffic. J Neurosci. 2000;20:8551–8565. doi: 10.1523/JNEUROSCI.20-23-08551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neely R, Landisman CE. Activation of Group I and Group II Metabotropic Glutamate Receptors Causes LTD and LTP of Electrical Synapses in the Rat Thalamic Reticular Nucleus. J Neurosci. 2015b;35:7616–7625. doi: 10.1523/JNEUROSCI.3688-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RL, Doeller JE, Verselis VK, Wittenberg BA. Gap junctional conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca++ JGenPhysiol. 1990;95:1061–1075. doi: 10.1085/jgp.95.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-L, Shih M-FM, Lai JS-Y, Yang H-T, Turner GC, Chen L, Chiang A-S. Heterotypic gap junctions between two neurons in the drosophila brain are critical for memory. Curr Biol. 2011;21:848–854. doi: 10.1016/j.cub.2011.02.041. [DOI] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Nicholson BJ. The role of connexins in ear and skin physiology — Functional insights from disease-associated mutations. Biochim Biophys Acta - Biomembr. 2013;1828:167–178. doi: 10.1016/j.bbamem.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Zolnik TA, Connors BW. Electrical synapses and the development of inhibitory circuits in the thalamus. J Physiol. 2016 doi: 10.1113/JP271880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–1815. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]