Abstract
The eye is innervated by neurons derived from both the central nervous system and peripheral nervous system. While much is known about retinal neurobiology and phototransduction, less attention has been paid to the innervation of the eye by the PNS and the roles it plays in maintaining a functioning visual system. The ophthalmic branch of the trigeminal ganglion contains somas of neurons that innervate the cornea. These nerves provide sensory functions for the cornea and are referred to as intraepithelial corneal nerves (ICNs) consisting of subbasal nerves and their associated intraepithelial nerve terminals. ICNs project for several millimeters within the corneal epithelium without Schwann cell support. Here, we present evidence for the hypothesis that corneal epithelial cells function as glial cells to support the ICNs. Much of the data supporting this hypothesis is derived from studies of corneal development and the reinnervation of the ICNs in the rodent and rabbit cornea after superficial wounds. Corneal epithelial cells activate in response to injury via mechanisms similar to those induced in Schwann cells during Wallarian Degeneration. Corneal epithelial cells phagocytize distal axon fragments within hours of ICN crush wounds. During aging, the proteins, lipids, and mitochondria within the ICNs become damaged in a process exacerbated by UV light. We propose that ICNs shed their aged and damaged termini and continuously elongate to maintain their density. Available evidence points to new unexpected roles for corneal epithelial cells functioning as surrogate Schwann cells for the ICNs during homeostasis and in response to injury.
Keywords: cornea, epithelium, Schwann cells, PNS, wound response, corneal nerves
The cornea and its innervation
Schwann cells are the primary glial cells of the peripheral nervous system (PNS) and have several functions. They produce myelin that surrounds and insulates neurons and increases their rate of neural transmission. They phagocytize axonal debris during development and after injury and coordinate cytokine signaling and inflammatory responses with macrophages. These functions are carried out via controlled dedifferentiation, proliferation, migration, and re-differentiation (Jessen and Mirsky, 2016). There are two types of Schwann cells in mature PNS nerves: myelinating and non-myelinating. Extensive research has been done on the differentiation and functions of myelinating Schwann cells; less is known about mature non-myelinating Schwann cells in unwounded nerves (Jessen and Mirsky, 2005; Griffin and Thompson, 2008; Gordon, 2015).
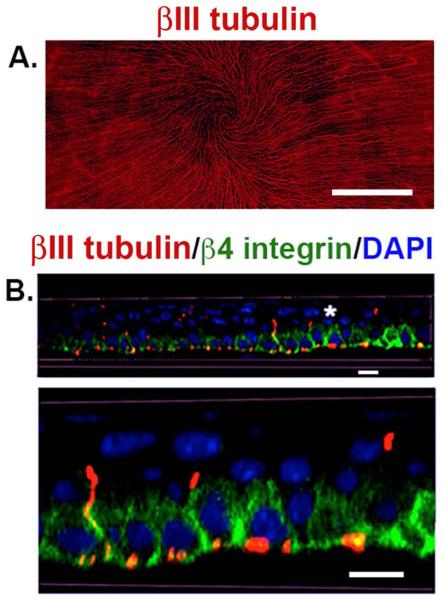
The only PNS nerves lacking Schwann cell support are free nerve endings (FNE) that play roles in sensory functions and penetrate the skin, cornea, and are present around hair follicles. In the skin and hair follicle, FNEs are typically shorter than 100 μm long. In the cornea, the FNEs extend for millimeters and are referred to as intraepithelial corneal nerves (ICNs), which consist of subbasal nerves (SBNs) and their associated intraepithelial nerve terminals (INTs). The density of ICNs in the rabbit cornea is 300-600 times that in the skin and 20-40 times that in the tooth pulp (Rózsa and Beuerman, 1982). High-resolution confocal images of the whole flat mounted mouse cornea en face show the density of sensory nerves in the cornea (Figure 1A); SBNs localize primarily within the β4 integrin-expressing corneal epithelial basal cells that make up the stratified squamous epithelium (Figure 1B) (Pajoohesh-Ganji, et al., 2015). INTs extend perpendicular to the basement membrane and terminate in the suprabasal and wing cell layers where β4 integrin is no longer expressed. When referring to corneal epithelial axon density determined experimentally using en facing imaging techniques, we use the term subbasal nerves (SBNs); apical axon extensions are referred to as INTs.
Figure 1. The corneal epithelial layer is densely innervated by subbasal nerves (SBNs).
A. This is a 21-panel projected and stitched spinning disk confocal image taken with a 25x objective showing the unwounded 8 week old Balb/c mouse flat mounted cornea stained to visualize the subbasal nerves using antibodies against βIII tubulin. The SBNs form a vortex at the apex of the cornea. The bar in A = 0.5 mm. B. Corneas from unwounded mice were stained to visualize the ICNs with βIII tubulin (red), β4 integrin (green), and nuclei with DAPI (blue) and imaged using a Zeiss 710 confocal microscope with a 60x oil objective. 3D confocal stacks were subjected to image processing using Volocity software and rotated to generate a cross section. The area identified by the asterisk was digitally enlarged and presented below. SBNs (red) localize adjacent to β4 integrin (green) at the basal and basolateral aspects of the corneal epithelial cells. β4 integrin expression is restricted primarily to the basal and basolateral membranes of the basal cells. Axons that project apically no longer interact with β4 integrin. Bars = 6 μm.
The corneal nerves originate from the trigeminal ganglion and enter the corneal stroma near the corneal limbus (Muller, et al., 2003; Guthoff, et al., 2005; He, et al., 2010; Marfurt, et al., 2010). They exit the corneal stroma through pores in the epithelial basement membrane and become surrounded by the plasma membranes of epithelial basal cells (Muller, et al., 2003). TEM studies have shown that the plasma membrane of corneal epithelial basal cells wraps around individual as well as groups of 1-40 subbasal axons (Muller, et al., 2003; Marfurt, et al., 2010) (Figure 2). When the nerves leave the stroma, associated Schwann cells do not accompany them into the epithelial compartment. The ICNs are derived from both myelinated and non-myelinated stromal nerves (Guthoff, et al., 2005). The sensory functions of these nerves have been studied extensively by Belmonte and colleagues (2004) and are of interest because of their involvement in neuropathic pain in dry eye and other diseases (Chao, et al., 2016).
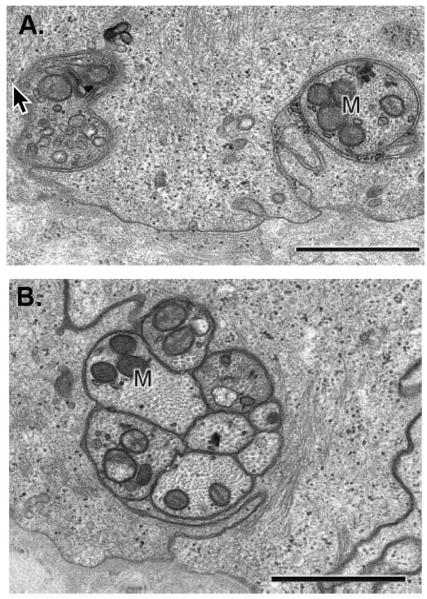
Figure 2. The SBNs contain variable numbers of individual axons bundled together.
A. Transmission electron micrograph of two cross-sectioned single nerve fibers cut in a plane perpendicular to the orientation of SBNs shown in Figure 1A. These nerves are wrapped in the basal membranes of the basal cells. B. Higher magnification of a cross-sectioned SBN bundle with 8 individual axons. This bundle is wrapped within the basolateral membranes of adjacent basal cells. Note in both A and B numerous mitochondria in the axons as indicated by the M. In addition, note that SBNs do not interact directly with the epithelial basement membrane. The cell membranes of the corneal epithelial basal cells wrap around the nerves giving the appearance in cross section that the nerves are being endocytosed by the basal cells. Images shown are taken from a paper by Muller and colleagues (2003) with permission from the publisher. Bars = 1 μm.
In vivo confocal imaging now allows for non-invasive imaging of the ICNs in the clinic (Malik, et al., 2003; Tervo and Moilanen, 2003; Guthoff, et al., 2009). Tervo and colleagues (2016) recently expressed concern over clinicians failing to acknowledge that en face in vivo confocal imaging visualizes SBNs well but can not quantify INTs. Evidence has accumulated to link changes in SBNs with various forms of neuropathy in both the central nervous system (CNS) and PNS (Ferrari, et al., 2013;Wang, et al., 2015). In the CNS, patients with Parkinson’s Disease (Kass-Iliyya, et al., 2015) and ALS (Ferrari, et al., 2014) have reduced density and increased tortuosity of their SBNs. Glaucoma patients also have reduced SBN density (Martone, et al., 2009; Labbé, et al., 2012); whether due to frequent application of topical medications containing preservatives or to pathology secondary to glaucoma deserves further study.
In the PNS, patients with a variety of pathologies that fall under the heading of small fiber neuropathy show defects in their corneal SBNs. These include patients with diabetes (Breiner, et al., 2014; Ziegler, et al., 2014; Maddaloni, et al., 2015), Wilson’s disease (Sturniolo, et al., 2015), fibromyalgia (Ramírez, et al., 2015), and Charcot-Marie-Tooth disease type 1A (Tavakoli, et al., 2012). Patients treated with specific chemotherapies for cancer can develop small fiber neuropathy with fewer SBNs (Campagnolo, et al., 2013). Non-invasive confocal imaging of the corneal SBNs may be useful to monitor progression of small fiber neuropathy (Jiang, et al., 2016).
The corneal epithelial basal cells that wrap around the SBNs adhere to the stroma via adhesion structures called hemidesmosomes that function to assure that the epithelium stays firmly adherent to the basement membrane and stroma (Stepp, et al., 1990; Zieske, et al., 1994). Corneal epithelial basal cells are renewed during homeostasis by proliferation coupled with differentiation; some basal cells remain and others leave the basement membrane and become suprabasal cells, wing cells, and apical squames. The cells that make up the apical surface of the corneal epithelium form tight junctions to act as barriers to infection by microorganisms and have surface specializations called microplicae that allow spreading and stabilization of the tear film (Gipson, 2004).
SBNs are typically millimeters long (He, et al., 2010; Marfurt, et al., 2010), raising the question of how they are maintained without the support of glial cells. At no other site in the PNS or CNS do nerves extend over such long distances without glial cell support. We propose that the basal, wing, and suprabasal cells of the corneal epithelium function as surrogate Schwann cells for the ICNs. Over time during evolution, the corneal epithelium appears to have taken over functions typically carried out by non-myelinating Schwann cells to maintain a dense collection of ICNs. In turn, the ICNs began providing the corneal epithelium with nutrients and raw materials to allow it to respond rapidly to eye injuries and preserve vision without vascular support.
The corneal epithelium surrounds SBNs, insulates, and stabilizes them via cell: cell and cell: substrate adhesions
Both myelinating and non-myelinating Schwann cells are associated with trigeminal nerves that innervate the corneal stroma; myelinated nerves are restricted to the periphery to minimize refractive error caused by myelin (Muller, et al., 2003). Although stromal nerves in the central cornea are not myelinated, they are supported by non-myelinating Schwann cells. Adhesion between sensory nerves and Schwann cells is mediated by cell: cell adhesion molecules including NCAM, L1CAM, and N-Cadherin (Doherty, et al., 1990; Letourneau, et al., 1990; Gess, et al., 2008; Zhang, et al., 2008; Jungnickel, et al., 2012) and integrins (Reichardt, et al., 1989; Niessen, et al., 1994; Berti, et al., 2006; van der Zee, et al., 2008; Afshari, et al., 2010; Pellegatta, et al., 2013) expressed on axon and Schwann cell plasma membranes. The majority of SBNs localize in close proximity to β4 integrin (Figure 1B); the apical branches of the INTs terminate in cells lacking β4 integrin.
In the PNS, small groups of axons are organized into bundles (fascicules) by a thin coating of extracellular matrix (ECM) that insulates and provides structural support (Esquisatto, et al., 2014). This ECM is produced by resident neural fibroblasts derived from neural crest cells (Joseph, et al., 2004). Neural-crest-derived corneal stromal cells, keratocytes, produce the stromal ECM (Hassell and Birk, 2010; Chen, et al., 2015). These cells may also make the ECM surrounding the stromal nerves or it may be made by another stromal cell type. Once nerves leave the stroma through the basement membrane and become SBNs, ECM made by fibroblasts can no longer support them.
We propose that corneal epithelial cells, which synthesize and secrete most of the proteins that make up the epithelial basement membrane zone (BMZ) (Ohji, et al., 1994; Zieske, et al., 1994; Torricelli, et al., 2013), also secrete ECM around the ICNs. The only non-epithelial cell-types present in the stratified squamous epithelium of the cornea are Langerhans cell-type dendritic cells (Hamrah, et al., 2002). At sites near where they leave the stroma, SBNs will retain the ECM produced by neural fibroblasts; in the center of the cornea, the corneal epithelial cells will produce the ECM coating around the SBNs. In addition, we predict that SBNs lose their ECM coating when they branch into INTs and extend apically since differentiated corneal epithelial suprabasal and wing cells down-regulate expression and function of integrins and ECM proteins (Stepp, 2006). Alternatively, the farther ICNs are from their location of entry into the epithelial compartment, the less ECM is present around the SBNs and INTs. TEM and functional studies will be needed to sort out whether corneal epithelial cells produce the ECM-coating for ICNs at the corneal center.
The organization of the ICNs revealed in TEM studies (Figure 2) is anatomically similar to that seen in the Remak bundles formed when non-myelinating Schwann cells wrap around non-myelinated C-fibers in the skin, gut, and corneal stroma (Yu, et al., 2009; Shaheen, et al., 2014). In the corneal epithelium, SBNs sit primarily between the basolateral and apical membranes of corneal epithelial basal and suprabasal cells and not beneath them as suggested by their name. A schematic diagram comparing cross sections of sensory axons in non-myelinating Schwann cells to SBNs in corneal epithelial cells is shown in Figure 3. From confocal images of the mouse cornea, we see fewer than 10% of INTs extend to the more apical squames; most are present within 1-5 μm from the basement membrane schematized in Figure 4; these values are similar to those reported by Rozsa and Beuerman (1982) in rabbit corneas. The diameters of SBNs vary depending on the number of individual axons bundled together. Their morphology varies as well; some SBNs are beaded while others are not. The beads are collections of mitochondria and endosomal vesicles (Muller, et al., 2003).
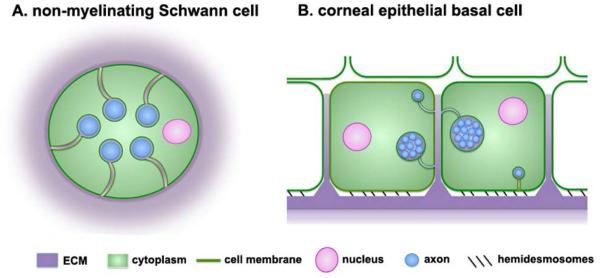
Figure 3. Non-myelinating Schwann cells and corneal epithelial cells wrap their cell membranes around sensory axons.
A. Schematic representation of a cross-section of a non-myelinating Schwann cell containing several axons. These cells are also referred to as Remak bundles or Remak Schwann cells. Axons are insulated from one another by the cytoplasm of the cell. Non-myelinating Schwann cells are embedded within the ECM under the basement membrane zone of the epidermis of the skin. B. Schematic representation of a cross section through two corneal epithelial basal cells whose cell membranes are wrapped around single axons or clusters containing several axons bundled together by ECM. Most axons are localized within the basolateral or apical membranes of the basal cells. Individual axons (blue) are the same diameter in both cell types. To make it easier to visualize the axons in the non-myelinating Schwann cell, the two schematic images are not shown at the same scale.
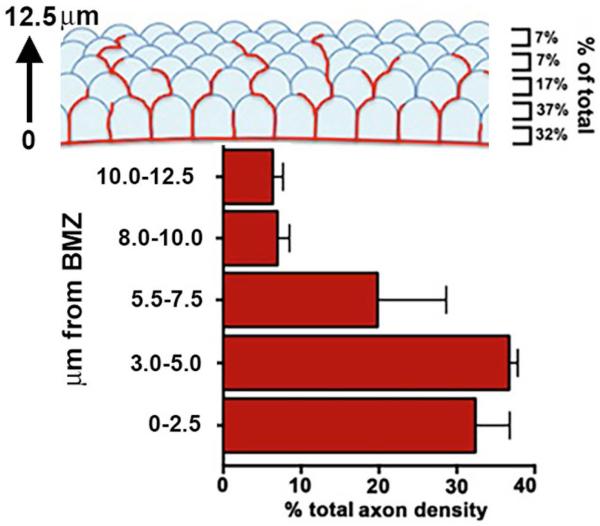
Figure 4. Intraepithelial corneal nerve density is greatest at the basolateral and apical aspects of the corneal basal cells.
Using confocal stacks of images acquired for assessment of axon density, we determined in unwounded mouse corneas, axon density as a function of distance from the basement membrane zone (BMZ). We defined the BMZ as the site where β4 integrin, the integrin present within hemidesmosomes, had its maximum intensity. ~70% of all axon density is seen within 5 μm of the BMZ and includes the basal cells and the basal-most aspect of the suprabasal cells. Only 7-10% of all ICN density is found within the apical squames.
Keeping SBNs near the basement membrane
Maintaining SBNs within basolateral and apical membranes of basal epithelial cells that differentiate every two to three weeks requires that SBNs transfer from the plasma membranes of differentiating cells to adjacent basal cells during corneal homeostasis. A SBN that is 1 mm long and 2-3 μm in diameter would interact with the plasma membranes of ~250 corneal epithelial basal cells if each basal cell is estimated to have a size of 4x4x4 μm. Thicker axon bundles, 5-10 μm in diameter, would associate with the plasma membranes of both corneal epithelial basal and suprabasal cells simultaneously.
Mechanisms must have evolved to keep SBNs in their anatomical niche near the BMZ. One likely mechanism would exploit differential integrin expression by corneal epithelial cells at various stages of their differentiation. As seen in Figure 1B, β4 integrin is restricted to the basal, basolateral, and apical membranes of basal cells in unwounded corneas. Corneal epithelial progenitor cells at the limbus have higher expression of α6β4 and α3β1 integrins (Pajoohesh-Ganji, et al., 2006; Stepp, 2006). Down-regulation of integrin expression occurs as basal cells differentiate, leave the BMZ, and become wing and suprabasal cells (Stepp, 2006; Stepp, et al., 2014).
While changes in integrin expression in basal corneal epithelial cells likely play a role in retaining SBNs near the basement membrane, other factors released by immune cells, stromal nerves, stromal cells (keratocytes), and/or the SBNs themselves are also probably involved. Such factors could be stored in the epithelial basement membrane and released over time to create chemokine gradients within the epithelium.
Corneal epithelial cells phagocytize axon debris
Although published almost a century ago in the Journal of Experimental Zoology (Matsumoto, 1918), the ability of corneal epithelial cells to phagocytize material in their environment received little attention for many years. Since then, corneal epithelial cells have been shown to phagocytize particulates, to mediate, in part, allergic responses at the ocular surface (Niederkorn, et al., 1989), and to engulf heat-killed yeast (Hua, et al., 2015). Corneal epithelial basal cells can ingest live and dead bacteria, reducing the numbers of bacteria present on the ocular surface after scratch injury, modulating the immune response to injury, and reducing corneal scarring (Fleiszig and Evans, 2002; Fleiszig, et al., 2003). Taken together, these data show that corneal epithelial cells are capable of phagocytosis of pathogens and other debris.
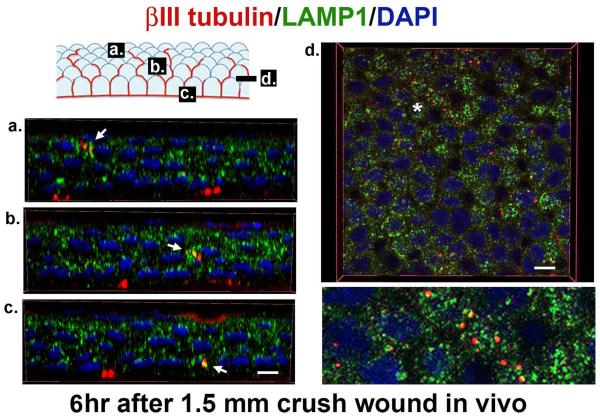
Schwann cells both produce and maintain myelin during homeostasis and phagocytize myelin and axonal debris after axon injury. We recently showed that SBNs disappear within three hours after the cornea is placed in organ culture (Stepp, et al., 2014). Since corneas were incubated ex vivo, it is unlikely that immune cells mediated the rapid destruction of SBNs. In Figure 5 we show that, in vivo, by six hours after crush wounds to the corneal SBNs, degenerating ICN fragments co-localize in corneal epithelial cells with LAMP1, a protein expressed in lysosomes where phagocytized debris is degraded. Corneal epithelial cells actively degrade axon fragments after they are severed or crushed.
Figure 5. Corneal epithelial cells phagocytize axon fragments.
3D confocal images using 100x oil immersion objectives were obtained to show fragments of ICNs (βIII-tubulin, red) accumulating within lysosomes (LAMP1, green) 6 hours after crush wounds to the cornea. When βIII-tubulin co-localizes with LAMP1+, structures are yellow. In the schematic, the letters a, b, and c correspond to the images presented below showing co-localization (arrows) of βIII-tubulin and LAMP1 in wing (a), suprabasal (b), and basal (c) corneal epithelial cells. These 3D images were generated using Volocity software. 3D images rotated and viewed en face in a section through the suprabasal cell layer in d. The area indicated by asterisk in d is magnified 3x and presented below. Nuclei are stained with DAPI (blue). Bars are 3 μm on the left and right, respectively.
Superficial injuries to the cornea similar to those studied in the mouse are the major cause of ophthalmology related emergency room visits (Channa, et al., 2016). Due to their proximity to the ocular surface and small diameter, ICNs are readily damaged along with corneal epithelial cells by superficial injuries. Phagocytosis of axonal debris would remove damaged lipids and proteins and provide corneal epithelial cells with a source of nutrients to assist in their activation and migration after injury.
Similarities between the roles played by Schwann cells and corneal epithelial cells after axon injury
Schwann cells and Wallerian Degeneration
Schwann cells play major roles in the immediate response of peripheral nerves to injury by inducing Wallerian Degeneration (WD). WD was first observed in 1850 (reviewed in Stoll et al., 2002); the term refers to the events that occur in the stump proximal to the crush injury and in the denervated distal nerve stump (Scheib and Hoke, 2013; Gordon, 2015; DeFrancesco-Lisowitz, et al., 2015). Schwann cells mediate many of the events in WD. Once myelinating Schwann cells are activated by axon injury, they de-differentiate into cells referred to as non-myelinating, immature, or repair Schwann cells (DeFrancesco-Lisowitz, et al., 2015; Jessen and Mirsky, 2016). WD is thought to be one of the major reasons injuries to nerves in the PNS heal better than those in the CNS where oligodendrocytes myelinate nerves. A promising line of investigation to improve recovery after spinal cord injury involves injections of repair Schwann cells to treat CNS injuries (Ban, et al., 2011; Bosse, 2012).
The prototype wound model used to define WD is crush or transection injury to the sciatic nerve in rodents (Wall, et al., 1979; Bosse, 2012, Scheib and Höke, 2013). Within seconds after injury, calcium ions from the extracellular space enter both the proximal and severed axon stump and activate calpain. This triggers the resealing of the plasma membrane of the proximal stump and fragmentation of the axon in the distal stump. These events release Toll-like receptor ligands that lead to activation of Schwann cells around both proximal and distal axon stumps and their dedifferentiation into repair Schwann cells. Within three to five hours after injury, repair Schwann cells secrete cytokines that induce recruitment of immune cells.
At the proximal nerve stump, severed axons undergo dieback leaving bulb or knob-like structures at their tips (Gordon, 2015). Dieback is mediated by proteases secreted by repair Schwann cells and leukocytes and extends to the first intact node of Ranvier, a distance of between 6-14 μm in adult mouse PNS nerves (Jaros and Jenkison, 1983). After dieback, it takes several hours before a growth cone reforms and the axon begins to regenerate. These events are accompanied by increased protein expression in the neuron soma to provide the raw materials needed to regrow axons. In the proximal stumps of severed non-myelinated nerves, it is not known whether dieback occurs.
Within 24 hours after sciatic nerve injury, repair Schwann cells activate cJun and begin to proliferate (Jessen and Mirsky, 2016). They secrete matrix metalloproteinases (MMPs) and, along with macrophages, phagocytize axon fragments. As WD proceeds, hollow tubes containing ECM (bands of Bunger) are left behind; Bunger bands guide regenerating axons and repair Schwann cells as they reinnervate (Shen, et al., 2001; Gordon, 2015). Whether structures similar to Bunger bands guide axon regeneration in regenerating non-myelinated nerves in the PNS has yet to be demonstrated. The cornea is an ideal site to look for these structures after crush wounds.
The response of corneal epithelial cells to injury shares features seen in Schwann cells during Wallerian Degeneration
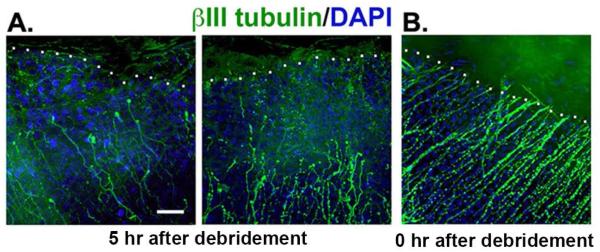
Corneal epithelial wound healing in humans and rodents has been well studied over the years (reviewed in Stepp, et al., 2014; Liu and Kao, 2015; Ljubimov and Saghizadeh, 2015). Many of the events mediated by activated Schwann cells during WD after sciatic nerve crush are seen in activated corneal epithelial cells after injuries that sever the ICNs (Table 1). We assessed the extent of SBN dieback in 1.5 mm mouse corneal debridement wounds that sever the SBNs. Within five hours, we observe dieback of the proximal nerve stumps from the wound margin by 16-54 μm with a mean of 39 μm (Figure 6A), compared to the 6-14 μm dieback in myelinated mouse PNS nerves (Jaros and Jenkison, 1983). As in the rest of the PNS, we also see knobs at the terminus of severed SBNs. The SBNs of corneas fixed within minutes of severing the axons remain at the cut edge of the tissue and show no retraction (Figure 6B). Proximal axon dieback is not restricted to myelinated nerves but also occurs in non-myelinated sensory nerves. How dieback is regulated and what signals SBNs to form a growth cone and initiate reinnervation as dieback is completed is not known.
Table 1.
Common responses to axon injury to the sciatic nerve and to intraepithelial corneal nerves (ICNs)
| time course | tissue response | sciatic nerve1 |
corneal ICNs2 |
|---|---|---|---|
| < 1 hour | Ca++ influx | + | + |
| 4–18 hours |
axon dieback,
TLR-release |
+ | + |
| 1–4 days |
MMP9 activation,
cell proliferation |
+ | + |
| 5–14 days |
macrophage
recruitment |
+ | + |
| 5 hours–28 days |
staggered axon
outgrowth |
+ | + |
taken from Gordon, et al., 2015 and Jessen and Mirsky, 2016
see text for specific citations
Figure 6. Proximal stubs of the SBNs dieback towards the periphery within 5 hours after injury.
Corneas wounded by 1.5 mm debridement injury and either sacrificed immediately or five hours after wounding. Tissues were stained to reveal the localization of the severed distal tips of the SBNs using an antibody against βIII tubulin (green) and the cell nuclei using DAPI (blue). A: Axons in corneas from mice sacrificed five hours after injury reveal significant retraction back from the leading edge. The white dotted line indicates the margin of the wound edge where epithelial cells are missing. The mean distance SBNs retracted was 39 μm but there is significant variation between corneas from different mice. SBNs, like myelinated PNS nerves, undergo dieback after wounding. B: Axons remain at the margin of the wound site in corneas from mice sacrificed within minutes of injury. Bar =10 μm.
Corneal epithelial cells are in an avascular environment and contain large stores of glycogen that acts as their primary energy source (Thoft and Friend, 1977). Glycogen storage in corneal epithelial cells is mediated by miRNA-31 and factor inhibiting hypoxia inducing factor (FIH-1) which in turn regulates corneal epithelial cell migration and wound repair (Peng, et al., 2012; Peng, et al., 2013; Wang, et al., 2009). Schwann cells in the PNS also store glycogen; during hypoglycemia, they release glycogen, in the form of the metabolite lactate, to support axonal function (Brown, et al., 2012; Stassart and Nave, 2015). It is not known what roles glycogen and lactate play in repair of nerves supported by non-myelinating Schwann cells. The close relationship between corneal epithelial cells and intraepithelial corneal nerves and the fact that glycogen can be visualized in corneal SBNs (Muller, et al., 1996) suggest that corneal epithelial cells may provide glycogen and/or lactate to SBNs during homeostasis and wound repair.
Cytokine mRNA and Toll-like receptor signaling increase after both sciatic nerve (Goethals, et al., 2010) and corneal injury leading to recruitment in the cornea of immune cells (Pearlman, et al., 2008; Eslani, et al., 2014). Overall protein synthesis (Zieske and Gipson, 1986) and MMP9 expression relative to total protein increase in corneal epithelial cells after injury (Pal-Ghosh, et al., 2011; Mauris, et al., 2014). Injured corneal epithelial cells secrete defensin proteins (Haynes, et al., 1999; McDermott, 2004). After migration is completed (20-22 hours after 1.5 mm injury), corneal epithelial cells proliferate to reestablish epithelial thickness (Stepp, et al., 2014). JNK signaling is also activated when corneas are injured or stressed by exposure to UV light (Block, et al., 2004; Black, et al., 2011). The majority of the severed axons take seven days to regenerate significant distances (Pajoohesh-Ganji, et al., 2015). Smaller (1 mm) mouse debridement wounds reinnervate more quickly and completely but even after four weeks, the SBNs at the center do not assume the morphology present before injury (Pal-Ghosh, et al, 2014).
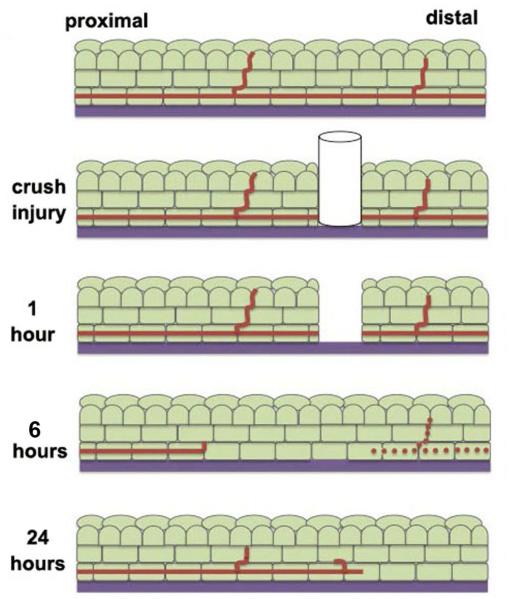
A second mouse corneal injury model used is the 1.5 mm crush injury (Pajoohesh-Ganji, et al. 2015; Reichard, et al., 2014). In this model, a 1.5 mm trephine is pressed onto the corneal epithelium and rotated to sever the SBNs. Epithelial cells are not removed. Within six hours after these wounds, SBNs in the corneal center start to degrade; 50% of the SBNs remain intact since they enter the epithelium from the stroma within the 1.5 mm site defined by the trephine (Pajoohesh-Ganji, et al., 2015). The morphology and density of SBNs return to levels seen before injury between one to four weeks. Figure 7 summarizes schematically the events that take place after corneal subbasal nerves are severed by crush wounding.
Figure 7. Changes in subbasal nerves in the cornea after crush injury.
At one hour after a crush wound, cells at the site of the injury die and the distal aspect of the axon is severed from the proximal aspect, which remains attached to the soma in the trigeminal ganglion. Between 5–18 hours after wounding, dieback of the proximal axon is observed and the distal axon loses it’s integrity. Axon fragments can be seen within lysosomes of the corneal epithelial cells. By 24 hours, axon regrowth can be observed.
By studying similarities and differences in the injury response programs followed by the cells that make up the sciatic nerve and those of the corneal epithelium after nerve injury we hope to develop new insight into the requirements needed for successful axonal regeneration.
Corneal epithelial cells express numerous Schwann cell markers
Schwann cells express specific sets of proteins that are used as markers for their isolation and characterization (Jessen and Mirsky, 2005; Liu, et al., 2015). These include GAP43, S100B, p75NTR, Sox2, Sox10, NCAM, MBP, and MPZ. GAP43 is expressed in the corneal epithelium (Martin and Bazan, 1992; Lin and Bazan, 1995) and upregulated in response to injury (Chaudhary, et al., 2012). S100B has been demonstrated in the chicken and mouse corneal epithelium (Conrad, et al., 2009) and increases in expression in response to fungal infection of the mouse cornea (Zhang, et al., 2016). p75NTR has been reported in the human corneal limbal epithelium (Qi, et al., 2007). Sox2 has been studied in the developing mouse cornea and found to be involved in mediating Pax6 function in the corneal epithelium (Aota, et al., 2003). Sox2 is expressed within corneal epithelial basal cells of Sox2 reporter mice (data not shown). NCAM is expressed in the developing and adult chick cornea (Mao, et al., 2012). Conrad and colleagues (2009) documented expression of Sox10, MBP, and MPZ in the chick corneal epithelium.
Based on their expression of numerous Schwann cell markers, it is not surprising that corneal epithelial cells can carry out many functions performed by non-myelinating Schwann cells supporting SBNs during homeostasis and facilitating reinnervation after injury. When, during evolution, corneal epithelium acquired the ability to function as surrogate non-myelinating Schwann cells for the SBNs and how the cells manage to carry out so many different functions is not known.
SBN loss stops corneal epithelial cell proliferation
Within 48 hours after denervation of PNS nerves by transection or crush injury, a subpopulation of the Schwann cells around the proximal and distal stumps convert into repair Schwann cells (DeFrancesco-Lisowitz, et al., 2015; Gordon, 2015; Jessen and Mirsky, 2016). In repair cells, genes encoding proteins involved in myelin synthesis are down regulated and those involving autophagy are upregulated. In addition, repair Schwann cells secrete cytokines to induce an immune response. After regeneration is complete, these cells differentiate back into mature Schwann cells. Prolonged denervation induces apoptosis of Schwann cells mediated by elevated expression of p75NTR (Ahmad, et al., 2015).
Neurotrophic keratitis is a potentially blinding condition whose corneal pathology (cloudiness, erosions, dry surface, increased risk of infections) results from reduced innervation of the cornea by stromal and ICNs (Sacchetti and Lambiase, 2014; Semeraro, et al., 2014; Shaheen, et al., 2014). It occurs secondary to neuropathy of the trigeminal nerve or after ocular surgery, trauma, and/or viral infections. Within 24 hours after surgical denervation of the trigeminal ganglion in mice, the corneal SBNs degenerate and the corneal surface becomes cloudy (Ferrari, et al., 2011). By day 7, corneal epithelial cells cease proliferating and begin to undergo apoptosis; surface markers for corneal epithelial stem cells in the limbus disappear and erosions form (Ueno, et al., 2012). These data demonstrate that during homeostasis, corneal epithelial cells require functional ICNs. Complete denervation induces many of the same changes in corneal epithelial cells (Ferrari, et al., 2011) induced in Schwann cells after sciatic nerve crush injury. The tonic release of neuropeptides and other trophic factors by ICNs may supply nutrients essential to maintaining corneal epithelial cell differentiation, cell proliferation, and turnover. Damaged ICN fragments could also be phagocytized and used as energy sources by corneal epithelial cells.
SBNs continually remodel and grow during homeostasis
The extension of growth cones and their targeting to specific sites during development and after injury have been studied extensively. In response to injury, nerves and their associated Schwann cells upregulate expression of a number of proteins including those referred to collectively as regeneration associated genes (RAGs) (Van Der Zee, et al., 1989; Frey, et al., 2000; Shaheen, et al., 2014). We have used QPCR to analyze mRNA expression for 22 different RAG genes in the corneal epithelium as a function of time after 1.5 mm debridement injuries that induce reinnervation of the ICNs. All 22 RAG mRNAs studied are expressed in mRNA isolated from the unwounded corneal epithelium; only those mediating netrin and ephrin: Eph signaling were upregulated during active reinnervation (Pajoohesh-Ganji, et al., 2015).
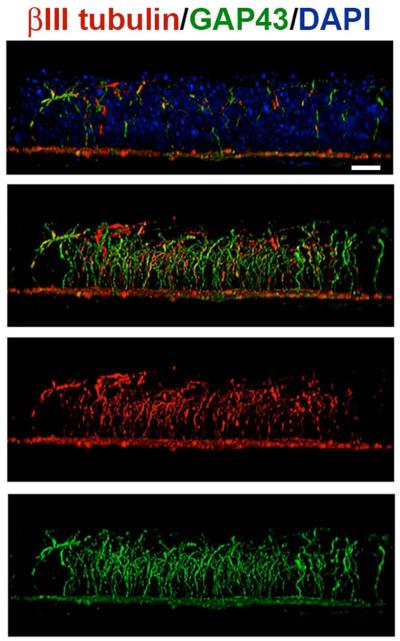
Growth associated protein 43 (GAP43) is one of the RAG proteins upregulated after corneal injury (Shaheen, et al., 2014). When we assessed unwounded and wounded corneal SBNs for GAP43 expression along with βIII tubulin, we found that GAP43 is strongly expressed in the stromal nerves as well as the apical axon extensions of the SBNs in unwounded corneas (Figure 8).
Figure 8. SBNs express the regeneration-associated protein GAP43 during homeostasis.
Unwounded corneas were stained to co-localize βIII tubulin (ρεδ) ανδ ΓΑΠ43(green); nuclei are indicated by staining with DAPI (blue) and used for whole mount imaging. Images were acquired with the 63x objective using confocal microscopy. Using Volocity, confocal image stacks are rotated to generate cross-sectional views. The field of view acquired in x and y is 135x135 μm; projection images show the full x or y field of view. The majority of the βIII tubulin+ SBNs in the unwounded cornea express GAP43 where it is localized within axons that extend apically. These data suggest that some axons are branching and growing more than others. Bar= 10 μm.
The expression of RAG mRNAs and the localization of the RAG protein GAP43 to intraepithelial nerve terminals during homeostasis suggest the axons are continuously growing. The growth and remodeling of corneal intraepithelial nerve terminals in live mice was first looked at by Harris and Purves (1989) using fluorescently tagged nerve fibers. They showed what was referred to as “remodeling” of nerve terminals in the uninjured corneal epithelium. These early studies led to others using transgenic mice expressing a fluorescent protein (Thy1-YFP) in intraepithelial corneal nerves (Yu and Rosenblatt, 2007; Namavari, et al., 2011; Sarkar, et al., 2013). Yu and Rosenblatt (2007) show the degeneration of distal stubs of nerve terminals within two days after transection. They also show that reinnervation occurs by successive rounds of nerve fiber extension and retraction. The study by Namavari and colleagues (2011) shows that the density of the intraepithelial nerves reaches adult levels six weeks after birth and that the arrangement of reinnervated nerve fibers after transection remains abnormal for times up to eight weeks after injury. Thy1-YFP is also expressed in immune cells; while fluorescent immune cells are observed adjacent to regenerating corneal stromal nerves in injured Thy-1 YFP mouse cornea (Sarkar, et al., 2013), they are not seen at sites where distal axon tips are degenerating in severed ICNs (Yu and Rosenblatt, 2007). The corneal epithelial cells must be responsible for the rapid clearance of debris generated by degenerating axon stubs.
A need for continuous growth of the ICNs becomes clear when we consider the damage done to lipids, proteins, and mitochondrial DNA over time with aging. Proteins, lipids, and organelles become damaged and cells are typically degraded via autophagy within specialized lysosomes called autophagosomes. While axons have mitochondria and endosomal vesicles, lysosomes are restricted to neuron cell bodies. Autophagy by Schwann cells would require that damaged proteins and lipids be transported within axons by retrograde transport to the stroma where debris could be degraded by resident myelinating and non-myelinating Schwann cells. Axon pruning via phagocytosis by glial cells has been well characterized during development in both the CNS and PNS.
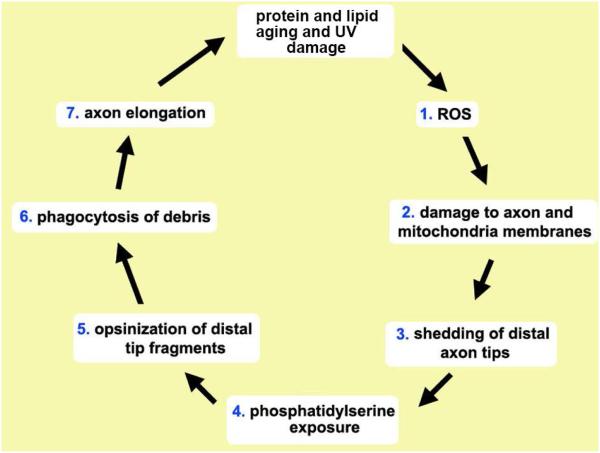
The ICNs are negatively impacted by direct exposure to UV light. Corneas exposed to excessive UVA and UVB show damage similar to that in the sunburned skin and develop a condition referred to as UV keratitis. Animal models of UV keratitis show that when exposed to excess UV light, corneal nerves are activated and become sensitized to cold (Acosta, et al., 2014). UV light induces DNA cross-links in corneal epithelial cell DNA (Shimmura, et al., 2004; Choy, et al., 2005). As seen in Figure 2, SBNs are rich in mitochondria; UV radiation damages mitochondria (Goyal, et al., 2015). The high density of ICNs on the cornea makes it more efficient for them to shed their termini and extend new axons. While we show in Figure 5 that corneal epithelial cells phagocytize axonal debris after injury, direct evidence documenting phagocytosis of axon fragments during homeostasis is not yet available. Figure 9 shows the sequence of events we propose mediates shedding of ICN distal axon tips and their phagocytosis by corneal epithelial cells. The phagocytic burden on corneal epithelial cells due to constant shedding of ICN fragments is high but could be offset by the nutrients and raw materials the digestion of axonal debris provides. There is precedence in the eye for epithelial cells phagocytizing shed distal axon fragments. Retinal Pigment Epithelial (RPE) cells phagocytize shed rod and cone outer segments.
Figure 9. Proposed mechanism for light induced SBN distal axon tip shedding and phagocytosis by corneal epithelial cells.
Protein and mitochondrial aging and light damage the activity of the enzymes responsible for maintaining (1) ROS balance inside axons and corneal epithelial cells. ROS leads to (2) DNA damage in SBN mitochondria and peroxidation of axonal proteins and lipids causing membrane fragmentation and leading to (3) the shedding of the SBN distal axon tips. Loss of membrane potential in SBN distal axon tips leads to (4) exposure of phosphatidylserine (PS) on the outer leaflet of their cell membranes. PS serves as a universal “eat me” signal and induces (5) opsinization of the axon fragments by galectin-3 and other glycoproteins present in the extracellular space between corneal epithelial basolateral membranes. Once opsinized, αvβ5 integrin mediated phagocytosis (6) takes place. To maintain subbasal nerve density, growth cones form on distal tips and SBNs elongate (7). In the retina, photoreceptor outer segments (POS) are shed and phagocytized by RPE cells via similar mechanism. Whether distal axon tip shedding and phagocytosis are subject to circadian regulation as seen in the shedding and phagocytosis of POS by RPE cells is not known.
In summary, we present evidence from the literature and our own studies showing that epithelial cells of the cornea wrap around and support the dense population of intraepithelial corneal nerves. The molecular and cellular responses of corneal epithelial cells to axonal injury are similar to those of Schwann cells during Wallarian Degeneration. These include expression of mRNAs for numerous RAGs as well as phagocytosis of degenerating axon tips shed from crushed or severed axons within hours after axon injury. We propose that the non-myelinated intraepithelial corneal nerves are not “free nerve endings” because corneal epithelial cells have taken over the functions typically provided by Schwann cells. Developing a better understanding of how corneal epithelial cells function as surrogate glial cells to maintain and support the intraepithelial corneal nerves and enhance their repair after injury will lead to new treatments for corneal pathologies and a better understanding of the causes of small fiber neuropathy.
Main points.
Intraepithelial corneal nerves (ICNs) of the cornea project for millimeters with no glial support.
Corneal epithelial cell (CEC) plasma membranes wrap around bundles of ICNs.
Axon fragments are phagocytized by CECs after crush injuries.
CECs function like Schwann cells in supporting ICNs.
Acknowledgements
This work was supported by a grant from the NIH to MAS (NEI R01-08512). Numerous colleagues and students contributed support, ideas, and helpful discussions over the years that impacted these studies and the data presented. These include Ahdeah Pajoohesh-Ganji, Anthony LaMantia, Thomas Maynard, Beverly Oakley, Stuart Yuspa, Maria Morasso, and Sally Moody. Imaging was done at the GWU Nanofabrication and Imaging Center.
References
- Acosta MC, Luna C, Quirce S, Gallar J. Corneal sensory nerve activity in an experimental model of UV keratitis. Invest Ophthalmol Vis Sci. 2014;55:3403–3412. doi: 10.1167/iovs.13-13774. C. [DOI] [PubMed] [Google Scholar]
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. GLIA. 2010;58:857–869. doi: 10.1002/glia.20970. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Fernando A, Gurgel R, Clark JJ, Xu L, Hansen MR. Merlin status regulates p75(NTR) expression and apoptotic signaling in Schwann cells following nerve injury. Neurobiol Dis. 2015;82:114–122. doi: 10.1016/j.nbd.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S-I, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol. 2003;257:1–13. doi: 10.1016/s0012-1606(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Ban D-X, Ning G-Z, Feng S-Q, Wang Y, Zhou X-H, Liu Y, Chen J-T. Combination of activated Schwann cells with bone mesenchymal stem cells: The best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6:707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Berti C, Nodari A, Wrabetz L, Feltri ML. Role of integrins in peripheral nerves and hereditary neuropathies. NeuroMol Med. 2006;8:191–204. doi: 10.1385/nmm:8:1-2:191. [DOI] [PubMed] [Google Scholar]
- Black AT, Gordon MK, Heck DE, Gallo MA, Laskin DL, Laskin JD. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochem Pharm. 2011;81:873–880. doi: 10.1016/j.bcp.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints. Role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Bosse F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349:5–14. doi: 10.1007/s00441-012-1389-5. [DOI] [PubMed] [Google Scholar]
- Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care. 2014;37:1418–1424. doi: 10.2337/dc13-2005. [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR. Schwann cell glycogen selectively supports myelinated axon function. Ann Neurol. 2012;72:406–418. doi: 10.1002/ana.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo M, Lazzarini D, Fregona I, Cacciavillani M, Bergamo F, Parrozzani R, Midena E, Briani C. Corneal confocal microscopy in patients with oxaliplatin-induced peripheral neuropathy. J Peri Ner System. 2013;18:269–271. doi: 10.1111/jns5.12036. [DOI] [PubMed] [Google Scholar]
- Channa R, Zafar SN, Canner JK, Haring RS, Schneider EB, Friedman DS. Epidemiology of eye-related emergency department visits. JAMA Ophthalmol. 2016;134:312–319. doi: 10.1001/jamaophthalmol.2015.5778. [DOI] [PubMed] [Google Scholar]
- Chao W, Belmonte C, Benitez Del Castillo JM, Bron AJ, Dua HS, Nichols KK, Novack GD, Schrader S, Willcox MD, Wolffsohn JS, Sullivan DA. Report of the Inaugural Meeting of the TFOS i2 = initiating innovation Series: Targeting the Unmet Need for Dry Eye Treatment. Ocular Surface. 2016;14:264–316. doi: 10.1016/j.jtos.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Namavari A, Yco L, Chang J-H, Sonawane S, Khanolkar V, Sarkar J, Jain S. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea. 2012;31:1460–1467. doi: 10.1097/ICO.0b013e318247b60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Mienaltowski MJ, Birk DE. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015;133:69–80. doi: 10.1016/j.exer.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy CK, Benzie IF, Cho P. UV-mediated DNA strand breaks in corneal epithelial cells assessed using the comet assay procedure. Photochem Photobiol. 2005;81:493–497. doi: 10.1562/2004-10-20-RA-347. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Albrecht M, Pettit-Scott M, Conrad GW. Embryonic corneal Schwann cells express some schwann cell marker mRNAs, but no mature Schwann cell marker proteins. Invest Ophthalmol Vis Sci. 2009;50:4173–4184. doi: 10.1167/iovs.08-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neurosci. 2015;302:174–203. doi: 10.1016/j.neuroscience.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Fruns M, Seaton P, Dickson G, Barton CH, Sears TA, Walsh FS. A threshold effect of the major isoforms of NCAM on neurite outgrowth. Nature. 1990;343:464–466. doi: 10.1038/343464a0. [DOI] [PubMed] [Google Scholar]
- Eslani M, Movahedan A, Afsharkhamseh N, Sroussi H, Djalilian AR. The role of toll-like receptor 4 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2014;55:6108–6115. doi: 10.1167/iovs.14-14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquisatto MAM, de Aro AA, Fôo HB, Gomes L. Changes in the connective tissue sheath of Wistar rat nerve with aging. Annals Anat. 2014;196:441–448. doi: 10.1016/j.aanat.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Chauhan SK, Ueno H, Nallasamy N, Gandolfi S, Borges L, Dana R. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011;52:2532–2539. doi: 10.1167/iovs.10-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Grisan E, Scarpa F, Fazio R, Comola M, Quattrini A, Comi G, Rama P, Riva N. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front Aging Neurosci v. 2014;6 doi: 10.3389/fnagi.2014.00278. article 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Nalassamy N, Downs H, Dana R, Oaklander AL. Corneal innervation as a window to peripheral neuropathies. Exp Eye Res. 2013;113:148–150. doi: 10.1016/j.exer.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Evans DJ. The pathogenesis of bacterial keratitis: Studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–278. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Fleiszig SMJ, Kwong MSF, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immunol. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D, Laux T, Xu L, Schneider C, Caroni P. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outrgrowth, and anatomical plasticity. J Cell Biol. 2000;149:1443–1453. doi: 10.1083/jcb.149.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess B, Halfter H, Kleffner I, Monje P, Athauda G, Wood PM, Young P, Wanner IB. Inhibition of N-cadherin and β-catenin function reduces axon-induced schwann cell proliferation. J Neurosci Res. 2008;86:797–812. doi: 10.1002/jnr.21528. [DOI] [PubMed] [Google Scholar]
- Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. GLIA. 2010;58:1701–1709. doi: 10.1002/glia.21041. [DOI] [PubMed] [Google Scholar]
- Gordon T, Tubbs RS, Rizk E, Shoja M, Loukas M, Spinner RJ, Barbaro N. Nerves and Nerve Injuries Volume 2: Pain, Treatment, Injury, Disease and Future Directions. Academic Press; New York: Apr 23, 2015. 2015. The Biology, Limits, and Promotion of Peripheral Nerve Regeneration in Rats and Humans; pp. 993–1019. [Google Scholar]
- Goyal S, Amar SK, Dubey D, Pal MK, Singh J, Verma A, Kushwaha HN, Ray RS. Involvement of cathepsin B in mitochondrial apoptosis by p-phenylenediamine under ambient UV radiation. J Hazard Mater. 2015;300:415–25. doi: 10.1016/j.jhazmat.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. GLIA. 2008;56:1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - A major review. Clin Exp Ophthalmol. 2009;37:100–117. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–646. [PubMed] [Google Scholar]
- Harris LW, Purves D. Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci. 1989;9:2210–2214. doi: 10.1523/JNEUROSCI.09-06-02210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Brit J Ophthalmol. 1999;83:737–741. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bazan NG, Bazan HEP. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Yuan X, Li Z, Coursey TG, Pflugfelder SC, Li D-QL. A novel innate response of human corneal epithelium to heat-killed Candida albicans by producing peptidoglycan recognition proteins. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0128039. article e0128039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaros E, Jenkison M. Quantitative studies of the abnormal axon-Schwann cell relationship in the peripheral motor and sensory nerves of the dystrophic mouse. Brain Res. 1983;258:181–196. doi: 10.1016/0006-8993(83)91141-1. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016 doi: 10.1113/JP270874. doi: 10.1113/JP270874 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol. 2016;100:9–14. doi: 10.1136/bjophthalmol-2014-306038. [DOI] [PubMed] [Google Scholar]
- Jungnickel J, Eckhardt M, Haastert-Talini K, Claus P, Bronzlik P, Lipokatic-Takacs E, Maier H, Gieselmann V, Grothe C. Polysialyltransferase overexpression in Schwann cells mediates different effects during peripheral nerve regeneration. Glycobiol. 2012;22:107–115. doi: 10.1093/glycob/cwr113. [DOI] [PubMed] [Google Scholar]
- Kass-Iliyya L, Javed S, Gosal D, Kobylecki C, Marshall A, Petropoulos IN, Ponirakis G, Tavakoli M, Ferdousi M, Chaudhuri KR, Jeziorska M, Malik RA, Silverdale MA. Small fiber neuropathy in Parkinson's disease: A clinical, pathological and corneal confocal microscopy study. Parkinson Rel Dis. 2015;21:1454–1460. doi: 10.1016/j.parkreldis.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V. Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol. 1990;138:430–442. doi: 10.1016/0012-1606(90)90209-2. [DOI] [PubMed] [Google Scholar]
- Lin N, Bazan HE. Protein kinase C substrates in corneal epithelium during wound healing: the phosphorylation of growth associated protein-43 (GAP-43) Exp Eye Res. 1995;61:451–459. doi: 10.1016/s0014-4835(05)80140-x. [DOI] [PubMed] [Google Scholar]
- Liu CY, Kao WW. Corneal Epithelial Wound Healing. Prog Mol Biol Transl Sci. 2015;134:61–71. doi: 10.1016/bs.pmbts.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jin YQ, Chen L, Wang Y, Yang X, Cheng J, Wu W, Qi Z, Shen Z. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE. 2015 doi: 10.1371/journal.pone.0123278. article e0123278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37:476–488. [PubMed] [Google Scholar]
- Maddaloni E, Sabatino F, Del Toro R, Crugliano S, Grande S, Lauria Pantano A, Maurizi AR, Palermo A, Bonini S, Pozzilli P, Manfrini S. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. 2015;32:262–266. doi: 10.1111/dme.12583. [DOI] [PubMed] [Google Scholar]
- Malik RA, Kallinikos P, Abbott CA, Van Schie CHM, Morgan P, Efron N, Boulton AJM. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- Mao X, Schwend T, Conrad GW. Expression and localization of neural cell adhesion molecule and polysialic acid during chick corneal development. Invest Ophthalmol Vis Sci. 2012;53:1234–1243. doi: 10.1167/iovs.11-8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Martin RE, Bazan NG. Growth-associated protein GAP-43 and nerve cell adhesion molecule in sensory nerves of cornea. Exp Eye Res. 1992;55:307–314. doi: 10.1016/0014-4835(92)90195-x. [DOI] [PubMed] [Google Scholar]
- Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, Pichierri P, Balestrazzi A, Motolese PA, Motolese I, Motolese E. An In Vivo Confocal Microscopy Analysis of Effects of Topical Antiglaucoma Therapy With Preservative on Corneal Innervation and Morphology. Am J Ophthalmol. 2009;147:725–735. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Matsumoto S. Demonstration of epithelial movement by the use of vital staining, with observations on phagocytosis in the corneal epithelium. J Exp Zoo. 1918;27:37–47. [Google Scholar]
- Mauris J, Woodward AM, Cao Z, Panjwani N, Argüeso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127:3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM. Defensins and other antimicrobial peptides at the ocular surface. Ocular Surface. 2004;2:229–247. doi: 10.1016/s1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavari A, Chaudhary S, Sarkar J, Yco L, Patel K, Han KY, Yue BY, Chang JH, Jain S. In vivo serial imaging of regenerating corneal nerves after surgical transection in transgenic thy1-YFP mice. Invest Ophthalmol Vis Sci. 2011;52:8025–8032. doi: 10.1167/iovs.11-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Peeler JS, Mellon J. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg Immun. 1989;2:83–90. [PubMed] [Google Scholar]
- Niessen CM, Cremona O, Daams H, Ferraresi S, Sonnenberg A, Marchisio PC. Expression of the integrin α6β4 in peripheral nerves: Localization in Schwann and perineural cells and different variants of the β4 subunit. J Cell Sci. 1994;107:543–552. doi: 10.1242/jcs.107.2.543. [DOI] [PubMed] [Google Scholar]
- Ohji M, SundarRaj N, Hassell JR, Thoft RA. Basement membrane synthesis by human corneal epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1994;35:479–483. [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075–1086. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Kyne BM, Saban DR, Stepp MA. Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea. Lab Invest. 2015;95:1305–1318. doi: 10.1038/labinvest.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Blanco T, Tadvalkar G, Pajoohesh-Ganji A, Parthasarathy A, Zieske JD, Stepp MA. MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice. J Cell Sci. 2011;124:2666–2675. doi: 10.1242/jcs.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Menko AS, Oh HY, Tadvalkar G, Saban DR, Stepp MA. Cytokine deposition alters leukocyte morphology and initial recruitment of monocytes and γδT cells after corneal injury. Invest Ophthalmol Vis Sci. 2014;55:2757–2765. doi: 10.1167/iovs.13-13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, Kester M, McMenamin PG. Toll-like receptors at the ocular surface. Ocular Surface. 2008;6:108–116. doi: 10.1016/s1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatta M, De Arcangelis A, D'Urso A, Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C, Georges-Labouesse E, Kreidberg J, Mayer U, Mckee KK, Yurchenco PD, Quattrini A, Wrabetz L, Feltri ML. α6β1 and α7β1 integrins are required in Schwann cells to sort axons. J Neurosci. 2013;33:17995–18007. doi: 10.1523/JNEUROSCI.3179-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Hamanaka RB, Katsnelson J, Hao LL, Yang W, Chandel NS, Lavker RM. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012;26:3140–3147. doi: 10.1096/fj.11-198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Katsnelson J, Yang W, Brown MA, Lavker RM. FIH-1/c-kit signaling: a novel contributor to corneal epithelial glycogen metabolism. Invest Ophthalmol Vis Sci. 2013;54:2781–2786. doi: 10.1167/iovs.12-11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Chuang EY, Yoon K-C, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, Li D-Q. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007;13:1934–1941. [PMC free article] [PubMed] [Google Scholar]
- Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Ramírez M, Martínez-Martínez L-A, Hernández-Quintela E, Velazco-Casapía J, Vargas A, Martínez-Lavín M. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Sem Arthrit Rheum. 2015;45:214–219. doi: 10.1016/j.semarthrit.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Reichard M, Hovakimyan M, Guthoff RF, Stachs O. In vivo visualisation of murine corneal nerve fibre regeneration in response to ciliary neurotrophic factor. Exp Eye Res. 2014;120:20–27. doi: 10.1016/j.exer.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Reichardt LF, Bixby JL, Hall DE, Ignatius MJ, Neugebauer KM, Tomaselli KJ. Integrins and cell adhesion molecules: Neuronal receptors that regulate axon growth on extracellular matrices and cell surfaces. Dev Neurosci. 1989;11:332–347. doi: 10.1159/000111910. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Chaudhary S, Jassim SH, Ozturk O, Chamon W, Ganesh B, Tibrewal S, Gandhi S, Byun YS, Hallak J, Mahmud DL, Mahmud N, Rondelli D, Jain S. CD11b+GR1+ myeloid cells secrete NGF and promote trigeminal ganglion neurite growth: implications for corneal nerve regeneration. Invest Ophthalmol Vis Sci. 2013;54:5920–5936. doi: 10.1167/iovs.13-12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, Di Iorio R, Costagliola C. Neurotrophic keratitis. Ophthalmologica. 2014;231:191–197. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z-L, Berger A, Hierner R, Allmeling C, Ungewickell E, Walter GF. A Schwann cell-seeded intrinsic framework and its satisfactory biocompatibility for a bioartificial nerve graft. Microsurg. 2001;21:6–11. doi: 10.1002/1098-2752(2001)21:1<6::aid-micr1001>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shimmura S, Tadano K, Tsubota K. UV dose-dependent caspase activation in a corneal epithelial cell line. Curr Eye Res. 2004;28:85–92. doi: 10.1076/ceyr.28.2.85.26237. [DOI] [PubMed] [Google Scholar]
- Stassart RM, Nave KA. Nerve regeneration: Specific metabolic demands? Exp Neurol. 2015;269:90–92. doi: 10.1016/j.expneurol.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. α6β4 integrin heterodimer is a component of hemidesmosomes. Proc Nat Acad Sci. USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Wounding the cornea to learn how it heals. Exp Eye Res. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: From Augustus Waller's observations to neuroinflammation. J Peri Nerv Sys. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Sturniolo GC, Lazzarini D, Bartolo O, Berton M, Leonardi A, Fregona IA, Parrozzani R, Midena E. Small fiber peripheral neuropathy in wilson disease: An in vivo documentation by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56:1390–1395. doi: 10.1167/iovs.14-15004. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Marshall A, Banka S, Petropoulos IN, Fadavi H, Kingston H, Malik RA. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve. 2012;46:698–704. doi: 10.1002/mus.23377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo T, Holopainen J, Belmonte C. Confocal Microscopy of Corneal Nerves-a Limited but Still Useful Technique to Evaluate Peripheral Neuropathies. JAMA Ophthalmol. 2016;134:990–991. doi: 10.1001/jamaophthalmol.2016.2178. [DOI] [PubMed] [Google Scholar]
- Tervo T, Moilanen J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog Ret Eye Res. 2003;22:339–358. doi: 10.1016/s1350-9462(02)00064-2. [DOI] [PubMed] [Google Scholar]
- Thoft RA, Friend J. Biochemical transformation of regenerating ocular surface epithelium. Invest Ophthalmol Vis Sci. 1977;16:14–20. [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53:867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Zee CEEM, Kreft M, Beckers G, Kuipers A, Sonnenberg A. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration. J Neurosci. 2008;28:11292–11303. doi: 10.1523/JNEUROSCI.3068-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Zee CEEM, Nielander HB, Vos JP, Lopes da Silva S, Verhaagen J, Oestreicher AB, Schrama LH, Schotman P, Gispen WH. Expression of growth-associated protein B-50 (GAP43) in dorsal root ganglia and sciatic nerve during regenerative sprouting. J Neurosci. 1989;9:3505–3512. doi: 10.1523/JNEUROSCI.09-10-03505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anesthesia dolorosa. Pain. 1979;7:103–113. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Wang EF, Misra SL, Patel DV. In Vivo Confocal Microscopy of the Human Cornea in the Assessment of Peripheral Neuropathy and Systemic Diseases. BioMed Res Int. 2015 doi: 10.1155/2015/951081. article: 951081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang H, Zhang F, Pan Z, Capó-Aponte J, Reinach PS. Dependence of EGF-induced increases in corneal epithelial proliferation and migration on GSK-3 inactivation. Invest Ophthalmol Vis Sci. 2009;50:4828–4835. doi: 10.1167/iovs.08-2983. [DOI] [PubMed] [Google Scholar]
- Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48:1535–1542. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]
- Yu W-M, Yu H, Chen Z-L, Strickland S. Disruption of laminin in the peripheral nervous system impedes nonmyelinating schwann cell development and impairs nociceptive sensory function. GLIA. 2009;57:850–859. doi: 10.1002/glia.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhao G-Q, Qu J, Che C-Y, Lin J, Jiang N, Zhao H, Wang X-J. Expression of S100B during the innate immune of corneal epithelium against fungi invasion. Int J Ophthalmol. 2016;9:191–197. doi: 10.18240/ijo.2016.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh J, Richardson PM, Bo X. Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Rest Neurol Neurosci. 2008;26:81–96. [PubMed] [Google Scholar]
- Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Köhler B, Stachs O, Guthoff RF, Roden M. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. German Diabetes Study (GDS) Group. Diabetes. 2014;63:2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Gipson IK. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986;27:1–7. [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Exp Cell Res. 1994;214:621–633. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]