Abstract
The prevalence of obesity in children has reached epidemic proportions. Concern about bone health in obese children, in part, derives from the potentially increased fracture risk associated with obesity. Additional risk factors that affect bone mineral accretion, may also contribute to obesity, such as low physical activity and nutritional factors. Consequences of obesity, such as inflammation, insulin resistance and non-alcoholic fatty liver disease, may also affect bone mineral acquisition, especially during the adolescent years when rapid increases in bone contribute to attaining peak bone mass. Further, numerous pediatric health conditions are associated with excess adiposity, altered body composition or endocrine disturbances that can affect bone accretion. Thus, there is a multitude of reasons for considering clinical assessment of bone health in an obese child. Multiple diagnostic challenges affect the measurement of bone density and its interpretation. These include greater precision error, difficulty in positioning, and the effects of increased lean and fat tissue on bone health outcomes. Future research is required to address these issues to improve bone health assessment in obese children.
Keywords: obesity, children, fracture, body composition, dual energy x-ray absorptiometry, peripheral quantitative computed tomography
Introduction
The prevalence of obesity in children has reached epidemic proportions; In the U.S., 17.5% of children ages 6 to 11years were obese in 2011–2014 and in the adolescent years (ages 12 to 19years), the prevalence reaches 20.5%[1]. This pattern is mirrored in other countries, and in 2010 it was estimated that 43 million children worldwide (35 million children in developing countries) were obese[2]. Orthopedic complications of childhood obesity include Blount’s disease and slipped capital femoral epiphysis[3]. The effect of childhood obesity on bone mineral accrual, current fracture risk, attainment of peak bone mass, and risk of osteoporosis later in life is less well understood. As we describe below, some factors contributing to obesity as well as some health consequences of obesity may be detrimental to bone mineral accretion during childhood. Increased fracture risk has been reported for obese children and adults. In addition, obesity occurs in patient populations with other risk factors for poor bone mineral accretion, such as immobility or glucocorticoid exposure, yet the unique technical challenges of bone health assessment in obese children are poorly understood. Here we highlight the key reasons for clinical concern for bone health in obese children, the technical challenges of clinical bone densitometry in the obese child, and outline a research agenda to address these challenges.
Patterns of bone mineral accretion in children
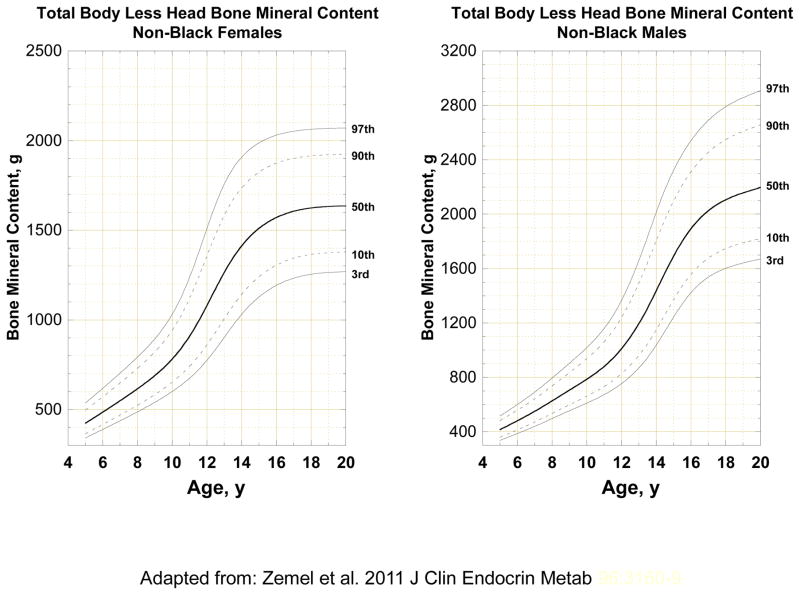
During the first two decades of life, the skeleton expands in length and breadth. Total body bone mineral accrual increases as a result of this expansion, and the thickness and density of bone also increases. These changes occur at a fairly steady pace through childhood, but rapid changes occur during the adolescent growth spurt with close to 40% of total body bone mineral accrual occurring within 2 years of the adolescent growth spurt[4]. The variability in bone outcomes also increases with age, as shown in Figure 1 for total body bone mineral content (BMC).
Figure 1.
Age and sex distribution of total body less head bone mineral content for children ages 5 to 20 years from the Bone Mineral Density in Childhood Study. Adapted from Zemel et al. [103].
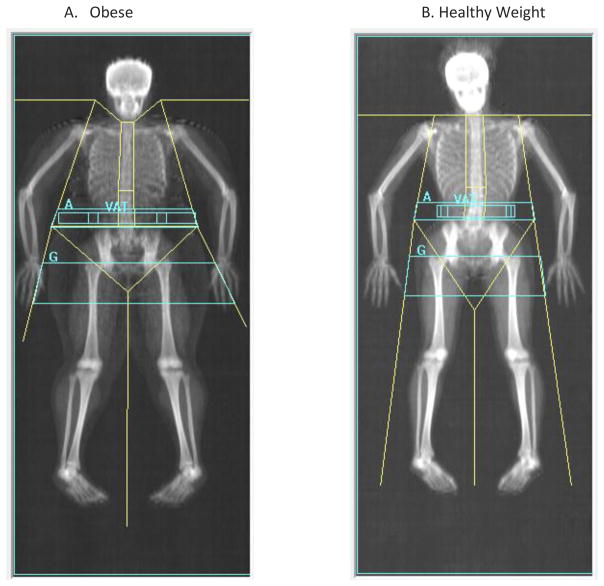
Bone mineral accretion in children is most commonly assessed by dual energy x-ray absorptiometry (DXA) because of it low radiation exposure, rapid scan time and widespread availability. DXA is the preferred method for clinical bone health assessment in children[5]. It generates a two-dimensional planar image and provides measures of bone mineral content (BMC, gm), areal-bone mineral density (aBMD, gm/cm2) and bone area (cm2). Standard scan sites include the total body, lumbar spine, proximal femur, and forearm. In addition, whole body scans provide measures of total lean mass, fat mass, percent body fat, and visceral adipose tissue area. Figure 2 shows whole body scans of an obese and a healthy weight teen of similar age and stature.
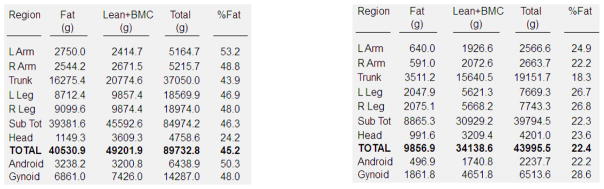
Figure 2. DXA Whole Body Scans in (A) an Obese Child and a (B) Healthy Weight Child of Similar Age and Height.
Whole body DXA scans of two 14 year old girls of similar height, one who is obese and the other who is healthy weight (BMI<85th percentile). Shown are the body composition results of the total body and sub-regions.
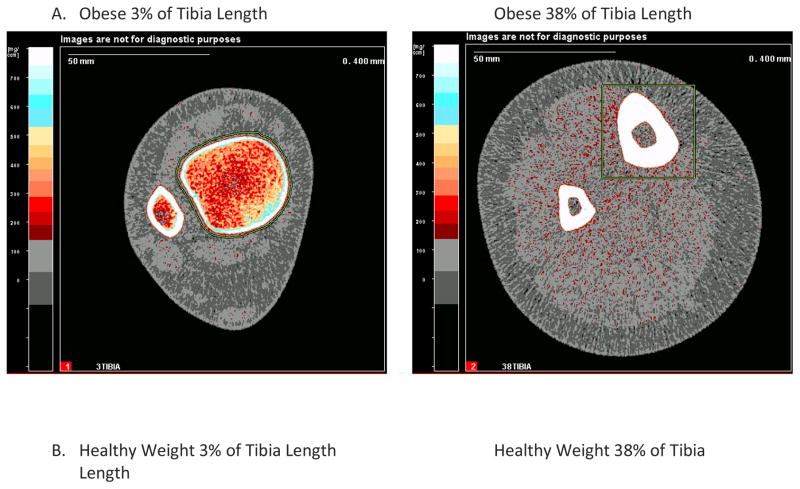
Unlike DXA, quantitative computed tomography (QCT) methods provide separate measures of cortical and trabecular volumetric BMD (vBMD), and in the long bones, measures of cortical bone geometry and structural strength such as cortical thickness, periosteal and endosteal circumference and section modulus. QCT methods are primarily used in the research setting, and have provided more precise insights into the nature of differences in bone structure between obese and non-obese children. A full-sized QCT can be used to measure the axial or peripheral skeleton, and dedicated tabletop peripheral QCT devices are used to measure the radius or tibia. Cross-sectional dimensions of lower limb fat and muscle area can also be obtained from these devices as indicators of body composition. High resolution peripheral QCT is a technology that has recently become available and provides more detailed measures of bone architecture, such as trabecular thickness and separation, and structural strength from finite element analysis[6]. Figure 3 illustrates peripheral QCT images in obese and healthy weight teens. Similar to DXA measures of BMC and aBMD, vBMD and cortical measures of structure and strength increase at a steady pace until mid-puberty and then increase rapidly until adult values are attained[7–9].
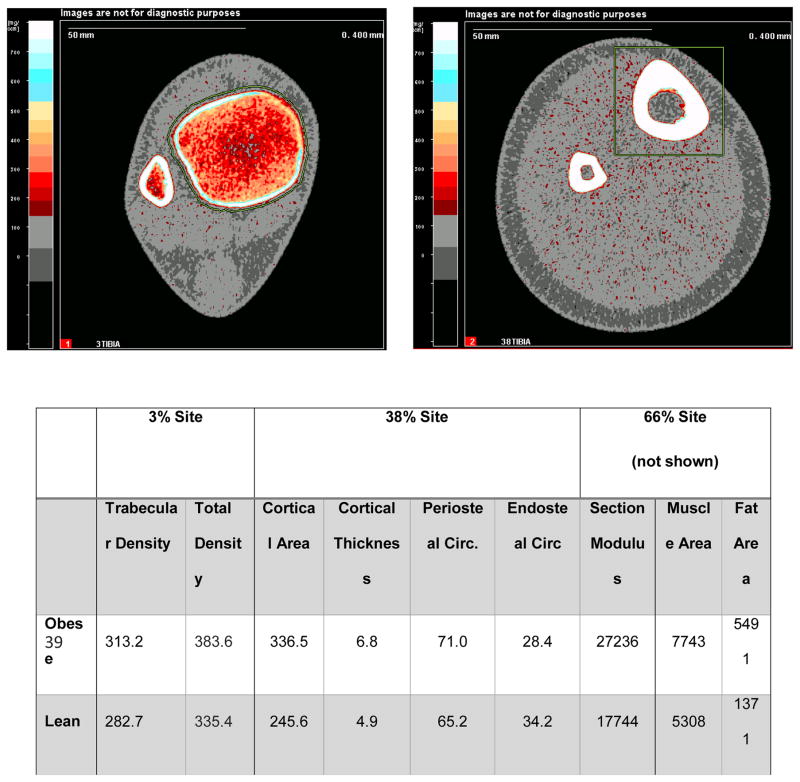
Figure 3. pQCT Scans of the Distal Tibia (3% and 38% of tibia length) in (A) an Obese Child and a (B) Healthy Weight Child of Similar Age and Height.
pQCT scans of the distal tibia at the 3% and 38% sites of two 14 year old girls of similar height (same as above), one who is obese and the other who is healthy weight (BMI<85th percentile). Shown are the bone and body composition results.
In general, regardless of the modality used to assess bone health, obese children tend to have larger bones and greater BMC, aBMD and vBMD than healthy weight children[10–12]. In addition, obese children tend to be taller, to mature earlier, and have greater lean mass[11, 13]. These are important considerations in evaluating bone health in the obese child, but as yet, we do not have adequate tools for accounting for excess body weight in clinical interpretation of bone health outcomes.
Body Composition and Bone Health in Obese Children
Lean Tissue
Elevated BMI during childhood and adolescence is accomplished by gains in both fat and lean mass. Evidence for the effect of increased BMI on bone mineral accretion is mixed and historically has been limited by cross-sectional design and lack of obese participants. In two recent studies using pQCT and obese participants, increased vBMD was demonstrated in obese adolescents compared to healthy weight controls[11, 13]. In the study conducted by Leonard et al, when the results were adjusted for the effect of lean mass on the association between BMI and vBMD, differences between the groups were attenuated [11]. This suggests that the positive effect of BMI on bone is at least partly mediated through the effect of greater lean mass.
Skeletal muscle and the forces it exerts on bone are a well-known determinant of bone strength and accretion. The functional muscle-bone unit model describes the adaptive process of bone accretion in response to exposure to mechanical stresses, in which muscle forces exerted on bone represent the greatest of these physiologic stresses. As muscle mass increases, bone is exposed to increased mechanical loads and responds through processes of modeling and remodeling that lead to osteogenesis[14, 15]. In adolescence, gains in lean mass during the pubertal growth spurt are associated with increased BMC and bone strength though the effect of lean mass is significantly influenced by differences in sex, maturation, race, and age[16–18]. In the evaluation of the effect of body composition on bone, surrogates of muscle force, including measures of whole body lean mass from DXA and cross-sectional muscle area from pQCT, have been used to evaluate the association of muscle forces and bone with the assumption that muscle forces are proportional to muscle mass[19].
Adipose Tissue
Studies examining the association of adiposity with bone mineral accrual and attainment of peak bone mass have yielded conflicting results[10, 20–27], perhaps as a consequence of differences in study design, skeletal sites, and statistical methods used to adjust for differences in lean body mass, maturation and stature.
The pattern of regional fat deposition, including visceral, subcutaneous, and intramuscular adipose tissue, may have important implications for bone outcomes. Gilsanz et al used QCT to evaluate the associations between abdominal visceral adipose tissue (VAT) and subcutaneous adipose (SAT) with femoral bone outcomes in a cross-sectional study in 100 healthy females, ages 15 to 25 years[22]. VAT and SAT had opposing effects on the appendicular skeleton. After adjusting for leg length and thigh musculature, SAT was positively associated with cortical bone area while VAT was negatively associated with cortical bone area (all p-values <0.01). These findings suggest that SAT may be beneficial to bone structure whereas VAT may be a unique pathogenic fat depot. Two studies have examined intramuscular adipose tissue (IMAT) and bone outcomes in cross-sectional samples of children who were predominantly of healthy weight. One study of 9 to 12 year old girls found an inverse association between IMAT and tibia cortical area (p=0.003) independent of SAT[24]. The other study of late adolescent males and females demonstrated IMAT and SAT were positively associated with tibia cortical periosteal circumference[28]. VAT and IMAT may have negative effects on bone, in part, due to an increased inflammatory cytokine profile and disordered metabolic regulators[29, 30]. Presently, pediatric bone health assessment guidelines[31] do not provide recommendations on how to account for body composition in clinical assessment.
Clinical considerations in the evaluation of bone health in obese children
Physical Activity and Diet
Obesity is a result of energy imbalance whereby energy intake exceeds energy expenditure, and the net difference is stored in the body. Obese children, in general, engage in less moderate-to-vigorous and vigorous physical activity and spend more time engaged in sedentary behaviors than non-obese peers[32, 33]. Indeed, in a prospective longitudinal study of children 11 years of age, followed to age 15, sedentary behavior time was positively associated with increases in BMI for children with BMI above the 50th percentile, but had no relationship to changes in BMI for children with BMI below the 50th percentile[34]. This finding is particularly concerning with respect to bone health and optimizing peak bone mass because of the known, beneficial effects of weight-bearing physical activity on bone mineral accretion[35, 36].
In addition to excess energy intake, other dietary characteristics common among obese children may be detrimental to bone mineral accretion. High consumption of carbonated sugar-sweetened beverages and low intakes of calcium rich foods, green vegetables, and fruit typify diets among overweight and obese children[37, 38]. Carbonated beverages may have a directly detrimental effect on bone mineral accretion or their effect may be indirect through displacement of milk as a beverage, resulting in lower calcium intake[39, 40]. Carbonated beverage consumption has also been associated with risk of fracture or recurrent fracture[41, 42]. Diets rich in dairy, green vegetables, resulting in greater intake of calcium, vitamin C and other nutrients, are associated with better bone mineral accretion in children[43, 44]. In addition, vitamin D deficiency is common in obese children[45, 46] and may contribute to lower calcium absorption. Thus, diet and physical activity may contribute to poor bone health in obese children, and their assessment is an important component in guiding treatment.
Health Consequences of Obesity That May Affect Bone
Obesity has frequently been associated with elevations in circulating levels of proinflammatory cytokines, acute phase reactants and a chronic, low-grade, systemic inflammatory state[30, 47]. Pro-inflammatory cytokines, in particular TNF-α, IL-1 and IL-6, are mediators of osteoclast differentiation and promote bone resorption[48], and are increased in obesity and obesity-related disease.[47] In adults, upregulation of these pro-inflammatory cytokines has been linked to accelerated bone loss and the development of osteopenia and osteoporosis[30, 48]. Chronic exposure to this proinflammatory cytokine profile during childhood and adolescence is a proposed mechanism for obesity-related deficits in bone development and may explain the relationship between increased intra-abdominal fat depots and lower cortical bone thickness in children as described above (see body composition).
Obesity-related alterations in adipokine secretion, including elevated concentration of pro-inflammatory leptin and reduced production of anti-inflammatory adiponectin, have been implicated in the relationship between obesity and bone. Adiponectin, inversely correlated with fat, is associated with both osteoblastic activity and bone formation as well as increased bone turnover. Elevated serum adiponectin concentration has been associated with low aBMD in in vivo studies[49], while in vitro studies suggest that adiponectin is associated with increased bone formation and decreased resorption[50]. The relationship between leptin concentrations, which increase proportionally to fat mass, and bone is also complex and not fully understood. Cross-sectional studies have suggested both positive and negative associations with aBMD in young men[51, 52]. Among children, leptin is higher among those who are obese, and is inversely related to aBMD[53], as well as cortical porosity of the radius and trabecular thickness of the tibia[54]. The relationship between adipokines and bone in obesity may also be affected by variable pattern of expression of these markers by regional fat deposition[55].
Abnormalities of glucose metabolism likely affect bone accretion. Increased fracture risk is reported among adults with T2DM, particularly at the hip, despite increased aBMD in adults with T2DM and metabolic syndrome[56]. When results are adjusted for BMI or height and weight, however, the aBMD differences are attenuated, and in some cases, show significantly lower aBMD in the participants compared to controls[57, 58]. Among children with T2DM, fracture risk is not known. Recent studies in children have established a potential negative effect of insulin resistance and hyperinsulinisim on bone outcomes[30, 59, 60]. In the Avon Longitudinal Study of Parents and Children, fasting insulin level was independently and negatively associated with tibia cortical vBMD and periosteal circumference in non-overweight children, mean age 15.5 years, after adjusting for age, height, muscle area, subcutaneous fat, and muscle density [60]. Recently, Kindler et al demonstrated that greater insulin resistance is inversely associated with the muscle-dependent relationship between IGF-1 and BMC in non-overweight girls and suggested that insulin resistance in children may compromise muscle development[61]. Additional proposed mechanisms for the detrimental effect of insulin include a possible lipotoxic effect on muscle and bone, altered insulin signaling among osteoblasts and impaired bone formation, Type 2 diabetes-related vitamin D deficiency, as well as impaired musculoskeletal development[60]. As described above, lean tissue is a key determinant of bone health in children, and further research is needed to determine the effects of abnormal glucose metabolism on the functional muscle-bone unit.
Special Pediatric Health Conditions Associated with Obesity and Poor Bone Health
Numerous patient populations have conditions whereby obesity is a disease consequence, and deficits in bone are often related to multiple factors related to the disease itself. These populations also present unique considerations in the assessment of bone health. Duchenne’s Muscular Dystrophy (DMD) and Prader-Willi Syndrome (PWS) and are two clinical conditions that exemplify special groups that require special handling for placement and positioning for bone density assessment due to factors such as extreme obesity, immobility, fragility, hypotonia and developmental delay.
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy of childhood and affects 1 in 3500 males. It is characterized by severe and persistent muscle weakness and loss of muscle mass, leading to immobility and long-term corticosteroid use is an approved and proven treatment[62]. A tendency toward obesity emerges by early adolescence; a cohort study of 252 steroid-naïve boys reported obesity in up to 54% of participants, followed by a decline in obesity in late adolescence[63]. Several prior studies have described deficiencies in aBMD in this population along with marked increase in fragility fractures[62, 64, 65]. These observed patterns in bone outcomes are due, to some degree, to immobility. The added consequences of corticosteroid treatment are less clear. There is good evidence that corticosteroids can prolong the period of independent ambulation with associated improvements in muscle strength and function[66]. However, the detrimental effects of long-term corticosteroids are cumulative; with greater increases over time being reported in central adiposity, reduced stature, pubertal delay and bone fragility [67, 68]. Despite the greater incidence of vertebral fracture [69, 70] in this population, there is only limited data demonstrating associated reductions in lumbar spine bone density [68, 71, 72]. In the study by Bianchi et al., greater losses were recorded in areal bone density in boys with DMD taking corticosteroids than those who were steroid naïve[71]. However, once the data were size adjusted the differences were no longer apparent, suggesting that the reductions were due to short stature rather than poor bone accrual. The lack of association between steroid exposure and vertebral fractures has been reported by others [73, 74]. In contrast, there is a strong relationship between reduced mobility, reduced bone density in the total body and distal femur and long bone fractures in boys with DMD [75]. The dichotomy between bone density and the relationship between the vertebral fractures and long bone fractures may reflect technical issues or other measures of bone quality not captured by DXA measurements. Further research in this area is needed.
PWS is a neurodevelopmental disorder characterized by severe hypothalamic obesity, hypotonia, growth hormone deficiency and hypogonadism, all of which have been associated with adverse musculoskeletal development[76]. Most experience incomplete pubertal development and significant deficits in aBMD have been described[77]. Treatment with growth hormone contributes to improved bone density in this group[78].
Other forms of hypothalamic or neuroendocrine-related obesity have also been evaluated. One study compared children with hypothalamic obesity to children with congenital hypopituitarism and those with simple obesity. All three groups had high bone mass, but were similar in bone density (BMD) after adjustment for body size[79]. Survivors of childhood cancer treated with cranial irradiation often have neuroendocrine complications that result in obesity or altered fat distribution. Anterior hypopituitarism from cranial irradiation can result in deficiencies in growth hormone, gonadotropins (luteinizing hormone/follicle-stimulating hormone [LH/FSH]), thyroid-stimulating hormone, and adrenocorticotropic hormone. A long-term follow-up of a large cohort (n=748) found that abdominal obesity was associated with untreated growth hormone and LH/FSH deficiencies, and untreated LH/FSH deficiency was associated with low trabecular volumetric BMD of the spine and muscle deficits[80]. A smaller cohort of allogeneic hematopoietic stem-cell transplantation (alloHSCT) survivors, ages 12 to 25 years, treated with total body irradiation (TBI) demonstrated increased visceral adipose tissue, marrow adiposity, fat infiltration of muscle and abnormal trabecular architecture despite being similar to controls in BMI values[81].
The evidence for increased fracture risk among obese children
The prevalence of fractures in children is high; 30 to 50% of healthy children are likely to experience a fracture by the time they reach adulthood and many children experience multiple fractures[82]. Fracture incidence increases with age during childhood, reaching a peak around ages 10 to 14 in boys and 10 to 13 in girls[83, 84] followed by an age-related decline in late adolescence. The timing of peak fracture incidence occurs just prior to the adolescent growth spurt, and may represent a period of temporal skeletal fragility. Coincidentally, obesity prevalence also begins to rise during this age range.
The suggestion of increased fracture risk in obese children arose in the earliest studies of bone density and fracture in children. Goulding et al.[85–88] observed that children who had experienced a forearm fracture had greater BMI and/or fat mass than controls. High BMI was also associated with occurrence of repeated forearm fractures. However, these early studies did not account for mechanism of injury, and may have been biased by the methods used to recruit controls. Cohort studies of healthy children show that sport participation increases the risk of fracture[89, 90]. A case-controlled study of forearm fracture among children presenting with forearm injuries at a single pediatric center did not find increased BMI or fatness among fracture cases[91]. However, a population-based survey of >900,000 children ages 2 to 19 found increased odds of lower extremity fractures among overweight, obese and extremely obese children[92]. To fully understand fracture risk in obese children, further study is needed that accounts for site of fracture, mechanism of injury and other exposures (e.g., glucocorticoid treatment), ideally in combination with bone size and density measures to improve clinical bone health assessment and fracture prevention.
Technical Considerations in the Assessment of the Obese Child
The technical considerations affecting bone health assessment in the obese child include those that pertain to all children, as well as considerations related to increased size and tissue thickness. The issues are summarized in Table 1 and described below.
Table 1.
Technical considerations in DXA use in obese children
| Issue | Comment | |
|---|---|---|
| Size related issues | Weight limits on DXA devices | Most contemporary devices limited to 204kg/450lb |
| Two-dimensional artefacts of DXA devices | Size correction using BMAD or height Z-score adjustment | |
| Projection errors | Increased tissue thickness may underestimate bone density | |
| Soft tissue artefacts | Magnitude in obese children is unknown | |
| Precision | Reduced precision in obese children | |
| Positional errors | Overlapping of adipose tissue and lack of air spaces for placement of subregion markers in analysis | Hemi-scan technique overcomes both these problems |
| Soft tissue inhomogeneity | Bone marrow composition | Impact in pediatric population unknown |
| Tissue thickness | Thicker tissues lower attenuation coefficients | May result in overestimation of fat mass |
| Cross calibration | Weight and body composition are positively associated with intermachine differences | Cross-calibration of DXA devices may require weight-based correction |
| Longitudinal follow-up | Changes in body composition can affect bone density | Conflicting reports from in vitro studies testing effect of additional fat on bone outcomes |
| Change in weight can result in change in scan acquisition parameters |
Size Related Issues
With an increase in childhood and adult obesity, contemporary DXA scanners now have larger active scan areas and increased maximum patient weight limits (204kg/450lb). In the growing child, the greatest technical challenge is the dependence of areal aBMD on size. DXA is a two dimensional technique which utilizes a planar image to make an assessment of a three-dimensional structure. As such areal bone density (g/cm2) overestimates true bone density (g/cm3) in taller children with larger bones and systematically underestimates true bone density in shorter children with smaller bones[93]. The impact of this size artefact was first identified clinically in 2004 by Gafni and colleagues[94]. They reviewed 34 pediatric DXA scans with an initial report of low bone mineral density and found that 88% of the scans had at least one error in interpretation and of these, 15% of the errors were due to inattention of short stature. Methodologies to overcome this issue go back over 20 years. Carter et al.[95] and Kroger et al.[96] both developed size adjustment approaches to use information within the DXA scan; either projected bone area[95] or measured bone width[96] to estimate of vertebral depth in order to calculate a bone mineral apparent density (BMAD g/cm3) which was considerably less dependent on bone size and body stature. Over the years there have been several attempts to adjust for DXA inaccuracies due to variations in body size and composition[97–102]. However, agreement on how to reduce the size dependence of aBMD and its clinical validity is still on going. Recent guidelines recommend using BMAD for the assessment of spine bone density and a height for age adjusted Z-score (HAZ) for the assessment of total body less head bone density[103]. Both techniques provide successful size adjustment of aBMD, but there is still limited data on whether size adjusted bone density will predict future fracture risk in children.
In adults, the effect of increased body weight on increased BMD is well-established, such that for one DXA manufacturers (GE Lunar) there is the ability to calculate weight adjusted Z-scores. The weight adjustment in the GE Lunar software increases the mean expected BMD in heavier individuals and lowers the mean expected BMD in lighter individuals. The magnitude of the change is small, roughly 0.003g/cm2 (approximately 0.025 SD) but can result in a whole standard deviation difference in a subject weighing 100kg. However, due to the complex interrelationships between BMI, aBMD and fracture risk, the 2013 ISCD guidelines do not recommend this adjustment.
In children, there is only limited data as to the impact of obesity and altered body composition on size adjustment techniques. Given that obese children tend to be taller and have larger bones[13], the technical size related issues of DXA are likely to further confound the accuracy of pediatric DXA measurements and therefore have a more pronounced effect on their clinical interpretation. Also, there is some evidence that changing body thickness can alter the measured projected bone area despite the true projected area remaining stable[104]. Accordingly, if technical inaccuracies in the measurement of obese children result in increased bone area measurements without a proportional increase in BMC, then they will have reduced areal BMD compared to their peers. The magnitude of soft tissue artefacts in the measurement of bone area, BMC and areal-BMD by DXA in obese children remains to be determined.
Precision
DXA is a quantitative technique often used to measure changes over time. Therefore, it is essential to know the precision of each site measured in order to calculate smallest detectable differences and accurate time monitoring intervals. Overall, precision of bone density and body composition by DXA in children is good with %CV values often falling below 1–2%[105]. Nevertheless, several researchers have reported that obesity results in poorer precision particularly for body composition parameters[106, 107]. Among 32 obese and 34 non-obese 6 to 19 year-olds, Wosje et al. found that the smallest detectable difference of absolute fat measured by DXA was 3.3 times higher in the obese versus non-obese child whereas the smallest detectable difference for percentage fat was 1.5 times lower, reflecting the high absolute values of body fat[107]. Among 144 women with BMI’s ranging from 15.5 to 45.9kg/m2, Knapp et al. found that the root mean standard deviation for total body BMD was 18% greater in obese women versus non-obese women[106]. These findings indicate that greater differences or longer time intervals between measurements are required to be certain of detecting any real change.
Positional Errors
One of the sources of poorer precision in obese children is that the larger body size inhibits ideal positioning. Often, trying to fit the obese child within the scan region of the DXA, results in an overlapping of adipose tissue and a lack of air spaces for the placement of regional analysis markers, most notably in the trunk region. More recently, a newer acquisition and analysis algorithm has been developed, called the ‘hemi-scan’. For DXA hemi-scan acquisition, the child is positioned such that one whole side is measured. The modified analysis tool then uses the data from the completely measured side (routinely the right) to estimate the bone and body composition values for the contra-lateral side. This technique has been shown to have no detrimental effect on machine precision and only a limited effect on measurement accuracy[108, 109].
Inhomogeneity of Soft Tissue
Bolotin et al. has suggested that the ubiquitous inaccuracies in DXA BMD methodologies are of in vivo anthropometric origin 2001[110]. Comparison of complex phantom configurations of red and yellow bone marrow mixes and extraosseous mixtures of fat and lean mass resulted in considerable fat related inaccuracies of DXA[111]. There was an increase in DXA inaccuracy of up to 20% with a decrease in fat mass and an increase in yellow marrow in adults. These inaccuracy errors likely have the greatest impact on older women with less lean mass and more yellow bone marrow, i.e. those most at risk of osteoporotic fractures[112]. The impact of these errors in a pediatric population is, as yet, unknown. The errors arise from the algorithms used by DXA systems to estimate bone and body composition, and the design of these dual energy systems[113]. The differential attenuation of low and high-energy photons in the x-ray beam allows for the determination of the relative amounts of bone, bone-free lean and fat mass in each pixel of the scan area. However, since only two photon energies are used, only two materials can be differentiated at any one pixel (either bone and soft-tissue or bone free lean mass and fat mass). As a consequence, DXA has to use material adjacent to bone tissue to estimate bone-free lean and fat mass within the pixels containing bone. The algorithms assume the adjacent material to be homogeneous for fat and lean tissue. However, when this is not the case inherent inaccuracies occur[114]. Greater yellow marrow will result in reduced bone density, whereas greater proportional fat mass will result in increased bone density.
Tissue Thickness and Beam Hardening
Beam hardening is the preferential attenuation of the lowest energy x-rays at greater tissue depth. Thicker tissues will have lower attenuation coefficients which translates into an over estimation of fat. The Hologic bone densitometer has an internal beam-filtering and calibration mechanism to try and overcome this problem but its effectiveness at high obesity levels is unknown[115].
Cross Calibration
For longitudinal assessment or multicenter research studies of bone and body composition it is ideal to obtain all measurements on the same device; however this is not always possible. Where machine change is necessary, cross-calibration between the old and new machine, or multiple machines, is recommended. Body weight can be a significant but varying source of error between scanners. In a pediatric cross-calibration study between a GE Lunar DPX-L device and a Lunar Prodigy scanner, machine differences were positively related to body weight[116]. In a similar adult study, Blake et al. found that percentage fat and body weight explained up to 40% of the variance in the differences between the two devices[117]. Intrinsically, this varying contribution of body weight to the cross-calibration of DXA devices can result in greater errors for obese than non-obese populations.
Longitudinal Follow-up
As with many of these issues, there is little published work on longitudinal BMD change in the presence of increasing or decreasing body fat. Several simulation studies have shown that the addition of lard, as a surrogate for additional adipose tissue, has a marked effect on bone density values. Evans et al. reported that weight change achieved using exogenous fat (lard) had relatively little effect on bone density measurement[118]. However, Tothill et al. reported greater increases in bone area and decreases in BMC and BMD dependent on the placement of the exogenous fat[104]. An additional consideration in longitudinal studies is that weight gain may result in a change in scan acquisitioning parameters. Generally, it is good practice to keep all scanning parameters the same for follow-up measurements. However, as children grow and gain weight there may be a need to adapt the scan mode accordingly to afford the most precise and reliable bone density assessment[119].
Summary and Future Considerations
The pediatric skeleton is sensitive to the health and behavior-related forces that influence bone mineral accrual, from physical activity to increased inflammatory cytokines. Obesity is associated with both increased fat mass and lean mass, which may have opposing effects on bone outcomes, and, along with the controversial issue of increased fracture risk in obese children, underscore the need for more research of the effects of pediatric obesity on lifelong skeletal health. The technical limitations of DXA and other modalities used to evaluate bone health of obese children are also paramount for improving clinical bone health assessment in the obese child. Consideration for a research agenda are as follows:
Obese children tend to be taller, mature earlier, and have greater lean and fat mass. How should these factors be considered in clinical interpretation of bone health outcomes?
Diet and physical activity may contribute to development of obesity as well as poor bone health in obese children. Do obese children require specialized diet and physical activity recommendations to maximize bone health and prevent fractures?
Health consequences of obesity such as increased circulating proinflammatory cytokines and abnormalities of glucose regulation likely have harmful effects on bone metabolism in the growing child. Are these characteristics useful in identifying the child at greatest risk for current or future bone fragility, and how are they best assessed?
Studies evaluating bone health outcomes and fracture risk in obese children have produced inconsistent results. Do obese children have greater risk for more serious fractures (such as lower extremity fractures)? Can fracture studies in obese children determine obesity-specific bone density thresholds associated with increased fracture risk? Do factors such as adipose tissue depots and muscle quality affect fracture risk and long-term bone health in the obese child?
Many children with health conditions that contribute to both obesity and poor bone mineral accrual present with challenges that can affect scan acquisition such as extreme obesity, immobility, and developmental delay. What are the optimal scan sites and positioning techniques for clinical bone health assessment in special groups?
Technical factors such as precision, inhomogeneity of soft tissue, effects of tissue thickness and beam hardening, cross-calibration and longitudinal monitoring are important considerations in clinical assessment, yet these effects are not well-understood or sufficiently quantified in general, and in particular, for obese children. For example, how do these technical factors influence bone density estimates in the context of excess weight loss or gain? Future research is needed to assess these effects, to advance clinical bone health assessment in the obese child.
Footnotes
Conflict of interest statement
Jennifer Kelley has nothing to disclose.
Nicola Crabtree has nothing to disclose.
Babette Zemel has nothing to disclose.
Contributor Information
Jennifer Kelley, Division of Endocrinology and Diabetes, Monroe Carell, Jr Children’s Hospital at Vanderbilt, Nashville, TN 37232.
Nicola Crabtree, Department of Endocrinology and Diabetes, Birmingham Children’s Hospital, Birmingham, UK.
Babette S. Zemel, Division of Gastroenterology, Hepatology and Nutrition, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 2.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obes Res. 2001;9(Suppl 4):239S–243S. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- 4.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–1739. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 5.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Adams JE, Engelke K, Zemel BS, Ward KA International Society of Clinical D. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:258–274. doi: 10.1016/j.jocd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–1600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 8.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. Journal of Clinical Endocrinology and Metabolism. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 11.Leonard MB, Zemel BS, Wrotniak BH, Klieger SB, Shults J, Stallings VA, Stettler N. Tibia and radius bone geometry and volumetric density in obese compared to non-obese adolescents. Bone. 2015;73:69–76. doi: 10.1016/j.bone.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stettler N, Berkowtiz RI, Cronquist JL, Shults J, Wadden TA, Zemel BS, Leonard MB. Observational study of bone accretion during successful weight loss in obese adolescents. Obesity. 2008;16:96–101. doi: 10.1038/oby.2007.17. [DOI] [PubMed] [Google Scholar]
- 13.Vandewalle S, Taes Y, Van Helvoirt M, Debode P, Herregods N, Ernst C, Roef G, Van Caenegem E, Roggen I, Verhelle F, Kaufman JM, De Schepper J. Bone size and bone strength are increased in obese male adolescents. J Clin Endocrinol Metab. 2013;98:3019–3028. doi: 10.1210/jc.2012-3914. [DOI] [PubMed] [Google Scholar]
- 14.Forwood MR, Turner CH. Skeletal adaptations to mechanical usage: results from tibial loading studies in rats. Bone. 1995;17:197S–205S. doi: 10.1016/8756-3282(95)00292-l. [DOI] [PubMed] [Google Scholar]
- 15.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5:232–238. [PubMed] [Google Scholar]
- 16.Ashby RL, Adams JE, Roberts SA, Mughal MZ, Ward KA. The muscle-bone unit of peripheral and central skeletal sites in children and young adults. Osteoporos Int. 2011;22:121–132. doi: 10.1007/s00198-010-1216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ausili E, Rigante D, Salvaggio E, Focarelli B, Rendeli C, Ansuini V, Paolucci V, Triarico S, Martini L, Caradonna P. Determinants of bone mineral density, bone mineral content, and body composition in a cohort of healthy children: influence of sex, age, puberty, and physical activity. Rheumatology international. 2012;32:2737–2743. doi: 10.1007/s00296-011-2059-8. [DOI] [PubMed] [Google Scholar]
- 19.Wetzsteon RJ, Petit MA, Macdonald HM, Hughes JM, Beck TJ, McKay HA. Bone structure and volumetric BMD in overweight children: a longitudinal study. Journal of Bone and Mineral Research. 2008;23:1946–1953. doi: 10.1359/jbmr.080810. [DOI] [PubMed] [Google Scholar]
- 20.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95:699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 24.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, Laudermilk M, Lohman TG, Going SB. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–2225. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46:977–984. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. Journal of Clinical Endocrinology and Metabolism. 2011;96:106–114. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity adventageous for bone strength? A peripheral quantitative computed tomography sudy in late adolescent females. Am J Clin Nutr. 2007;86:1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 28.Deere K, Sayers A, Viljakainen HT, Lawlor DA, Sattar N, Kemp JP, Fraser WD, Tobias JH. Distinct relationships of intramuscular and subcutaneous fat with cortical bone: findings from a cross-sectional study of young adult males and females. J Clin Endocrinol Metab. 2013;98:E1041–1049. doi: 10.1210/jc.2013-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utsal L, Tillmann V, Zilmer M, Maestu J, Purge P, Jurimae J, Saar M, Latt E, Maasalu K, Jurimae T. Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-gamma levels in 10- to 11-year-old boys with increased BMI. Hormone research in paediatrics. 2012;78:31–39. doi: 10.1159/000339831. [DOI] [PubMed] [Google Scholar]
- 30.Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol Cell Endocrinol. 2015;410:52–63. doi: 10.1016/j.mce.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Rowlands AV. Physical Activity, Inactivity, and Health During Youth. Pediatr Exerc Sci. 2016;28:19–22. doi: 10.1123/pes.2016-0007. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JA, Mattocks C, Ness AR, Leary SD, Pate RR, Dowda M, Blair SN, Riddoch C. Sedentary behavior and obesity in a large cohort of children. Obesity (Silver Spring) 2009;17:1596–1602. doi: 10.1038/oby.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell JA, Pate RR, Beets MW, Nader PR. Time spent in sedentary behavior and changes in childhood BMI: a longitudinal study from ages 9 to 15 years. Int J Obes (Lond) 2013;37:54–60. doi: 10.1038/ijo.2012.41. [DOI] [PubMed] [Google Scholar]
- 35.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48:1032–1036. doi: 10.1136/bjsports-2014-093574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36:250–257. doi: 10.1136/bjsm.36.4.250. discussion 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasnain SR, Singer MR, Bradlee ML, Moore LL. Beverage intake in early childhood and change in body fat from preschool to adolescence. Child Obes. 2014;10:42–49. doi: 10.1089/chi.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kavey RE. How sweet it is: sugar-sweetened beverage consumption, obesity, and cardiovascular risk in childhood. J Am Diet Assoc. 2010;110:1456–1460. doi: 10.1016/j.jada.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen M, Jensen M, Kudsk J, Henriksen M, Molgaard C. Short-term effects on bone turnover of replacing milk with cola beverages: a 10-day interventional study in young men. Osteoporos Int. 2005;16:1803–1808. doi: 10.1007/s00198-005-1935-z. [DOI] [PubMed] [Google Scholar]
- 40.Libuda L, Alexy U, Remer T, Stehle P, Schoenau E, Kersting M. Association between long-term consumption of soft drinks and variables of bone modeling and remodeling in a sample of healthy German children and adolescents. Am J Clin Nutr. 2008;88:1670–1677. doi: 10.3945/ajcn.2008.26414. [DOI] [PubMed] [Google Scholar]
- 41.Manias K, McCabe D, Bishop N. Fractures and recurrent fractures in children; varying effects of environmental factors as well as bone size and mass. Bone. 2006;39:652–657. doi: 10.1016/j.bone.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Wyshak G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch Pediatr Adolesc Med. 2000;154:610–613. doi: 10.1001/archpedi.154.6.610. [DOI] [PubMed] [Google Scholar]
- 43.Wosje KS, Khoury PR, Claytor RP, Copeland KA, Hornung RW, Daniels SR, Kalkwarf HJ. Dietary patterns associated with fat and bone mass in young children. The American journal of clinical nutrition. 2010;92:294–303. doi: 10.3945/ajcn.2009.28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vatanparast H, Baxter-Jones A, Faulkner RA, Bailey DA, Whiting SJ. Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: the University of Saskatchewan Pediatric Bone Mineral Accrual Study. The American journal of clinical nutrition. 2005;82:700–706. doi: 10.1093/ajcn.82.3.700. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, Willett WC, Villamor E. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. The American journal of clinical nutrition. 2010;92:1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace TC, Reider C, Fulgoni VL., 3rd Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J Am Coll Nutr. 2013;32:321–330. doi: 10.1080/07315724.2013.839905. [DOI] [PubMed] [Google Scholar]
- 47.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 49.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanazawa I. Adiponectin in metabolic bone disease. Current medicinal chemistry. 2012;19:5481–5492. doi: 10.2174/092986712803833146. [DOI] [PubMed] [Google Scholar]
- 51.Papadopoulou F, Krassas GE, Kalothetou C, Koliakos G, Constantinidis TC. Serum leptin values in relation to bone density and growth hormone-insulin like growth factors axis in healthy men. Archives of andrology. 2004;50:97–103. [PubMed] [Google Scholar]
- 52.Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21:1871–1878. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]
- 53.do Prado WL, de Piano A, Lazaretti-Castro M, de Mello MT, Stella SG, Tufik S, do Nascimento CM, Oyama LM, Lofrano MC, Tock L, Caranti DA, Damaso AR. Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab. 2009;27:613–619. doi: 10.1007/s00774-009-0082-6. [DOI] [PubMed] [Google Scholar]
- 54.Dimitri P, Jacques RM, Paggiosi M, King D, Walsh J, Taylor ZA, Frangi AF, Bishop N, Eastell R. Leptin may play a role in bone microstructural alterations in obese children. J Clin Endocrinol Metab. 2015;100:594–602. doi: 10.1210/jc.2014-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campos RM, de Mello MT, Tock L, da Silva PL, Corgosinho FC, Carnier J, de Piano A, Sanches PL, Masquio DC, Tufik S, Damaso AR. Interaction of bone mineral density, adipokines and hormones in obese adolescents girls submitted in an interdisciplinary therapy. J Pediatr Endocrinol Metab. 2013;26:663–668. doi: 10.1515/jpem-2012-0336. [DOI] [PubMed] [Google Scholar]
- 56.de L, II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 57.Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk--the MINOS study. J Bone Miner Res. 2010;25:1446–1454. doi: 10.1002/jbmr.13. [DOI] [PubMed] [Google Scholar]
- 58.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 59.Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28:372–378. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- 60.Sayers A, Lawlor DA, Sattar N, Tobias JH. The association between insulin levels and cortical bone: findings from a cross-sectional analysis of pQCT parameters in adolescents. J Bone Miner Res. 2012;27:610–618. doi: 10.1002/jbmr.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kindler JM, Pollock NK, Laing EM, Jenkins NT, Oshri A, Isales C, Hamrick M, Lewis RD. Insulin Resistance Negatively Influences the Muscle-Dependent IGF-1-Bone Mass Relationship in Premenarcheal Girls. J Clin Endocrinol Metab. 2016;101:199–205. doi: 10.1210/jc.2015-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckner JL, Bowden SA, Mahan JD. Optimizing Bone Health in Duchenne Muscular Dystrophy. Int J Endocrinol. 2015;2015:928385. doi: 10.1155/2015/928385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willig TN, Carlier L, Legrand M, Riviere H, Navarro J. Nutritional assessment in Duchenne muscular dystrophy. Dev Med Child Neurol. 1993;35:1074–1082. doi: 10.1111/j.1469-8749.1993.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 64.King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, Mendell JR, Kissel JT. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 65.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. Journal of Pediatric Orthopedics. 2000;20:71–74. [PubMed] [Google Scholar]
- 66.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016:CD003725. doi: 10.1002/14651858.CD003725.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, Muntoni F NorthStar Clinical N. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84:698–705. doi: 10.1136/jnnp-2012-303902. [DOI] [PubMed] [Google Scholar]
- 68.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop. 2000;20:71–74. [PubMed] [Google Scholar]
- 69.Singh A, Schaeffer EK, Reilly CW. Vertebral Fractures in Duchenne Muscular Dystrophy Patients Managed With Deflazacort. J Pediatr Orthop. 2016 doi: 10.1097/BPO.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 70.Vestergaard P, Glerup H, Steffensen BF, Rejnmark L, Rahbek J, Moseklide L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J Rehabil Med. 2001;33:150–155. [PubMed] [Google Scholar]
- 71.Bianchi ML, Mazzanti A, Galbiati E, Saraifoger S, Dubini A, Cornelio F, Morandi L. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos Int. 2003;14:761–767. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 72.Bianchi ML, Morandi L, Andreucci E, Vai S, Frasunkiewicz J, Cottafava R. Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: response to calcium and vitamin D treatment. Osteoporos Int. 2011;22:529–539. doi: 10.1007/s00198-010-1275-5. [DOI] [PubMed] [Google Scholar]
- 73.Crabtree NJ, Roper H, McMurchie H, Shaw NJ. Regional changes in bone area and bone mineral content in boys with duchenne muscular dystrophy receiving corticosteroid therapy. J Pediatr. 2010;156:450–455. doi: 10.1016/j.jpeds.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Tian C, Wong BL, Hornung L, Khoury JC, Miller L, Bange J, Rybalsky I, Rutter MM. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:760–767. doi: 10.1016/j.nmd.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Henderson RC, Berglund LM, May R, Zemel BS, Grossberg RI, Johnson J, Plotkin H, Stevenson RD, Szalay E, Wong B, Kecskemethy HH, Harcke HT. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J Bone Miner Res. 2010;25:520–526. doi: 10.1359/jbmr.091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Longhi S, Grugni G, Gatti D, Spinozzi E, Sartorio A, Adami S, Fanolla A, Radetti G. Adults with Prader-Willi syndrome have weaker bones: effect of treatment with GH and sex steroids. Calcif Tissue Int. 2015;96:160–166. doi: 10.1007/s00223-014-9949-1. [DOI] [PubMed] [Google Scholar]
- 77.Bakker NE, Wolffenbuttel KP, Looijenga LH, Hokken-Koelega AC. Testes in infants with Prader-Willi syndrome: human chorionic gonadotropin treatment, surgery and histology. J Urol. 2015;193:291–298. doi: 10.1016/j.juro.2014.07.113. [DOI] [PubMed] [Google Scholar]
- 78.Carrel AL, Myers SE, Whitman BY, Allen DB. Sustained benefits of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome are dose-dependent. J Pediatr Endocrinol Metab. 2001;14:1097–1105. doi: 10.1515/jpem-2001-0805. [DOI] [PubMed] [Google Scholar]
- 79.Shaikh MG, Crabtree N, Kirk JM, Shaw NJ. The relationship between bone mass and body composition in children with hypothalamic and simple obesity. Clin Endocrinol (Oxf) 2014;80:85–91. doi: 10.1111/cen.12263. [DOI] [PubMed] [Google Scholar]
- 80.Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM, Barnes N, Armstrong GT, Krasin MJ, Srivastava DK, Pui CH, Merchant TE, Kun LE, Gajjar A, Hudson MM, Robison LL, Sklar CA. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mostoufi-Moab S, Magland J, Isaacoff EJ, Sun W, Rajapakse CS, Zemel B, Wehrli F, Shekdar K, Baker J, Long J, Leonard MB. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. J Bone Miner Res. 2015;30:1657–1666. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clark EM. The epidemiology of fractures in otherwise healthy children. Curr Osteoporos Rep. 2014;12:272–278. doi: 10.1007/s11914-014-0227-y. [DOI] [PubMed] [Google Scholar]
- 83.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. Jama. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 84.Mayranpaa MK, Makitie O, Kallio PE. Decreasing incidence and changing pattern of childhood fractures: A population-based study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25:2752–2759. doi: 10.1002/jbmr.155. [DOI] [PubMed] [Google Scholar]
- 85.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 86.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 87.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 88.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 89.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wren TA, Shepherd JA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Dorey FJ, Winer KK, Gilsanz V. Racial Disparity in Fracture Risk between White and Nonwhite Children in the United States. J Pediatr. 2012;161:1035–1040. doi: 10.1016/j.jpeds.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalkwarf HJ, Laor T, Bean JA. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA) Osteoporos Int. 2011;22:607–616. doi: 10.1007/s00198-010-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471:1199–1207. doi: 10.1007/s11999-012-2621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fewtrell MS. Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child. 2003;88:795–798. doi: 10.1136/adc.88.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy x-ray absorptiometry (DEXA) J Pediatr. 2004;144:253–257. doi: 10.1016/j.jpeds.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 95.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 96.Kroger H, Kotaniemi A, Vainio P, Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner. 1992;17:75–85. doi: 10.1016/0169-6009(92)90712-m. [DOI] [PubMed] [Google Scholar]
- 97.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–972. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, Abrams SA. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res. 2001;16:1658–1664. doi: 10.1359/jbmr.2001.16.9.1658. [DOI] [PubMed] [Google Scholar]
- 99.Horlick M, Wang J, Pierson RN, Jr, Thornton JC. Prediction models for evaluation of total-body bone mass with dual-energy X-ray absorptiometry among children and adolescents. Pediatrics. 2004;114:e337–345. doi: 10.1542/peds.2004-0301. [DOI] [PubMed] [Google Scholar]
- 100.Molgaard C, Thomsen BL, Michaelsen KF. Influence of weight, age and puberty on bone size and bone mineral content in healthy children and adolescents. Acta Paediatr. 1998;87:494–499. doi: 10.1080/08035259850158173. [DOI] [PubMed] [Google Scholar]
- 101.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. The Journal of Clinical Endocrinology and Metabolism. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 103.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tothill P, Laskey MA, Orphanidou CI, van Wijk M. Anomalies in dual energy X-ray absorptiometry measurements of total-body bone mineral during weight change using Lunar, Hologic and Norland instruments. Br J Radiol. 1999;72:661–669. doi: 10.1259/bjr.72.859.10624323. [DOI] [PubMed] [Google Scholar]
- 105.Shepherd JA, Wang L, Fan B, Gilsanz V, Kalkwarf HJ, Lappe J, Lu Y, Hangartner T, Zemel BS, Fredrick M, Oberfield S, Winer KK. Optimal monitoring time interval between DXA measures in children. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26:2745–2752. doi: 10.1002/jbmr.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knapp KM, Welsman JR, Hopkins SJ, Shallcross A, Fogelman I, Blake GM. Obesity increases precision errors in total body dual-energy x-ray absorptiometry measurements. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2015;18:209–216. doi: 10.1016/j.jocd.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Wosje KS, Knipstein BL, Kalkwarf HJ. Measurement error of DXA: interpretation of fat and lean mass changes in obese and non-obese children. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2006;9:335–340. doi: 10.1016/j.jocd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 108.International Atomic Energy Agency. Dual Energy X Ray Absorptiometry for Bone Mineral Density and Body Composition Assessment. IAEA; Vienna: 2011. [Google Scholar]
- 109.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. The American journal of clinical nutrition. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 110.Bolotin HH. Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral densitometry may flaw osteopenic/osteoporotic interpretations and mislead assessment of antiresorptive therapy effectiveness. Bone. 2001;28:548–555. doi: 10.1016/s8756-3282(01)00423-9. [DOI] [PubMed] [Google Scholar]
- 111.Bolotin HH, Sievanen H, Grashuis JL, Kuiper JW, Jarvinen TL. Inaccuracies inherent in patient-specific dual-energy X-ray absorptiometry bone mineral density measurements: comprehensive phantom-based evaluation. J Bone Miner Res. 2001;16:417–426. doi: 10.1359/jbmr.2001.16.2.417. [DOI] [PubMed] [Google Scholar]
- 112.Bolotin HH. A new perspective on the causal influence of soft tissue composition on DXA-measured in vivo bone mineral density. J Bone Miner Res. 1998;13:1739–1746. doi: 10.1359/jbmr.1998.13.11.1739. [DOI] [PubMed] [Google Scholar]
- 113.Webber CE. The effect of fat on bone mineral measurements in normal subjects with recommended values of bone, muscle and fat attenuation coefficients. Clin Phys Physiol Meas. 1987;8:143–158. doi: 10.1088/0143-0815/8/2/005. [DOI] [PubMed] [Google Scholar]
- 114.Svendsen OL, Hassager C, Skodt V, Christiansen C. Impact of soft tissue on in vivo accuracy of bone mineral measurements in the spine, hip, and forearm: a human cadaver study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1995;10:868–873. doi: 10.1002/jbmr.5650100607. [DOI] [PubMed] [Google Scholar]
- 115.LaForgia J, Dollman J, Dale MJ, Withers RT, Hill AM. Validation of DXA body composition estimates in obese men and women. Obesity. 2009;17:821–826. doi: 10.1038/oby.2008.595. [DOI] [PubMed] [Google Scholar]
- 116.Crabtree NJ, Shaw NJ, Boivin CM, Oldroyd B, Truscott JG. Pediatric in vivo cross-calibration between the GE Lunar Prodigy and DPX-L bone densitometers. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:2157–2167. doi: 10.1007/s00198-005-2021-2. [DOI] [PubMed] [Google Scholar]
- 117.Blake GM, Harrison EJ, Adams JE. Dual X-ray absorptiometry: cross-calibration of a new fan-beam system. Calcif Tissue Int. 2004;75:7–14. doi: 10.1007/s00223-004-0169-y. [DOI] [PubMed] [Google Scholar]
- 118.Evans EM, Mojtahedi MC, Kessinger RB, Misic MM. Simulated change in body fatness affects Hologic QDR 4500A whole body and central DXA bone measures. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2006;9:315–322. doi: 10.1016/j.jocd.2006.04.117. [DOI] [PubMed] [Google Scholar]
- 119.Laskey MA, Murgatroyd PR, Prentice A. Comparison of narrow-angle fan-beam and pencil-beam densitometers: in vivo and phantom study of the effect of bone density, scan mode, and tissue depth on spine measurements. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2004;7:341–348. doi: 10.1385/jcd:7:3:341. [DOI] [PubMed] [Google Scholar]