Abstract
Angiogenin is a member of the ribonuclease A superfamily of proteins that has been implicated in stimulating angiogenesis but whether angiogenin can directly affect ovarian granulosa or theca cell function is unknown. Therefore, the objective of these studies was to determine the effect of angiogenin on proliferation and steroidogenesis of bovine granulosa and theca cells. In experiments 1 and 2, granulosa cells from small (1 to 5 mm diameter) follicles and theca cells from large (8 to 22 mm diameter) follicles were cultured to evaluate the dose-response effect of recombinant human angiogenin on steroidogenesis. At 30 and 100 ng/ml, angiogenin inhibited (P < 0.05) granulosa cell progesterone production and theca cell androstenedione production but did not affect (P > 0.10) granulosa cell estradiol production or theca cell progesterone production, and did not affect numbers of granulosa or theca cells. In experiments 3 and 4, granulosa and theca cells from both small and large follicles were cultured with 300 ng/ml of angiogenin to determine if size of follicle influenced responses to angiogenin. At 300 ng/ml, angiogenin increased large follicle granulosa cell proliferation but decreased small follicle granulosa cell progesterone and estradiol production and large follicle theca cell progesterone production. In experiments 5 and 6, angiogenin stimulated (P < 0.05) proliferation and DNA synthesis in large follicle granulosa cells. In experiment 7, 300 ng/ml of angiogenin increased (P < 0.05) CYP19A1 messenger RNA (mRNA) abundance in granulosa cells but did not affect CYP11A1 mRNA abundance in granulosa or theca cells and did not affect CYP17A1 mRNA abundance in theca cells. We conclude that angiogenin appears to target both granulosa and theca cells in cattle, but additional research is needed to further understand the mechanism of action of angiogenin in granulosa and theca cells, as well as its precise role in folliculogenesis.
Keywords: angiogenin (ANG), granulosa cells, theca cells, IGF1, tumor necrosis factor α (TNFα)
Introduction
Angiogenesis or the formation of blood vessels from pre-existing blood vessels is controlled by many different angiogenic factors (Lee et al., 1999; Ucuzian et al., 2010). Angiogenesis is involved in a variety of physiological processes such as wound repair and embryological development (Redmer and Reynolds, 1996; Przybylski, 2009; Appelmann et al., 2010), and is under the influence of anti-angiogenic and pro-angiogenic molecular mediators. One of the pro-angiogenic factors is angiogenin (ANG), a member of the ribonuclease A superfamily of 10 to 28 kDa proteins ascribed as a group of enzymes that have inherent substrate specificity and differing functional capacities (for review see: Tello-Montoliu et al., 2006; Gupta et al., 2013; Premzl, 2014). However, unlike many of the RNase A superfamily members, ANG has been shown to have its own specific membrane receptor that mediates its effects (Hatzi and Badet, 1999; Xu et al., 2001). Angiogenin was first isolated from medium conditioned by human colon carcinoma (HT-29) cells (Fett et al., 1985) and later found in normal human serum (Shapiro et al., 1987), bovine milk (Maes et al., 1988) and other mammalian serum (Bond et al., 1993). Specific angiogenic effects of ANG are multifaceted (Gao and Xu, 2008) and includes induction of endothelial cell proliferation (Hu et al., 1997; Tsuji et al., 2005), tumor cell adhesion (Soncin et al., 1994), and tumor cell invasion (Gho et al., 2002).
In domestic animals, ovarian vasculature surrounding follicles increases during normal follicular development (Yamada et al., 1995; Jiang et al., 2002, 2003; Moonmanee et al., 2013), but the molecular mechanisms that initiate ovarian angiogenesis are not well defined. In the bovine and human ovary, using in situ hybridization, ANG messenger RNA (mRNA) was localized in granulosa cells and oocytes (but not theca cells) of secondary and tertiary follicles, luteal cells of developing corpora lutea, and vascular endothelial and smooth muscle cells (Lee et al., 1999; Koga et al., 2000). Malamitsi-Puchner et al. (2001) reported that follicular fluid levels of ANG were significantly greater in human follicles yielding mature v. immature oocytes. Moreover, follicular fluid concentrations of ANG (ranging from 100 to 800 ng/ml) and progesterone were positively correlated in women (Koga et al., 2000). Therefore, we hypothesized that ANG, in addition to its angiogenic effects, would stimulate steroidogenesis and increase proliferation of both granulosa and theca cells. Therefore, the objectives of this study were to determine the effect of ANG on granulosa and theca cell proliferation and steroid production in vitro.
Materials and methods
Hormones and reagents
The hormones and reagents used in cell culture were: ovine FSH (NIDDK-oFSH-20; activity: 175 X NIH-FSH-S1 U/mg) and ovine LH (NIDDK-oLH-26; activity: 1.0 X NIH-LH-S1 U/mg) from the National Hormone & Pituitary Program (Torrance, CA, USA), recombinant human ANG and IGF-1 (IGF1) from R&D Systems (Minneapolis, MN, USA), testosterone from Steraloids (Wilton, NH, USA), fetal calf serum (FCS) from EquiTech-Bio Inc. (Kerrville, TX, USA), and collagenase and DNase from Sigma-Aldrich Corp. (St. Louis, MO, USA). Human ANG and bovine ANG proteins share 66% amino acid homology (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Cell culture
Ovaries from non-pregnant beef cows were collected from a local slaughterhouse, and based on surface diameter, granulosa cells were collected from small (1 to 5 mm) and large (8 to 22 mm) follicles as previously described (Langhout et al., 1991; Stewart et al., 1995; Spicer et al., 2008). Small and large follicles were bisected after aspiration of follicular fluid and granulosa cells separated from the theca interna via blunt dissection, and the theca interna was torn into small pieces, rinsed with basal serum-free medium (1 : 1 Dulbecco’s modified Eagle’s medium and Ham’s F-12; 0.12 mM gentamycin, 2.0 mM glutamine, and 38.5 mM sodium bicarbonate; all obtained from Sigma-Aldrich Corp.) and enzymatically digested for 1 h at 37°C as previously described (Stewart et al., 1995; Spicer and Chamberlain, 1998; Spicer et al., 2008). Briefly, after digestion, the non-digested tissue was filtered out, and theca cells were then centrifuged at 50 × g for 5 min, supernatant discarded and pellet washed with serum-free medium. The purity of theca cells prepared this way was >90% (Spicer and Stewart, 1996; Spicer et al., 2008). The theca and granulosa cells were re-suspended in serum-free media containing collagenase and DNase at 1.25 and 0.5 mg/ml, respectively, to prevent cell clumping before plating.
Viable cells (2 × 105 in 25 to 45 µl of medium/well) were plated in each well of 24-well Falcon multiwell plates (Becton Dickinson, Lincoln Park, NJ, USA) containing 1 ml of basal medium with 10% FCS (v/v). Cells were cultured at 38.5°C in 10% FCS (v/v) for the first 48 h with a medium change at 24 h. Cells were then washed twice with serum-free medium and the various treatments (see below) applied in serum-free medium (1 ml per well) for 48 h with a medium change at 24 h. After 48 h, medium was collected for steroid quantification via radioimmunoassays (RIA) and cells were collected for cell enumeration via Coulter counting (see below). The concentrations of LH, FSH and IGF1 were selected based on previous studies (Spicer and Stewart, 1996; Spicer and Chamberlain, 1998; Spicer et al., 2002). Because steroid production in this culture system is maximized with combined gonadotropins and growth factor treatment, only weakly responsive to gonadotropins alone, and not responsive to growth factors alone (Stewart et al., 1995; Spicer and Chamberlain, 1998; Spicer et al., 2002), gonadotropins were used in combination with IGF1 for most experiments. The concentrations of ANG used for experiments were selected based on studies indicating that concentrations of ANG in bovine plasma (Bond and Vallee, 1988; Chang et al., 1997) and human follicular fluid (Koga et al., 2000; Malamitsi-Puchner et al., 2001; Kawano et al., 2003) range between 0.4 and 800 ng/ml.
Experimental design
Experiment 1 was designed to determine the dose-response effects of ANG on steroidogenesis and number of granulosa cells of small follicles. Granulosa cells from small follicles were selected for this experiment because we have shown that they grow and respond well to growth factor treatments (Spicer et al., 2002). Granulosa cells from small follicles were cultured for 48 h in the presence of 10% FCS, and then cells were washed and incubated in serum-free medium in the presence of 0, 30 or 100 ng/ml of ANG without or with 30 ng/ml of FSH and/or 30 ng/ml of IGF1 for 48 h. Medium was changed every 24 h and contained 500 ng/ml of testosterone as an estrogen precursor (Spicer and Chamberlain, 1998). Cells were counted and medium samples were collected for progesterone (P4) and estradiol (E2) determinations via RIA (see below).
Experiment 2 was designed to determine the dose-response effects of ANG on steroidogenesis and number of theca cells of large follicles. Theca cells from large follicles were selected for this experiment because we have shown that they grow and respond well to growth factor treatments (Spicer et al., 2008). Theca cells from large follicles were cultured for 48 h in the presence of 10% FCS, and then cells were washed and incubated in serum-free medium in the presence of 0, 30 or 100 ng/ml of ANG without or with 30 ng/ml of LH and/or 30 ng/ml of IGF1 for 48 h. Medium was changed every 24 h. Cells were counted and medium samples were collected for P4 and androstenedione determinations via RIA (see below).
Experiment 3 was designed to compare the effects of high dose (i.e. 300 ng/ml) ANG on steroidogenesis and number of granulosa cells isolated from small and large follicles. Because experiment 1 showed minor effects of 30 and 100 ng/ml of ANG on small follicle granulosa cell numbers or steroid production, studies were conducted using 300 ng/ml of ANG with both small and large follicle granulosa cells. Cells from small and large follicles were cultured for 48 h in the presence of 10% FCS, and then cells were washed and incubated in serum-free medium in the presence of 0 or 300 ng/ml of ANG without or with 30 ng/ml of FSH and/or 30 ng/ml of IGF1 for 48 h. Medium was changed every 24 h and contained 500 ng/ml of testosterone as an estrogen precursor (Spicer and Chamberlain, 1998). Cells were counted and medium samples were collected for P4 and E2 determinations via RIA (see below).
Experiment 4 was designed to compare the effects of high dose (i.e. 300 ng/ml) ANG on steroidogenesis and number of theca cells isolated from small and large follicles. Because experiment 2 showed minor effects of 30 and 100 ng/ml of ANG on large follicle theca cell numbers or steroid production, studies were conducted using 300 ng/ml of ANG with both small and large follicle theca cells. For this experiment, small follicle theca cells were obtained from follicles 3 to 6 mm in diameter as previously described (Spicer et al., 2008). Cells from small and large follicles were cultured for 48 h in the presence of 10% FCS, and then cells were washed and incubated in serum-free medium in the presence of 0 or 300 ng/ml of ANG without or with 30 ng/ml of LH and/or 30 ng/ml of IGF1 for 48 h. Medium was changed every 24 h. Cells were counted and medium samples were collected for P4 and androstenedione determinations via RIA (see below).
Experiment 5 was designed to test the effect of ANG on large follicle granulosa cell numbers. Cells were cultured for 48 h in 10% FCS medium, and then treated for an additional 24 h or 48 h with either 0 or 300 ng/ml of ANG in the presence of 10% FCS. Separate cultures were terminated at 0, 24 and 48 h post-treatment, and cells were counted. Dose of ANG was selected based on results from experiments 1 and 3.
Experiment 6 was designed to determine the effect of ANG on large follicle granulosa and theca cell proliferation as measured by 3H-thymidine incorporation into DNA. Because experiments 1 to 4 showed an effect of ANG on cell numbers only in the presence of IGF1, this experiment evaluated the effect of ANG in the presence of IGF1. After 48 h in 10% FCS, cells were serum-starved for 24 h by culturing in serum-free medium, medium changed, and then cells cultured for an additional 40 h in serum-free medium with either 0 or 300 ng/ml of angiogenin in the presence of 1 µCi of 3H-thymidine and 30 ng/ml of IGF1 to assess DNA synthesis as previously described (Spicer and Aad, 2007; Spicer et al., 2008).
Experiment 7 was conducted to determine the effect ANG on steroidogenic gene expression in granulosa and theca cells. Granulosa cells from small follicles and theca cells from large follicles were cultured for 48 h in 10% FCS, medium changed, and then cells cultured for an additional 24 h in serum-free medium with either no treatment or 300 ng/ml of ANG. Granulosa cells were concomitantly treated with 30 ng/ml of FSH and IGF1 and theca cells were treated concomitantly with 30 ng/ml of LH and IGF1. Doses of FSH, LH and IGF1 were selected based on previous studies (Spicer et al., 2002; Lagaly et al., 2008). Gonadotropins were added to all treatments because IGF1 alone has little or no effect on steroid production (Lagaly et al., 2008; Spicer et al., 2008). After 24 h of treatment, cells were lysed in 0.5 ml of TRIzol for RNA extraction (see below) and quantification of CYP11A1, CYP19A1 or CYP17A1 mRNA.
RNA extraction and quantification
Total RNA was extracted using TRIzol reagent protocol (Life Technologies, Carlsbad, CA, USA), and RNA was quantitated by spectrophotometry at 260 nm using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA) as previously described (Voge et al., 2004; Aad et al., 2006; Schreiber and Spicer, 2012). Quantification of gene expression was conducted by fluorescent one-step real-time PCR as previously described (Spicer and Aad, 2007; Grado-Ahuir et al., 2011). The bovine CYP11A1, CYP17A1 and CYP19A1 primer and probe sequences and information are described by Lagaly et al. (2008). The internal standard was 18S ribosomal RNA. Data analysis was done using the comparative threshold cycle (Ct) method as previously described (Voge et al., 2004; Aad et al., 2006). Briefly, the ΔCt was determined by subtracting the 18S Ct value from the target gene unknown value. For each target gene and within each experiment, the ΔΔCt was determined by subtracting the higher ΔCt (the least expressed unknown) from all other ΔCt values. Fold changes in target gene mRNA abundance were calculated as being equal to 2−ΔΔCt.
Determination of steroid concentrations and cell numbers
Medium was collected from individual wells and frozen at −20°C for subsequent determination of concentrations of P4, E2 and/or androstenedione via RIA as previously described (Langhout et al., 1991; Stewart et al., 1995; Spicer and Chamberlain, 1998). The intra- and interassay coefficients of variation were 5.6% and 11% for the progesterone RIA, 6.5% and 12% for the estradiol RIA, and 4.1% and 7.3% for the androstenedione RIA.
Numbers of cells were determined via Coulter counting as previously described (Langhout et al., 1991; Stewart et al., 1995; Spicer et al., 2008) and used to calculate steroid production on a ng or pg per 105 cell basis. Briefly, cells were gently washed twice with 0.9% saline (500 µl; w/v), exposed to 500 µl of trypsin solution (0.25%, w/v) for 20 min at 25°C, scraped from each well, and counted using a Coulter counter (model Z2; Beckman Coulter Inc., Miami, FL, USA).
Cell aggregates were disrupted via pipetting the cell suspension back and forth through a 500 µl pipette tip three to five times, and diluted in 9 ml of 0.9% saline (w/v) before counting.
Statistical analysis
Data are presented as the least squares means (± SE) of measurements from three or more individual pools of large and small follicle granulosa/theca cells used as experimental replicates. Each biological replicate (i.e. pool of cells) was conducted on independent pools of cells. Each pool of small follicle granulosa cells were collected from 10 to 30 ovaries (n = 5 to 15 cattle) yielding 6 to 8 ml of follicular fluid. Each of the large follicle granulosa/theca cell pools was obtained from 7 to 10 follicles from at least five animals. Small follicle theca cells were obtained from 6 to 20 ovaries (n = 3 to 10 animals). Within each replicated experiment, treatments were applied to each pool of cells in duplicate or triplicate culture wells. Steroid production was expressed as ng or pg/105 cells per 24 h, and cell numbers at the termination of each experiment were used for this calculation. Specific differences in cell numbers and steroid production among treatments were determined via ANOVA using GLM procedure of SAS (Statistical Analysis System, Cary, NC, USA) and Fisher’s protected least significant difference procedure (Ott, 1977). Significance was declared at P < 0.05.
Results
Experiment 1: dose response of ANG on cell numbers and steroidogenesis of small follicle granulosa cells
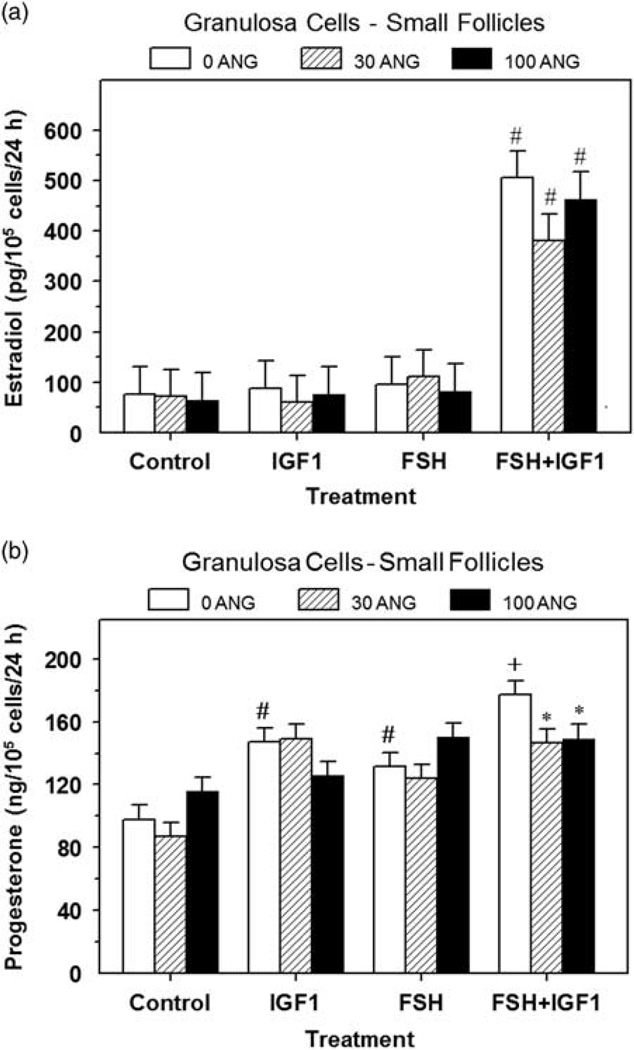
Treatment of granulosa cells with IGF1 alone increased (P < 0.05) cell numbers by 54% to 73% (Table 1), however none of the doses of ANG (i.e. 30 or 100 ng/ml) affected (P > 0.10) control or IGF1-induced granulosa cell numbers (Table 1). Alone FSH had no effect (P > 0.05) on cell numbers but FSH significantly enhanced the IGF1-induced increase (P < 0.001) in cell numbers (Table 1). Dose of ANG had no significant effect on E2 production (Figure 1a). FSH and IGF1 synergized to stimulate (P < 0.01) E2 production by 6.6-fold, and ANG had no significant effect on this FSH plus IGF1-induced E2 production (Figure 1a); alone neither FSH nor IGF1 affected (P > 0.10) E2 production. Both IGF1 and FSH increased P4 production and 100 ng/ml of ANG reduced (P < 0.05) the FSH plus IGF1-induced P4 production by 16% (Figure 1b).
Table 1.
Effect of recombinant human angiogenin on granulosa and theca cell numbers in experiments 1 and 2
| Angiogenin (ng/ml) (cell numbers (×105/well)) |
||||||
|---|---|---|---|---|---|---|
| Experiments | FSH or LH | IGF1 | 0 | 30 | 100 | SEM1 |
| Experiment 1 – GC FSH | – | – | 1.10a | 1.03a | 1.02a | 0.08 |
| Experiment 2 – TC LH | – | – | 0.78a | 0.85a | 0.83a | 0.07 |
| Experiment 1 – GC FSH | + | – | 1.07a | 1.29a | 1.24a | 0.08 |
| Experiment 2 – TC LH | + | – | 0.84a | 0.77a | 0.93a | 0.07 |
| Experiment 1 – GC FSH | – | + | 1.69b | 1.74b | 1.76b | 0.08 |
| Experiment 2 – TC LH | – | + | 1.20b | 1.18b | 1.24b | 0.07 |
| Experiment 1 – GC FSH | + | + | 2.15c | 2.29c | 2.42c | 0.08 |
| Experiment 2 – TC LH | + | + | 1.21b | 1.20b | 1.22b | 0.07 |
Granulosa cells (GC) from small (1 to 5 mm; experiment 1) and theca cells (TC) from large (8 to 22 mm; experiment 2) bovine follicles were cultured as described in ‘Materials and methods’ section, and treated for 48 h with FSH (for GC), LH (for TC) and/or IGF1 (0 or 30 ng/ml) and 0, 30 or 100 ng/ml of angiogenin.
Within an experiment, means without a common letter differ (P < 0.05).
SEM for n = 6.
Figure 1.
Effect of angiogenin on basal, FSH- and IGF1-induced estradiol (a) and progesterone (b) production by small-follicle granulosa cells (experiment 1). Cells were cultured for 48 h as described in ‘Materials and methods’ section, and then treated for an additional 48 h with 0, 30 or 100 ng/ml of angiogenin (ANG) and: Control (no IGF1 or FSH), IGF1 (30 ng/ml), FSH (30 ng/ml) or FSH plus IGF1. Values are means ± SEM of three separate experiments (n = 6). *Within a panel and treatment, mean differs (P < 0.05) from its respective 0 ANG mean. #Within a panel, mean differs (P < 0.05) from its respective Control mean. +Within a panel, mean differs (P < 0.05) from its respective IGF1 alone or FSH alone treatment mean.
Experiment 2: dose response of ANG on steroidogenesis of large follicle theca cells
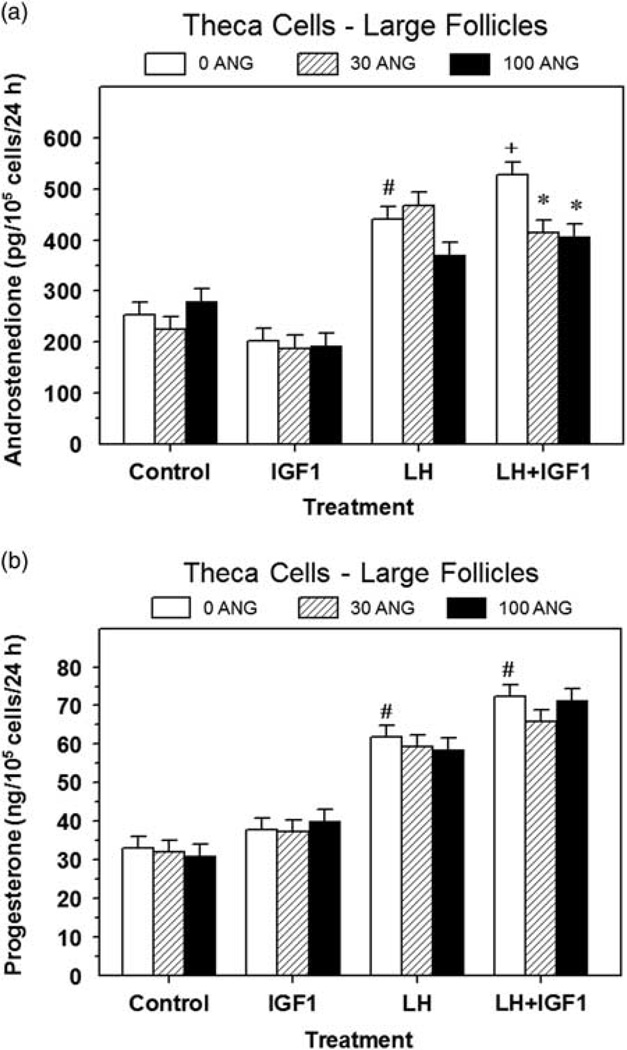
Treatment of theca cells with IGF1 increased (P < 0.05) cell numbers by 31% to 56% (Table 1). Neither LH nor ANG (30 or 100 ng/ml) affected (P > 0.10) theca cell numbers induced by IGF1 (Table 1). LH increased (P < 0.05) both androstenedione and P4 production, and ANG (30 and 100 ng/ml) decreased (P < 0.05) IGF1 plus LH-induced androstenedione production by 22% to 23%, but ANG had no effect (P > 0.10) on basal, LH-induced or IGF1-induced androstenedione production (Figure 2a). None of the doses of ANG affected (P > 0.10) P4 production by theca cells (Figure 2b).
Figure 2.
Effect of angiogenin on basal, LH- and IGF1-induced androstenedione (a) and progesterone (b) production by large follicle theca cells (experiment 2). Cells were cultured for 48 h as described in ‘Materials and methods’ section, and then treated for an additional 48 h with 0, 30 or 100 ng/ml of angiogenin (ANG) and: Control (no IGF1 or LH), IGF1 (30 ng/ml), LH (30 ng/ml) or LH plus IGF1. Values are means ± SEM of three separate experiments (n = 6). *Within a panel and treatment, mean differs (P < 0.05) from its respective 0 ANG mean. #Within a panel, mean differs (P < 0.05) from its respective Control mean. +Within a panel, mean differs (P < 0.05) from its respective LH alone treatment mean.
Experiment 3: effect of high-dose of ANG on steroidogenesis of small and large follicle granulosa cells
Angiogenin had no effect (P > 0.10) on numbers of small follicle granulosa cells, but increased (P < 0.05) cell numbers in FSH + IGF1-treated large follicle granulosa cells (Table 2). Treatment with FSH and IGF1 increased (P < 0.05) cell numbers by 2.3-fold and 4.8-fold above controls in small and large follicle granulosa cell cultures, respectively. Angiogenin decreased (P < 0.05) P4 production by 12% in small follicle granulosa cells treated with FSH + IGF1 and by 30% in control (untreated) large follicle granulosa cells (Table 2). In small follicle granulosa cells, FSH and IGF1 stimulated (P < 0.01) E2 production by 5.7-fold, and ANG decreased this hormone-induced E2 production by 27% (Table 2). In large follicle granulosa cells, ANG had no effect (P > 0.10) on E2 production (Table 2). To compare across granulosa cell experiments, data (basal and FSH plus IGF1 treatment) from experiments 1 and 3 were expressed as fold of controls and shown in Supplementary Figure S1.
Table 2.
Effect of 2-day treatment of 300 ng/ml of angiogenin (ANG) on basal and IGF1 plus FSH-induced progesterone production, estradiol production and numbers of granulosa cells from small (1 to 5 mm) and large (≥8 mm) bovine follicles of experiment 3
| Size of follicle | Treatment | Dose of ANG (ng/ml) | Progesterone (ng/105 cells/24 h) |
Estradiol (ng/105 cells/24 h) |
Cell number (×105 per well) |
|---|---|---|---|---|---|
| Small | Control | 0 | 58.0a | 0.05a | 0.64a |
| Small | Control | 300 | 52.0a | 0.05a | 0.69a |
| Small | FSH+IGF1 | 0 | 85.6c | 0.29c | 1.46b |
| Small | FSH+IGF1 | 300 | 75.3b | 0.21b | 1.61b |
| SEM1 | 2.6 | 0.01 | 0.07 | ||
| Large | Control | 0 | 203.0b | 0.58a | 0.15a |
| Large | Control | 300 | 142.0a | 0.36a | 0.20a |
| Large | FSH+IGF1 | 0 | 215.0b | 2.63b | 0.75b |
| Large | FSH+IGF1 | 300 | 188.0b | 2.26b | 0.82c |
| SEM1 | 13.0 | 0.27 | 0.02 |
Within a column, means without a common superscript differ (P <0.05).
SEM for n = 6.
Experiment 4: effect of high-dose ANG on steroidogenesis of small and large follicle theca cells
LH decreased (P < 0.05) small follicle theca cell numbers induced by IGF1 but had no effect (P > 0.10) on numbers of large follicle theca cells (Table 3). Angiogenin (300 ng/ml) restored this decrease induced by LH (Table 3) in small follicle theca cells, and increased (P < 0.05) numbers of IGF1-treated large follicle theca cells by 23% but had no effect on LH + IGF1-treated large follicle theca cells (Table 3). Angiogenin had no effect (P > 0.10) on P4 production by small follicle theca cells (Table 3), but decreased (P < 0.05) P4 production in IGF1-treated large follicle theca cells by 17% (Table 3). In both small and large follicle theca cells, ANG had no effect (P > 0.10) on IGF1-induced or LH + IGF1-induced androstenedione production (Table 3). To compare across theca cell experiments, data (basal and LH plus IGF1 treatment) from experiments 2 and 4 were expressed as fold of controls and shown in Supplementary Figure S2.
Table 3.
Effect of 2-day treatment of 300 ng/ml of angiogenin (ANG) on IGF1- and IGF1 plus LH-induced progesterone production, androstenedione production and numbers of theca cells from small (3 to 6 mm) and large (≥8 mm) bovine follicles of experiment 4
| Size of follicle | Treatment | Dose of ANG (ng/ml) | Progesterone (ng/105 cells/24 h) |
Androstenedione (ng/105 cells/24 h) |
Cell number (×105 per well) |
|---|---|---|---|---|---|
| Small | +IGF1 | 0 | 42.9a | 1.15a | 1.17b |
| Small | +IGF1 | 300 | 42.0a | 1.07a | 1.16b |
| Small | LH +IGF1 | 0 | 205.3b | 31.2b | 0.90a |
| Small | LH +IGF1 | 300 | 190.4b | 28.0b | 1.11b |
| SEM1 | 6.0 | 0.9 | 0.04 | ||
| Large | +IGF1 | 0 | 28.8b | 1.06a | 1.13a |
| Large | +IGF1 | 300 | 24.0a | 0.91a | 1.28b |
| Large | LH +IGF1 | 0 | 44.0c | 4.00b | 1.21ab |
| Large | LH +IGF1 | 300 | 40.8c | 3.94b | 1.20ab |
| SEM1 | 1.6 | 0.17 | 0.05 |
Within a column, means without a common superscript differ (P < 0.05).
SEM for n = 9.
Experiment 5: effect of ANG on granulosa and theca cell proliferation induced by 10% FCS
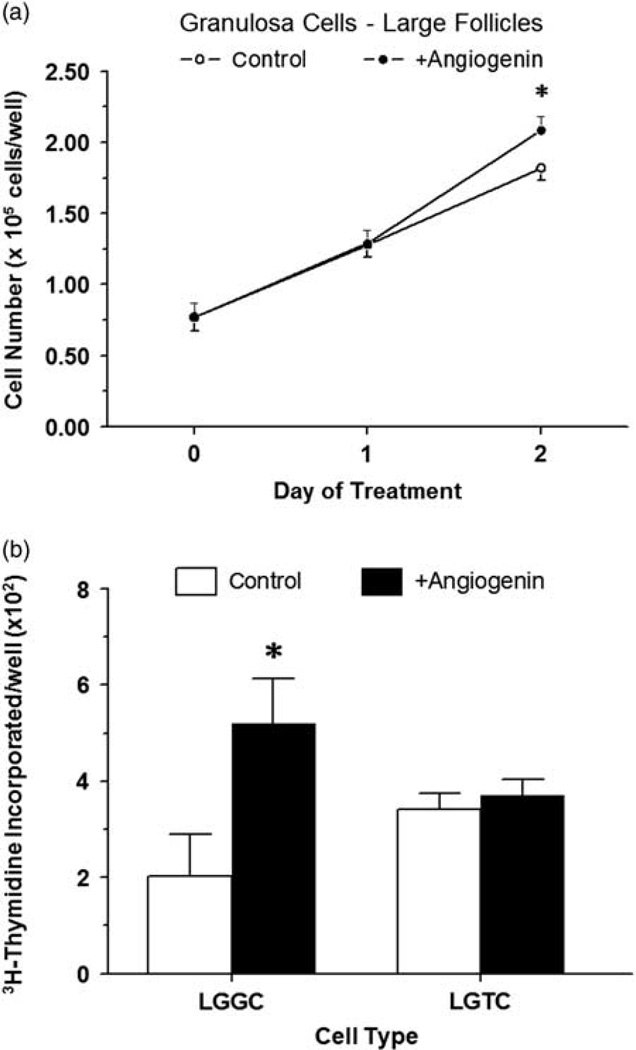
After 2 days of treatment, ANG (300 ng/ml) further enhanced (P < 0.05) proliferation of large follicle granulosa cells stimulated by 10% FCS (Figure 3a). Granulosa cells grew 2.4- and 2.7-fold between day 0 and 2 in Control and ANG-treated cultures, respectively (P < 0.05; Figure 3a). Cell numbers in ANG-treated cells did not differ (P > 0.10) from controls on day 1 of treatment (Figure 3a).
Figure 3.
Effect of angiogenin on proliferation of large follicle granulosa cells induced by 10% FCS (a) (experiment 5) and 3H-thymidine incorporation into large follicle granulosa and theca cells (b) treated with IGF1 (experiment 6). (a) Cells were cultured for 48 h as described in ‘Materials and methods’ section, and then treated for an additional 48 h with 0 or 300 ng/ml of angiogenin in the presence of 10% FCS; cells were enumerated via Coulter counting. (b) Cells were cultured for 48 h as described in ‘Materials and methods’ section, serum-starved for 24 h, and then treated for an additional 40 h with 3H-thymidine and IGF1 (30 ng/ml) and either 0 or 300 ng/ml of angiogenin. *Within a panel, asterisk (*) indicates mean differs (P < 0.05) from its respective control (0 ANG) mean. Values are means ± SEM of three separate experiments (n = 6).
Experiment 6: effect of ANG on granulosa and theca cell proliferation induced by IGF1
Treatment of bovine granulosa cells from large follicles with 300 ng/ml of ANG increased (P < 0.05) IGF1-induced 3H-thymidine incorporation into DNA by 2.6-fold (Figure 3b). Angiogenin (300 ng/ml) had no effect (P > 0.10) on large follicle theca cell proliferation as measured by 3H-thymidine incorporation into DNA (Figure 3b).
Experiment 7: effect of ANG on steroidogenic gene expression in granulosa and theca cells
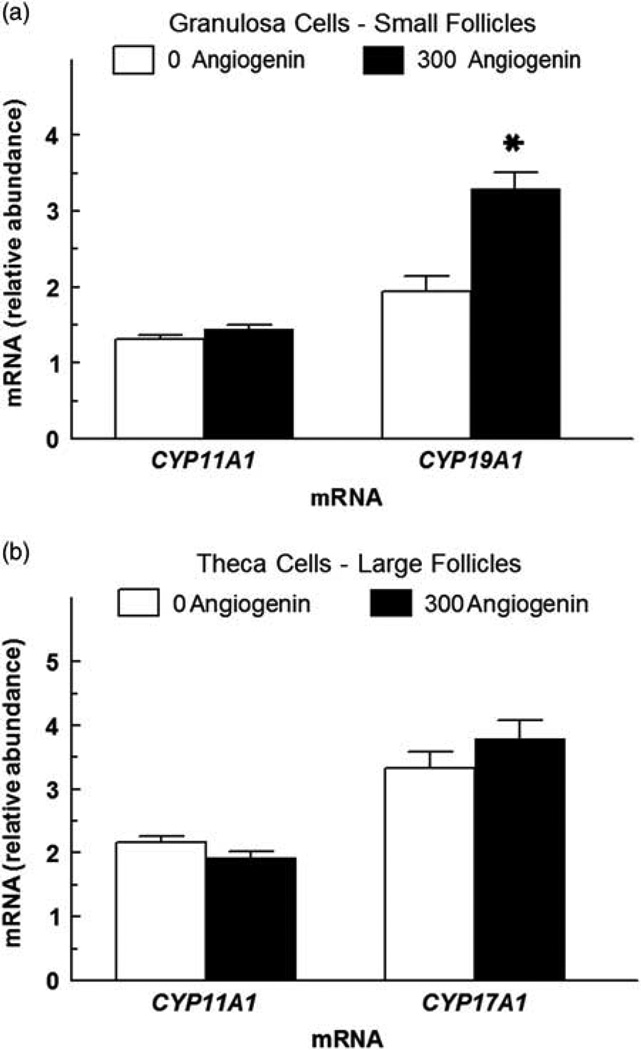
Treatment of 300 ng/ml of ANG to granulosa cells from small follicles and theca cells from large follicles had no effect (P > 0.10) on CYP11A1 mRNA abundance (Figure 4). However, ANG at 300 ng/ml increased (P < 0.05) CYP19A1 mRNA abundance in granulosa cells and had no effect (P > 0.10) on CYP17A1 mRNA abundance in theca cells (Figure 4).
Figure 4.
Effect of angiogenin (ANG) on steroidogenic enzyme gene expression in granulosa and theca cells (experiment 7). (a) Effect of effect of ANG (300 ng/ml) on CYP19A1 (aromatase) and CYP11A1 (side-chain cleavage enzyme) mRNA abundance in small follicle granulosa cells. (b) Effect of ANG (300 ng/ml) on CYP17A1 and CYP11A1 mRNA abundance in large follicle theca cells. Granulosa cells from small follicles and theca cells from large follicles were cultured for 48 h in the presence of 10% FCS, and then treated in serum-free medium with ANG for 24 h. Granulosa cells were also concomitantly treated with 30 ng/ml of IGF1 and 30 ng/ml of FSH, and theca cells were concomitantly treated with 30 ng/ml of IGF1 and 30 ng/ml of LH. Values are means of three separate experiments (± SEM) and normalized to constitutively expressed 18S ribosomal RNA. *Mean differs (P <0.05) from its respective control (0 ng/ml of ANG).
Discussion
Results of the present study revealed that ANG stimulated large follicle granulosa cell proliferation and inhibited small follicle granulosa cell P4 and E2 production and large follicle theca cell P4 and androstenedione production, whereas ANG had no effect on large follicle theca cell proliferation.
For the first time, the present experiments showed that presumed physiological concentrations of ANG (i.e. 30 to 300 ng/ml) were bioactive in both granulosa and theca cell cultures. Although not known for cattle, follicular fluid levels of ANG in women range from 0.4 to 800 ng/ml (Koga et al., 2000; Malamitsi-Puchner et al., 2001; Kawano et al., 2003). Within this concentration range, ANG inhibited granulosa and theca cell steroidogenesis but stimulated granulosa cell proliferation, suggesting a possible regulatory role in follicular development. We hypothesize that ANG, while stimulating blood vessel development, may also promote proliferation of large follicle granulosa cells, while slowing differentiation of small follicle granulosa cells. Presumably, this ANG-induced suppression of small follicle differentiation would allow small follicles to grow and develop its vasculature before they are able to respond to increased gonadotropins during the preovulatory period.
Angiogenin effects on numbers of granulosa or theca cells have not been reported previously and varied with dose, cell type and hormone treatment. At 30, 100 and 300 ng/ml, ANG had no significant effect on numbers of small follicle granulosa cells or numbers of large follicle theca cells. However, at 300 ng/ml, ANG increased proliferation of large follicle granulosa cells in the presence of IGF1 and 10% FCS. These results indicate a change in ANG response as follicles develop. A previous study indicated that ANG stimulates 3H-thymidine incorporation into human umbilical venous endothelial cells grown in medium containing 5% FCS and 5 ng/ml of basic fibroblast growth factor (Hu et al., 1997; Liu et al., 2001). Cell survival and anti-apoptotic effects of ANG have been reported in other cell models (Li and Hu, 2012; Saikia et al., 2014). Thus, effects of ANG on follicular atresia should be investigated. Also, further research will be required to determine the mechanisms by which differentiated granulosa cells from large follicles are more responsive to the stimulatory effect of ANG on proliferation than less differentiated granulosa cells of small follicles, but likely involve changes in the IGF1 intracellular response system, and/or a change in numbers of ANG receptors.
In the present study, ANG had weak inhibitory effects on P4 production by small follicle granulosa cells concomitantly treated with FSH and IGF1, a condition in which cyclic adenosine monophosphate is dramatically elevated (Davoren et al., 1985; Zhang et al., 2000). In untreated large follicle granulosa cells and in IGF1-treated large follicle theca cells, ANG also weakly inhibited P4 production. However, these small decreases in P4 production were not accompanied with any change in abundance of CYP11A1 mRNA suggesting that the mechanism for P4 inhibition by ANG is not mediated via a change in CYP11A1 expression. Nonetheless, the present studies indicate for the first time that ANG, in addition to its purported role in inducing angiogenesis, may regulate steroidogenesis in granulosa and theca cells. In small follicle granulosa cells, the paradoxical increase in CYP19A1 mRNA induced by ANG while E2 production remained unchanged at the same dose of ANG will require further elucidation. ANG did not affect E2 production by large follicle granulosa cells indicating that its effect on steroidogenesis in large follicle granulosa cells is exclusive to P4 production. In large follicle theca cells, 30 and 100 ng/ml of ANG decreased LH plus IGF1-induced androstenedione production but when tested at 300 ng/ml, ANG had no effect on androstenedione production or abundance of CYP17A1 mRNA, suggesting abi-phasic response of theca cells to ANG. However, this inhibitory effect of ANG on androstenedione production was weak (i.e. 22% to 23% inhibition) and theca cell P4 production was only inhibited by 17% in large follicle theca cells treated with 300 ng/ml of ANG, suggesting a minor role for ANG in regulating theca cell steroidogenesis. Immunohistochemical staining and in situ hybridization in cattle showing that theca cells localize ANG protein but not ANG mRNA provides further support of the idea that ANG produced by granulosa cells communicates with theca cells (Lee et al., 1999). Further studies will be required to elucidate the developmental cell-specific effects of ANG on ovarian cell proliferation and steroidogenesis.
The mechanism of action and intracellular signaling pathway of ANG that affect granulosa and theca cell mitosis is unknown. In a recent study, ANG-induced proliferation of human glioblastoma U87MG cells was reported to act via a nuclear factor-κB pathway (Xia et al., 2015). In another study, the protein kinase B/Akt pathway was reported to be induced by ANG in human umbilical vein endothelial cells (Kim et al., 2007). Moreover, Saikia et al. (2014) showed that ANG-generated transfer RNA halves (tiRNAs) and survival of mouse embryonic cortical neuronal cells involves a cytochrome c interaction that inhibits apoptosome formation and activity. Whether any of these pathways are sensitive to ANG in bovine granulosa and theca cells will require further study.
In summary, results of the present study provide new evidence for ANG-dependent regulation of proliferation and steroidogenesis in granulosa and thecal cells that may influence follicle development. In particular, results indicate that ANG inhibits steroidogenesis of undifferentiated (small follicles) granulosa cells and stimulates mitogenesis of differentiated (large follicle) granulosa cells. The steroidogenic effects of ANG on large follicle theca cells although weak were also inhibitory. We conclude that ANG may target granulosa and theca cells in cattle, stimulating proliferation and inhibiting steroidogenesis, but additional research is needed to understand the mechanism of action of ANG in granulosa and theca cells, as well as its precise role in folliculogenesis.
Supplementary Material
Implications.
Angiogenin is a member of the ribonuclease A superfamily of proteins implicated in inducing angiogenesis. The present study provides new evidence for angiogenin-dependent regulation of follicle development. In particular, results indicate that angiogenin inhibits steroidogenesis of undifferentiated (small follicles) granulosa cells and stimulates mitogenesis of differentiated (large follicle) granulosa cells. The steroidogenic effects of angiogenin on large follicle theca cells were also inhibitory. Further studies will be required to elucidate cell-specific effects of angiogenin on ovarian cell proliferation and steroidogenesis during follicular development.
Acknowledgments
The authors thank the Oklahoma State University Wentz Project scholarship program (Lew Wentz Foundation) for financial support of A. Burress; the Bill & Melinda Gates Foundation for financial support of J. Dentis; Dr. A. F. Parlow, National Hormone & Pituitary Program, (Torrance, CA, USA) for purified LH and FSH; and Creekstone Farms (Arkansas City, KS, USA) for their generous donations of bovine ovaries. Approved for publication by the Director, Oklahoma Agric. Exp. Sta., and supported in part under project H-2510. This work was supported by project grant #R15-HD066302-01 from the National Institutes of Health.
Footnotes
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1751731116002044
References
- Aad PY, Voge JL, Santiago CA, Malayer JR, Spicer LJ. Real-time RT-PCR quantification of pregnancy-associated plasma protein-A mRNA abundance in bovine granulosa and theca cells: effects of hormones in vitro. Domestic Animal Endocrinology. 2006;31:357–372. doi: 10.1016/j.domaniend.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Appelmann I, Liersch R, Kessler T, Mesters RM, Berdel WE. Angiogenesis inhibition in cancer therapy: platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and their receptors: biological function and role in malignancy. Recent Results in Cancer Research. 2010;180:51–81. doi: 10.1007/978-3-540-78281-0_5. [DOI] [PubMed] [Google Scholar]
- Bond MD, Strydom DJ, Vallee BL. Characterization and sequencing of rabbit pig and mouse angiogenins. Biochimica et Biophysica Acta. 1993;1162:177–186. doi: 10.1016/0167-4838(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Bond MD, Vallee BL. Isolation of bovine angiogenin using a placental ribonuclease inhibitor binding assay. Biochemistry. 1988;27:6282–6287. doi: 10.1021/bi00417a013. [DOI] [PubMed] [Google Scholar]
- Chang S-I, Jeong G-B, Park S-H, Ahn B-C, Choi J-D, Chae Q, Namgoong SK, Chung S-I. Detection, quantitation, and localization of bovine angiogenin by immunological assays. Biochemical and Biophysical Research Communications. 1997;232:323–327. doi: 10.1006/bbrc.1997.6280. [DOI] [PubMed] [Google Scholar]
- Davoren JB, Hsueh JW, Li CH. Somatomedin C augments FSH-induced differentiation of cultured rat granulosa cells. Am. J. Physiol. 1985;249:E26–E33. doi: 10.1152/ajpendo.1985.249.1.E26. [DOI] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochimica et Biophysica Sinica. 2008;40:619–624. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Gho YS, Yoon WH, Chae CB. Antiplasmin activity of a peptide that binds to the receptor-binding site of angiogenin. Journal of Biological Chemistry. 2002;277:9690–9694. doi: 10.1074/jbc.M105526200. [DOI] [PubMed] [Google Scholar]
- Grado-Ahuir JA, Aad PY, Spicer LJ. New insights into the pathogenesis of cystic follicles in cattle: microarray analysis of gene expression in granulosa cells. Journal of Animal Science. 2011;89:1769–1786. doi: 10.2527/jas.2010-3463. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Haigh BJ, Griffin FJ, Wheeler TT. The mammalian secreted RNases: mechanisms of action in host defence. Innate Immunology. 2013;19:86–97. doi: 10.1177/1753425912446955. [DOI] [PubMed] [Google Scholar]
- Hatzi E, Badet J. Expression of receptors for human angiogenin in vascular smooth muscle cells. European Journal of Biochemistry. 1999;260:825–832. doi: 10.1046/j.1432-1327.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Macchiarelli G, Miyabayashi K, Sato E. Follicular microvasculature in the porcine ovary. Cell and Tissue Research. 2002;310:93–101. doi: 10.1007/s00441-002-0565-4. [DOI] [PubMed] [Google Scholar]
- Jiang JY, Macchiarelli G, Tsang BK, Sato E. Capillary angiogenesis and degeneration in bovine ovarian antral follicles. Reproduction. 2003;125:211–223. [PubMed] [Google Scholar]
- Kawano Y, Zeineh Hasan K, Fukuda J, Mine S, Miyakawa I. Production of vascular endothelial growth factor and angiogenic factor in human follicular fluid. Molecular and Cellular Endocrinology. 2003;202:19–23. doi: 10.1016/s0303-7207(03)00056-x. [DOI] [PubMed] [Google Scholar]
- Kim HM, Kang DK, Kim HY, Kang SS, Chang SI. Angiogenin-induced protein kinase B/Akt activation is necessary for angiogenesis but is independent of nuclear translocation of angiogenin in HUVE cells. Biochemical and Biophysical Research Communications. 2007;352:509–513. doi: 10.1016/j.bbrc.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Koga K, Osuga Y, Tsutsumi O, Momoeda M, Suenaga A, Kuga K, Fujiwara T, Takai Y, Yano T, Taketani Y. Evidence for the presence of angiogenin in human follicular fluid and the up-regulation of its production by human chorionic gonadotropin and hypoxia. Journal of Clinical Endocrinology and Metabolism. 2000;85:3352–3355. doi: 10.1210/jcem.85.9.6837. [DOI] [PubMed] [Google Scholar]
- Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian granulosa and theca cell function. Molecular and Cellular Endocrinology. 2008;284:38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Langhout DJ, Spicer LJ, Geisert RD. Development of a culture system for bovine granulosa cells: effects of growth hormone, estradiol, and gonadotrophins on cell proliferation, steroidogenesis, and protein synthesis. Journal of Animal Science. 1991;69:3321–3334. doi: 10.2527/1991.6983321x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Lee I-S, Kang T-C, Jeong GB, Chang S-I. Angiogenin is involved in morphological changes and angiogenesis in the ovary. Biochemical and Biophysical Research Communications. 1999;257:182–186. doi: 10.1006/bbrc.1999.0359. [DOI] [PubMed] [Google Scholar]
- Li S, Hu GF. Emerging role of angiogenin in stress response and cell survival under adverse conditions. Journal of Cellular Physiology. 2012;227:2822–2826. doi: 10.1002/jcp.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yu D, Xu ZP, Riordan JF, Hu GF. Angiogenin activates Erk1/2 in human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications. 2001;287:305–310. doi: 10.1006/bbrc.2001.5568. [DOI] [PubMed] [Google Scholar]
- Maes P, Damart D, Rommens C, Montreuil J, Spik G, Tartar A. The complete amino acid sequence of bovine milk angiogenin. FEBS Letters. 1988;241:41–45. doi: 10.1016/0014-5793(88)81027-5. [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Sarandakou A, Baka SG, Tziotis J, Rizos D, Hassiakos D, Creatsas G. Concentrations of angiogenic factors in follicular fluid and oocyte-cumulus complex culture medium from women undergoing in vitro fertilization: association with oocyte maturity and fertilization. Fertility and Sterility. 2001;76:98–101. doi: 10.1016/s0015-0282(01)01854-4. [DOI] [PubMed] [Google Scholar]
- Moonmanee T, Navanukraw C, Uriyapongson S, Kraisoon A, Aiumlamai S, Guntaprom S, Rittirod T, Borowicz PP, Redmer DA. Relationships among vasculature, mitotic activity, and endothelial nitric oxide synthase (eNOS) in bovine antral follicles of the first follicular wave. Domestic Animal Endocrinology. 2013;45:11–21. doi: 10.1016/j.domaniend.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Ott L. An Introduction to Statistical Methods and Data Analysis. North Scituate, MA: Duxbury Press; 1977. [Google Scholar]
- Premzl M. Comparative genomic analysis of eutherian ribonuclease A genes. Molecular Genetics and Genomics. 2014;289:161–167. doi: 10.1007/s00438-013-0801-5. [DOI] [PubMed] [Google Scholar]
- Przybylski M. A review of the current research on the role of bFGF and VEGF in angiogenesis. Journal of Wound Care. 2009;12:516–519. doi: 10.12968/jowc.2009.18.12.45609. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Reynolds LP. Angiogenesis in the ovary. Reviews of Reproduction. 1996;1:182–192. doi: 10.1530/ror.0.0010182. [DOI] [PubMed] [Google Scholar]
- Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao XH, Guan BJ, Yuan Y, Jankowsky E, Feng Z, Hu GF, Pusztai-Carey M, Gorla M, Sepuri NB, Pan T, Hatzoglou M. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells fromapoptosis during osmotic stress. Molecular and Cellular Biology. 2014;34:2450–2463. doi: 10.1128/MCB.00136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber NB, Spicer LJ. Effects of fibroblast growth factor 9 (FGF9) on steroidogenesis and gene expression and control of FGF9 mRNA in bovine granulosa cells. Endocrinology. 2012;153:4491–4501. doi: 10.1210/en.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Strydom DJ, Olson KA, Vallee BL. Isolation of angiogenin from normal human plasma. Biochemistry. 1987;26:5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Soncin F, Shapiro R, Fett JW. A cell-surface proteoglycan mediates human adenocarcinoma HT-29 cell adhesion to human angiogenin. Journal of Biological Chemistry. 1994;269:8999–9005. [PubMed] [Google Scholar]
- Spicer LJ, Aad P. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biology of Reproduction. 2007;77:18–27. doi: 10.1095/biolreprod.106.058230. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biology of Reproduction. 2008;78:243–253. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Chamberlain CS. Influence of cortisol on insulin- and insulin-like growth factor 1 (IGF-1)-induced steroid production and on IGF-1 receptors in cultured bovine granulosa cells and thecal cells. Endocrine. 1998;9:153–161. doi: 10.1385/ENDO:9:2:153. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Chamberlain CS, Maciel SM. Influence of gonadotropins on insulin- and insulin-like growth factor-I (IGF-I)-induced steroid production by bovine granulosa cells. Domestic Animal Endocrinology. 2002;22:237–254. doi: 10.1016/s0739-7240(02)00125-x. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Stewart RE. Interaction among bovine somatotropin, insulin, and gonadotropins on steroid production by bovine granulosa and thecal cells. Journal of Dairy Science. 1996;79:813–821. doi: 10.3168/jds.S0022-0302(96)76429-9. [DOI] [PubMed] [Google Scholar]
- Stewart RE, Spicer LJ, Hamilton TD, Keefer BE. Effects of insulin-like growth factor I and insulin on proliferation and on basal and luteinizing hormone-induced steroidogenesis of bovine thecal cells: involvement of glucose and receptors for insulin-like growth factor I and luteinizing hormone. Journal of Animal Science. 1995;73:3719–3731. doi: 10.2527/1995.73123719x. [DOI] [PubMed] [Google Scholar]
- Tello-Montoliu A, Patel JV, Lip GYH. Angiogenin: a review of the pathophysiology and potential clinical applications. Journal of Thrombosis and Haemostasis. 2006;4:1864–1874. doi: 10.1111/j.1538-7836.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Research. 2005;65:1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. Journal of Burn Care and Research. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voge JL, Santiago CA, Aad PY, Goad DW, Malayer JR, Spicer LJ. Effect of insulin-like growth factors (IGF), FSH, and leptin on IGF-binding-protein mRNA expression in bovine granulosa and theca cells: quantitative detection by real-time PCR. Peptides. 2004;25:2195–2203. doi: 10.1016/j.peptides.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Xia W, Fu W, Cai X, Wang M, Chen H, Xing W, Wang Y, Zou M, Xu T, Xu D. Angiogenin promotes U87MG cell proliferation by activating NF-κB signaling pathway and downregulating its binding partner FHL3. PLoS One. 2015;10:e0116983. doi: 10.1371/journal.pone.0116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Monti DM, Hu G. Angiogenin activates human umbilical artery smooth muscle cells. Biochemical and Biophysical Research Communications. 2001;285:909–914. doi: 10.1006/bbrc.2001.5255. [DOI] [PubMed] [Google Scholar]
- Yamada O, Abe M, Takehana K, Hiraga T, Iwasa K, Hiratsuka T. Microvascular changes during the development of follicles in bovine ovaries: a study of corrosion casts by scanning electron microscopy. Archives of Histology Cytology. 1995;58:567–574. doi: 10.1679/aohc.58.567. [DOI] [PubMed] [Google Scholar]
- Zhang G, Garmey JC, Veldhuis JD. Interactive stimulation by luteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17α-hydroxylase/17,20-lyase (CYP17) genes in porcine theca cells. Endocrinology. 2000;141:2735–2742. doi: 10.1210/endo.141.8.7595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.