Abstract
IMPORTANCE
Several factors are associated with increased hepatocellular carcinoma (HCC) recurrence after liver transplantation (LT), but no reliable risk score has been established to determine the individual risk for HCC recurrence.
OBJECTIVE
We aimed to develop and validate a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score for patients with HCC meeting Milan criteria by imaging.
DESIGN, SETTING, AND PARTICIPANTS
Predictors of recurrence were tested in a development cohort of 721 patients who underwent LT between 2002 and 2012 at 3 academic transplant centers (University of California–San Francisco; Mayo Clinic, Rochester; and Mayo Clinic, Jacksonville) to create the RETREAT score. This was subsequently validated in a cohort of 341 patients also meeting Milan criteria by imaging who underwent LT at the University of Toronto transplant center using the C concordance statistic and net reclassification index.
MAIN OUTCOMES AND MEASURES
Characteristics associated with post-LT HCC recurrence.
RESULTS
A total of 1061 patients participated in the study; 77.8%(825) were men, and the median (IQR) age was 58.2 (53.3–63.9) years in the development cohort and 56.4 (51.7–61.0) years in the validation cohort (P < .001). In the development cohort of 721 patients (542 men), median α-fetoprotein (AFP) level at the time of LT was 8.3 ng/mL; 9.4% had microvascular invasion (n = 68), and 22.1% were beyond Milan criteria on explant (n = 159) owing to understaging by pretransplantation imaging. Cumulative probabilities of HCC recurrence at 1 and 5 years were 5.7% and 12.8%, respectively. On multivariable Cox proportional hazards regression, 3 variables were independently associated with HCC recurrence: microvascular invasion, AFP at time of LT, and the sum of the largest viable tumor diameter and number of viable tumors on explant. The RETREAT score was created using these 3 variables, with scores ranging from 0 to 5 or higher that were highly predictive of HCC recurrence (C statistic, 0.77). RETREAT was able to stratify 5-year post-LT recurrence risk ranging from less than 3%with a score of 0 to greater than 75% with a score of 5 or higher. The validation cohort (n = 340; 283 men) had significantly higher microvascular invasion (23.8% [n = 81], P < .001), explant beyond Milan criteria (37.3% [n = 159], P < .001), and HCC recurrence at 5 years (17.9% [n = 159], P = .03). RETREAT showed good model discrimination (C statistic, 0.82; 95%CI, 0.77–0.86) and superior recurrence risk classification compared with explant Milan criteria (net reclassification index, 0.40; P = .001) in the validation cohort.
CONCLUSIONS AND RELEVANCE
We have developed and validated a simple and novel prognostic score that may improve post-LT HCC surveillance strategies and help identify patients who may benefit from future adjuvant therapies.
For 2 decades, the Milan criteria (1 lesion of ≤5 cm, 2–3 lesions of ≤3 cm)1 have been the benchmark for the selection of candidates with hepatocellular carcinoma (HCC) for liver transplantation (LT).2,3 Treatment of HCC now accounts for more than 20% of all LTs performed in the United States.4 Despite physician adherence to the Milan criteria, HCC recurrence still occurs in about 10% to 15% of patients,5–7 with a median survival of only about a year after HCC recurrence.2,3,8 Only 10% to 30% of recurrent HCCs are eligible for resection or ablation.8,9
The impact of tumor size and number on HCC recurrence risk is best illustrated in the “Metro-ticket forecast,”10 which follows the paradigm of “the further the distance (from conventional limits defined by Milan criteria), the higher the price (paid in terms of HCC recurrence).” Microvascular invasion is a well-established predictor of HCC recurrence after LT.5,11–13 Other factors implicated in HCC recurrence include elevated α-fetoprotein (AFP) levels14–17 and possibly des-gamma-carboxyprothrombin,18 poorly differentiated tumor grade,12 tumor progression despite locoregional therapy (LRT),19,20 as well as short waiting time before LT.21,22
Despite known risk factors for HCC recurrence after LT, no validated risk score is available to provide quantifiable and reliable measurements of an individual’s risk of post-LT HCC recurrence. The lack of a reliable model to estimate the risk for HCC recurrence after LT may explain why there is no standardized approach to HCC surveillance after LT2,5 and wide variation in this practice across LT centers.5 In the present large multicenter study, we aimed to develop and validate a recurrence risk score, the Risk Estimation of Tumor Recurrence After Transplant (RETREAT), for patients with HCC who meet the Milan criteria by imaging at the time of LT.
Methods
Study Design and Patient Population
This multicenter study was approved by the institutional review boards of all participating institutions, and all boards waived patient written informed consent. The study included adult patients (age ≥18 years) with HCC always meeting Milan criteria on imaging who underwent LT with Model for End-Stage Liver Disease (MELD) score exception from June 2002 to December 2012. Patients requiring tumor downstaging to Milan criteria and those with intrahepatic cholangiocarcinoma or mixed HCC–cholangiocarcinoma on explant were excluded. Patients with incidental HCC were also excluded mainly because wait time to LT was 1 of the variables evaluated as a predictor of post-LT HCC recurrence. The development cohort consisted of 721 patients who underwent LT at 3 centers with different waiting times: short (Mayo Clinic, Jacksonville), medium(Mayo Clinic, Rochester), and long (University of California, San Francisco [UCSF]). The validation cohort consisted of 340 patients also within Milan criteria on imaging who underwent LT with MELD exception over the same time period at the University of Toronto.
The variables collected included age, sex, size and number of HCC tumors at time of diagnosis, AFP at time of listing and LT, LRT, cause of the liver disease, MELD score, and waiting time (defined as the time from HCC diagnosis to LT). All patients underwent contrast-enhanced computed tomography or magnetic resonance imaging at a minimum of once every 3 months after listing for LT.
Key Points.
Question
What is an individual’s risk for recurrence of hepatocellular carcinoma (HCC) after liver transplant based on their tumor characteristics?
Findings
Using a multicenter retrospective cohort study approach, we have developed and validated a simple and novel score, the Risk Estimation of Tumor Recurrence After Transplant (RETREAT), that incorporates 3 variables: explant tumor burden, microvascular invasion, and α-fetoprotein level. The RETREAT score was highly predictive of HCC recurrence risk after liver transplantation.
Meaning
The RETREAT risk score will help identify patients who would potentially derive benefit from future adjuvant therapies and also assist in determining posttransplant surveillance strategies.
Explant pathology reports were reviewed to determine histologic grade based on the modified Edmondson criteria,23 presence of vascular invasion, and tumor stage. Explant tumor staging was based on size and number of only viable tumors. The following categories of tumor stage were evaluated: within vs outside Milan criteria, total tumor diameter, number of viable lesions, and the sum of the largest diameter of viable tumor plus the number of viable tumors on explant.
Statistical Analysis and Creation of the RETREAT Score
The study end points were 5-year post-LT HCC recurrence and survival. Recurrence and survival probabilities were estimated by the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariable hazard ratios (HRs) for predictors of post-LT HCC recurrence were determined by Coxproportional hazards regression models and reported with 95% confidence intervals (CIs). Potential cutoffs were evaluated using the Akaike information criterion (AIC), with lower AIC values indicating better model fit. Predictors of HCC recurrence with a univariate P < .10 were included in the multivariable analysis, and the final model was selected by backward step wise elimination (P > .05 for removal). The RETREAT score was then created based on the final multivariable model coefficients.24 Model coefficients were scaled to the coefficient for AFP and rounded to the nearest integer. This produced a simplified point scale reflecting the relative impact of model covariables. The integer value for each model component was then summed to calculate the RETREAT score.
In the validation cohort, the RETREAT score was tested and compared with Milan criteria (based on explant pathology findings). The overall C statistic was used to assess model discrimination, and net reclassification index25 to evaluate improvement in model performance by quantifying the proportion of correct risk reclassification for HCC recurrence. Net reclassification improvement was estimated using a priori 1-and 5-year recurrence risk groups (<5%, 5% to <10%, 10% to <20%, and ≥20%). Correct risk reclassification was indicated by RETREAT score predicted probabilities that reclassified patients with recurrence into higher risk groups and patients without recurrence into lower risk groups compared with predicted probabilities estimated by explant Milan criteria.
Results
Patient Characteristics
The baseline characteristics of the 721 patients in the development cohort and the 340 patients in the validation cohort are summarized in Table 1. Compared with the development cohort, the validation cohort was younger, had a higher percentage of men, and more frequently had hepatitis B or alcohol abuse as the cause of the liver disease. White patients made up 62.0% of the development cohort (n = 447); Asians, 19.1% (n = 138); Hispanics, 11.2%(n = 81);and African Americans 5.1% (n = 37). Patients in the validation cohort were more likely to have a single tumor and less likely to receive LRT prior to LT. Median wait time from HCC diagnosis to LT ranged from 4.3 months in the center with the shortest wait time to 12.9 months in the center with the longest wait time. Overall, wait time in the development cohort was shorter than in the validation cohort (8.1 vs 10.5 months).
Table 1.
Clinical Characteristics of the Study Participantsa
| Characteristic | Cohort | P Value | |
|---|---|---|---|
| Development (n = 721) | Validation (n = 340) | ||
| At the Time of HCC Diagnosis | |||
| Age, median (IQR), y | 58.2 (53.3–63.9) | 56.4 (51.7–61.0) | <.001 |
| Male sex | 542 (75.2) | 283 (83.2) | <.001 |
| Liver disease | |||
| Hepatitis C | 424 (58.8) | 191 (56.2) | <.001 |
| Hepatitis B | 116 (16.1) | 78 (22.9) | |
| Fatty liver disease | 63 (8.7) | 11 (3.2) | |
| Alcoholic liver disease | 62 (8.6) | 45 (13.2) | |
| Others | 56 (7.8) | 15 (4.4) | |
| MELD score, median (IQR) | 11 (9–14) | 11 (8–14) | .79 |
| HCC | |||
| 1 | 498 (69.1) | 260 (76.5) | .02 |
| 2 | 167 (23.2) | 53 (15.6) | |
| 3 | 56 (7.8) | 27 (7.9) | |
| AFP, median (IQR), ng/mL | 12.0 (5.0–54.5) | 11.0 (5.0–41.0) | .82 |
| While on Wait List | |||
| Received LRT | 660 (91.5) | 221 (65.0) | <.001 |
| Wait time to LT, median (IQR), mo | 8.1 (4.1–14.2) | 10.5 (6.0–18.0) | <.001 |
| At the Time of LT | |||
| AFP, median (IQR), ng/mLb | 8.3 (4.0–29.0) | 9.4 (4.5–36.0) | .21 |
| ≤20 | 482 (70.0) | 221 (65.0) | |
| 21–99 | 126 (18.2) | 72 (21.2) | |
| 100–999 | 66 (9.6) | 36 (10.6) | |
| ≥1000 | 15 (2.2) | 11 (3.2) | |
| Pathologic stage | |||
| No residual tumor | 199 (27.6) | 48 (14.1) | <.001 |
| Within Milan criteria | 363 (50.3) | 165 (48.5) | |
| Beyond Milan criteria | 159 (22.1) | 127 (37.3) | |
| Largest viable tumor diameter (cm) + No. of tumors, median (IQR) | 3.5 (0–5.3) | 4.5 (3.0–6.7) | <.001 |
| Microvascular invasion | 68 (9.4) | 81 (23.8) | <.001 |
| Histologic gradec | |||
| Completely necrotic | 199 (28.0) | 48 (14.7) | <.001 |
| Well differentiated | 200 (28.1) | 28 (8.6) | |
| Moderately differentiated | 238 (33.4) | 218 (66.9) | |
| Poorly differentiated | 75 (10.5) | 32 (9.8) | |
Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma; IQR, interquartile range; LRT, locoregional therapy; LT, liver transplantation; MELD, Model for End-Stage Liver Disease.
Unless otherwise noted, data are reported as number (percentage) of participants
For development cohort, n = 689.
For development cohort, n = 712; for validation cohort, n = 326.
Compared with the developmental cohort, the validation cohort had significantly higher proportions of microvascular invasion (23.8% [n = 81] vs 9.4% [n = 32]), moderately or poorly differentiated tumor grade (76.7% [n = 260] vs 44.0% [n = 150]), and explant tumor stage beyond Milan criteria (37.3% [n = 127] vs 22.1% [n = 75]). The median for the sum of the largest viable tumor diameter (cm) plus the number of viable tumors on explant was 4.5 in the validation cohort vs 3.5 in the development cohort (P < .001).
Post-LT Outcomes—Development Cohort
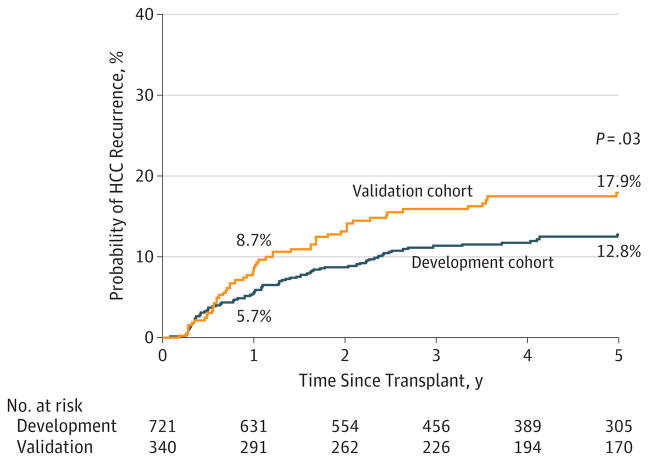
Median post-LT follow-up was 4.5 years (interquartile range [IQR], 2.5–6.8 years) in the development cohort. Follow-up included a combination of AFP and cross-sectional imaging every 4 to 6 months for the first 2 years after LT. This was performed in all HCC LT recipients at 2 centers and in patients deemed to be at high risk for HCC recurrence (AFP >300, explant tumor burden beyond Milan criteria, and/or microvascular invasion) at a third center. Overall post-LT survival was 93.1% (95% CI, 91.0%–94.8%) at 1 year and 77.0% (95% CI, 73.4%–80.1%) at 5 years. Recurrence of HCC was found in 11.6%(84 of 721) at a median of 13.0 months (IQR, 5.4–26.7 months) after LT. The most common sites of HCC recurrence were the lung(44.0% [n = 37]), bone (29.8% [n = 25]), liver (26.2% [n = 22]), and peritoneum (26.2% [n = 22]). The majority (75.0% [n = 63]) had a single site of HCC recurrence; 20.2% [n = 17] had 2 sites; and 4.8%[n = 4] had 3 or more sites of recurrence at diagnosis. Overall post-LT HCC recurrence at 1, 2, and 5 years was 5.7% (95% CI, 4.2%–7.8%), 8.7%(95% CI, 6.8%–11.1%), and 12.8%(95% CI, 10.3%–15.7%), respectively (Figure 1). There were no significant center-specific differences in rates of HCC recurrence.
Figure 1.
Kaplan-Meier Probability of Hepatocellular Carcinoma (HCC) Recurrence Within 5 Years for Liver Transplant Recipients in the Development and Validation Cohorts
Predictors of Post-LT HCC Recurrence and Creation of the RETREAT Score
Predictors of post-LT HCC recurrence in the development cohort on univariate analysis included microvascular invasion, moderately or poorly differentiated tumor grade, AFP at the time of LT as a continuous variable and at all tested cutoffs, and the sum of the largest diameter of viable tumor (cm) plus the number of tumors (Table 2). The tumor sum criterion had the lowest AIC compared with other assessments of tumor burden and therefore the best model fit. Age, sex, race/ethnicity, MELD score, cause of the liver disease, number of lesions at HCC diagnosis, and LRT(vs no LRT)were not predictive of HCC recurrence on univariate analysis. On multivariable analysis, predictors of HCC included (1) microvascular invasion, (2)sum of the largest diameter of viable tumor and number of viable tumors (at the following cutoffs: 1.1–4.9, 5–9.9, and ≥10), and (3) AFP at LT (at the following cutoffs: 20–99, 100–999, and ≥1000 ng/mL). The multivariable model coefficients for these 3 significant variables were then used to calculate a simplified RETREAT score. An individual patient’s RETREAT score is calculated by adding the individual points for each of the 3 variables (Table 3). The actual statistical model and the coefficients are also summarized in Table 3.
Table 2.
Univariate Analysis of Predictors of Posttransplant HCC Recurrence in the Development Cohort by Cox Proportional Hazards Regression
| Predictora | Univariate HR (95% CI) | P Value |
|---|---|---|
| AFP at LT, ng/mL [Reference, ≤20] | ||
| 21–99 | 2.03 (1.19–3.49) | .01 |
| 100–999 | 3.26 (1.82–5.85) | <.001 |
| ≥1000 | 11.63 (5.61–24.09) | <.001 |
| Microvascular invasion | 7.82 (4.96–12.33) | <.001 |
| Largest viable tumor diameter (cm) + No. of tumors [Reference, 0]b | ||
| 1–4.9 | 1.99 (0.93–4.24) | .08 |
| 5–9.9 | 4.60 (2.21–9.56) | <.001 |
| ≥10 | 22.51 (9.57–52.94) | <.001 |
| Tumor differentiation [Reference, completely necrotic tumor] | ||
| Well | 1.78 (0.84–3.73) | .13 |
| Moderate | 3.02 (1.54–5.96) | .001 |
| Poor | 5.51 (2.62–11.58) | <.001 |
| HCC lesions, No. at diagnosis | ||
| 2 vs 1 | 1.06 (0.62–1.78) | .84 |
| 3 vs 1 | 1.84 (0.94–3.61) | .08 |
| Wait time from HCC diagnosis to LT | ||
| <6 Months | 1.44 (0.90–2.31) | .13 |
| >18 Months | 1.84 (0.99–3.41) | .06 |
Abbreviations: AFP, α-fetoprotein; HCC, hepatocellular carcinoma; HR, hazard ratio; LRT, loco-regional therapy; LT, liver transplantation; MELD, Model for End-Stage Liver Disease.
Age, sex, race/ethnicity, MELD score, cause of liver disease, number of lesions at HCC diagnosis, and LRT (vs no LRT) were not predictive of HCC recurrence on univariate analysis.
Largest viable tumor diameter (cm) + No. of viable tumors = 0 if no viable tumor is identified.
Table 3.
Multivariable Analysis of Predictors of HCC Recurrence and Creation of the RETREAT Score
| Predictor | Multivariable HR (95% CI) | P Value | β Coefficient | RETREAT Pointsa |
|---|---|---|---|---|
| AFP at LT, ng/mL | ||||
| 0–20 | 1 [Reference] | NA | NA | 0 |
| 21–99 | 1.80 (1.05–3.10) | .03 | 0.59 | 1 |
| 100–999 | 2.56 (1.42–4.62) | .002 | 0.94 | 2 |
| ≥1000 | 4.45 (1.98–10.00) | <.001 | 1.49 | 3 |
| Microvascular invasion | 3.80 (2.23–6.47) | <.001 | 1.34 | 2 |
| Largest viable tumor diameter (cm) plus No. of viable tumorsb | ||||
| 0 | 1 [Reference] | NA | NA | 0 |
| 1.1–4.9 | 1.58 (0.73–3.39) | .25 | 0.45 | 1 |
| 5.0–9.9 | 2.69 (1.24–5.83) | .01 | 0.99 | 2 |
| ≥10 | 6.75 (2.55–17.88) | <.001 | 1.91 | 3 |
Abbreviations: AFP, α-fetoprotein level; HCC, hepatocellular carcinoma; HR, hazard ratio; LT, liver transplantation; NA, not applicable; RETREAT, risk estimation of tumor recurrence after transplant.
The RETREAT score is obtained by adding the total number of points scored in each of the 3 variables (range, 0–8). RETREAT score = 0 if a patient has an AFP of 0 to 20 ng/mL at LT, no microvascular invasion, and no viable tumor in the explant.
For example, if there are 3 lesions on explant, 2 viable lesions measuring 4 cm and 3 cm and a single completely necrotic lesion measuring 5 cm, the completely necrotic lesion is not counted, and the sum of the largest diameter of viable tumor (cm) and number of viable tumors would be 6 (4 = diameter of the largest lesion + 2 = No. of viable tumors). Explant largest viable tumor diameter + No. = 0 if no viable tumor is identified.
RETREAT Score and Predicted HCC Recurrence Risk
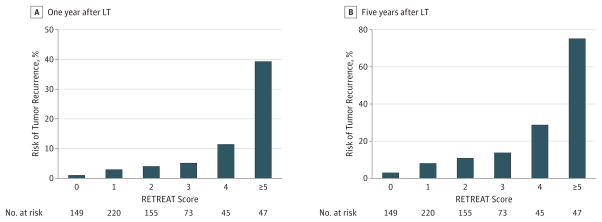
Calculated RETREAT scores ranged from 0 to 8, with the most common scores being 1 (31.9% [n = 220]) and 2 (22.5% [n = 149]). A patient with completely necrotic tumor on explant after LRT, no microvascular invasion on explant, and an AFP level lower than 20 ng/mL at LT would have a RETREAT score of 0 (21.6% of the cohort [n = 149]), predicting 1- and 5-year recurrence risk of only 1.0% (95% CI, 0.0%–2.1%) and 2.9%(95% CI, 0.0%–5.6%), respectively. Predicted risk of 1-and 5-year HCC recurrence rose with each point scored (Figure 2) such that a patient with a RETREAT score of 5 or higher (6.8% of the cohort [n = 47]) had a predicted 1- and 5-year recurrence risk of 39.3%(95% CI, 25.5%–50.5%) and 75.2%(95% CI, 56.7%–85.8%), respectively. The RETREAT score C statistic was 0.77 (95% CI, 0.71–0.82) in the development cohort for predicting HCC recurrence compared with 0.70 (95% CI, 0.63–0.76) for Milan criteria by explant. The C statistics for the individual components of RETREAT were 0.65 (95% CI, 0.58–0.72) for AFP,0.74 (95% CI, 0.68–0.80) for the sum of the largest viable lesion plus number of lesions on explant, and 0.68 (95% CI, 0.64–0.73) for microvascular invasion.
Figure 2.
Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Scores
The C statistic was 0.77 for the development cohort and 0.82 for the validation cohort. LT indicates liver transplant.
Validation of the RETREAT Score
For purposes of comparison, data for the development cohort are reported in the Post-LT Outcomes—Development Cohort subsection of the Results section. In the validation cohort, the median post-LT follow-up was 5.1 years (IQR, 2.6–7.7 years). The overall post-LT survival in this cohort was 91.8% (95% CI, 88.3%–94.2%) at 1 year and 71.5% (95% CI, 66.2%–76.2%) at 5 years (P = .06 vs development cohort). Higher HCC recurrence rates at 1 and 5 years after LT were observed in the validation cohort vs 8.7% (95% CI, 6.1%–7.8%) and 17.9% (95% CI, 14.0%–22.8%), respectively (P = .08 at 1 year, P = .03 at 5 years) (Figure 1). The RETREAT score performed well in the validation cohort with a C statistic of 0.82(95% CI 0.77–0.86). Using the net reclassification index, we found that the RETREAT score improved prediction of HCC recurrence after LT compared with the Milan criteria at 1 year (0.40, P = .001) and 5 years after LT (0.31, P < .001).
Discussion
There is a growing body of evidence that the Milan criteria represent just 1 of many factors that predict post-LT survival and HCC recurrence.26 Factors commonly used to define high risk for HCC recurrence after LT have included explant tumor stage beyond Milan criteria, tumor vascular invasion, and poorly differentiated tumor grade. These factors, however, are binary (yes or no), with each factor separately providing only a very crude estimation of HCC recurrence risk after LT. Even patients classified as low risk for HCC recurrence based on explant tumor burden within Milan criteria still carry an estimated 10% to 15% risk for HCC recurrence at 5 years after LT.5–7 A reliable and validated prognostic scoring system to provide an accurate quantification of individual HCC recurrence risk would be helpful to guide HCC surveillance strategies and determine the need for post-LT adjuvant therapy if available in the future.
In this large, multicenter study involving over 1000 patients with HCC always within Milan criteria by imaging before LT, we developed and validated the RETREAT score using 3 variables that were highly predictive of HCC recurrence: AFP at LT, microvascular invasion, and the sum of the largest diameter of viable tumor (cm) plus the number of viable tumors on explant (C statistic, 0.77). The RETREAT score was able to stratify 5-year HCC recurrence risks ranging from less than 3% in those with a risk score of 0 to higher than 75% with a risk score of 5 or higher. Despite higher rates of microvascular invasion, explant tumor burden, and overall HCC recurrence in the validation cohort, the RETREAT score still performed well with an even higher C statistic of 0.82.
One of the strengths of the RETREAT score is that it accounts for the effects of preoperative LRT, and only viable tumors are considered. Those with no viable tumors in the explant receive a score of 0 in assessing pathologic tumor burden, reflecting a very low risk for HCC recurrence. This finding is entirely consistent with 2 recent studies demonstrating a very low probability of HCC recurrence (0%to 2.4%)in patients who achieve complete response to LRT based on explant pathologic findings.27,28 In the present study, the sum of the largest diameter of viable tumor (cm) plus the number of viable tumors in the explant predicted HCC recurrence better than other categories in assessing tumor burden, namely tumors outside (vs within) Milan criteria and the total tumor diameter. Similarly, Mazzaferro et al10 proposed using a combination of the size of the largest tumor and the total number of tumors on explant to better estimate post-LT survival in the Metroticket forecast.10 Mazzaferro and colleagues used a web-based registry in their data collection and relied on overall patient survival rather than HCC recurrence as the end point. The present study used HCC recurrence as the primary end point and provided further evidence supporting the combination of maximum size and number of viable tumors as a reliable predictor of tumor recurrence after LT. The multicenter study design also allowed us to include a wide range of races/ethnicities and causes of liver disease. While viral hepatitis and non-white race appear to be strong risk factors for HCC development,29 we did not observe such an association in terms of increased post-LT HCC recurrence.
Recently, the University of California, Los Angeles (UCLA) group reported a prognostic nomogram for HCC recurrence after LT.12 This was constructed over a span of 30 years and did not include a validation set. One of the strengths of the RETREAT score is its simplicity in contrast to the UCLA nomogram, which involves 7 variables, including 1 that must be calculated. Furthermore, the UCLA study included 38%of patients with HCC either outside the Milan criteria by imaging or discovered incidentally in the explant, and participants an overall 5-year post-LT survival of only 60%. One would therefore question the generalizability of the UCLA nomogram in the current MELD era based on Milan criteria, with expected 5-year post-LT survival of 70% to 80%.2,5,30 We have included centers with short, medium, and long waiting times in our study, thus avoiding potential bias related to length of waiting time in a single-center study and improving the generalizability of our results.
How does the RETREAT score potentially affect clinical practice after LT? The RETREAT score helps determine whether HCC surveillance after LT is warranted, taking into consideration a recent study suggesting that long-term survival can be achieved with tumor-directed therapy in a subset with local HCC recurrence.9 Since the majority of HCC recurrence in the present study occurred within the first 2 years after LT, we propose the following guidelines for HCC surveillance strategy that has recently been instituted at UCSF: HCC surveillance every 6 months for 2 years for a RETREAT score of 1 to 3 and every 6 months for 5 years for a RETREAT score of 4. Patients with a RETREAT score of 5 or higher should undergo HCC surveillance every 3 to 4 months for 2 years followed by every 6 months for years 2 through 5. We further propose using multiphasic abdominal computed tomographic ormagnetic resonance imaging, chest computed tomography, and AFP for surveillance at the recommended interval. Those with a RETREAT score of 0 should receive no surveillance, given their 5-year predicted recurrence risk of less than 3%. Identifying this subgroup( 20% of study cohort [n = 149]) that does not require surveillance is cost saving.
The RETREAT score could potentially affect the use of post-LT immunosuppression. Calcineurin inhibitors may increase the risk for post-LT HCC recurrence,31,32 and the mammalian target of rapamycin (mTOR) inhibitors may have antineoplastic properties.33,34 Anumber of studies have evaluated post-LT outcomes with them TOR-inhibitors sirolimus35–37 and everolimus.38–41 Despite early optimism, a prospective randomized controlled phase 3 international trial (SiLVER trial) failed to demonstrate an overall benefit of sirolimus in improving long-term recurrence-free survival beyond 5 years.42 Regarding possible adjuvant therapies, sorafenib is being investigated in a phase 1 trial to prevent post-LT HCC recurrence in high-risk candidates with tumors outside the Milan criteria or microvascular invasion and/or poorly differentiated tumor grade in the explant.43 Other potential therapies such as liver allograft-derived natural killer cells44 and radioimmunologic agents45 hold promise. The RETREAT scoremay be helpful in the design of future clinical trials—identifying candidates at high risk for HCC recurrence and providing a reference for the expected incidence of HCC recurrence. Patients with a RETREAT score of 4 or higher are at high risk for HCC recurrence and are therefore appropriate candidates for future adjuvant therapies.
Limitations
There are limitations of the present study, including missing information on AFP at the time of LT in 4% of the developmental cohort [n = 29] and explant histologic tumor grade in 2% of the combined developmental and validation cohorts [n = 21]. We also did not assess lack of response to LRT or tumor progression despite LRT as potential risk factors for HCC recurrence. This analysis would have been difficult owing to multiple time points for evaluation in the center with prolonged LT waiting time and insufficient duration to observe the full effects of LRT in the center with short LT waiting time. Given the limitations of the retrospective study design, a multicenter study is planned to prospectively evaluate the application of RETREAT in post-LT HCC surveillance and to confirm the prognostic power of the RETREAT score.
Conclusions
In conclusion, we have developed and validated a novel score (RETREAT) for predicting post-LT HCC recurrence in patients always meeting the Milan criteria by pre-LT imaging. RETREAT stratifies 5-year HCC recurrence risk from less than 3% with a risk score of 0 to greater than 75% with a risk score of 5 or higher. The RETREAT scoremay help improve post-LT HCC surveillance strategies and identify patients who may benefit from future adjuvant therapies.
Acknowledgments
Funding/Support: This work was supported by the Biostatistics Core of the UCSF Liver Center (P30 DK026473).
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The funder played a role in the collection, management, analysis, and interpretation of the data. The funder had no role in the design and conduct of the study; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Previous Presentation: This research was presented at the American Association for the Study of Liver Diseases Liver Meeting; November 13, 2016; Boston, Massachusetts.
Author Contributions: Drs Mehta and Yao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Mehta, Heimbach, Harnois, Sapisochin, Burns, Greig, Roberts, Yao.
Acquisition, analysis, or interpretation of data: Mehta, Heimbach, Harnois, Sapisochin, Dodge, Lee, Sanchez, Grant, Yao.
Drafting of the manuscript: Mehta, Harnois, Sapisochin, Dodge, Roberts, Yao.
Critical revision of the manuscript for important intellectual content: Mehta, Heimbach, Harnois, Sapisochin, Dodge, Lee, Burns, Sanchez, Greig, Grant, Yao.
Statistical analysis: Mehta, Harnois, Dodge.
Administrative, technical, or material support: Heimbach, Sapisochin, Sanchez, Grant.
Supervision: Greig.
References
- 1.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60(6):1957–1962. doi: 10.1002/hep.27272. [DOI] [PubMed] [Google Scholar]
- 5.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16(3):262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi DM, Tzakis AG, Martin P, et al. Liver transplantation for hepatocellular carcinoma in the model for end-stage liver disease era. J Am Coll Surg. 2010;210(5):727–734. 735–736. doi: 10.1016/j.jamcollsurg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143(2):182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 9.Sapisochin G, Goldaracena N, Astete S, et al. Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series. Ann Surg Oncol. 2015;22(7):2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Llovet JM, Miceli R, et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 12.Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220(4):416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(suppl 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 14.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19(6):634–645. doi: 10.1002/lt.23652. [DOI] [PubMed] [Google Scholar]
- 15.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20(8):945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–994. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35(9):987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21(5):599–606. doi: 10.1002/lt.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai Q, Avolio AW, Graziadei I, et al. European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19(10):1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 20.Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant. 2014;14(6):1383–1390. doi: 10.1111/ajt.12684. [DOI] [PubMed] [Google Scholar]
- 21.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transpl. 2014;20(9):1045–1056. doi: 10.1002/lt.23917. [DOI] [PubMed] [Google Scholar]
- 22.Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2014;20(8):937–944. doi: 10.1002/lt.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 26.Mehta N, Yao FY. Moving past “One size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19(10):1055–1058. doi: 10.1002/lt.23730. [DOI] [PubMed] [Google Scholar]
- 27.Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262(3):536–545. doi: 10.1097/SLA.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 28.Montalti R, Mimmo A, Rompianesi G, et al. Absence of viable HCC in the native liver is an independent protective factor of tumor recurrence after liver transplantation. Transplantation. 2014;97(2):220–226. doi: 10.1097/TP.0b013e3182a8607e. [DOI] [PubMed] [Google Scholar]
- 29.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. 2014;120(22):3485–3493. doi: 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Vivarelli M, Cucchetti A, La Barba G, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248(5):857–862. doi: 10.1097/SLA.0b013e3181896278. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59(6):1193–1199. doi: 10.1016/j.jhep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Chen K, Man K, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q. Rationale of personalized immunosuppressive medication for hepatocellular carcinoma patients after liver transplantation. Liver Transpl. 2014;20(3):261–269. doi: 10.1002/lt.23806. [DOI] [PubMed] [Google Scholar]
- 34.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60(4):855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerman MA, Trotter JF, Wachs M, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14(5):633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 36.Chinnakotla S, Davis GL, Vasani S, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15(12):1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 37.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51(4):1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 38.Junge G, Saliba F, De Simone P, et al. Everolimus impact on hepatocellular carcinoma recurrence after liver transplantation—12, 24, and 36 months data from 719 LTx recipients [Abstract] Am J Transplant. 2014;14(S3):694. [Google Scholar]
- 39.Duvoux C, Toso C. mTOR inhibitor therapy: does it prevent HCC recurrence after liver transplantation? Transplant Rev (Orlando) 2015;29(3):168–174. doi: 10.1016/j.trre.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Ferreiro AO, Vazquez-Millán MA, López FS, Gutiérrez MG, Diaz SP, Patiño MJ. Everolimus-based immunosuppression in patients with hepatocellular carcinoma at high risk of recurrence after liver transplantation: a case series. Transplant Proc. 2014;46(10):3496–3501. doi: 10.1016/j.transproceed.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 41.Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27(10):1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 42.Geissler EK, Schnitzbauer AA, Zülke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100(1):116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel AB, El-Khoueiry AB, Finn RS, et al. Phase I trial of sorafenib following liver transplantation in patients with high-risk hepatocellular carcinoma. Liver Cancer. 2015;4(2):115–125. doi: 10.1159/000367734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanimine NTY, Ishiyama K, Ohira M, Shimizu S, Yano T, Ohdan H. Adoptive Immunotherapy with liver allograft-derived NK Cells improves recurrence-free survival after living-donor liver transplantation in patients with hepatocellular carcinoma [Abstract] Am J Transplant. 2015;15:S3. [Google Scholar]
- 45.Xu J, Shen ZY, Chen XG, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45(2):269–276. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]