The results of the study show that in this obese patient population, both MR elastography and vibration-controlled transient elastography (VCTE) had excellent diagnostic performance for assessing liver fibrosis; MR elastography had a greater examination success rate than VCTE and a higher interobserver agreement than liver biopsy.
Abstract
Purpose
To evaluate the diagnostic performance and examination success rate of magnetic resonance (MR) elastography and vibration-controlled transient elastography (VCTE) in the detection of hepatic fibrosis in patients with severe to morbid obesity.
Materials and Methods
This prospective and HIPAA-compliant study was approved by the institutional review board. A total of 111 patients (71 women, 40 men) participated. Written informed consent was obtained from all patients. Patients underwent MR elastography with two readers and VCTE with three observers to acquire liver stiffness measurements for liver fibrosis assessment. The results were compared with those from liver biopsy. Each pathology specimen was evaluated by two hepatopathologists according to the METAVIR scoring system or Brunt classification when appropriate. All imaging observers were blinded to the biopsy results, and all hepatopathologists were blinded to the imaging results. Examination success rate, interobserver agreement, and diagnostic accuracy for fibrosis detection were assessed.
Results
In this obese patient population (mean body mass index = 40.3 kg/m2; 95% confidence interval [CI]: 38.7 kg/m2, 41.8 kg/m2]), the examination success rate was 95.8% (92 of 96 patients) for MR elastography and 81.3% (78 of 96 patients) or 88.5% (85 of 96 patients) for VCTE. Interobserver agreement was higher with MR elastography than with biopsy (intraclass correlation coefficient, 0.95 vs 0.89). In patients with successful MR elastography and VCTE examinations (excluding unreliable VCTE examinations), both MR elastography and VCTE had excellent diagnostic accuracy in the detection of clinically significant hepatic fibrosis (stage F2–F4) (mean area under the curve: 0.93 [95% CI: 0.85, 0.97] vs 0.91 [95% CI: 0.83, 0.96]; P = .551).
Conclusion
In this obese patient population, both MR elastography and VCTE had excellent diagnostic performance for assessing hepatic fibrosis; MR elastography was more technically reliable than VCTE and had a higher interobserver agreement than liver biopsy.
© RSNA, 2016
An earlier incorrect version of this article appeared online. This article was corrected on January 25, 2017.
Introduction
Chronic liver disease and cirrhosis remain a leading cause of mortality in the United States (1). These trends are expected to increase because of an aging population, the growing epidemic of obesity and nonalcoholic fatty liver disease, and the continued burden of disease from chronic hepatitis C infection (2). For the majority of affected patients, the morbidity and mortality from chronic liver disease and cirrhosis are directly related to progressive hepatic fibrosis.
Therefore, it is very important to detect hepatic fibrosis for disease treatment and management. On the one hand, although liver biopsy is currently the standard of reference for detecting liver fibrosis, it has some important limitations—such as its sampling error and invasive nature (3–8). On the other hand, many investigators have found that hepatic stiffness measured with magnetic resonance (MR) elastography (9–12) and ultrasonography (US)–based elastography (13–15) is an accurate biomarker for detecting hepatic fibrosis. The diagnostic accuracy (area under the curve [AUC]) for detecting hepatic fibrosis (stage F2–F4) has been reported to be 90.9%–99.4% for MR elastography and 83.7%–91.4% for US-based vibration-controlled transient elastography (VCTE) (16,17). US-based shear-wave elastography and US-based acoustic radiation force imaging have also shown promising results (14,18,19) but still require further investigation (20–24).
In 2015, 65% of the adult American population and one-third of children were reported to be overweight or obese (25,26). This growing epidemic has placed tremendous technical challenges on imaging technologies and equipment (25). For elastography techniques, VCTE failed in 54.5% of obese patients (mean body mass index [BMI] ± standard deviation = 40.5 kg/m2 ± 7.3) with the conventional M probe, so an XL probe was designed specifically for obese patients. The use of a combination of M and XL probes has reduced the technical failure rate to 23.2% (27). For MR elastography, although our previous study (12) did not find that BMI was a statistically significant risk factor for unsuccessful MR elastography examinations (P = .2) in 1287 patients with a large range of BMIs (range, 15.9–61.3 kg/m2; mean, 29.9 kg/m2), another study found that obesity (patients could not fit into the magnet bore) caused two unsuccessful MR elastography examinations in 141 patients (16).
In view of the growing obese population and the resultant technical challenges to both MR elastography and VCTE, the aim of our study was to evaluate the diagnostic performance and examination success rate of MR elastography and VCTE in the detection of hepatic fibrosis in obese patients. Our hypothesis was that MR elastography is more technically reliable and diagnostically accurate than VCTE for detecting hepatic fibrosis in obese patients.
Materials and Methods
The Mayo Clinic and authors J.C., M.Y., K.J.G., and R.L.E. have intellectual property rights and a financial interest through receipt of royalties and equity from licensing of MR elastography technology. R.L.E. serves as uncompensated chief executive officer of Resoundant (Rochester, Minn), which is majority-owned by the Mayo Clinic. J.O., V.M., and L.S. are employees of Echosens (Paris, France). L.S. serves as chief technology officer of and holds equity in Echosens. The authors affiliated with the Mayo Clinic had control of the data. This research was conducted under the oversight of, and in compliance with, the Mayo Clinic Conflict of Interest Review Board.
Patients
The institutional review board at the Mayo Clinic approved this prospective Health Insurance Portability and Accountability Act–compliant study, and all enrolled patients provided written informed consent. Between March 2010 and May 2013, patients referred for liver biopsy after clinical evaluation for known or suspected chronic liver disease were considered for enrollment consecutively in our study. The inclusion criteria were as follows: (a) age of at least 18 years; (b) diagnosis of chronic hepatitis C, nonalcoholic fatty liver disease, and/or alcoholic liver disease verified with liver histologic examination within at least 48 hours, but no later than 1 month, of study enrollment; and (c) verbal and written fluency of the English language. We also intended to include patients with obesity and morbid obesity because, at our institution, the number of patients who undergo liver biopsy has decreased as a result of the increase in the number of clinical MR elastography examinations being prescribed; however, patients who undergo bariatric surgery still undergo liver biopsy. The criteria for bariatric surgery is a BMI of at least 40 kg/m2 (morbidly obese) or a BMI of at least 35 kg/m2 (severely obese), with comorbidities according to the National Institutes of Health guidelines (28). Thus, most patients recruited for our study were obese patients who had undergone bariatric surgery. The exclusion criteria are listed in Appendix E1 (online). Patients fasted for at least 6 hours before they underwent MR elastography and VCTE.
Histologic Assessment
Liver biopsy was performed by using either a percutaneous needle biopsy or subcapsular wedge biopsy in our clinical practice. Liver specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin and Masson trichrome stains. The METAVIR scoring system or Brunt classification (when appropriate) was used for histopathologic interpretations, with fibrosis classified as stage F0, F1, F2, F3, or F4, as described in Appendix E1 (online). The grade of necroinflammatory activity for all histologic liver specimens was evaluated semiquantitatively on a scale of A0 to A3 according to location as either portal or lobular (29). A group of staff hepatopathologists performed the first interpretation of the liver biopsy specimens as part of the clinical service, and one independent hepatopathologist (T.C.S.) provided a second interpretation while blinded to the results of the first interpretation and elastography. For discrepancies in fibrosis stage, the independent hepatopathologist reviewed the cases again and provided a final interpretation. In addition, the length of the liver biopsy sample (in millimeters), the number of portal tracts in the biopsy sample, and biopsy type were recorded. The first and second interpretations were used to assess interobserver agreement of liver biopsy. The final interpretations from the independent hepatopathologist were used for the statistical analysis.
MR Elastography and MR Imaging of Fat
MR elastography uses a pneumatic driver system to send mechanical shear waves into the patient’s liver and uses MR imaging to produce images of wave propagation in the liver that are processed to create cross-sectional maps of liver stiffness (shear modulus, in kilopascals) (9–11). In our study, patients underwent MR elastography in the supine position, with a 19-cm-diameter passive drum driver placed against the anterior body wall close to the right hepatic lobe. The passive driver was secured with an elastic belt wrapped around the body and connected to an MR elastography active driver system via a 7.6-m-long flexible vinyl chloride tube with an inside diameter of 1.9 cm. A 1.5-T whole-body MR imaging unit with a 60-cm bore (Signa; GE Medical Systems, Milwaukee, Wis) was used with an eight-channel torso radiofrequency coil array. A record was made of patients who could not complete the MR elastography examination. The MR elastography parameters are described in Appendix E1 (online). Wave images were processed on the imager automatically by the proprietary multimodel direct inversion algorithm (Mayo Clinic, Rochester, Minn), producing four sections showing the stiffness distribution in the liver (elastograms) and associated wave confidence maps. The confidence maps report the correlation coefficient of local polynomial fits to the wave images and reflect the wave image signal-to-noise ratio (including wave amplitude and magnetization signal-to-noise ratio) and the presence of artifacts (eg, blood vessels). Two readers (M.Y. and J.C., with 11 and 7 years of experience, respectively) drew regions of interest inside the hepatic parenchyma, about 5–10 pixels away from the liver boundary, excluding regions with major blood vessels and wave confidence below 95%. The readers were blinded to the clinical history and results of liver biopsy. The mean and standard deviation of liver stiffness in the regions of interest were reported. Patients also underwent in-phase and out-of-phase hepatic fat imaging with the parameters described in Appendix E1 (online).
US-based VCTE Examination
Patients underwent VCTE (Fibroscan; Echosens, Paris, France) to measure liver stiffness (Young modulus, in kilopascals) on the same day as MR elastography with use of a standard M probe or an XL probe that is designed for patients with a high BMI. The M probe and XL probe have different central ultrasound frequencies (3.5 MHz vs 2.5 MHz) and measurement depths (2.5–6.5 cm vs 3.5–7.5 cm). VCTE was performed with the patient in the supine position with the right arm in maximal abduction. The operators used a portable US scanner (Vscan, GE Medical Systems) in the abdominal mode to identify a spot on the chest between the ribs at the level of the right hepatic lobe where the subcutaneous fat was thin and the liver was free of large vascular structures. The skin-to-capsule distance (SCD) was measured first with the portable US scanner. Then the operators positioned one of the two VCTE probes (M or XL) on the designated intercostal spot with appropriate pressure and launched the VCTE measurements. The M probe was chosen for patients with an SCD of 2.5 cm or less, and the XL probe was used for patients with an SCD of more than 2.5 cm. If the M probe failed, however, the XL probe was used even if the SCD was less than 2.5 cm.
Three authors are manufacturer-certified operators (J.C., M.Y., and D.M.S., all with 3 years of experience), and they performed the VCTE examinations while blinded to clinical history and the results of liver biopsy. At least two of the authors attempted multiple trials for achieving 10 valid measurements in each patient; if it was not possible to obtain 10 valid measurements, the examination was recorded as a technique failure. Details of the procedure is described in Appendix E1 (online).
The median value of the 10 valid measurements was recorded as the patient liver stiffness. In addition, the interquartile range of the valid measurements and the valid measurement rate (the number of valid measurements divided by the number of attempted measurements) of the examination were recorded. For our study, we applied the most commonly used reliability criteria for VCTE proposed by Castera et al (30): A set of stiffness values are considered reliable if at least 60% of the individual measurements are reported as valid by the instrument and the interquartile range–to-median ratio is 30% or less. Recently, a somewhat less strict standard has been proposed by Boursier et al (31): Stiffness values are considered to be reliable if the interquartile range–to-median ratio is 30% or less or the interquartile range–to-median ratio is greater than 30% with a median liver stiffness of less than 7.1 kPa. The total examination time was about 5–15 minutes. In conjunction with the VCTE examination, the patient’s chest and waist circumferences were measured with a tape measure.
With VCTE, the liver stiffness is reported by using the Young modulus for VCTE and the shear modulus for MR elastography. For nearly incompressible and isotropic material, the Young modulus was three times the shear modulus (32).
Statistical Analysis
The patient characteristics were summarized according to sex by using descriptive statistics. Data were reported as means (and 95% confidence intervals [CIs]) for items with continuous values and as numbers of patients for categoric items. The success rate of MR elastography or VCTE was calculated as the number of patients who underwent successful MR elastography or VCTE examinations divided by the total number of patients who underwent examinations with both methods. Six factors (age, chest and waist circumference, SCD, BMI, and sex) were examined as possible indicators of successful and unsuccessful MR elastography examinations. In addition to those six factors, the type of VCTE probe was also examined between successful and unsuccessful VCTE examinations.
Diagnostic performance in the detection of fibrosis was assessed by using a 2 × 2 table classic receiver operator characteristic curve (ROC) analysis in patients who had successful MR elastography and reliable VCTE examinations; the VCTE ROC was also analyzed with the unreliable VCTE examinations included to evaluate the effect of unreliable examinations on the diagnostic accuracy of VCTE. To detect a difference of 0.05 in the AUC between MR elastography and VCTE, assuming 0.1 for the standard deviation of AUC for each method, .05 for the probability of type I error (α), and 0.85 for the statistical power, each method requires at least 73 patients. Sensitivity, specificity, positive predictive value, and negative predictive value were also calculated, and stiffness thresholds were chosen to provide a sensitivity of at least 80% (33).
In addition to the classic 2 × 2 table ROC analysis where nonevaluable MR elastography and VCTE examinations were excluded because of technical failures, we performed a 3 × 2 table ROC analysis, including all nonevaluable MR elastography cases and nonevaluable and unreliable VCTE cases. According to the intention-to-diagnosis design or “worst case” scenario, if a failed MR elastography or VCTE examination (nonevaluable examination) had negative findings at biopsy, then this nonevaluable examination would be included as a “false-positive” case for that examination and a positive biopsy case would be included as a “false-negative” case for that examination (34).
Software (JMP Pro 11; SAS, Cary, NC) was used for the statistical analysis. AUC comparison was performed by the Model Comparison function. To compare the difference between any two groups, one-way analysis of variance (for continuous values) or the Fisher exact test (for categoric values) was performed to calculate P values. P ≤ .05 was indicative of a statistically significant difference.
The method for analyzing interobserver agreement and confounding effects on liver stiffness are described in Appendix E1 (online).
Results
In total, 111 patients participated in our study. The mean overall BMI was 40.3 kg/m2 (95% CI: 38.7 kg/m2, 41.8 kg/m2), the time between MR elastography and/or VCTE and biopsy was 23.6 days (95% CI: 22.0 days, 25.2 days), and the average region of interest size per section at MR elastography was 28.6 cm2 (95% CI: 25.7 cm2, 31.5 cm2). Tables 1 and 2 show a summary of patient characteristics according to sex.
Table 1.
Summary of Patient Characteristics
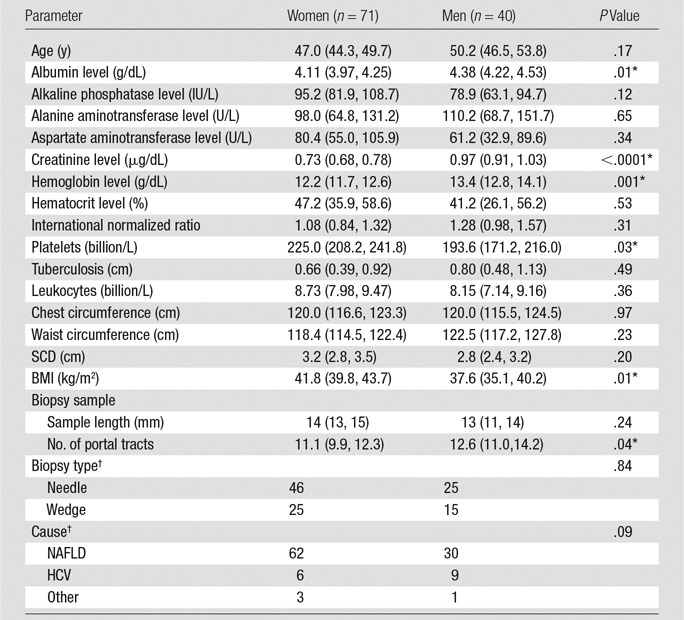
Note.—Except where indicated, data are means, with 95% CIs in parentheses.
*Statistically significantly difference between men and women (P ≤ .05).
†Data are numbers of patients. HCV = hepatitis C virus, NAFLD = nonalcoholic fatty liver disease.
Table 2.
Fibrosis Stage according to Inflammation Grade and Sex
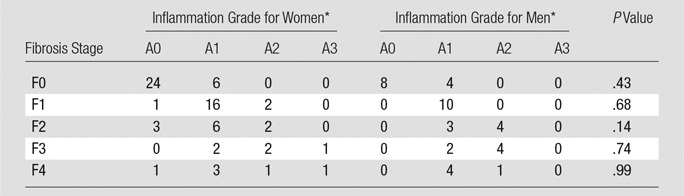
*Data are numbers of patients.
Examination Success Rates
Figure 1 (right) shows the patient flowchart for VCTE examinations. One patient refused to undergo MR elastography but underwent a successful VCTE examination. A different patient refused to undergo VCTE but underwent a successful MR elastography examination. For 13 other patients, the VCTE machine was unavailable at the time of the examination because machine delivery was delayed at the beginning of our study; in addition, the two probes were sent out for calibration every 6 months. Five of the 110 patients who underwent MR elastography were not able to complete the MR elastography examination: Two patients were too large to fit into the MR imager bore and three became claustrophobic when placed inside the imager bore. VCTE failed (<10 valid measurements) in four of the 97 patients who underwent VCTE, and stiffness measurements were considered unreliable (not reliable according to the reliability criteria) in 14 patients.
Figure 1:
Patient flowcharts for ROC analysis (left) and VCTE examinations (right). MRE = MR elastography.
Therefore, 105 of 110 patients (95.5%) had successful MR elastography examinations and 79 of 97 patients (81.4%) had successful VCTE examinations. Ninety-six patients underwent both MR elastography and VCTE examinations. Of those 96 patients, 77 patients had successful VCTE and MR elastography examinations, three patients had unsuccessful VCTE and MR elastography examinations, 15 patients had successful MR elastography but unsuccessful VCTE examinations, and one patient had a successful VCTE examination but an unsuccessful MR elastography examination. The examination success rate of MR elastography was 95.8% (92 of 96 patients). The success rate of VCTE was 81.3% (78 of 96 patients) with the criteria used by Castera et al (30) and 88.5% (85 of 96 patients) with the criteria used by Boursier et al (31), both of which are lower than that of MR elastography (P = .02 and .39, respectively).
Table 3 shows factors that affect MR elastography and VCTE success status. Among the six risk factors studied (age, chest and waist circumference, SCD, BMI, and sex), only waist circumference was significantly different between patients with successful MR elastography examinations and those with unsuccessful MR elastography examinations (mean waist circumference: 118.9 cm [95% CI: 115.7 cm, 122.1 cm] for successful examinations and 139.5 cm [95% CI: 123.6 cm, 155.4 cm] for unsuccessful examinations, P = .02; P ≥ .06 for the other factors). Among the seven risk factors that possibly affect VCTE (the six mentioned earlier plus the type of VCTE probe), three factors were significantly different between patients with successful VCTE examinations and those with unsuccessful VCTE examinations: BMI (P = .01), chest circumference (P = .02), and waist circumference (P = .03; P ≥ .07 for the other factors).
Table 3.
Factors That Affect MR Elastography and VCTE Success Status
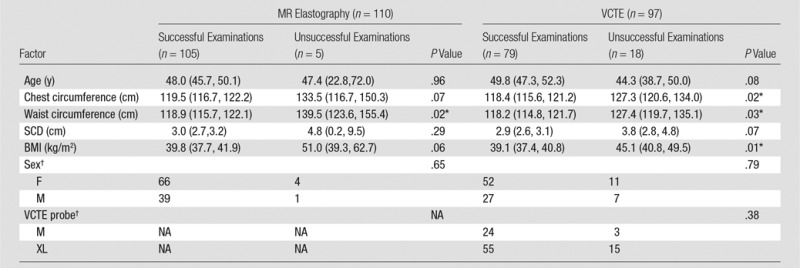
Note.—Except where indicated, data are means, with 95% CIs in parentheses.
*Indicates a statistically significant difference (P ≤ .05) between successful examinations and unsuccessful examinations.
†Data are numbers of patients. NA = not applicable.
Diagnostic Performance in the Detection of Fibrosis
Figure 1 (left) shows the patient flowchart for the classic 2 × 2 table and 3 × 2 table ROC analysis. Of the 77 patients who underwent successful VCTE (after excluding unreliable examinations) and MR elastography examinations, 51 were women and 26 were men. The demographic characteristics for these 77 patients according to sex were as follows: mean age, 48.3 years (95% CI: 45.2 years, 51.4 years) versus 51.5 years (95% CI: 47.2 years, 55.8 years) for women and men, respectively, P = .23; chest circumference, 117.8 cm (95% CI: 114.2 cm, 121.4 cm) versus 119.6 cm (95% CI: 114.6 cm, 124.6 cm), P = .56; waist circumference, 115.6 cm (111.5 cm, 119.8 cm) versus 121.9 cm (95% CI: 116.0 cm, 127.9 cm), P = .09; SCD, 3.0 cm (95% CI: 2.7 cm, 3.2 cm) versus 2.7 cm (95% CI: 2.3 cm, 3.1 cm), P = .24; and BMI, 39.8 kg/m2 (95% CI: 37.7 kg/m2, 41.9 kg/m2) versus 37.8 kg/m2 (95% CI: 34.9 kg/m2, 40.7 kg/m2), P = .29.
Figures 2 and 3 show examples of MR elastography and VCTE liver stiffness measurements for a patient (BMI = 41.0 kg/m2) with no fibrosis and no inflammation and another patient (BMI = 39.4 kg/m2) with fibrosis and inflammation, respectively. With 2 × 2 table ROC analysis, Table 4 shows AUCs (and 95% CIs) and their comparisons for differentiating different fibrosis grades with MR elastography (reader J.C.) and VCTE (with unreliable examinations both excluded and included). The AUC for detecting clinically significant hepatic fibrosis (stage, ≥F2) was 0.91 (95% CI: 0.83, 0.96) with VCTE (excluding unreliable examinations) and 0.93 (95% CI: 0.85, 0.97) with MR elastography (P = .551); however, with the VCTE unreliable examinations included, the AUC for VCTE decreased statistically significantly to 0.83 (95% CI: 0.73, 0.90; P = .005), which was also statistically significantly less than that of MR elastography (P = .018). There were no AUC differences between the two MR elastography readers. Table 5 shows the cutoff stiffness values, sensitivities, specificities, positive predictive values, and negative predictive values for detecting different stages of hepatic fibrosis.
Figure 2a:
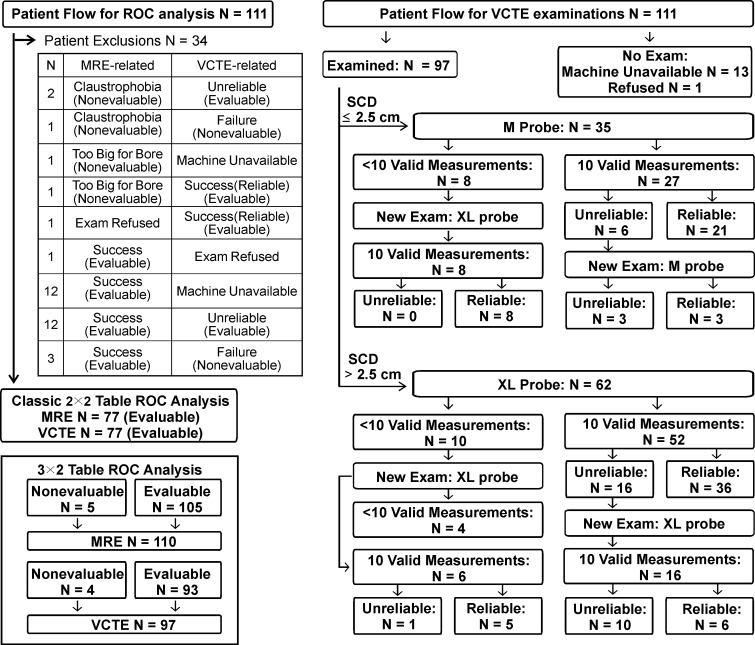
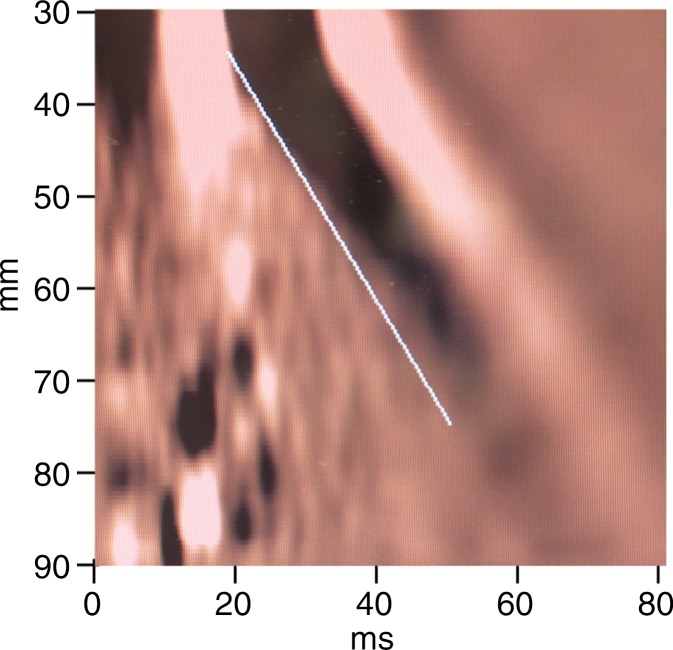
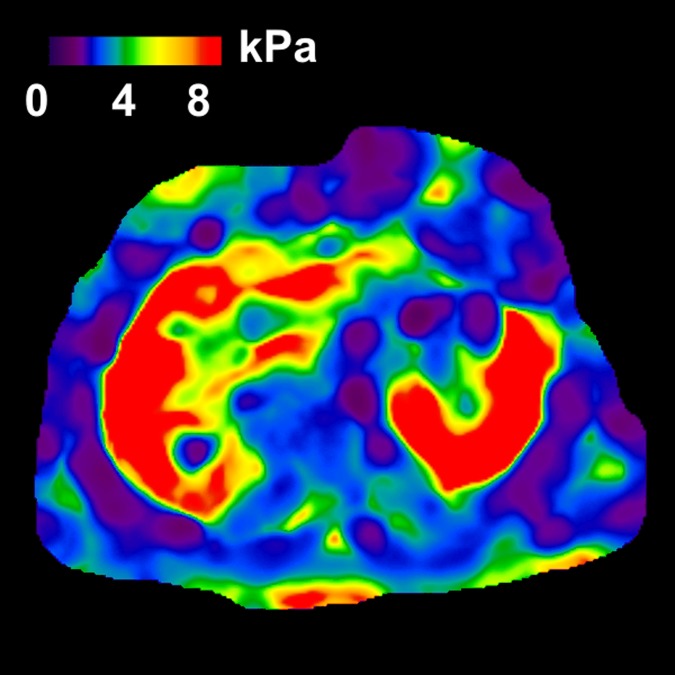
MR elastography and VCTE liver stiffness measurements in 63-year-old woman with nonalcoholic fatty liver disease (BMI, 41.0 kg/m2 ). (a) MR elastography magnitude image (field of view, 40 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 2.45 kPa with MR elastography and 5.5 kPa with VCTE (XL probe). Liver biopsy was performed with wedge biopsy, sample length was 9 mm, number of portal tracts in biopsy sample was 10, fibrosis stage was 0, and inflammation grade was 0.
Figure 3a:
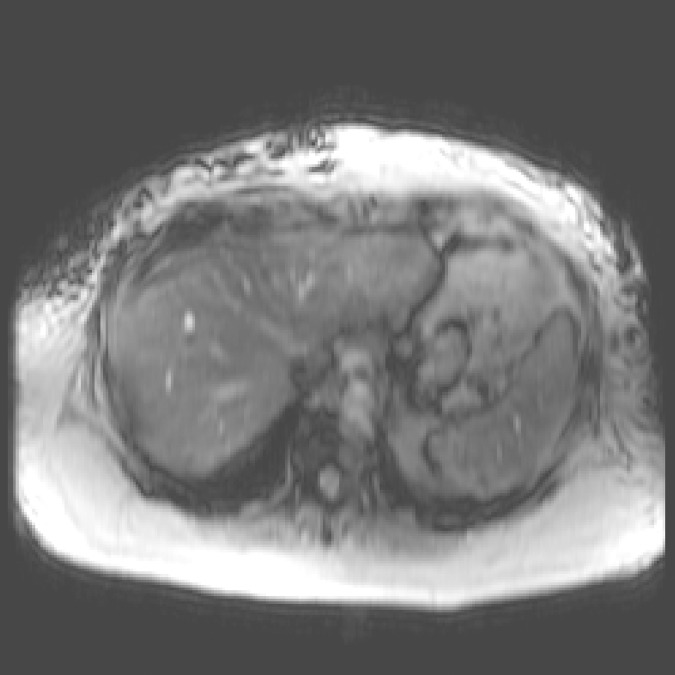
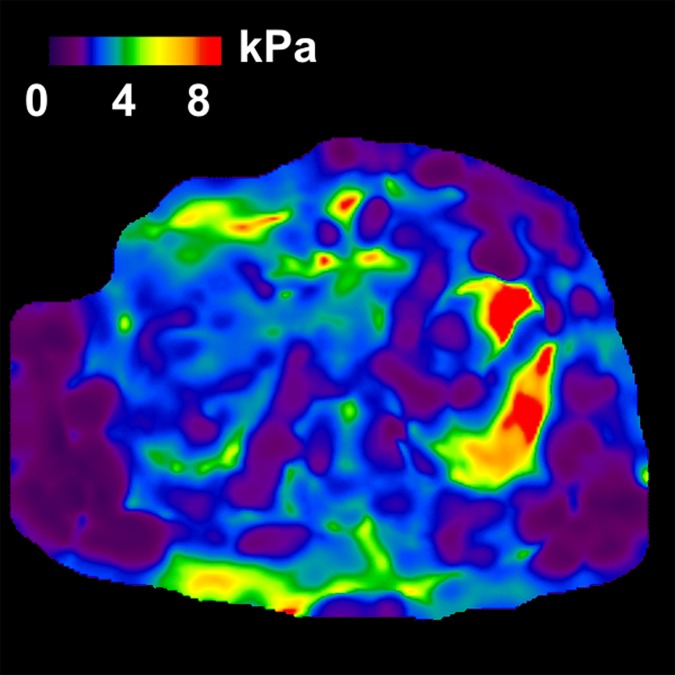
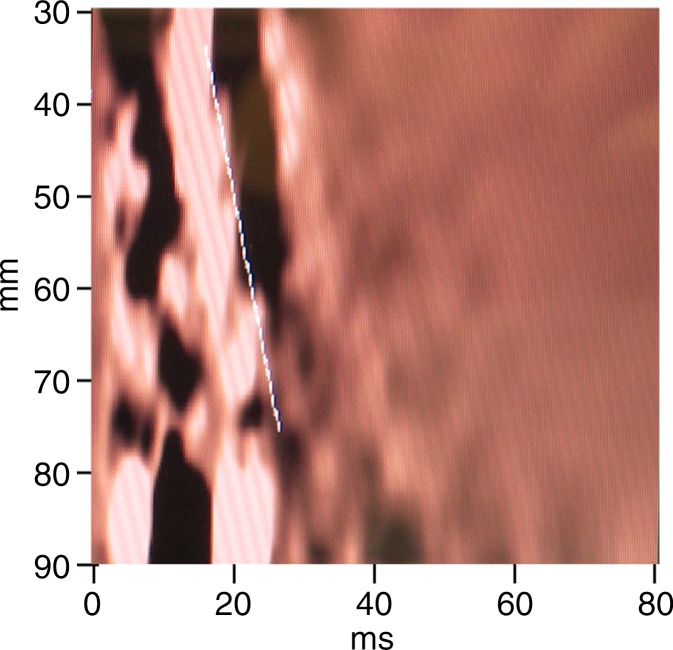
MR elastography and VCTE measurements of liver stiffness in 58-year-old woman with nonalcoholic steatohepatitis (BMI, 39.4 kg/m2 ). (a) MR elastography magnitude image (field of view, 42 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 8.97 kPa with MR elastography and 66.4 kPa with VCTE (XL probe). Liver biopsy was performed with needle biopsy, sample length was 20 mm, number of portal tracts in biopsy sample was eight, fibrosis stage was 4, and inflammation grade was 3.
Table 4.
Performance in the Differentiation of Hepatic Fibrosis Grades
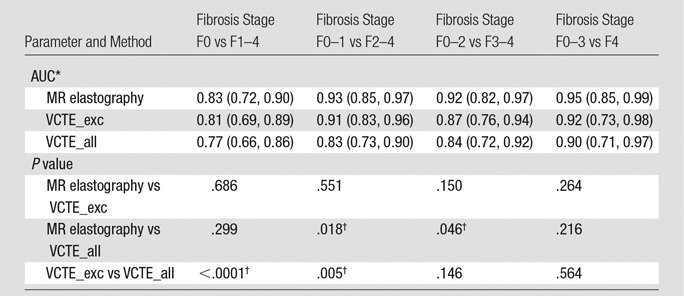
Note.—For the VCTE_exc group, we excluded patients who had unreliable VCTE examinations and included only those who had both successful MR elastography and successful (reliable) VCTE examinations. For the VCTE_all group, we included patients who had both unreliable and reliable VCTE examinations. The MR elastography group comprised only patients who had both successful MR elastography and successful (reliable) VCTE examinations.
*Data are means, with 90% CIs in parentheses.
†Statistically significantly difference (P ≤ .05).
Table 5.
Diagnostic Performance in the Detection of Hepatic Fibrosis
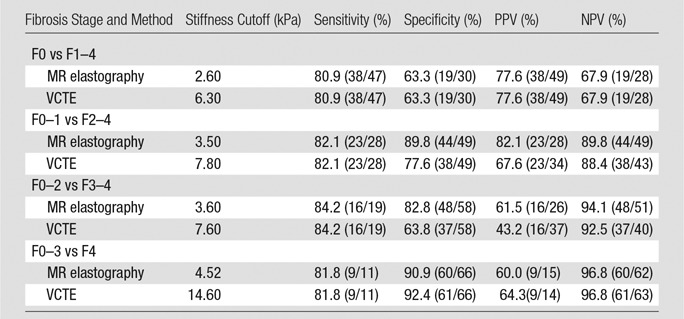
Note.—MR elastography and VCTE included patients who had both successful MR elastography and successful (reliable) VCTE examinations. Numbers in parentheses are raw data. NPV = negative predictive value, PPV = positive predictive value.
Figure 2b:
MR elastography and VCTE liver stiffness measurements in 63-year-old woman with nonalcoholic fatty liver disease (BMI, 41.0 kg/m2 ). (a) MR elastography magnitude image (field of view, 40 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 2.45 kPa with MR elastography and 5.5 kPa with VCTE (XL probe). Liver biopsy was performed with wedge biopsy, sample length was 9 mm, number of portal tracts in biopsy sample was 10, fibrosis stage was 0, and inflammation grade was 0.
Figure 2c:
MR elastography and VCTE liver stiffness measurements in 63-year-old woman with nonalcoholic fatty liver disease (BMI, 41.0 kg/m2 ). (a) MR elastography magnitude image (field of view, 40 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 2.45 kPa with MR elastography and 5.5 kPa with VCTE (XL probe). Liver biopsy was performed with wedge biopsy, sample length was 9 mm, number of portal tracts in biopsy sample was 10, fibrosis stage was 0, and inflammation grade was 0.
Figure 3b:
MR elastography and VCTE measurements of liver stiffness in 58-year-old woman with nonalcoholic steatohepatitis (BMI, 39.4 kg/m2 ). (a) MR elastography magnitude image (field of view, 42 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 8.97 kPa with MR elastography and 66.4 kPa with VCTE (XL probe). Liver biopsy was performed with needle biopsy, sample length was 20 mm, number of portal tracts in biopsy sample was eight, fibrosis stage was 4, and inflammation grade was 3.
Figure 3c:
MR elastography and VCTE measurements of liver stiffness in 58-year-old woman with nonalcoholic steatohepatitis (BMI, 39.4 kg/m2 ). (a) MR elastography magnitude image (field of view, 42 cm). (b) MR elastography stiffness map. (c) VCTE stiffness measurement. Liver stiffness was 8.97 kPa with MR elastography and 66.4 kPa with VCTE (XL probe). Liver biopsy was performed with needle biopsy, sample length was 20 mm, number of portal tracts in biopsy sample was eight, fibrosis stage was 4, and inflammation grade was 3.
Table 6 shows the results of the 3 × 2 table with intention-to-diagnosis ROC analysis after including patients with nonevaluable (failed) MR elastography and VCTE examinations as false-positive or false-negative cases. As expected, the 3 × 2 table ROC analysis shows considerably lower diagnostic performance when compared with classic 2 × 2 table ROC analysis. For example, in the detection of stage F2–F4 fibrosis, MR elastography AUC decreased from 0.93 (95% CI: 0.85, 0.97) to 0.80 (95% CI: 0.69, 0.88) and VCTE AUC from 0.91 (95% CI: 0.83, 0.96) to 0.76 (95% CI: 0.64, 0.84).
Table 6.
Results of Intention-to-Diagnosis ROC Analysis with 3 × 2 Table, with Inclusion of Nonevaluable Examinations
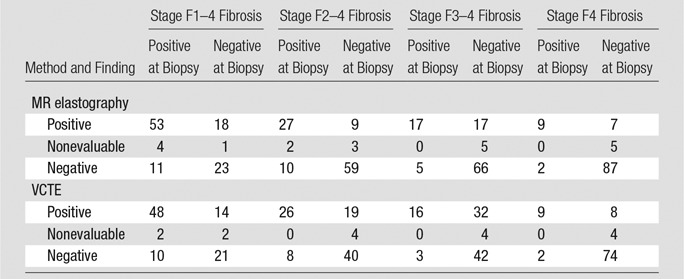
Note.—Data are numbers of patients. According to the intention-to-diagnosis design or “worst case” scenario, if a failed MR elastography or VCTE examination (nonevaluable) had a negative finding at biopsy, then this nonevaluable examination would be included as a false-positive case for that examination and a positive biopsy case would be included as a false-negative case for that examination. For MR elastography, the stiffness threshold and AUC were 2.6 and 0.73 (95% CI: 0.62, 0.81), respectively, for stage F1–4 fibrosis, 3.5 and 0.82 (95% CI: 0.71, 0.89) for stage F2–4 fibrosis, 3.6 and 0.85 (95% CI: 0.75, 0.91) for stage F3–4 fibrosis, and 4.52 and 0.91 (95% CI: 0.81, 0.96) for stage F4 fibrosis. For VCTE, the stiffness threshold and AUC were 6.3 and 0.71 (95% CI: 0.59, 0.81), respectively, for stage F1–4 fibrosis, 7.8 and 0.78 (95% CI: 0.67, 0.86) for stage F2–4 fibrosis, 7.6 and 0.80 (95% CI: 0.68, 0.88) for stage F3–4 fibrosis, and 14.6 and 0.86 (95% CI: 0.70, 0.95) for stage F4 fibrosis.
The interobserver agreement intraclass correlation coefficients of MR elastography and biopsy were 0.95 and 0.89, respectively. Detailed results of interobserver agreement and confounding effects on liver stiffness are described in Appendixes E2 and E3 (online).
Discussion
In the patients who underwent both MR elastography and VCTE, the success rate of MR elastography was 95.8% and the success rate of VCTE was 81.3% with the criteria used by Castera et al (30) and 88.5% with the criteria used by Boursier et al (31). This finding is in agreement with that of a previous study of 141 patients with a lower average BMI (mean BMI, 25.9 kg/m2 ± 4.0) than our patients, where the examination success rate was 94% with MR elastography and 84% with VCTE (P = .016) (16).
For MR elastography, high BMI is not necessarily the direct cause of an unsuccessful examination. For example, we found that waist circumference, and not BMI, was the only statistically significant risk factor for unsuccessful MR elastography examinations. The mean waist circumference was 118.9 cm (95% CI: 115.7 cm, 122.1 cm) for successful examinations and 139.5 cm (95% CI: 123.6 cm, 155.4 cm) for unsuccessful examinations (P = .02). We found that as long as the patient can fit into the bore comfortably, MR elastography can be performed successfully independent of his or her BMI. In our previous study of 1287 patients with a large range of BMIs (range, 15.9–61.3 kg/m2; mean, 29.9 kg/m2), we also found that BMI was not a statistically significant risk factor for unsuccessful MR elastography examinations (P = .2), whereas MR elastography had a technical failure rate of 5.6% (77 of 1377 examinations) owing to hepatic iron overload, execution errors, software glitches, and respiratory artifacts (12). Other investigators also found that claustrophobia, obesity (could not fit into the magnet bore), and hemochromatosis were the causes for unsuccessful MR elastography examinations (rate, 4.65% [six of 129 examinations] to 5.67% [eight of 141 examinations]) (16,35). In practice, the use of sedation for claustrophobia, wide-bore imagers for patients with obesity, and spin echo–based MR elastography sequences for those with iron overload (36) can improve the success rate of MR elastography.
For VCTE, conversely, high BMI (P = .01) and chest (P = .02) and waist (P = .03) circumference were responsible for unsuccessful VCTE examinations. Surprisingly, neither SCD nor the type of VCTE probe (M and XL) was a statistically significant risk factor for unsuccessful VCTE examinations in our study. This suggests that the type of VCTE probe can continue to be chosen according to the patient’s SCD. Previous VCTE studies have shown that obesity had a great effect on VCTE technique failures, which ranged from 23.2% to 54.5% (27).
In our study, the technical failure rate of VCTE (4.1% [four of 97 patients]; <10 valid measurements) was much lower than that in previous studies, although 14 of 97 patients (14.4%) had unreliable examinations. We used a separate US machine to guide the positioning of the VCTE probes, and we had multiple trained operators present to perform multiple examinations with the best effort on difficult patients as needed. This strategy may explain why the VCTE failure rate in our study was lower than that in previous studies.
Overall, it is suggested that if an obese patient can fit into the imager bore, then MR elastography is preferred; otherwise, VCTE should be performed as the initial approach for liver stiffness measurement.
In our study, the AUCs for detecting clinically significant hepatic fibrosis (stage, ≥F2) by using successful VCTE (excluding unreliable examinations) and MR elastography examinations were very high: 0.91 (95% CI: 0.83, 0.96) for VCTE and 0.93 (95% CI: 0.85, 0.97) for MR elastography. There was no statistically significant difference between the two methods (P = .551). However, when unreliable VCTE examinations were included, the AUC of VCTE decreased significantly to 0.83 (95% CI: 0.73, 0.90; P = .005).
Our findings are in agreement with those from previous studies. For example, in a study of 146 patients with chronic liver diseases (mean BMI, 25.9 kg/m2 ± 4.0), where unreliable VCTE examinations were not identified or excluded, the diagnostic accuracy (AUC) for detecting hepatic fibrosis (stage F2–F4) was 99.4% for MR elastography and 83.7% for VCTE (16). In another study of 103 patients (BMI, 25.5 kg/m2 ± 3.9) with viral hepatitis B and C, where unreliable VCTE examinations were actually identified or excluded, the AUC for detecting advanced fibrosis (stage F2–F4) was 90.9% for MR elastography and 91.4% for VCTE (17). Therefore, we anticipate that increasing the number of reliable VCTE examinations—either by means of training or technical improvements—will improve VCTE AUCs and its success rate.
Our study had nonevaluable (failed) MR elastography and VCTE examinations, which were excluded from the classic 2 × 2 table ROC analysis. We included them as false-positive or false-negative cases according the intention-to-diagnosis design or “worst case” scenario in the 3 × 2 table ROC analysis. The 2 × 2 table ROC analysis is the approved approach that has been most widely used in the literature, whereas the 3 × 2 table analysis provides a more conservative assessment.
Our study had several limitations. First, our patient population was unevenly distributed in terms of fibrosis stages and inflammation grades. Second, 40 of our 111 patients (36%) underwent wedge biopsy instead of needle biopsy during bariatric surgery. A wedge biopsy could cause the disease stage to be overestimated (37). Finally, our biopsy sample lengths were less than 2 cm, and we did not consider other possible confounding factors that could create false-positive cases, such as portal hypertension, esophageal varices (37), congestive heart failure (38), and venous pressure (39).
The results of our study showed that, in this obese patient population, both MR elastography and VCTE had excellent diagnostic performance in the assessment of liver fibrosis; MR elastography had a greater examination success rate than VCTE and a higher interobserver agreement than liver biopsy.
Advances in Knowledge
■ For detecting clinically significant hepatic fibrosis (stage F2–F4) in patients with successful MR elastography and vibration-controlled transient elastography (VCTE) examinations, both MR elastography and VCTE had excellent diagnostic accuracy, with a mean area under the curve of 0.93 (95% confidence interval [CI]: 0.85, 0.97) and 0.91 (95% CI: 0.83, 0.96), respectively (P = .551).
■ In patients with obesity (mean body mass index [BMI] = 40.3 kg/m2; 95% CI: 38.7 kg/m2, 41.8 kg/m2), the examination success rate of MR elastography (95.8%) was higher than that of VCTE (81.3% or 88.5%).
■ Interobserver agreement was higher with MR elastography than with biopsy (intraclass correlation coefficient: 0.95 vs 0.89, respectively).
Implication for Patient Care
■ In patients with obesity, MR elastography can be performed successfully, independent of BMI, as long as they can fit into the magnet bore.
APPENDIX
SUPPLEMENTAL FIGURE
Acknowledgments
Acknowledgments
We thank Diane M. Sauter, RT, for assistance in scanning patients; Stephanie M. Johnson, RN, and Janice M. Zimmerman, CCRPII, for coordinating the study; and Stephen S. Cha, BS, and Rickey E. Carter, PhD, for assistance with the statistical analysis.
Received April 13, 2016; revision requested May 26; revision received July 20; accepted August 23; final version accepted September 13.
Supported by National Institute of Biomedical Imaging and Bioengineering (grants EB 001981, EB 10393, and EB 017197).
Disclosures of Conflicts of Interest: J.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives royalties from Resoundant; institution receives royalties from Resoundant; has stock/stock options in Resoundant; institution has stock/stock options in Resoundant. Other relationships: the Mayo Clinic and J.C. have intellectual property rights and a financial interest in MRE technology; the VCTE device in this study was on loan from Echosens and returned to the company after the study. M.Y. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives royalties from Resoundant; institution receives royalties from Resoundant; has stock/stock options in Resoundant; institution has stock/stock options in Resoundant. Other relationships: the Mayo Clinic and M.Y. have intellectual property rights and a financial interest in MRE technology; the VCTE device in this study was on loan from Echosens and returned to the company after the study. J.A.T. disclosed no relevant relationships. J.O. disclosed no relevant relationships. K.J.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives royalties from Resoundant; institution receives royalties from Resoundant; has stock/stock options in Resoundant; institution has stock/stock options in Resoundant. Other relationships: disclosed no relevant relationships. T.C.S. disclosed no relevant relationships. V.M. disclosed no relevant relationships. L.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is an employee of Echosens. Other relationships: disclosed no relevant relationships. R.L.E. Activities related to the present article: received provision of writing assistance, medicines, equipment, or administrative support from Echosens. Activities not related to the present article: receives royalties from Resoundant; institution receives royalties from Resoundant; has stock/stock options in Resoundant; institution has stock/stock options in Resoundant; is the CEO of Resoundant (uncompensated). Other relationships: the Mayo Clinic and R.L.E. have intellectual property rights and a financial interest in MRE technology; the VCTE device in this study was on loan from Echosens and returned to the company after the study.
Abbreviations:
- AUC
- area under the curve
- BMI
- body mass index
- CI
- confidence interval
- ROC
- receiver operating characteristic
- SCD
- skin-to-capsule distance
- VCTE
- vibration-controlled transient elastography
References
- 1.Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project online database. U.S. Department of Health and Human Services. http://www.hcup-us.ahrq.gov/home.jsp. Published 2009. Accessed November 3, 2016. [Google Scholar]
- 2.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol 2004;2(12):1048–1058. [DOI] [PubMed] [Google Scholar]
- 3.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344(7):495–500. [DOI] [PubMed] [Google Scholar]
- 4.Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006;55(4):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut 1995;36(3):437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99(5):1396–1400. [DOI] [PubMed] [Google Scholar]
- 7.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000;32(3):477–481. [DOI] [PubMed] [Google Scholar]
- 8.Sporea I, Popescu A, Sirli R. Why, who and how should perform liver biopsy in chronic liver diseases. World J Gastroenterol 2008;14(21):3396–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huwart L, Sempoux C, Salameh N, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase–to-platelet ratio index. Radiology 2007;245(2):458–466. [DOI] [PubMed] [Google Scholar]
- 10.Asbach P, Klatt D, Hamhaber U, et al. Assessment of liver viscoelasticity using multifrequency MR elastography. Magn Reson Med 2008;60(2):373–379. [DOI] [PubMed] [Google Scholar]
- 11.Rustogi R, Horowitz J, Harmath C, et al. Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging 2012;35(6):1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology 2016;278(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography. III. Liver. Ultrasound Med Biol 2015;41(5):1161–1179. [DOI] [PubMed] [Google Scholar]
- 14.Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013;33(8):1138–1147. [DOI] [PubMed] [Google Scholar]
- 15.Dhyani M, Gee MS, Misdraji J, Israel EJ, Shah U, Samir AE. Feasibility study for assessing liver fibrosis in paediatric and adolescent patients using real-time shear wave elastography. J Med Imaging Radiat Oncol 2015;59(6):687–694; quiz 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135(1):32–40. [DOI] [PubMed] [Google Scholar]
- 17.Bohte AE, de Niet A, Jansen L, et al. Non-invasive evaluation of liver fibrosis: a comparison of ultrasound-based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur Radiol 2014;24(3):638–648. [DOI] [PubMed] [Google Scholar]
- 18.Yap WW, Kirke R, Yoshida EM, Owen D, Harris AC. Non-invasive assessment of liver fibrosis using ARFI with pathological correlation: a prospective study. Ann Hepatol 2013;12(4):608–615. [PubMed] [Google Scholar]
- 19.Yoon JH, Lee JM, Woo HS, et al. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol 2013;14(2):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med 2013;34(3):260–265. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Song P, Urban MW, et al. Bias observed in time-of-flight shear wave speed measurements using radiation force of a focused ultrasound beam. Ultrasound Med Biol 2011;37(11):1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potthoff A, Attia D, Pischke S, et al. Influence of different frequencies and insertion depths on the diagnostic accuracy of liver elastography by acoustic radiation force impulse imaging (ARFI). Eur J Radiol 2013;82(8):1207–1212. [DOI] [PubMed] [Google Scholar]
- 23.Fontanilla T, Cañas T, Macia A, et al. Normal values of liver shear wave velocity in healthy children assessed by acoustic radiation force impulse imaging using a convex probe and a linear probe. Ultrasound Med Biol 2014;40(3):470–477. [DOI] [PubMed] [Google Scholar]
- 24.Bota S, Sporea I, Sirli R, et al. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of acoustic radiation force impulse (ARFI) elastography: analysis of a cohort of 1,031 subjects. Eur J Radiol 2014;83(2):268–272. [DOI] [PubMed] [Google Scholar]
- 25.Carucci LR. Imaging obese patients: problems and solutions. Abdom Imaging 2013;38(4):630–646. [DOI] [PubMed] [Google Scholar]
- 26.Gurnani M, Birken C, Hamilton J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am 2015;62(4):821–840. [DOI] [PubMed] [Google Scholar]
- 27.de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int 2010;30(7):1043–1048. [DOI] [PubMed] [Google Scholar]
- 28.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992;55(2 Suppl):615S–619S. [DOI] [PubMed] [Google Scholar]
- 29.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47(4):598–607. [DOI] [PubMed] [Google Scholar]
- 30.Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51(3):828–835. [DOI] [PubMed] [Google Scholar]
- 31.Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57(3):1182–1191. [DOI] [PubMed] [Google Scholar]
- 32.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal 2001;5(4):237–254. [DOI] [PubMed] [Google Scholar]
- 33.Obuchowski NA. ROC analysis. AJR Am J Roentgenol 2005;184(2):364–372. [DOI] [PubMed] [Google Scholar]
- 34.Schuetz GM, Schlattmann P, Dewey M. Use of 3×2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ 2012;345:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon JH, Lee JM, Joo I, et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 2014;273(3):772–782. [DOI] [PubMed] [Google Scholar]
- 36.Mariappan YK, Venkatesh SK, Glaser K, McGee KP, Ehman RL. MR elastography of liver with iron overload: development, evaluation and preliminary clinical experience with improved spin echo and spin echo EPI sequences [abstr]. In: Proceedings of the Twenty-First Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2013; 278. [Google Scholar]
- 37.Ronot M, Lambert S, Elkrief L, et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 2014;24(6):1394–1402. [DOI] [PubMed] [Google Scholar]
- 38.Millonig G, Friedrich S, Adolf S, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol 2010;52(2):206–210. [DOI] [PubMed] [Google Scholar]
- 39.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and MR elastography for assessing liver fibrosis. II. Diagnostic performance, confounders, and future directions. AJR Am J Roentgenol 2015;205(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.