Abstract
Background
Myocardial tolerance to ischemia is influenced by age and preoperative cyanosis through unknown mechanisms and significantly affects postoperative outcomes. Cytochrome c oxidase (CcOx), the terminal enzyme of the mitochondrial electron transport chain, may play a role in the susceptibility to ischemic-reperfusion (IR) injury. Our study aimed at investigating changes in human myocardial CcOx activity based on age and preoperative oxygen saturation to understand its role in transition from neonatal to mature myocardium and hypoxic conditions.
Methods
The right atrial appendage from patients undergoing first time surgical repair/palliation of congenital heart defects was analyzed for steady state CcOx activity by oxidation of ferrocytochrome c via spectrophotometry and steady state CcOx subunit I protein content by protein immunoblotting. Student’s t-test compared CcOx activity and protein levels between patients with preoperative hypoxia and normoxia. Multiple linear regression analysis was used to assess the effects of age and preoperative arterial oxygen saturations (SaO2) on CcOx protein activity and protein content.
Results
Thirty-two patients with a median (interquartile range) age of 83 days (8–174) and preoperative oxygen saturation 98% (85–100%) were enrolled. Independent of age, preoperative SaO2 ≤90% was associated with significantly greater CcOx steady state activity (p=0.004). Additionally, older age itself was associated with increased CcOx steady state activity (p=0.022); the combination of preoperative SaO2 and age account for 33% of the variation in CcOx steady state activity (R2=0.332). There was no increase in the CcOx subunit I protein content with either age or preoperative hypoxia.
Conclusions
In patients with congenital heart disease, an increase in CcOx steady state activity is seen with increasing age. Hypoxia leads to upregulation of CcOx steady state activity without an increase in the amount of enzyme protein itself. Higher CcOx activity in older and cyanotic patients may indicate CcOx-dependent reactive oxygen species as the mechanism for IR injury.
Keywords: myocardial protection, ischemia reperfusion, cyanotic congenital heart disease, neonatal cardiac surgery, cytochrome oxidase
Introduction
Immature neonatal myocardium has greater tolerance to ischemia-reperfusion (IR) injury, which decreases with age to mature myocardium.1–3 Preoperative hypoxemia in cyanotic congenital heart disease also increases the susceptibility to IR injury and negatively impacts outcomes, as evidenced by higher perioperative morbidity, longer ventilation time, greater need for inotropic support, lower cardiac output and longer length of intensive care unit stay.2,4–6 Myocardial protective strategies against IR injury during cardiac surgery are not uniformly effective for neonatal hearts.3 Increased activity of the individual enzymes involved in oxidative phosphorylation may be an adaptive cellular mechanism to preserve organ function and minimize hypoxic injury in chronic hypoxia.7,8
Cytochrome c oxidase (CcOx), or complex IV, catalyzes the final step in the mitochondrial electron transfer chain and is regarded as one of the major regulation sites for oxidative phosphorylation. It is made up of 13 subunits, where ten structural subunits are encoded by nuclear deoxyribonucleic acid (DNA) and subunits I, II and III, which make up the catalytic center of the enzyme, are encoded by mitochondrial DNA. CcOx subunit I contains the heme a,a3 binuclear center and is the active site of the enzyme. CcOx activity is increased in conditions with hypoxia9 and plays an important role in the generation of reactive oxygen species, resulting in IR injury.10,11 However, its role in human myocardium and its relation to IR injury has not yet been demonstrated.
We hypothesized that CcOx activity and content is increased with age and preoperative hypoxia. The study aims to measure the preoperative cytochrome c oxidase activity and cytochrome c oxidase protein subunit I content in patients with congenital heart disease and identify its relationship to age and preoperative hypoxia.
Materials and Methods
After Institutional Review Board (IRB) approval and appropriate parental/patient consent, patients undergoing first-time surgical repair/palliation for congenital heart diseases via a median sternotomy between July 2011 and December 2013 were prospectively enrolled. All re-operative patients were excluded from the study.
The age of the patient and average preoperative arterial oxygen saturations (SaO2) (averaged value over 12 hours prior to surgery) were obtained from medical records. For patients having elective procedures requiring inpatient admission on the morning of the surgery, an average of the SaO2 measured at the preoperative visit (1 day prior to the surgery) and the 1st SaO2 measured in the operating room prior to administration of supplemental oxygen and anesthesia induction was used.
The average measured SaO2 rather than the morphology of the cardiac defect was used to define preoperative hypoxemia or cyanosis as this study aimed, specifically, to look at the response of myocardial mitochondrial CcOx to hypoxemia, irrespective of the morphological cardiac defect. While the patient’s anatomical defect influenced the presence of preoperative hypoxemia or cyanosis, there was significant cross-over of morphological cyanotic congenital heart disease patients to the acyanotic group due to pulmonary over-circulation and mixing physiology. An arbitrary cut-off of ≤90% was labeled as ‘Preoperative Hypoxia’ and all remaining patients were grouped as ‘Preoperative Normoxia’.
Intraoperatively, the tip of the right atrial appendage was excised during venous cannulation for cardiopulmonary bypass and immediately placed in phosphate buffered saline at 4° Celsius for rapid transport to the research laboratory for analysis.
Mitochondrial isolation
The myocardial tissue was minced before homogenizing in H medium (70 mmol/L sucrose, 220 mmol/L mannitol, 2.5 mmol/L Hepes [pH 7.4] and 2 mmol/L ethylenediamine tetra acetic acid).12 The mitochondria were isolated by means of differential centrifugation as previously described and mitochondrial protein concentration was determined by using the method of Lowry, using bovine serum albumin as standard.12,13 All steps were carried out at 4° Celsius.
CcOx steady-state kinetics/activity
CcOx kinetics was assayed by using the method of Smith, in which the rate of oxidation of ferrocytochrome c was measured by following the decrease in absorbance at 550 nm.12,13 Assays were executed in a 1-mL reaction volume containing 50 mmol/L PO4−2 (pH 7.0), 2% lauryl maltoside and 1µg of mitochondrial protein. Ferrocytochrome c was added at a concentration of 40 mmol/L to initiate the reaction. Reaction rates were measured using a Cary-1E spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Specific activity was calculated from the mean values of 3 to 4 measurements, using 21.1 mM−1·cm−1 as the extinction coefficient of ferrocytochrome c at 550 nm.
Protein immunoblotting for CcOx subunit I protein content
Samples (10 µg) of mitochondrial protein were subjected to sodium dodecylsulfate–acrylamide gel electrophoresis and immunoblotting, as previously described.9 Blots were labeled with a primary polyclonal antibody to mouse CcOx subunit I (Molecular Probes, Eugene, OR, USA) and secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The signal was detected with enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and density was measured by means of scanning densitometry. The mean protein density measured in the normoxemic group was normalized to the protein content of 1 unit and the relative protein content of each sample calculated accordingly.
Statistical analysis
Student’s t-test was used to measure the differences between CcOx steady state activity and CcOx subunit I protein content in patients with preoperative hypoxia (SaO2 ≤90%, n=11) vs. preoperative normoxia (n=21). Pearson correlation (r) and coefficient of determination (R2) were used to examine the linear association between changes in CcOx activity and CcOx subunit I protein content. Multiple linear regression analysis was used to assess the independent effects of age in days on the Log10 scale and preoperative SaO2 on CcOx activity and CcOx subunit I protein content. IBM SPSS version 21.0 was used for statistical analysis (IBM, Armonk, NY, USA) with two-tailed p<0.05 considered to be statistically significant.
Results
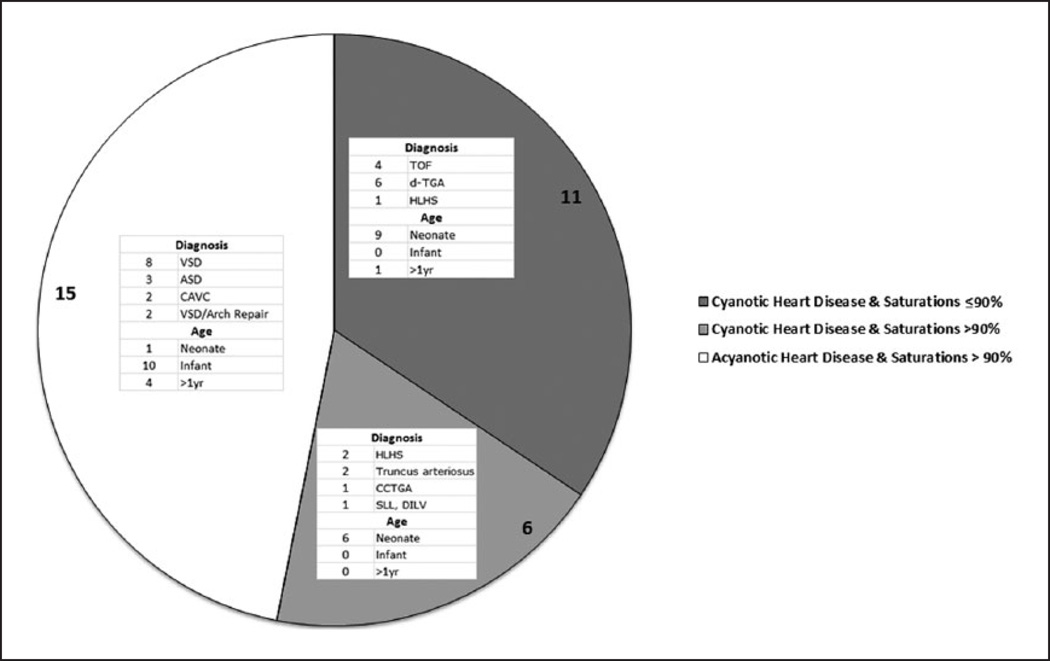
Thirty two (N=32) patients met the enrollment criteria and were included in the study. The details of the anatomical diagnoses of the patients are provided in Table 1 and Figure 1. The median age of the patients was 83 days (IQR 8–174 days). The group was comprised of 17 males (53%) and 15 females (47%). Eleven (11) patients had oxygen saturations (SaO2) ≤90% and comprised the ‘Preoperative Hypoxia’ group and the remaining twenty-one (21) patients comprised the ‘Preoperative Normoxia’ group. Eleven patients with anatomical cyanotic heart defects had oxygen saturations of ≤90%, concordant with their anatomical diagnosis. Six patients with anatomical disease defined as cyanotic heart defects physiologically had clinical pictures consisted with congestive heart failure, radiological evidence of pulmonary plethora and arterial oxygen saturations >90% and crossed over into the physiologically normoxemic group. All patients with acyanotic heart defects had oxygen saturations >90%.
Table 1.
Study Population.
| Primary Diagnosis | Patients |
|---|---|
| ASD | 3 |
| ASD, VSD, PDA | 1 |
| CAVC | 2 |
| CCTGA | 1 |
| d-TGA | 6 |
| HLHS | 3 |
| Complex Single Ventricle | 1 |
| TOF | 4 |
| Truncus arteriosus | 2 |
| VSD | 7 |
| Ebstein’s Anomaly | 1 |
| VSD, PS | 1 |
| Patients with preoperative hypoxia (oxygen saturation ≤90%) |
11 (34%) |
| Male Gender | 17 (53%) |
| Age (days), Median (interquartile range) | 83 (8–174) |
ASD: atrial septal defect; CAVC: complete atrioventricular canal defect; CCTGA: congenitally corrected transposition of great arteries; d-TGA: transposition of great arteries; HLHS: hypoplastic left heart syndrome; PDA: patent ductus arteriosus; PS: pulmonary stenosis; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
Figure 1.
Distribution of patients into ‘Preoperative Hypoxia’ vs. ‘Preoperative Normoxia’ groups by anatomical diagnosis and age. Six patients with anatomical disease defined as cyanotic heart defects crossed over to the ‘Normoxic Group’ for oxygen saturations >90%.
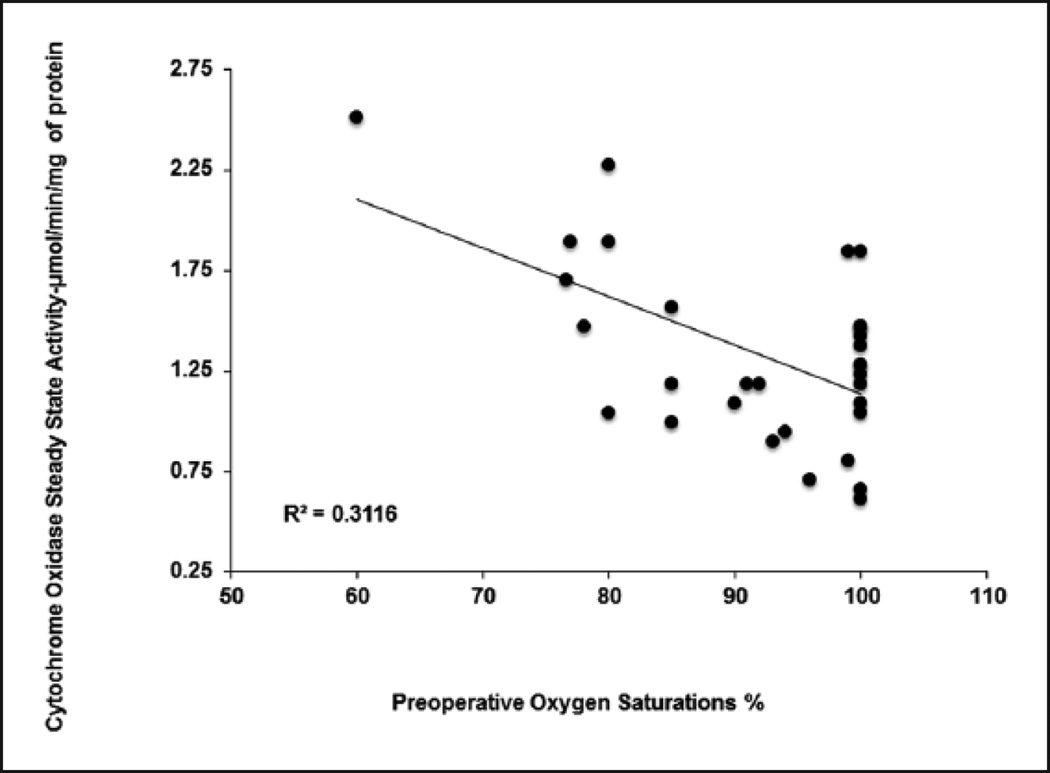
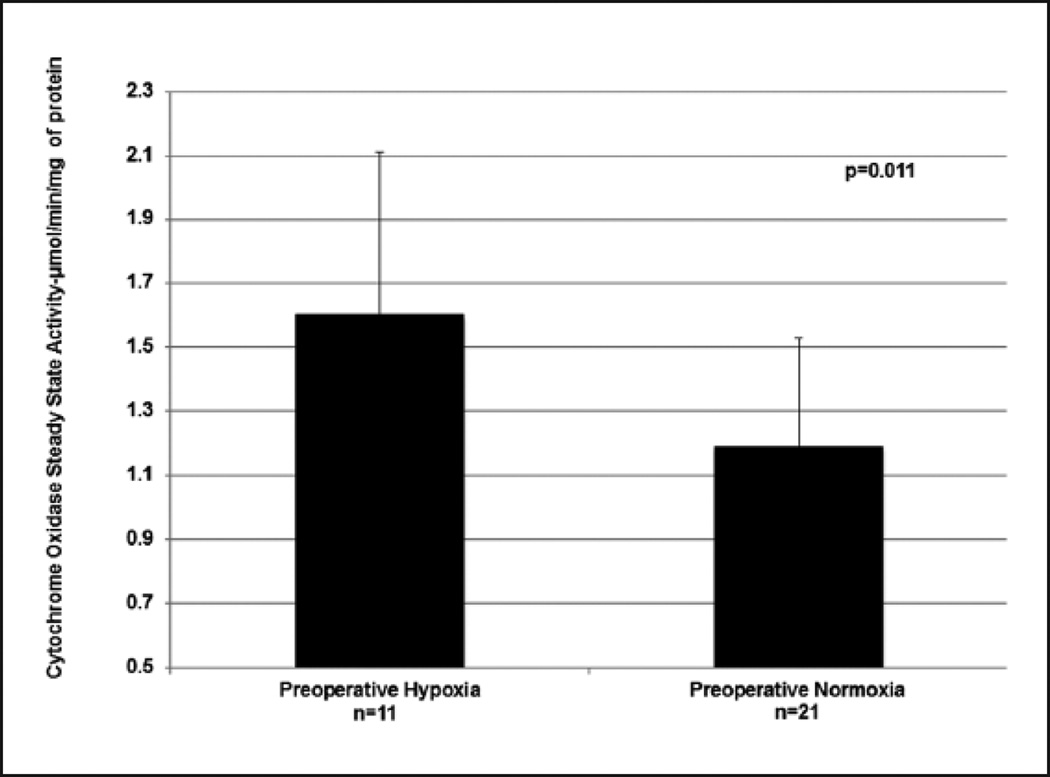
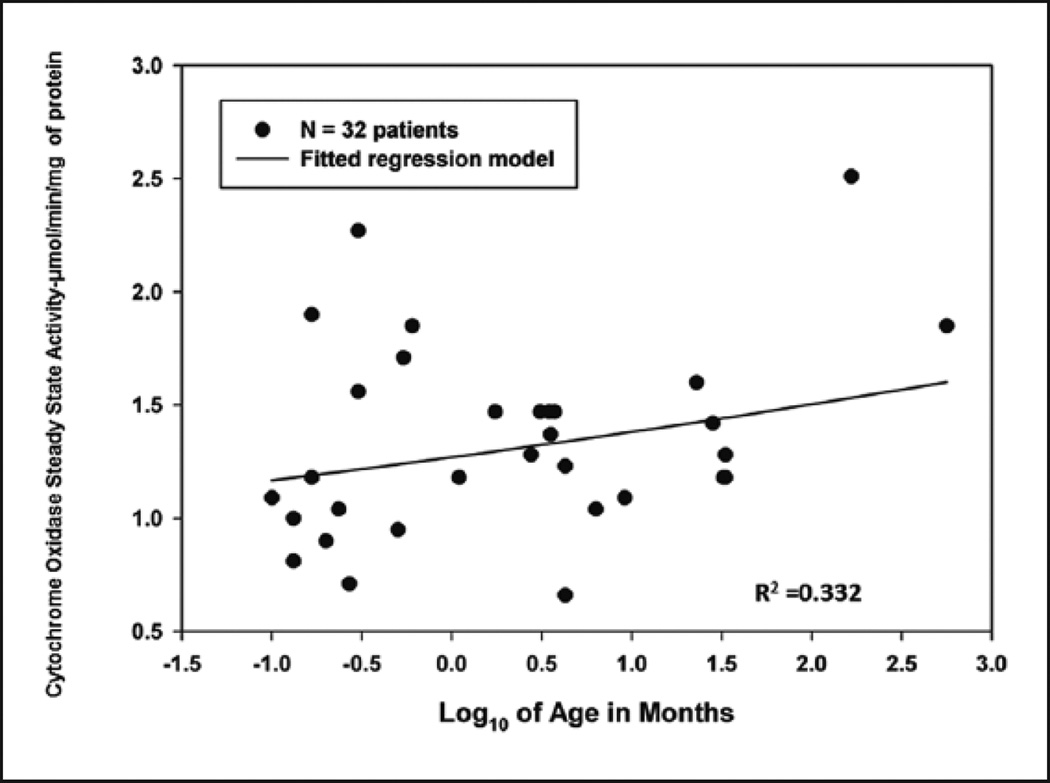
Mitochondrial CcOx steady state activity inversely correlated with the patient’s baseline arterial oxygen saturation (Figure 2) (R2=0.3116), with increased enzyme activity noted in patients with baseline preoperative hypoxia (mean ± SD, 1.60 ± 0.51 µmol/min/mg of protein) compared to patients with preoperative normoxia (mean ± SD, 1.19 ± 0.34 µmol/min/mg of protein) (p=0.011) (Figure 3) (Table 2). There was also a significantly greater CcOx steady state activity observed in the atrial myocardial tissue with increased age (Figure 4). The combination of preoperative SaO2 and age provides an R2 of 0.332 as evaluated by multiple linear regression, accounting for approximately 33% of the variation in CcOx activity.
Figure 2.
Effect of preoperative hypoxia on cytochrome c oxidase steady state activity; stratified by ‘Preoperative Hypoxia’ vs. ‘Preoperative Normoxia’ groups.
Figure 3.
Cytochrome c oxidase steady state activity is increased in the‘Preoperative Hypoxia’ group compared to the ‘Preoperative Normoxia’ group.
Table 2.
Enzyme specific activity and subunit I protein level with preoperative oxygenation.
| Preoperative Hypoxia (n=11) | Preoperative Normoxia (n=21) | p-value | |
|---|---|---|---|
|
Cytochrome c oxidase steady state specific activity (µmol/min/mg of protein) |
1.60 ± 0.51 | 1.19 ± 0.34 | 0.011* |
|
Cytochrome oxidase steady subunit 1 protein content |
1.05 ± 0.13 | 1.00 ± 0.11 | 0.271 |
Values presented as mean ± SD.
Statistically significant difference between Preoperative Hypoxia vs. Preoperative Normoxia.
Figure 4.
Effect of age on cytochrome c oxidase steady state activity. Age in months is plotted on the Log10 scale and has a positive relationship with CcOx activity. Age along with preoperative SaO2 as evaluated by multiple linear regression provides a moderate R2 value of 0.332, accounting for 33% of the variation in CcOx activity.
Multiple linear regression analysis revealed significantly greater CcOx steady state activity in the atrial myocardial tissue for patients with preoperative hypoxia (p=0.004) and increasing age (p=0.022). (Table 3).
Table 3.
Multiple linear regression analysis (association between cytochrome c oxidase activity and content with age and preoperative hypoxia).
| Increasing Age | Preoperative Hypoxia | |
|---|---|---|
|
Cytochrome c oxidase activity |
p=0.022* | p=0.004* |
|
Cytochrome c oxidase content |
p=0.90 | p=0.27 |
Statistically significant independent predictors of CcOx activity, R2=0.332 for age and 0.3116 for preoperative hypoxia.
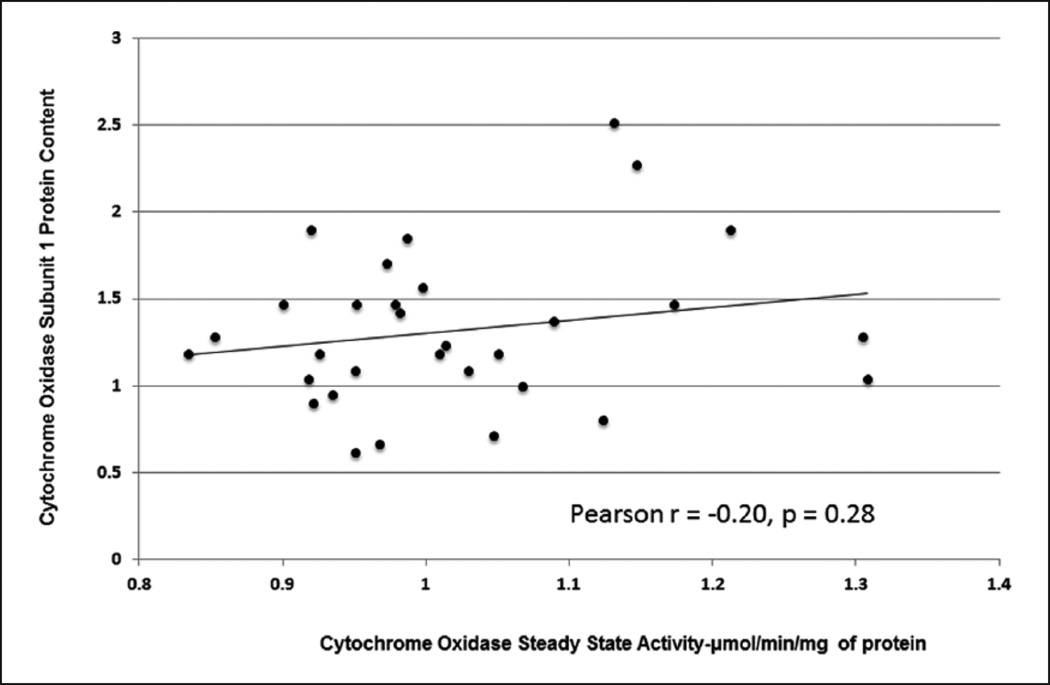
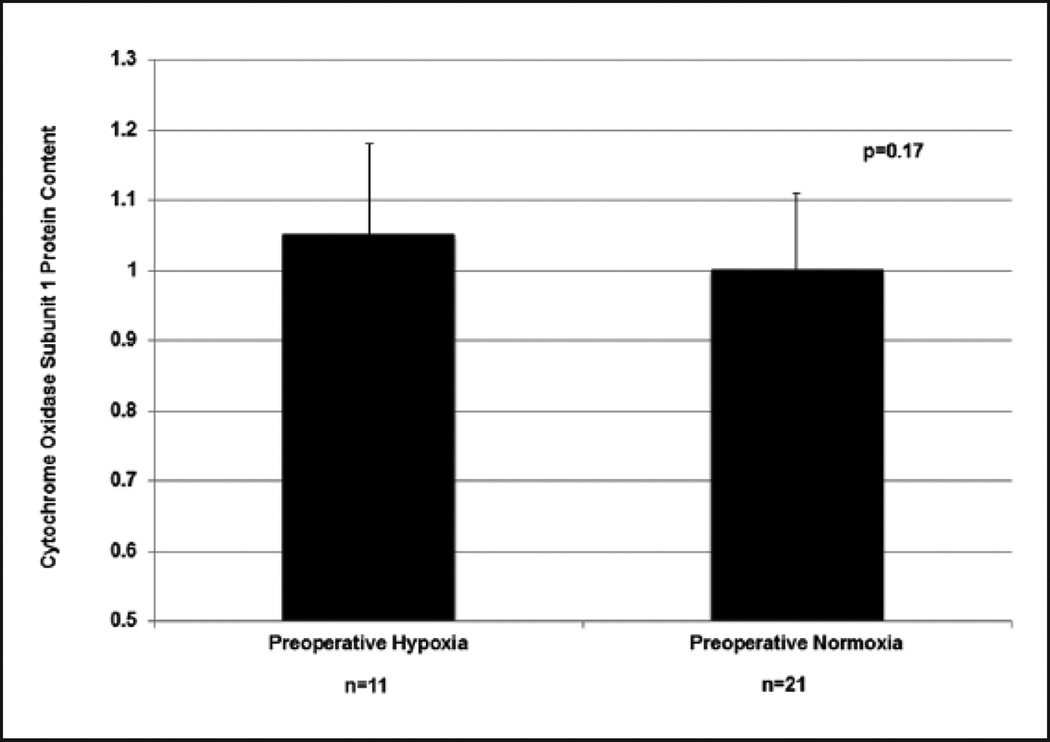
The increase in CcOx steady state activity, however, was not associated with an increase in steady state CcOx subunit I protein content. (Pearson r=−0.20, p=0.28) (Figure 5). CcOx subunit I content (mean±SD) was comparable in patients with preoperative hypoxia (n=11, 1.05±0.131) and those without preoperative hypoxia (n=21, 1.00±0.111) (p=0.27) (Figure 6) (Table 2).
Figure 5.
Lack of correlation between cytochrome c oxidase activity and cytochrome c oxidase subunit I protein content.
Figure 6.
Effect of preoperative hypoxia on cytochrome c oxidase subunit I protein content, ‘Preoperative Hypoxia’ vs. ‘Preoperative Normoxia’ groups.
Multiple linear regression revealed that neither age nor preoperative hypoxia was independently associated with any change in CcOx subunit I protein content (p=0.90 for age, p=0.27 for preoperative hypoxia or normoxia) (Table 3).
Comment
While age and preoperative cyanosis are well known determinants of myocardial tolerance to global ischemia in patients with congenital heart disease (CHD),2,5 the exact mechanisms for this phenomenon are poorly understood.
Cytochrome c oxidase (CcOx) is the terminal rate-limiting enzyme of the mitochondrial respiratory chain, which reacts directly with molecular oxygen and its activity has been shown to be increased in models of chronic hypoxia.9 This increase in CcOx steady state activity/kinetics in the setting of chronically decreased tissue oxygen tension might be an adaptive mechanism to preserve function and minimize hypoxic injury;9 however, upon reperfusion, it may be counterproductive and lead to reperfusion injury by the generation of reactive oxygen species.10,11 While adult myocardial protection studies show a clear advantage with the use of blood cardioplegia, studies in pediatric patients, especially neonates, have shown comparable3 or even superior results with crystalloid cardioplegia.14 Reperfusion injury may be responsible for these findings and needs to be further investigated.
Our study shows significantly lower CcOx steady state activity in younger patients, which increases with age. An increase in CcOx steady state activity was also seen with preoperative hypoxia. This is the first study in humans to demonstrate an increase in CcOx kinetics in a clinical patient population that is known to have greater vulnerability to ischemia reperfusion injury. Finding lower CcOx steady state activity in the ischemia-resistant younger myocardium and a rise in enzyme activity seen with age and hypoxia/cyanosis parallels the clinical findings of higher susceptibility to IR injury in older patients and those with cyanotic heart disease. While the exact mechanism remains to be defined, this finding suggests the potential role of changes in CcOx function in this clinical scenario with regard to reactive oxygen species generation and subsequent myocardial IR injury.
While an increase in CcOx steady state activity was observed, this was not associated with an increase in total enzyme protein as measured by CcOx subunit I protein content. This is in contrast to chronic hypoxia animal model studies9 where CcOx subunit I content paralleled the CcOx steady state activity with hypoxia. While these findings indicate a yet unstudied mechanism for CcOx increase in humans and highlight interspecies physiological differences, perhaps a longer duration of hypoxemic state than the natural history of cyanotic congenital heart disease in the newborn would allow is required for transcriptional or translational changes that lead to enzyme production and an increase in the enzyme protein content.
The focus of myocardial preservation in congenital cardiac surgery, so far, has been on the ischemia period, with measures such as hypothermia and myocardial protection strategies with various cardioplegia compositions and additives and the varying use of hot shots before aortic cross-clamp removal and reperfusion.15 Reperfusion strategies after oxygen deprivation have been shown to be particularly beneficial in patients after cardiac arrest.16–19 Alteration of the reperfusion of the ischemic myocardium after the removal of the aortic cross-clamp has not been pursued as a strategy yet. This largely unstudied phase of cardiopulmonary bypass could hold important answers to further reducing myocardial IR injury by simple alteration in reperfusion strategy to counter the increase in activity of the enzymes involved in aerobic energy production, such as CcOx.
Our study has the limitation of being a prospective, non-randomized series of patients of heterogeneous diagnoses referred for surgery. While we were able to demonstrate the changes with age, the study also was underpowered to detect the exact transition time from neonatal to mature myocardium. As this is a study of clinical patients who underwent standard cardiac surgical treatment, tissue collection was limited to the tip of the right atrial appendage, which is routinely excised for venous cannulation in this patient population. As significant variation in the myocardial mitochondrial CcOx activity may be seen between tissues of various chambers from the same heart,13 this may not be an accurate representation of the ventricular myocardial CcOx activity and metabolism. Our study was limited to patients with congenital heart defects who required surgical intervention. Therefore, our findings may reflect disease-specific changes and may not be representative of the normal population.
Conclusion
In patients with congenital heart disease, an increase in CcOx steady state activity is seen with increasing age. Hypoxia leads to the upregulation of CcOx steady state activity without an increase in the amount of enzyme protein itself. Higher CcOx activity in older and cyanotic patients may indicate CcOx-dependent reactive oxygen species as a mechanism for IR injury. Further studies to delineate the exact role of CcOx in myocardial IR injury are required.
Units of Measurement
Cytochrome c oxidase steady state kinetics/activity: micromoles/minute/milligram (µmol/min/mg) of protein
Cytochrome c oxidase subunit I protein content: all values calculated relative to the mean normoxic density value, set as 1 unit of protein.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by National Institute of Health/National Institute of General Medical Sciences Grant NIH R01GM103842-01 (RJL), NIH P30 ES009089 (RJL).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jonas RA. Myocardial protection for neonates and infants. Thorac Cardiov Surg. 1998;46:288–291. doi: 10.1055/s-2007-1013087. [DOI] [PubMed] [Google Scholar]
- 2.Imura H, Caputo M, Parry A, Pawade A, Angelini GD, Suleiman MS. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation. 2001;103:1551–1556. doi: 10.1161/01.cir.103.11.1551. [DOI] [PubMed] [Google Scholar]
- 3.Sinha P, Zurakowski D, Jonas RA. Comparison of two cardioplegia solutions using thermodilution cardiac output in neonates and infants. Ann Thorac Surg. 2008;86:1613–1619. doi: 10.1016/j.athoracsur.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Merante F, Mickle DA, Weisel RD, et al. Myocardial aerobic metabolism is impaired in a cell culture model of cyanotic heart disease. Am J Physiol. 1998;275:H1673–H1681. doi: 10.1152/ajpheart.1998.275.5.H1673. [DOI] [PubMed] [Google Scholar]
- 5.Choi YH, Cowan DB, Wahlers TC, Hetzer R, Del Nido PJ, Stamm C. Calcium sensitisation impairs diastolic relaxation in post-ischaemic myocardium: implications for the use of Ca(2+) sensitising inotropes after cardiac surgery. Eur J Cardio-Thorac. 2010;37:376–383. doi: 10.1016/j.ejcts.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modi P, Suleiman MS, Reeves B, et al. Myocardial metabolic changes during pediatric cardiac surgery: a randomized study of 3 cardioplegic techniques. J Thorac Cardiovasc Surg. 2004;128:67–75. doi: 10.1016/j.jtcvs.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 7.Bitar FF, el Sabban M, Bitar H, et al. Lack of apoptosis in the hypoxic brain of a rat model mimicking cyanotic heart disease. Brain Injury. 2002;16:891–900. doi: 10.1080/02699050210147194. [DOI] [PubMed] [Google Scholar]
- 8.Dernevik L, Bylund-Fellenius AC, Ekroth R, Holm J, Idstrom JP, Schersten T. Enzymatic activities in heart and skeletal muscle of children with cyanotic and non-cyanotic congenital heart disease. Thorac Cardiov Surg. 1988;36:310–312. doi: 10.1055/s-2007-1022971. [DOI] [PubMed] [Google Scholar]
- 9.Piel DA, Khan AR, Waibel R, et al. Chronic hypoxemia increases myocardial cytochrome oxidase. J Thorac Cardiovasc Surg. 2005;130:1101–1106. doi: 10.1016/j.jtcvs.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radical Bio Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Misiak M, Beyer C, Arnold S. Cytochrome c oxidase isoform iv-2 is involved in 3-nitropropionic acid-induced toxicity in striatal astrocytes. Glia. 2009;57:1480–1491. doi: 10.1002/glia.20864. [DOI] [PubMed] [Google Scholar]
- 12.Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome c oxidase in sepsis. Shock. 2004;21:110–114. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi K, Cao-Danh H, Kawai A, et al. Prolonged preservation of the blood-perfused canine heart with glycolysis-promoting solution. Ann Thorac Surg. 1999;68:903–907. doi: 10.1016/s0003-4975(99)00534-2. [DOI] [PubMed] [Google Scholar]
- 14.Giordano R, Arcieri L, Cantinotti M, et al. Custodiol solution and cold blood cardioplegia in arterial switch operation: retrospective analysis in a single center. Thorac Cardiovasc Surg. 2016;64:53–58. doi: 10.1055/s-0035-1566235. [DOI] [PubMed] [Google Scholar]
- 15.del Nido PJ. Myocardial protection and cardiopulmonary bypass in neonates and infants. Ann Thorac Surg. 1997;64:878–879. doi: 10.1016/s0003-4975(97)00696-6. [DOI] [PubMed] [Google Scholar]
- 16.Friehs I, Cao-Danh H, Takahashi S, et al. Adenosine prevents protein kinase c activation during hypothermic ischemia. Circulation. 1997;96:II-221–II-225. discussion II-225–226. [PubMed] [Google Scholar]
- 17.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra-Corpor Technol. 2012;44:98–103. [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkado A, Cao-Danh H, Sommers KE, del Nido PJ. Buffering capacity of histidine in cardioplegia analyzed by myocardial production of lactate and alanine. Kyobu Geka (Japanese) 1993;46:1021–1024. [PubMed] [Google Scholar]
- 19.Lin JJ, Hsia SH, Wang HS, Chiang MC, Lin KL. Therapeutic hypothermia associated with increased survival after resuscitation in children. Pediatr Neurol. 2013;48:285–290. doi: 10.1016/j.pediatrneurol.2012.12.021. [DOI] [PubMed] [Google Scholar]