Abstract
Background
The goal of this study was to detect the predictors of chronic pain at 6 months after thoracic surgery from a comprehensive evaluation of demographic, psychosocial, and surgical factors.
Methods
Thoracic surgery patients were enrolled 1 week before surgery and followed-up 6 months post-surgery in this prospective, observational study. Comprehensive psychosocial measurements were assessed before surgery. The presence and severity of pain was assessed at 3 and 6 months after surgery. One-hundred seven patients were assessed during the first 3 days after surgery and 99 (30 thoracotomy and 69 video-assisted thoracoscopic surgery, thoracoscopy) patients completed the 6 months follow-up. Patients with vs without chronic pain related to thoracic surgery at 6 months were compared.
Results
Both incidence (p = 0.37) and severity (p = 0.97) of surgery-related chronic pain at 6 months were similar after thoracotomy (33%, 95% confidence interval [CI]: 17% to 53%, 3.3 ± 2.1) and thoracoscopy (25%, 95% CI: 15% to 36%, 3.3 ± 1.7). Both frequentist and Bayesian multivariate models revealed that severity of acute pain (numerical rating scale, 0–10) is the measure associated with chronic pain related to thoracic surgery. Psychosocial factors and quantitative sensory testing were not predictive.
Conclusions
There was no difference in the incidence and severity of chronic pain at 6 months in patients undergoing thoracotomy versus thoracoscopy. Unlike other post-surgical pain conditions, none of the pre-operative psychosocial measurements were associated with chronic pain after thoracic surgery.
Keywords: chronic pain, thoracic surgery, thoracotomy, VATS, predictors, multivariate model
INTRODUCTION
Chronic pain is the most common symptom for which patients seek medical care (26% in the US),1,2 and surgery is the cause of chronic pain for 22.5% of these patients.3 The International Association for the Study of Pain (IASP) defines “chronic post-surgical pain” as pain persisting at least 3 months after surgery.4
Despite advances in medical care, meta-analyses of prospective studies on chronic pain at 3 months (17 studies, 1439 patients) and 6 months (15 studies, 1354 patients) after thoracotomy demonstrate that the incidence of chronic pain at 3 and 6 months after thoracotomy were 57% (95% confidence interval [CI]: 51 to 64%) and 47% (95% CI: 39 to 56%), respectively.5 A few studies evaluated results for chronic pain after video-assisted thoracic surgery (VATS, thoracoscopy);5,6 however, the data were not sufficient to summarize.
The goal of this study was to detect the predictors of chronic pain at 6 months after thoracic surgery, both from thoracotomy and VATS, from a comprehensive evaluation of demographic, psychosocial, and surgical factors. Our hypothesis was that the chronic pain related to thoracic surgery is not only related to surgery- and anesthesia-related factors, but also associated with psychosocial measures assessed before the surgery. The primary outcome of the current study was the presence of chronic pain (yes/no) at 6 months after thoracic surgery. This is the primary analysis of this prospective, observational study.
MATERIALS AND METHODS
Study Design and Subjects
This prospective, observational study was approved by the University of Iowa Institutional Review Board (IRB #201202796, Iowa City, IA). Thoracic surgery patients were recruited from the University of Iowa Hospitals and Clinics (UIHC) and the Iowa City Veterans Affairs Medical Center (VAMC), both located in Iowa City, IA. The Iowa City VAMC thoracic surgery clinic was staffed by the UIHC thoracic surgery clinic, and patient care was similar in these 2 hospitals. Patients were approached for consent during their pre-operative visit approximately 1 week prior to their thoracic surgery.
To be eligible, patients must have spoken English, been 18 to 80 years old, and been scheduled for thoracic surgery. The surgical procedures included thoracotomy, pneumonectomy, removal of lung other than bilobectomy, pneumonectomy, completion pneumonectomy, sleeve lobectomy, segmentectomy, single- or multiple-wedge resection, or thoracoscopy with excision-plication of bullae, with lobectomy, with partial or total pulmonary decortication or with wedge resection of lung. Patients with limitations of self-expression or visual dysfunction, or having emergency surgery, a severe psychiatric illness, or having chronic pain problems in the chest area for longer than 2 months prior to the thoracic surgery, were excluded. Pregnant women and prisoners were also excluded.
Pre-operative Psychosocial Assessments
Following informed consent, the research nurse asked patients to complete 5 computer-adaptive PROMIS7 questionnaires for 1) anxiety (PROMIS Bank v1.0 - anxiety), 2) depression (PROMIS Bank v1.0 - depression), 3) physical role function (PROMIS Bank v1.0 – physical function), 4) fatigue (PROMIS Bank v1.0 - fatigue), and 5) sleep disturbance (PROMIS Bank v1.0 – sleep disturbance). These computer-adaptive questionnaires contain a large collection of items measuring one characteristic; each subsequent question is chosen depending on the patient’s responses to previous queries, thus limiting the number of questions to approximately 6 per questionnaire. The results of the PROMIS questionnaires are presented as standardized T-scores with a mean of 50 and a standard deviation (SD) of 10. Therefore, a patient with a physical function T-score of 40 is 1 SD below the U.S. general population mean.8 Patients then completed 3 additional computer questionnaires examining 1) post-traumatic stress disorder (PCL-C9), 2) catastrophizing (Pain Catastrophizing Scale, PCS 10), and 3) psychological acceptance (AAQ-II.)11
Pre-operative Pain Assessments
During the pre-operative visit, patients were asked to rate their current pain at rest using the numerical rating scale (NRS, 0–10) based on the following question: “Please rate your current pain at rest by indicating the number that best describes your pain where 0 means no pain and 10 is the worst imaginable pain.” Next, we asked patients to cough strongly 2 times and rate pain with coughing using the NRS (0–10) by asking “Please rate your current pain during coughing by indicating the number that best describes your pain where 0 means no pain and 10 is the worst imaginable pain.” In addition, they were asked to rate their average expected post-operative pain severity for each of their first 3 post-operative days (NRS, 0–10) (“Please rate your expected average pain on day 1 (2 or 3) after surgery”). Severities of pain are reported as overall NRS when it includes all patients’ assessments, even if pain severities were 0 (zero).
Quantitative Sensory Testing
Pre-operative pain threshold to cold was measured by the TSA—II (Thermal Sensory Analyzer, Medoc Inc., Israel) via 30 × 30 mm contact thermode. During this procedure, a probe starting from 30 °C (86 °F) was applied to the forearm and the temperature was decreased by 1 °C/s (1.8°F/s) increments until the patient experiences pain. The patient was asked to click a button when the nonpainful cold sensation changes to a painful cold sensation. At this time, the temperature of the probe returned to 30°C (86°F). If the patient did not stop the test, the lowest temperature reached would be 10 °C (50 °F), and at this time the temperature rapidly returns to 30 °C (86 °F). This process was repeated 3 times, and the average of 3 measurements (°C) was presented as the pain threshold to cold.
Pain magnitude to suprathreshold cold was estimated in terms of a NRS (0–10). This time, the thermal testing device cooled to 10 °C (50 °F) and stayed at that temperature for 15 seconds. At the end of the 15 seconds, patients were asked to rate their pain in terms of the NRS (0–10). This process was repeated 3 times, and the average of 3 measurements (NRS) was presented as the pain magnitude to suprathreshold cold.
Anesthesia and Surgery
The type of surgery was determined by surgeons based on their usual practice. Surgeons and anesthesiologists performed their usual intraoperative care. The usual postoperative pain management for patients undergoing VATS was patient-controlled analgesia using hydromorphone or morphine. Generally, when patients tolerated oral nutrition, oral opioid pain medications were initiated. For open thoracotomy, unless contraindicated, a thoracic epidural catheter was placed at approximately T5–T6, and 0.05% or 0.10% bupivacaine was infused at 8–14 ml/hour as tolerated, based on blood pressure and pain scores.12 Patient controlled analgesia was utilized for rescue in patients receiving epidural local analgesia. If converted from VATS to open thoracotomy, a patient was given patient-controlled analgesia and offered thoracic epidural analgesia in the recovery room or on postoperative day 1. Postoperative opioid analgesic use during the first 24 hours was recorded in oral morphine equivalents.
Electronic Medical Records
Age, American Society of Anesthesiologists physical status (general medical condition), pre-existing medical conditions, pre-operative opioid usage, pre-operative radiation or chemotherapy within 6 weeks of the surgery, duration and type of surgery, post-operative analgesic use and post-operative radiation or chemotherapy within 6 months after surgery were obtained from the electronic medical records.
First 3 Post-operative Days
Research assistants visited patients during the first 3 days after surgery to collect the severity of average pain during the previous 24 hours (NRS, 0–10) (“Please rate your pain by indicating the number that best describes your pain on average in the last 24 hour”) and the number of chest tubes present for each day. The chest tube management was at the discretion of the surgical team and it was counted present if the patient had a chest tube at 6:00 AM that day. When patients were discharged over the weekend, the data collection form to measure the severity of average pain during the previous 24 hours and the number of chest tubes present for each day for the first 3 post-operative days was sent with the patient with a stamped return envelope.
Follow-up Assessment
At 3 and 6 months after thoracic surgery, patients were mailed 3 questionnaires. The intensity of their average pain during the previous week (NRS, 0–10) was assessed based on the following question: “Please rate your thoracic surgery pain only by indicating the number that best describes your pain on average in the last one week using the numerical rating scale (0–10, 0: no pain, 10: worst possible pain).” Forms for physical functioning and chronic pain acceptance (CPAQ13) were also sent. In addition, the presence of pain from thoracic surgery and extent of pain limiting daily activities were identified by telephone interview with the following questions: 1) “Do you currently have pain related to your thoracic surgery?” and 2) “Does the pain limit your daily activities?” (yes/no).
Statistical Analyses
The primary outcome variable and the statistical analysis plan were defined prior to data collection. The primary outcome variable of the study is chronic pain related to thoracic surgery at 6 months after surgery, based on the following question: “Do you currently have pain related to your thoracic surgery?” Those patients with chronic pain related to thoracic surgery at 6 months after surgery were compared to those patients without such pain at that time.
The two-sided 95% confidence interval (CI) for the incidence of chronic pain related to thoracic surgery at 6 months after surgery was calculated according to Clopper and Pearson.14
The normality of the continuous data was statistically tested by the Shapiro-Wilk test and by examining the quantile-quantile plot. Normally distributed continuous variables were presented as mean ± SD and univariately compared using a two-sample t test. When the distribution is not normal, median along with first (Q25) and third quartiles (Q75) were presented and the groups were compared using a Wilcoxon rank sum test. Categorical data was presented as frequency and percentage, and were statistically tested using the chi-square test or the Fisher’s exact test, where appropriate. Those subjects with any response to the phone interview at 6 months were included in the analyses. Missing data points were not imputed.
Analyses of repeated measures were performed with mixed effects models with unstructured covariance structures.
Univariate and multivariate statistical analyses are performed with both traditional frequentist and Bayesian analyses. In Bayesian analyses, unknown parameters are random variables and therefore prior probability distributions should be defined. The Bayesian model combines the prior distribution with data and produces a posterior distribution. Inferences are made from the posterior distribution. Prior distributions used for the overall mean, and the coefficients for the fixed effect terms such as severity of acute pain and presence of pre-operative pain at rest were assumed Normal, as usual for these types of analyses, and were not very informative (see Appendix 1, Supplemental Digital Content 1).
Those covariates with a univariate p-value of less than 0.20 were examined in the frequentist multivariate model. The corresponding Bayesian analogue for the p-values of 0.20 is to examine whether the 80% two-sided posterior credible interval of the slope term excludes zero. This enables us to work with a smaller subset of the covariates that are most likely to be significant in the multivariate model. The covariates considered for the frequentist and Bayesian multivariate models were: age at surgery, pre-operative pain at rest (or pre-operative pain with coughing), pre-operative opioid usage, average expected pain severity, any chest tube on day 3 after surgery, severity of acute post-operative pain during the first 3 days after surgery, standardized sleep disturbance score and the pain catastrophizing scale total score.
A model with all potential covariates can be written as follows:
Where μ is the intercept in the logit scale and β1 to β8 are coefficients to adjust for the potential model covariates that were introduced before. Note that, since pre-operative pain with coughing and pre-operative pain at rest are correlated (weighted kappa = 0.47, p < 0.0001), we separately examined these 2 variables in the model.
Those covariates with multivariate p-values of less than 0.01 were included in the final multivariate models. Backward model selection techniques were applied to find the most parsimonious model. Models were compared with a stepwise approach based on their Akaike’s Information Criterion (AIC15) for frequentist models and Deviance Information Criterion (DIC16) for Bayesian models. Relative risks and associated 99% confidence and credible intervals were provided for key covariates of chronic pain. Relative risk estimates and associated confidence intervals were calculated using the modified Poisson regression approach17–19 for the frequentist multivariate model and using the log-binomial model20 for the Bayesian multivariate model. Sensitivity to prior distributions was examined to test the robustness of the model for Bayesian models.
The goodness-of-fit of the final multivariate logistic regression model was evaluated by the Hosmer-Lemeshow test,21 where a p-value of less than 0.05 indicates lack of fit. The area under the receiver operating characteristics curve (c-statistic) for the multivariate logistic regression model was provided.
The sample size of the study was assessed based on the logistical challenges, instead of a priori power calculations, in this study. The main goal of the study was to collect preliminary data for a future larger study. We aimed to have a complete data from 100 patients in this prospective longitudinal study.
Frequentist analyses were performed by using SAS 9.4 software (SAS Inst., Cary, NC). Plots were created using SigmaPlot version 12.5 (Systat Software, San Jose, CA) and R version 3.2.5 (The R Foundation, Austria).22 Bayesian analysis were performed in WinBUGS 1.4.3 software.23 WinBUGS uses Markov chain Monte Carlo methods. To represent the extreme regions of the parameter space, three parallel chains of equal lengths with disperse initial values were used in WinBUGS analyses. Convergence was judged by Brooks, Gelman, Rubin diagnostics plots,24 density and history plots and autocorrelations. Bayesian results were based on 5,000 iterations after a burn-in period of 5,000 iterations in each chain.
RESULTS
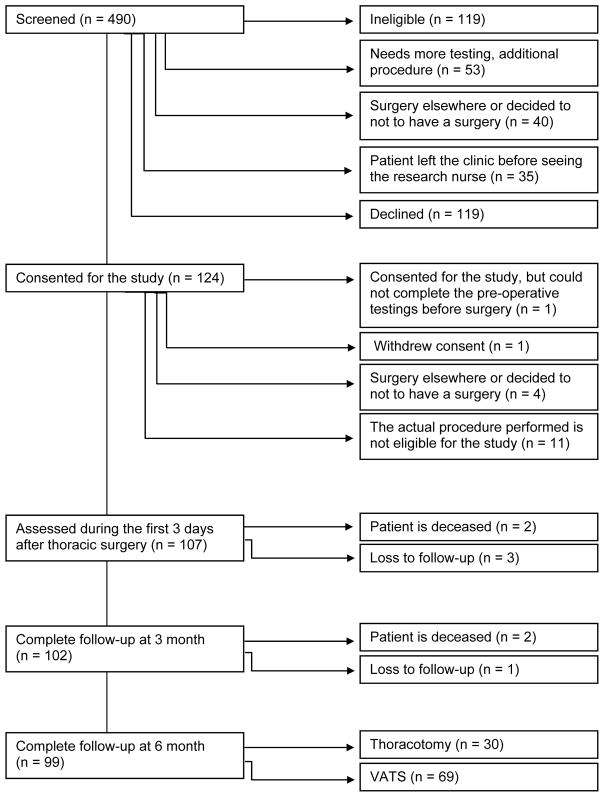
Four-hundred ninety patients were screened between March 8, 2013 and December 21, 2015. From these, 124 patients were consented and 107 patients were assessed during the first 3 days after surgery. One hundred two patients completed the follow-up interviews at 3 months and 99 patients completed the follow-up interviews at 6 months (Figure 1). From these, 30 patients had thoracotomy (19 scheduled for thoracotomy and 11 converted from VATS) and 69 patients had VATS. Two surgeons (KP and JK) performed 74% of the operations. Data from 99 patients with 6 month follow-up are presented.
Figure 1.
Flowchart of patients.
VATS: video-assisted thoracic surgery
Acute and Chronic Pain — Thoracotomy vs VATS
The overall NRS during the acute postoperative period for all patients is plotted from pre-operative period to the third post-operative day in Figure 2 for both thoracotomy vs VATS patients. There were no differences between the average NRS preoperatively and for the 3 days after surgery in thoracotomy patients compared to patients undergoing VATS.
Figure 2.
Numerical rating scale (NRS, 0–10) for patients during the 6 months of the study separately for thoracotomy vs VATS patients. Note that those patients with zero pain scores were included in this plot. When analyzed longitudinally, the time effect was significant (p<0.0001). Neither surgery (p = 0.13), nor the interaction (p=0.53) effects were significant.
One-sided error bars represent standard deviation.
POD: post-operative day, pre-op: pre-operative, VATS: video-assisted thoracic surgery.
Incidences of chronic pain at 3 and 6 months after thoracotomy were 47% (14/30, 95% CI: 28% to 66%) and 33% (10/30, 95% CI: 17% to 53%), respectively. The incidences of chronic pain after VATS at 3 and 6 months were 29% (21/72, 95% CI: 19% to 41%) and 25% (17/69, 95% CI: 15% to 36%), respectively. The incidences of chronic pain both at 3 (p = 0.09) and 6 months (p = 0.37) were not different for thoracotomy and VATS patients (Figure 3).
Figure 3.

Incidences of chronic pain related to thoracotomy and VATS ant 3 and 6 months after surgery.
VATS: video-assisted thoracic surgery
When the average NRS is calculated for those patients with chronic pain related to thoracic surgery, the severities of pain at 3 and 6 months after thoracotomy were 3.9 ± 2.3 (n = 9) and 3.3 ± 2.1 (n = 9), respectively. Similarly, for VATS patients, the NRS for those patients with chronic pain related to thoracic surgery at 3 and 6 months were 2.8 ± 2.2 (n = 17) and 3.3 ± 1.7 (n = 16), respectively. For those patients with chronic pain related to thoracic surgery, differences between thoracotomy and VATS patients at 3 (p = 0.18) and 6 (p = 0.93) months were not statistically significant. Severities of pain at 3 and 6 months after surgery are also presented as mild (NRS, 0–2), moderate (3–5) and severe (>5) in Table 1.
Table 1.
3 and 6 month follow-up for pain assessments
| Pain at 6 months (n = 27 ) | No Pain at 6 months (n = 72 ) | p-value | ||
|---|---|---|---|---|
|
| ||||
| Severity of pain at 3 months | Severity of pain at 3 m | 3.0 (2.0, 5.0) (n = 26) | 0.0 (0.0, 2.3) (n = 68) | 0.0002 |
| Thoracotomy | 3.0 (3.0, 6.0) (n = 9) | 1.5 (0.0, 3.0) (n = 18) | ||
| VATS | 2.0 (2.0, 5.0) (n = 17) | 0.0 (0.0, 1.5) (n = 50) | ||
|
| ||||
| Thoracotomy | 0.39≠ | |||
| Mild (0–2) | 3 (18.8%) | 13 (81.3) | ||
| Moderate (3–5) | 4 (23.4%) | 7 (63.6%) | ||
|
| ||||
| VATS | 0.07≠ | |||
| Mild (0–2) | 9 (17.7%) | 42 (82.4%) | ||
| Moderate (3–5) | 6 (42.9%) | 8 (57.1%) | ||
|
| ||||
| Severity of pain at 6 months | Severity of pain at 6 m | 4.0 (2.0, 4.5) (n = 25 ) | 0.0 (0.0, 1.0) (n = 66 ) | |
| Thoracotomy | 4.0 (1.0, 4.0) (n = 9) | 0.0 (0.0, 1.0) (n = 18) | ||
| VATS | 3.0 (2.0, 4.8) (n = 16) | 0.0 (0.0, 1.0) (n = 48) | ||
|
| ||||
| Thoracotomy | ||||
| Mild (0–2) | 4 (18.2%) | 18 (81.8%) | 0.016≠ | |
| Moderate (3–5) | 5 (71.4%) | 2 (28.6%) | ||
|
| ||||
| VATS | ||||
| Mild (0–2) | 7 (13.7%) | 44 (86.3%) | 0.003≠ | |
| Moderate (3–5) | 8 (53.3%) | 7 (46.7%) | ||
There was no patient with severe pain (NRS >5) at 3 or 6 months after thoracic surgery.
Data is presented as median (first and third quartiles), and Wilcoxon rank sum test p-value or frequency (%), and Fisher’s exact test (≠) p-value was provided.
m: month, VATS: video-assisted thoracic surgery
Because there were no differences between thoracotomy and VATS patients for both incidences of chronic pain and the average NRS at 3 and 6 months after thoracic surgery, we present results by combining thoracotomy and VATS patients for the remainder of the paper.
Chronic Pain Related to Thoracic Surgery, Thoracotomy and VATS Combined
Altogether, the incidence of chronic pain at 3 months after either type of thoracic surgery was 34% (35/102, 95% CI: 25% to 44%) and the severity of pain among those patients with chronic pain related to thoracic surgery at 3 months was 3.3 ± 2.4 (n = 35). Pain limited the daily activities of 16% (16/102) of patients at 3 months after surgery. For the 6-month assessment, the incidence was 27% (27/99, 95% CI: 19% to 37%), and the severity of pain among those patients with chronic pain related to thoracic surgery was 3.3 ± 1.8. Pain limited the daily activities of 8.2% (8/98) of the patients.
The severity of acute and chronic pain (NRS) is plotted for those patients with and without thoracic surgery–related chronic pain at 6 months after surgery in Figure 4. Those patients with chronic pain related to thoracic surgery at 6 months consistently reported higher NRS pre-operatively, for 3 days postoperatively, and at 3 and 6 months (all p < 0.05). When analyzed longitudinally, both the time effect (p < 0.0001) and the group effect (chronic thoracic surgery pain at 6 months) (p < 0.0001) were significant, the interaction (p=0.62) term was not.
Figure 4.
Severity of pain for those patients with and without chronic pain related to thoracic surgery at 6 months after surgery. Note that those patients with zero pain scores were included in this plot. When analyzed longitudinally, both the time effect (p<0.0001) and the presence of pain at 6 months (p<0.0001) were significant, but not the not the interaction (p= 0.62) term. Note that, one patient (¥) was sedated during the first 3 days. Some (5 patients at 3 months, and 8 patients at 6 months) patients completed phone interviews at 3 and 6 months, but did not return the mail including the NRS.
One-sided error bars represent standard deviation.
NRS: numerical rating scale, POD: post-operative day, pre-op: pre-operative, *: p≤ 0.05, ***: p≤0.001.
At 6 months after surgery, the NRS of patients without chronic pain related to thoracic surgery was 0.5 (95% CI: 0.05 to 0.98) units higher compared to pre-operative pain at rest (p = 0.03). On the other hand, for those patients with chronic pain related to thoracic surgery, the NRS at 6 months after surgery was 2.3 units (95% CI: 1.4 to 3.2) higher compared to pre-operative pain at rest (p < 0.0001). Therefore, statistically, neither group returned to baseline pain levels at 6 months after their surgery.
Note that, because of the exclusion criteria, preoperatively, no patients had chronic pain in their chest region, but many had a non-zero NRS (Figure 5). Similarly, some patients without chronic pain related to thoracic surgery at 6 months still reported a non-zero NRS at that time. Even if we specifically asked for the “presence of thoracic surgery–related pain” for the primary outcome variable, the NRS may reflect other symptoms or be affected by other conditions.
Figure 5.
Severity of pre-operative pain at rest (NRS, 0–10) for those patients with (red triangles) vs without (black circles) chronic pain related to thoracic surgery at 6 months.
NRS: numerical rating scale
Pre-operative Evaluations
Pre-operative evaluations comparing patients with and without chronic pain related to thoracic surgery at 6 months are presented in Table 2. Based on the pre-operative evaluations, those patients who report higher pre-operative pain scores at rest (p = 0.025, Figure 5) and pain upon coughing (p = 0.014, see Figure 1, Supplemental Digital Content 2), as well as those patients who expect to have higher severity of acute pain after the surgery (p = 0.026), tend to have higher likelihood of reporting chronic pain related to thoracic surgery at 6 months. Other demographics, quantitative sensory testing measurements, or pre-operative radiation or chemotherapy evaluated during the pre-operative visit were not associated with the presence of chronic pain at 6 months after surgery.
Table 2.
Pre-operative evaluation, approximately 1 week before surgery.
| Pain at 6 months (n = 27) | No Pain at 6 months (n = 72) | p-value | |
|---|---|---|---|
|
| |||
| Age at surgery (mean ± SD) | 58 ± 10 | 62 ± 11 | 0.13 |
|
| |||
| Male | 16 (31.4%) | 35 (68.6%) | 0.34 |
| Female | 11 (22.9%) | 37 (77.1%) | |
|
| |||
| ASA PS | |||
| 2 | 8 (25.0%) | 24 (75.0%) | |
| 3 | 18 (31.0%) | 40 (69.0%) | 0.58 |
| 4 | 1 (14.3%) | 6 (85.7%) | |
|
| |||
| History of tobacco use | |||
| Never smoker | 6 (21.4%) | 22 (78.6%) | |
| Former | 14 (28.0%) | 36 (72.0%) | 0.64 |
| Current | 7 (33.3%) | 14 (66.7%) | |
|
| |||
| History of alcohol use | |||
| <2 drinks a day | 24 (28%) | 61 (72%) | 0.75≠ |
| ≥2 drinks a day | 3 (21%) | 11 (79%) | |
|
| |||
| QST Cold degree 0C | 0 (0, 2.9) | 0 (0, 3.2) | 0.66 |
|
| |||
| QST supra NRS (0–10) | 0.3 (0, 3.3) | 1.0 (0, 3.6) | 0.49 |
|
| |||
| Pre-operative pain at rest (NRS, 0–10) | 1.00 ± 1.57 | 0.40 ± 1.00 | 0.025 |
| Median (Q25, Q75) [min, max] (see Fig 5) | 0 (0, 2) [0, 6] | 0 (0, 0) [0, 5] | |
|
| |||
| Pre-operative pain at rest >0 | |||
| Yes | 11 (44.0%) | 14 (56.0%) | 0.03 |
| No | 16 (21.6%) | 58 (78.4%) | |
|
| |||
| Pre-operative pain with cough (NRS, 0–10) | 0.9 ± 1.4 | 0.4 ± 1.0 | 0.014 |
| Median (Q25, Q75) [min, max] | 0 (0, 2) [0, 4] | 0 (0, 0) [0, 6] | |
|
| |||
| Pre-operative pain with coughing >0 | |||
| Yes | 11 (45.8%) | 13 (54.2%) | 0.019 |
| No | 16 (21.3%) | 59 (78.7%) | |
|
| |||
| Expected pain severity (NRS, 0–10) | |||
| Day 1 | 8.0 (5.0, 9.0) | 6.5 (5.0, 8.0) | 0.031 |
| Day 2 | 6.0 (4.5, 8.0) | 5.0 (3.0, 6.0) | 0.058 |
| Day 3 | 5.0 (3.0, 7.0) | 3.0 (2.0, 5.0) | 0.02 |
| Average | 6.0 (4.0, 7.0) | 4.7 (3.2, 6.2) | 0.026 |
|
| |||
| Pre-operative chemotherapy within 6 weeks of the surgery | |||
| Yes | 2 (40%) | 3 (60%) | 0.61≠ |
| No | 25 (27%) | 69 (73%) | |
|
| |||
| Pre-operative radiation therapy within 6 weeks of the surgery | |||
| Yes | 2 (100%) | 0 (0%) | 0.07≠ |
| No | 25 (26%) | 72 (74%) | |
|
| |||
| Opioid user at the time of last pre-operative clinic visit | |||
| Yes | 5 (45%) | 6 (55%) | 0.17≠ |
| No | 22 (25%) | 66 (75%) | |
Data is presented as median (first and third quartiles), and Wilcoxon rank sum test p-value or frequency (%), and chi-square test or Fisher’s exact test (≠) p-value was provided.
ASA PS: American Society of Anesthesiologists physical status, min: minimum, max: maximum, NRS: numerical rating scale, Q25: 25th percentile, Q75: 75th percentile, QST: quantitative sensory testing, SD: standard deviation.
Expected vs Observed Post-operative Pain
Scatterplots, Pearson correlation coefficients, and the associated p-values between the expected and observed pain scores (NRS) for each post-operative day for those patients with vs without chronic pain related to thoracic surgery at 6 months are presented in Figure 2, Supplemental Digital Content 2. Correlation coefficients among those patients having chronic pain related to thoracic surgery at 6 months were all ≤0.21 and were all non-significant (p ≥ 0.3). On the other hand, correlation coefficients among those patients not having chronic pain related to thoracic surgery at 6 months were all ≥0.4 and were significant (p ≤ 0.001). The differences between observed and expected acute pain scores for those patients with chronic pain related to thoracic surgery at 6 months were not different (p = 0.70).
Early Post-operative Evaluations
Assessments measured during the day of surgery and the first 3 days after the surgery as well as the post-operative radiation and chemotherapy within 6 months of surgery are presented in Table 3. Most of the patients had lobectomy (49%) or wedge resection (43%). Note that, 83% (25/30) of thoracotomy patients had epidural analgesia compared to 12% (8/69) of VATS patients. In addition to higher severity of average acute pain (p = 0.001), the presence of chest tube on the third post-surgical day (p = 0.02) was also univariately associated with the higher chance of developing chronic pain at 6 months.
Table 3.
Intra-operative and early post-operative evaluation (days 0 to 3 following surgery) and post-operative therapy.
| Variable | Pain at 6 months (n = 27) | No Pain at 6 months (n = 72) | p-value |
|---|---|---|---|
|
| |||
| Type of Surgery | |||
| Thoracotomy | 10 (33.3%) | 20 (66.7%) | 0.37 |
| VATS | 17 (24.6%) | 52 (75.4%) | |
|
| |||
| Procedure Type ¥ | |||
| Lobectomy | 18 (37%) | 31 (63%) | |
| Wedge Resection | 10 (23%) | 33 (77%) | |
| Pneumonectomy | 2 (50%) | 2 (50%) | |
| biopsy/resection of lung nodule(s) or infiltrate | 0 (0%) | 6 (100%) | |
| Other | 1 (25%) | 3 (75%) | |
|
| |||
| Number of Ports (VATS) | |||
| 0–2 | 2 (28.6%) | 5 (71.4%) | 1.00≠ |
| 3+ | 15 (24.2%) | 47 (75.8%) | |
|
| |||
| Incision size (for thoracotomy patients) (median, Q25, Q75) | 17.0 (13.0,18.0) | 16.0 (15.0, 23.5) | 0.94 |
|
| |||
| Duration of surgery | |||
| Thoracotomy | 152 (129, 191) | 140 (105, 203) | 0.86 |
| VATS | 148 (44, 198) | 64.0 (47, 121) | 0.11 |
|
| |||
| Oral morphine equivalent (Post-operative opioid use) | |||
| Thoracotomy | 103 (79, 354) | 80 (40, 161) | 0.30 |
| VATS | 161 (88, 201) | 87 (30, 143) | 0.090 |
|
| |||
| Epidural use (starting the day of surgery and at least 2 days after surgery) | |||
| Yes | 11 (33.3%) | 22(66.7%) | 0.33 |
| No | 16 (24.2%) | 50 (75.8%) | |
|
| |||
| Any chest tube on POD 1 | |||
| Yes | 26 (27%) | 70 (73%) | 1.00≠ |
| No | 1 (33.3%) | 2 (66.7%) | |
|
| |||
| Any chest tube on POD 2 | |||
| Yes | 19 (34.5%) | 36 (65.5%) | 0.069 |
| No | 8 (18.2%) | 36 (81.8%) | |
|
| |||
| Any chest tube on POD 3 | |||
| Yes | 14 (42.4%) | 19 (57.6%) | |
| No | 13 (19.7%) | 53 (80.3%) | 0.017 |
|
| |||
| Acute post-operative pain (median, Q25, Q75) | |||
| Day1 | 7.0 (5.0, 8.0) | 5.0 (3.0, 7.0) | 0.008 |
| Day2 | 6.0 (4.0, 8.0) | 3.0 (2.0, 6.0) | 0.0008 |
| Day3 | 4.5 (3.0, 6.0) | 2.5 (1.0, 6.0) | 0.009 |
| Average | 5.7 (4.3, 6.5) | 4.0 (2.7, 5.3) | 0.001 |
|
| |||
| Post-operative chemotherapy within 6 months of the surgery | |||
| Yes | 5 (23%) | 17 (77%) | 0.79 |
| No | 22 (29%) | 55 (71%) | |
|
| |||
| Post-operative radiation therapy within 6 months of the surgery | |||
| Yes | 3 (25%) | 9 (75%) | 1.0≠ |
| No | 24 (28%) | 63 (72%) | |
Data is presented as median (first and third quartiles), and Wilcoxon rank sum test p-value or frequency (%), and chi-square test or Fisher’s exact test (≠) p-value was provided unless indicated otherwise.
some patients had more than one type of procedure. Therefore, the sum of procedure types is greater than 99.
Q25: 25th percentile, Q75: 75th percentile, VATS: video-assisted thoracic surgery.
Pre-operative Psychosocial Measurements
Associations of pre-operative psychosocial measurements with the presence of chronic pain related to thoracic surgery at 6 months are presented in Table 4. Even if scales, such as pain catastrophizing, indicated some trend, when the type I error rate of 0.05 level is used, none of the pre-operative psychosocial evaluations indicated a difference for those patients with versus without chronic pain related to thoracic surgery at 6 months.
Table 4.
Pre-operative psychosocial assessments.
| Variable | Pain at 6 months (n = 27) | No Pain at 6 months (n = 72) | p-value |
|---|---|---|---|
|
| |||
| Anxiety T | 53.5 ± 9.2 | 52.7 ± 7.9 | 0.68 |
|
| |||
| Depression T | 49.5 ± 7.3 | 49.0 ± 7.5 | 0.78 |
|
| |||
| Fatigue T | 50.2 ± 8.5 | 48.7 ± 8.2 | 0.43 |
|
| |||
| Physical Function T | 45.1 ± 9.5 | 46.8 ± 8.6 | 0.43 |
|
| |||
| Sleep T | 51.8 ± 9.4 | 48.0 ± 9.4 | 0.10 |
|
| |||
| PCS total score | 23 (14, 29) | 17 (14, 22) | 0.15 |
|
| |||
| PCS total score | |||
| > 30 | 5 (33.3%) | 10 (66.7%) | 0.51 |
| ≤30 | 22 (26%) | 62 (74%) | |
|
| |||
| PCS rumination | 9 (4, 12) | 5 (4, 8) | 0.20 |
|
| |||
| PCS magnification | 4 (3, 6) | 4 (3, 5) | 0.24 |
|
| |||
| PCS helplessness | 8 (6, 10) | 7 (6, 10) | 0.099 |
|
| |||
| PTSD total score | 23 (19, 35) | 21 (19, 24) | 0.27 |
|
| |||
| AAQ total score | 10 (7, 16) | 10 (7, 13) | 0.74 |
The results of the PROMIS questionnaires are presented as standardized T-scores with a mean of 50 and a standard deviation (SD) of 10. Therefore, a patient with a physical function T-score of 40 is 1 SD below the U.S. general population mean.
Data is presented as median (first and third quartiles), and Wilcoxon rank sum test p-value or frequency (%), and chi-square test p-value was provided.
AAQ: acceptance and action questionnaire (higher scores indicate greater emotional distress), PCS: pain catastrophizing scale (lower score is better. For the normative dataset, the 75th percentile was 30), PTSD: post-traumatic stress disorder (lower score is better).
Psychosocial Measurements at 3 and 6 Month Follow-ups
As a part of the follow-up, at 3 and 6 months after surgery, patients were also asked to complete the short form PROMIS physical function and chronic pain acceptance questionnaires (CPAQ25), Table 5. Even if at the baseline both groups of patients reported similar standardized physical function scores (p = 0.43, Table 4), at 6 months after surgery, the standardized physical function scores for those patients with chronic pain related to thoracic surgery was 5.7 units (95% CI of the difference: 2.4 to 9.1) lower compared to those patients without such pain at that time (p = 0.007). However, within the chronic pain and no chronic pain related to thoracic surgery groups, the changes in physical function scores from baseline to 3 and 6 months were not statistically significant (p > 0.05 for each). For example, for those patients with thoracic surgery–related pain at 6 months, the reductions on standardized physical function scores from baseline to 6 months was 4.3 units (95% CI: −0.5 to 9.0).
Table 5.
3 and 6 month follow-up for psychosocial assessments
| Pain at 6 months (n = 27 ) | No Pain at 6 months (n = 72 ) | p-value | |
|---|---|---|---|
| CPAQ total score (3 months) (n = 58 ) | 71 (61, 85) | 76.5 (62.0, 87.5) | 0.51 |
| CPAQ activity (3 months) (n = 58) | 41.5 (36, 53) | 40.5 (33.5, 51) | 0.71 |
| CPAQ pain willing (3 months) (n = 61) | 29 (24, 36) | 36.5 (28, 42) | 0.079 |
| CPAQ total score (6 months) (n = 53) | 77 (61, 83) | 80 (65, 91) | 0.34 |
| CPAQ activity (6 months) (n = 57) | 47 (38, 55) | 47 (37, 51.5) | 0.46 |
| CPAQ pain willing (6 months) (n = 54) | 28 (23, 32) | 38 (27, 47) | 0.015 |
| T-score for physical function at 3 months | 42.6 ± 7.5 N=20 |
46.5 ± 9.7 N=66 |
0.0648 |
| T-score for physical function at 6 months | 41.3 ± 5.6 N=23 |
47.1 ± 9.4 N=62 |
0.0073 |
|
Pre-op physical Function to physical function at 3 months |
Mean diff= 4.5 95% CI: −1.1 to 10.1 |
Mean = 0.1 95% CI: −2.2 to 2.4 |
|
|
Pre-op physical Function to physical function at 6 months |
Mean = 4.3 95% CI: −0.5 to 9.0 |
Mean = −0.7 95% CI: −3.2 to 1.9 |
The results of the PROMIS questionnaires are presented as standardized T-scores with a mean of 50 and a standard deviation (SD) of 10. Therefore, a patient with a physical function T-score of 40 is 1 SD below the U.S. general population mean.
Normally distributed continuous variables were presented as mean ±SD and two-sample t-test p-value or median (first and third quartiles), and Wilcoxon rank sum test p-value was provided.
CPAQ: chronic pain acceptance questionnaire, pre-op: pre-operative.
Frequentist Multivariate Model
When those covariates with univariate p-values of less than 0.2 were included in the multivariate model and stepwise backward model selection procedures were applied, a reduced frequentist multivariate model was obtained (Table 6). According to the multivariate model, the only factor associated with the presence of chronic pain related to thoracic surgery at 6 months is the severity of acute post-operative pain during the first 3 days after surgery (NRS, 0–10).
Table 6.
Frequentist and Bayesian multivariate models for the presence of chronic pain at 6 months after thoracic surgery.
| Frequentist | Bayesian | |||
|---|---|---|---|---|
| Effect | Multivariate p-val | Multivariate Relative risk (99% confidence interval) | Posterior Mean (99% credible interval) | Relative risk (99% credible interval) |
| Average severity of acute pain during the first 3 days (NRS, 0–10) | 0.001 | 1.27 (1.12, 1.44) | 0.19 (0.04, 0.41) | 1. 22 (1.04, 1.50) |
The covariates considered for the frequentist and Bayesian multivariate models were: age at surgery, pre-operative pain at rest (or pre-operative pain with coughing), pre-operative opioid usage, average expected pain severity, any chest tube on day 3 after surgery, severity of acute post-operative pain during the first 3 days after surgery, standardized sleep disturbance score and the pain catastrophizing scale total score.
The final frequentist multivariate model using the modified Poisson regression approach:
Logit(probability of chronic pain) = −2.56 + 0.25 acute pain.
The final Bayesian multivariate model:
Logit(probability of chronic pain) = −2.33 + 0.19 acute pain.
NRS: numerical rating scale.
Based on the multivariate model, those patients with higher severity of acute pain during the first 3 days after surgery have higher likelihood of developing chronic pain related to thoracic surgery. Each point of increase on the severity of the acute post-operative pain (NRS, 0–10) score increased the chance of developing chronic pain related to thoracic surgery 1.3 times (99% CI: 1.1 to 1.4). The Hosmer-Lemeshow test indicated the model’s adequacy for the data (p = 0.24). The area under the curve (AUC, c-statistic) of the multivariate model is 0.73.
Bayesian Multivariate Model
When those covariates in which 80% two-sided posterior credible interval of the slope term excludes zero (age at surgery, pre-operative pain at rest (or pre-operative pain with coughing), pre-operative opioid usage, average expected pain severity, any chest tube on day 3 after surgery, severity of acute post-operative pain during the first 3 days after surgery, standardized sleep disturbance score and the pain catastrophizing scale total score) were included in the Bayesian logistic regression model and stepwise backward model selection procedures were applied, a reduced Bayesian multivariate model was obtained (Table 6). Parallel to the frequentist multivariate model, the Bayesian multivariate model also only included the severity of acute post-operative pain during the first 3 days after surgery (NRS, 0–10) as a significant covariate associated with the presence of chronic pain related to thoracic surgery at 6 months. Relative risk estimates from the Bayesian model were similar to frequentist estimates and are presented in last 2 columns of Table 6. Different prior distributions provided similar results (see Appendix 1, Supplemental Digital Content 1).
Type of Surgery
Even though there were not univariate differences between thoracotomy and VATS patients for developing chronic pain, to inspect the impact of surgery on outcome, the surgery effect was examined in the final Bayesian and frequentist multivariate models. There was no increase on the area under the curve, nor differences on the inferences from the multivariate models.
In addition, model selection was repeated within the open surgery and VATS groups separately. Within the VATS group (n = 69), the severity of acute post-operative pain during the first three days after surgery was the only predictor for both frequentist (p = 0.001) and Bayesian models (99% credible interval: 0.21 to 1.17).
Because of the small sample size (n = 30), none of the covariates were significant within the open thoracotomy group either with the frequentist or the Bayesian models.
DISCUSSION
This is the first prospective study to consider a comprehensive list of pre-operative, demographic, psychosocial and surgical variables for thoracic surgery patients. We surveyed patients through 6 months with a small (7.5%) loss to follow-up rate and almost no missing data. Our broad inclusion criteria allowed us to generalize our results to all thoracic surgery patients, including those patients converted from VATS to thoracotomy. There was no difference in the incidence and severity of chronic pain 6 months after thoracic surgery in patients undergoing VATS versus thoracotomy. Based on both the frequentist and the Bayesian multivariate models, the covariate associated with the chronic pain related to thoracic surgery was higher severity average acute pain during the first 3 days after surgery. Preoperative psychosocial factors were not associated with the development of chronic pain.
Thoracotomy vs VATS
Several studies propose that nerve injury is associated with chronic postsurgical pain;26 yet, both chronic neuropathic (20% to 30%) as well as non-neuropathic pain occur after thoracic surgery,27,28 suggesting that post-thoracic surgery pain is not simply due to direct nerve injury. VATS is considered less invasive than open thoracotomy, and would seem to be less likely to cause nerve injury. However, accumulating data indicate that VATS and thoracotomy have similar rates of chronic pain.28–33 Also, consistent with a previous study,32 during the course of 6 months follow-up, among those patients with chronic pain related to thoracic surgery, NRS scores for thoracotomy patients were not different compared to those of VATS patients.
Even though some patients reported that they do not have chronic pain related to thoracic surgery at 3 and 6 months, some did report a positive NRS at those times. NRS scores at 3 and 6 months may reflect other sources of pain that patients undergoing thoracic surgery experience.
Pre-operative Pain at Rest
The majority (75%) of the patients reported zero pain at the pre-operative assessment.
Pre-operative pain is identified as a risk factor to chronic pain after total knee replacement34 as well as other surgeries.35,36 In our study, we excluded those patients with pre-existing chronic pain in the chest area, and the indication for surgery does not usually contribute to preoperative pain. However, we do not have detailed information regarding other pre-existing pain conditions. Approximately 25% of the patients in our study had a preoperative NRS > 0. If those patients with pre-existing pain conditions such as back pain and fibromyalgia were excluded from the study, the generalizability of study results to the patient population would be diminished.
Acute Pain
Consistent with the thoracic surgery37,38 and other post-surgical chronic pain conditions,35,39,40 we report that a higher severity of acute pain is associated with a greater likelihood of developing chronic pain. Much chronic pain can be initiated by an inciting event like surgery, trauma, or infection and begins as acute pain. In general, studies show that reducing pain with various analgesic regimens has been successful in the acute postoperative period.41,42 When those patients were followed up to examine the long-term effect, incidences of chronic pain were usually not different.43–48 Regional techniques like epidural analgesia continue to have a role in reducing acute pain and acute postoperative morbidity. Although many assume that good treatment of acute pain will prevent chronic pain, this association with acute pain may be a marker for a patient prone to poor longer term outcomes that may not be prevented through better acute inpatient management. Reducing acute pain using either regional anesthesia or other techniques, and examining the incidence and severity of chronic pain after surgery remains as important areas of investigation.49,50
Pre-operative Psychosocial Assessments
In general, chronic pain after surgery is associated with pre-operative psychosocial factors.51–53 A previous study reported higher anxiety and/or depression scores among those patients with vs without chronic pain related to thoracic surgery.54 However, it is currently unknown if the psychosocial factors were different before the surgery, or if those patients with chronic pain after surgery developed anxiety or depression after surgery or after developing chronic pain. Measuring psychosocial factors before surgery enabled us to test this hypothesis in our study. Contrary to our expectations, and consistent with a recent observational study on VATS patients,6 none of the pre-operative psychosocial variables were significantly associated with the presence of chronic pain. Our negative results may be due to the small sample size in our study and the large number of associations tested. To examine the role of psychosocial factors, in future studies batteries of psychosocial factors could be assessed both before and after the surgery in larger samples. In addition, measures of neuropathic pain, mood and function can be added to longitudinally assess the impact of these factors on the pain outcome and impact of pain on the psychosocial factors.
Quantitative Sensory Testing
A few studies have examined quantitative sensory testing to predict chronic pain for thoracic surgeries. Wildgaard et al6 pre-operatively enrolled 47 patients and followed them up at 3 months after VATS. Pre-operative sensory thresholds to warmth, cool and heat pain on the thorax were not predictive of chronic pain. Yarnitsky38 et al. pre-operatively enrolled 62 patients and followed them up around 29 weeks after thoracotomy. During the pre-operative period, heat pain threshold, supra-threshold pain magnitude to heat and noxious inhibitory control (DNIC) were measured. They showed that pain threshold and supra-threshold pain scores were not associated with the chronic pain. Only DNIC and acute pain were predictors of chronic pain. Not finding cold pain threshold and supra-threshold pain magnitude to cold in our study is consistent with Yarnitsky et al’s results.38
Multivariate Model
Both frequentist and Bayesian multivariate models revealed that severity of average acute pain during the first 3 days after surgery (NRS, 0–10) is the only measure associated with the presence of chronic pain related to thoracic surgery at 6 months. Because we examined a large number of associations with a sample size of only 99 patients, we used a type I error rate of 0.01 instead of 0.05. Despite a small sample size in this study, the area under the curve for the final multivariate model discriminating between patients with and without chronic pain is 0.73. Larger, multicenter studies are needed to examine if the severity of acute pain remains as the only predictor associated with the presence of chronic pain related to thoracic surgery.
Observational Study
During the last 2 decades, the number of surgeons preferring less invasive VATS to open thoracotomy has increased.31 Recently, Bendixen et al31 completed a randomized, controlled trial for patients undergoing lobectomy for stage I lung cancer. They reported episodes of moderate to severe pain being more frequent after anterolateral thoracotomy compared to VATS at 52 weeks after surgery. They reported strict exclusion criteria that limited the eligible patient pool. Using strict exclusion criteria and randomizing only a subgroup of patients is remarkably difficult. On the other hand, with a prospective, observational study, all thoracic surgery patients can be included, which likely increases the generalizability of the study results.
Study Limitations
First, because of the observational nature of the study, numbers of patients in the thoracotomy (n = 30) and VATS (n = 69) groups are different. Type of surgery is based on the surgical preference. However, the type of surgery was not a significant factor affecting the incidence and severity of chronic pain. Second, since the choices of anesthetic and postoperative analgesic regimens utilized during the first few days after surgery are not likely to influence the development of chronic pain after thoracotomy,43–48 they were not rigorously standardized but were usual care. For example, the use of acetaminophen and nonsteroidal anti-inflammatory drugs was not standardized. Third, we do not have detailed information about other comorbid chronic pain problems that were present before surgery or developed during the 6-month follow-up. Fourth, we did not examine specifically for evidence of nerve injury during the 3- and 6-month follow-up assessments. Fifth, during the follow-up interview at 3 and 6 months after surgery, we asked patients if thoracic surgery related pain limits their daily activities. Pain limited daily activities of 16% and 8.2% of the patients at 3 and 6 months after surgery, respectively. We do not have detailed information using a validated questionnaire in a United States population33 about specific functions that were limited.
Conclusions
The incidence of chronic pain related to thoracic surgery at 6 months is 27%, and pain limits daily activities of 8.2% of the patients at that time. The incidence of chronic pain is similar for thoracotomy and VATS patients. Patients with higher severity of pain during the first 3 days after surgery have higher likelihood of developing chronic pain related to thoracic surgery at 6 months. Unlike other post-surgical pain conditions, none of the pre-operative psychosocial measurements were associated with the presence of chronic pain. Psychosocial factors may become evident after surgery or as chronic pain develops. Therefore, future studies should examine psychosocial factors not only pre-operatively, but also at other time points during the follow-up.
Supplementary Material
Acknowledgments
We appreciate the help of Alicia Manning, ADN (Department of Anesthesia, University of Iowa, Iowa City, IA, USA), Pam Jacobs, RN (Department of Anesthesia, University of Iowa, Iowa City, IA, USA) and Joan Ricks-McGillin, RN, BSN, (Department of Cardiothoracic Surgery, University of Iowa, Iowa City, IA, USA) for the patient enrollment. We also appreciate edits suggested by Mr. Paul Casella, MFA (Department of Internal Medicine, University of Iowa, Iowa City, IA, USA).
Funding Source: The study was supported by the grant number NS080110–01A1 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Bethesda, Maryland). Support was also provided by the Department of Anesthesia at the University of Iowa (Iowa City, IA, USA).
Footnotes
Conflicts of Interest: The authors declare no competing interests.
Contributor Information
Emine Ozgur Bayman, Departments of Anesthesia and Biostatistics, University of Iowa, Iowa City, IA, USA. Contribution: Conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content.
Kalpaj R. Parekh, Department of Cardiothoracic Surgery, University of Iowa, Iowa City, IA, USA. Contribution: Revising the article critically for important intellectual content and final approval of the version to be published.
John Keech, Department of Cardiothoracic Surgery, University of Iowa, Iowa City, IA, USA, Contribution: Revising the article critically for important intellectual content and final approval of the version to be published.
Atakan Selte, Medical student in Istanbul University, Cerrahpasa Medical Faculty, Istanbul, Turkey. Contribution: Data entry, retrieval and cleaning and final approval of the version to be published.
Timothy J. Brennan, Departments of Anesthesia and Pharmacology, University of Iowa, Iowa City, IA, USA. Contribution: Conception and design, interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published.
References
- 1.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain medicine (Malden, Mass) 2008;9:803–12. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics, Health United States; Services USDoHaH, editor. With Chartbook on Trends in the Health of Americans. 2006. Hyattsville, MD: 2006. p. 559. [PubMed] [Google Scholar]
- 3.Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76:167–71. [PubMed] [Google Scholar]
- 4.Harold Merskey NB. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2. Seattle, WA: IASP Press; 1994. Classification of Chronic Pain. [Google Scholar]
- 5.Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15:887–97. doi: 10.1016/j.jpain.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Kehlet H. Persistent postsurgical pain after video-assisted thoracic surgery--an observational study. Acta Anaesthesiol Scand. 2016;60:650–8. doi: 10.1111/aas.12681. [DOI] [PubMed] [Google Scholar]
- 7.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, Group PC. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 8.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–82. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. The Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- 10.Sullivan M, JL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;7:524–32. [Google Scholar]
- 11.Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Waltz T, Zettle RD. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behav Ther. 2011;42:676–88. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011;115:181–8. doi: 10.1097/ALN.0b013e318220847c. [DOI] [PubMed] [Google Scholar]
- 13.McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107:159–66. doi: 10.1016/j.pain.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 15.Akaike H. A New Look at the Statistical-Model Identification. Current Contents/Engineering Technology & Applied Sciences. 1981:22. [Google Scholar]
- 16.Gelman A, Carlin JB, Stern HS, Rubin D. Bayesian data analysis. 3. Chapman & Hall/CRC texts in statistical science; 2004. [Google Scholar]
- 17.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 18.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 19.Fang J. Statistics and Data Analysis. 2011. Using SAS® Procedures FREQ, GENMOD, LOGISTIC, and PHREG to Estimate Adjusted Relative Risks – A Case Study. [Google Scholar]
- 20.Torman VB, Camey SA. Bayesian models as a unified approach to estimate relative risk (or prevalence ratio) in binary and polytomous outcomes. Emerging themes in epidemiology. 2015;12:1–10. doi: 10.1186/s12982-015-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Goodness of Fit Tests for the Multiple Logistic Regression-Model. Commun Stat a-Theor. 1980;9:1043–69. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 23.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Stat Comput. 2000;10:325–37. [Google Scholar]
- 24.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–55. [Google Scholar]
- 25.McCracken LM, Spertus IL, Janeck AS, Sinclair D, Wetzel FT. Behavioral dimensions of adjustment in persons with chronic pain: pain-related anxiety and acceptance. Pain. 1999;80:283–9. doi: 10.1016/s0304-3959(98)00219-x. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti F, Vighetti S, Ricco C, Amanzio M, Bergamasco L, Casadio C, Cianci R, Giobbe R, Oliaro A, Bergamasco B, Maggi G. Neurophysiologic assessment of nerve impairment in posterolateral and muscle-sparing thoracotomy. J Thorac Cardiovasc Surg. 1998;115:841–7. doi: 10.1016/S0022-5223(98)70365-4. [DOI] [PubMed] [Google Scholar]
- 27.Guastella V, Mick G, Soriano C, Vallet L, Escande G, Dubray C, Eschalier A. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011;152:74–81. doi: 10.1016/j.pain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9:955–61. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Li WW, Lee TW, Lam SS, Ng CS, Sihoe AD, Wan IY, Yim AP. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest. 2002;122:584–9. doi: 10.1378/chest.122.2.584. [DOI] [PubMed] [Google Scholar]
- 30.Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg. 1997;12:82–7. doi: 10.1016/s1010-7940(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 31.Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. The Lancet Oncology. 2016;17:836–44. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 32.Rizk NP, Ghanie A, Hsu M, Bains MS, Downey RJ, Sarkaria IS, Finley DJ, Adusumilli PS, Huang J, Sima CS, Burkhalter JE, Park BJ, Rusch VW. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98:1160–6. doi: 10.1016/j.athoracsur.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayman EO, Lennertz R, Brennan T. Pain-related Limitations in Daily Activities Following Thoracic Surgery in a United States Population. Pain physician. 2017 [PubMed]
- 34.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114:551–61. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Guyatt GH, Kennedy SA, Romerosa B, Kwon HY, Kaushal A, Chang Y, Craigie S, de Almeida CP, Couban RJ, Parascandalo SR, Izhar Z, Reid S, Khan JS, McGillion M, Busse JW. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ. 2016;188:E352–E61. doi: 10.1503/cmaj.151276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins F, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 37.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–8. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Tasmuth T, Kataja M, Blomqvist C, von Smitten K, Kalso E. Treatment-related factors predisposing to chronic pain in patients with breast cancer--a multivariate approach. Acta Oncol. 1997;36:625–30. doi: 10.3109/02841869709001326. [DOI] [PubMed] [Google Scholar]
- 40.Callesen T, Bech K, Kehlet H. Prospective study of chronic pain after groin hernia repair. Br J Surg. 1999;86:1528–31. doi: 10.1046/j.1365-2168.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- 41.Behera BK, Puri GD, Ghai B. Patient-controlled epidural analgesia with fentanyl and bupivacaine provides better analgesia than intravenous morphine patient-controlled analgesia for early thoracotomy pain. J Postgrad Med. 2008;54:86–90. doi: 10.4103/0022-3859.40772. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee M, Goswami A, Gupta SD, Sarbapalli D, Pal R, Kar S. Analgesia in post-thoracotomy patients: Comparison between thoracic epidural and thoracic paravertebral blocks. Anesth Essays Res. 2010;4:75–80. doi: 10.4103/0259-1162.73511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochroch EA, Gottschalk A, Augostides J, Carson KA, Kent L, Malayaman N, Kaiser LR, Aukburg SJ. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology. 2002;97:1234–44. doi: 10.1097/00000542-200211000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009;36:170–80. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Grosen K, Drewes AM, Hojsgaard A, Pfeiffer-Jensen M, Hjortdal VE, Pilegaard HK. Perioperative gabapentin for the prevention of persistent pain after thoracotomy: a randomized controlled trial. Eur J Cardiothorac Surg. 2014;46:76–85. doi: 10.1093/ejcts/ezu032. [DOI] [PubMed] [Google Scholar]
- 46.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert review of neurotherapeutics. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 47.Yang MK, Cho CH, Kim YC. The effects of cryoanalgesia combined with thoracic epidural analgesia in patients undergoing thoracotomy. Anaesthesia. 2004;59:1073–7. doi: 10.1111/j.1365-2044.2004.03896.x. [DOI] [PubMed] [Google Scholar]
- 48.Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. European journal of pain (London, England) 2008;12:378–84. doi: 10.1016/j.ejpain.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111:711–20. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buvanendran A. Regional anesthesia and analgesia: prevention of chronic pain. Techniques in Regional Anesthesia and Pain Managemen. 2008;12:199–202. [Google Scholar]
- 51.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14:307–11. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masselin-Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J, Bouhassira D, Baudic S. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain. 2013;14:854–64. doi: 10.1016/j.jpain.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Katz J, Asmundson GJ, McRae K, Halket E. Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. European journal of pain (London, England) 2009;13:870–8. doi: 10.1016/j.ejpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Springer JS, Karlsson P, Madsen CS, Johnsen B, Finnerup NB, Jensen TS, Nikolajsen L. Functional and structural assessment of patients with and without persistent pain after thoracotomy. European journal of pain (London, England) 2017;21:238–49. doi: 10.1002/ejp.919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.