Abstract
BACKGROUND
Narcolepsy, a disorder of Rapid Eye Movement (REM) sleep, is characterized by excessive daytime sleepiness and cataplexy, a loss of muscle tone triggered by emotional stimulation. Current narcolepsy pharmacotherapeutics include controlled substances with abuse potential or drugs with undesirable side effects. Since partial agonists at trace amine-associated receptor 1 (TAAR1) promote wakefulness in mice and rats, we evaluated whether TAAR1 agonism had beneficial effects in two mouse models of narcolepsy.
METHODS
In the first experiment, male homozygous B6-Taar1tm1(NLSLacZ)Blt (Taar1 KO) and wildtype mice were surgically implanted to record EEG, EMG, locomotor activity (LMA) and body temperature (Tb) and the efficacy of the TAAR1 agonist, RO5256390, on sleep/wake and physiological parameters was determined. In the second experiment, the effects of the TAAR1 full agonist RO5256390 and partial agonist RO5263397 on sleep/wake, LMA, Tb and cataplexy were assessed in two mouse narcolepsy models.
RESULTS
RO5256390 profoundly reduced REM sleep in wildtype mice; these effects were eliminated in Taar1 KO mice. The TAAR1 partial agonist RO5263397 also promoted wakefulness and suppressed NREM sleep. Both compounds reduced Tb in the two narcolepsy models at the highest doses tested. Both TAAR1 compounds also mitigated cataplexy, the pathognomonic symptom of this disorder, in the mouse narcolepsy models. The therapeutic benefit was mediated through a reduction in the number of cataplexy episodes and time spent in cataplexy.
CONCLUSIONS
These results suggest TAAR1 agonism as a new therapeutic pathway for the treatment of this orphan disease. The common underlying mechanism may be the suppression of REM sleep.
Keywords: Cataplexy, sleep, hypocretin, orexin, mouse models, trace amines
INTRODUCTION
The sleep disorder narcolepsy afflicts 1 in 2000 individuals in the U.S. and is characterized by excessive daytime sleepiness (EDS), abnormalities of rapid-eye-movement (REM) sleep, and cataplexy, the sudden loss of muscle tone triggered by emotional stimulation (1). Specific degeneration of neurons that contain hypocretin (Hcrt, also known as orexin) is the likely cause of narcolepsy (2, 3). Cerebrospinal fluid Hcrt1 levels are reduced in narcoleptic patients relative to controls (4-6), presumably from the loss of Hcrt neurons (2, 3). A number of animal models of narcolepsy exist that are characterized by cataplexy, sleep/wake fragmentation and increased REM sleep propensity (7-10); some models exhibit both construct and face validity (11, 12)
Current treatments for narcolepsy involve symptomatic management of EDS and cataplexy. Amphetamines and other stimulants with abuse potential treat EDS through presynaptic stimulation of dopaminergic (DA) transmission (1, 13). Modafinil has become the first-line treatment for sleepiness because it has fewer side effects than amphetamine-like stimulants (14). The wake-promoting mechanism of action of modafinil may be through inhibition of the DA transporter (15), but could also be through enhancement of trace amine activity (16). Antidepressants, both tricyclic and selective monoaminergic reuptake inhibitors, alleviate cataplexy to the extent that they, or their metabolites, inhibit NA uptake (17, 18). The only approved drug that treats both cataplexy and EDS is γ-hydroxybutyrate (GHB) (19, 20).
Trace amine-associated receptor 1 (TAAR1) (21, 22) is a negative modulator of monoaminergic neurotransmission (23, 24) and may present a novel therapeutic pathway for the treatment of narcolepsy. Whereas Taar1 KO mice appear similar to wildtype (WT) littermates in most neurological and behavioral analyses (23, 24), when challenged with d-amphetamine, Taar1 KO mice show enhanced hyperlocomotion and exaggerated striatal release of DA, NA and 5-HT (23, 24). Conversely, TAAR1-overexpressing mice show little response to amphetamine (25). The spontaneous firing rate of dopaminergic ventral tegmental area (VTA) and serotonergic neurons in the dorsal raphe nuclei (DRN) is greatly increased in the absence of TAAR1 (23); TAAR1 tonically activates GIRK channels to reduce basal firing activity of these neurons (26, 27). TAAR1 may also regulate glutamatergic activity in the cerebral cortex (27, 28). The endogenous ligands for TAAR1 are trace amines (TAs) such as β-phenylethylamine (PEA), p-tyramine (pTyr), octopamine and tryptamine, molecules that are closely related to the classical biogenic amines (29, 30), and the thyronamines thyronamine (T0AM) and 3-iodothyronamine (T1AM) (31), which are structurally related to thyroid hormones triiodothyronine and thyroxine. Although thyroid hormones have been hypothesized to be regulated by Hcrt, circulating levels are associated with changes in sleep/wake in both narcoleptic patients and controls (32). Abnormal levels of TAs have been associated with neuropathological disorders (29, 30, 33-35).
Several small molecule TAAR1 ligands have been described. The TAAR1 selective antagonist EPPTB (26) increased the firing frequency of DA neurons in vitro, suggesting that TAAR1 either exhibits constitutive activity or is tonically activated by endogenous agonist(s). Conversely, the full agonist RO5166017 inhibited the firing of dopaminergic VTA and serotonergic DRN neurons but did not affect locus coeruleus neurons, an area devoid of Taar1 expression (27). We recently described the effects of partial TAAR1 agonists in rodent and primate neurobehavioral paradigms (36-38) that suggested therapeutic potential for TAAR1 agonism in psychosis, mood disorders and substance abuse. These studies also revealed dose-dependent wake-promoting and REM sleep-inhibiting properties of TAAR1 partial agonists. Unlike psychostimulants, however, the wakefulness produced by TAAR1 partial agonists was not accompanied by hyperlocomotion and increased body temperature (36, 37). Consequently, we hypothesized that TAAR1 agonism might be a novel therapeutic pathway for treatment of narcolepsy and report here the efficacy of TAAR1 agonists to promote wakefulness and alleviate cataplexy in two models of murine narcolepsy.
METHODS AND MATERIALS
All experimental procedures were approved by the Institutional Animal Care and Use Committee at SRI International and were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals. Detailed methods are given in Supplement 1.
Protocol 1: Efficacy of TAAR1 full and partial agonists in WT and Taar1 KO mice
Animals
To evaluate wake-promoting efficacy of RO5256390, adult male homozygous B6-Taar1tm1(NLSLacZ)Blt mice (Taar1 KO) (23) (n=11) and their WT littermates (n=13) were used. As a comparison to RO5256390, data are presented from a second cohort of Taar1 KO (n=8) and a pooled group of 5 WT littermates and 7 WT B6-Tg(Taar1)27 (TAAR1-overexpressing) mice (25) treated with RO5363397, as described previously (38). Both strains were maintained on a C57BL/6 background. Mice were obtained from F. Hoffmann-LaRoche, Basel, Switzerland or were bred at SRI International using founders obtained from Roche.
Surgical Procedures and Data Recording
Mice were instrumented for tethered recording of electroencephalogram (EEG) and electromyogram (EMG) and telemetered monitoring of locomotor activity (LMA) and core body temperature (Tb) as described (38-40). EEG/EMG data were continuously recorded using iox2 (v2.8.0.11; EMKA Technologies, France) and analyzed as described (38-40).
Drugs
RO5256390 and RO5263397 (synthesized at Roche) and caffeine (Sigma) were prepared fresh as solutions or suspensions with 1 h sonication and serial dilutions using 0.3% Tween-80 in water as the vehicle (Veh). Doses (37) were delivered at 10 mL/kg final volume and were administered p.o.
Experimental design
One group of mice (n=11 Taar1 KO and 13 WT littermates) received p.o. RO5256390 (1, 3, and 10 mg/kg, T½=3.7 h (37), Caf (10 mg/kg, positive control) or Veh. As described previously (38), the other group (n=8 Taar1 KO and 12 WT) received p.o. RO5263397 (0.1, 0.3, and 1 mg/kg, T½=6 h), Caf (10 mg/kg, positive control) or Veh. All mice were dosed at ZT6 (ZT0=08:00) in balanced order with at least 3 d between treatments.
Data analysis and statistics
EEG and EMG data were visually scored offline in 10 s epochs as wakefulness, NREM, and REM using ecgAUTO (EMKA Technologies) by expert scorers blind to drug treatment and genotype. Time spent in each state and LMA and Tb were assessed for 3 h postdosing. Drug efficacy was evaluated using 2-way mixed ANOVA comparing genotype and drug treatment followed by Bonferroni t-tests, where appropriate.
Protocol 2: TAAR1 full and partial agonists in mouse narcolepsy models
Animals
Male, hemizygous transgenic C57BL/6-Tg(orexin/ataxin-3)/Sakurai mice (Atax) mice (11) and transgenic C57BL/6-Tg(orexin/tTA; TetO diphtheria toxin A fragment)/Yamanaka (DTA) mice (12) bred at SRI International were used. DTA mice were studied after dietary doxycycline withdrawal (Dox(−)) for 28 d, which produces degeneration of Hcrt cells and severe cataplexy (12).
Surgical Procedures and Data Recording
Mice were surgically implanted with transmitters for EEG, EMG, Tb and locomotor activity recording as described (41, 42). Physiological and video-recorded behavioral data were simultaneously acquired with DataQuest Art 4.2 (Data Sciences Inc., St. Paul, MN).
Drugs
Almorexant (Alm, (2R)-2-[(1S)-6,7-Dimethoxy-1-[2-(4-trifluoromethyl-phenyl)-ethyl]-3,4-dihydro-1H-isoquinolin-2-yl]-N-methyl-2-phenyl-acetamide) (43) was synthesized at SRI International (>99% purity as determined by NMR, mass spectrometry and HPLC) according to procedures described in the patent literature (44). RO5263397 and RO5256390 were synthesized at Roche. Desipramine (Des, 10-11-Dihydro-N-methyl-5H-dibenz(Z)[b,f]azepine-5-propanamine hydrochloride) was purchased from R&D Systems (Minneapolis, MN) and modafinil (Mod, 2-[(Diphenylmethyl)sulfinyl]acetamide) was purchased from Waterstone Technology (Carmel, IN). Drugs were prepared as described above using 1.25% hydroxypropyl methyl cellulose/0.1% dioctyl sodium sulfosuccinate in water (w/w) as the Veh. Doses (37, 41, 45, 46) were delivered at 10 mL/kg final volume i.p. (Alm) or p.o. (RO5263397, RO5256390, Des, Mod or Veh).
Experimental design
To assess the potential of TAAR1 agonists to promote wakefulness and reduce cataplexy, RO5263397 and RO5256390 were tested in mice at the time of day corresponding to when these effects would be most needed in narcoleptic humans (i.e., first half of the active phase). Therefore, Atax and DTA mice were dosed at ZT12.5 (ZT0=3:00) in a counter-balanced crossover design with RO5263397 (0.3, 1, 3 mg/kg), RO5256390 (1, 3, 10 mg/kg), Des (5 mg/kg), Mod (100 mg/kg), or Veh. Desipramine was used as a positive control for cataplexy reduction (46) while Mod, an FDA-approved medication for EDS, served as a positive control for wake promotion (45). To increase the occurence of cataplexy, Atax mice were pre-dosed on experimental days with a non-sedating dose of Alm (30 mg/kg) at ZT12 (41, 42). At least 3 d separated treatments. Physiological and video-recorded behavioral data were collected from ZT12-18 on each dosing day. Primary outcomes included time spent in wakefulness, NREM, REM, and cataplexy. Gross motor activity and Tb were also assessed.
Data analysis and statistics
Data were classified in 10-s epochs as wakefulness, NREM, REM, or cataplexy. Criteria for cataplexy were ≥ 10 s of EMG atonia, theta-dominated EEG, and video-confirmed behavioral immobility preceded by ≥ 40 s of wakefulness (47). Data were analyzed as time spent in classification per 30 min bin. Cumulative time spent in cataplexy and cataplexy density (number of cataplexy bouts/min of wakefulness) was calculated for 5.5 h post-dosing.
Wakefulness, NREM and REM sleep were compared between drug conditions using twoway repeated measures (RM) ANOVA on factors “drug condition” and “30 min bin”. Cataplexy time was evaluated between drug conditions using one-way ANOVA. When appropriate, post hoc Bonferroni t tests (α=0.05) were performed after ANOVA. Data that did not meet the assumptions of normality and equal variance as required for ANOVA were subjected to analysis using Friedman RM-ANOVA on Ranks with post hoc tests using Dunnett's Method (α=0.05). Cataplexy density data were pooled between mouse models and linear regressions were performed to determine the relationship between cataplexy density after Veh vs. the therapeutic response (cataplexy density change from Veh). Significant relationships were detected with Pearson product-moment correlation.
RESULTS
Protocol 1: Efficacy of full and partial TAAR1 agonists in Taar1 KO and WT mice
We previously showed that the TAAR1 partial agonist RO5263397 promoted wakefulness and inhibited REM sleep in mice and that these effects were TAAR1-dependent (38). To determine the generality of this response, we evaluated the efficacy of another TAAR1 agonist, RO5256390, on sleep/wake when administered to WT and Taar1 KO mice in the mid-light phase when mice normally spend a majority of time asleep. Although we have previously characterized RO5256390 as a full agonist in rat and monkey, its intrinsic activity (IA) in mice (79%) is considerably lower than that found in rat, monkey or human (~100%), although still higher than the 59% value for the partial agonist RO5263397 determined in mice (37). Although the IA values suggest that RO5256390 may be a partial agonist in mice, to be consistent with the existing literature, we will refer to RO5263397 as a partial TAAR1 agonist and RO5256390 as a full TAAR1 agonist.
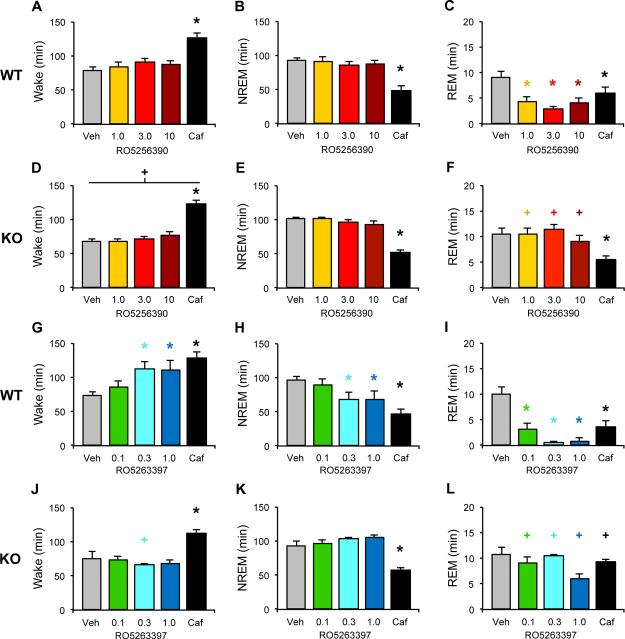
RO5256390 did not significantly affect the amount of Wake or NREM sleep in WT (Figure 1A,B) or Taar1 KO (Figure 1D,E) mice. By contrast, RO5256390 significantly decreased REM sleep at all three doses in WT (Figure 1C); this effect was absent in Taar1 KO mice (Figure 1F; interaction: F4,88=8.296, P<0.001). Caf significantly increased wake time (Figure 1A,D; main effect of drug: F4,88=42.671, P<0.001) and decreased NREM time (Figure 1B,E; main effect of drug: F4,88=50.275, P<0.001) and REM time in both genotypes (Figure 1C,F). While there was a significant main effect of genotype on Wake time (Figure 1A,D; main effect of genotype: F1,22=4.394, P=0.048), a t-test comparing Wake time between WT and KO mice was negative for the Veh treatment. Thus, the observed genotype effect likely reflects small increases in wake time corresponding to the REM suppression following RO5256390 (Figure 1C,F). Supplementary Figure S1 provides the hourly amounts for the effects of RO5256390 on all 3 states in both genotypes.
Figure 1.
TAAR1 agonists suppress REM sleep in WT mice. Total time in wake (A, D), NREM (B, E) and REM sleep (C, F) was summed for 3 h following dosing with the full agonist RO5256390 (RO5256390, 1-10 mg/kg, warm colors), caffeine (Caf, 10 mg/kg, black) or vehicle (Veh, gray) at zeitgeber time (ZT) 6 in WT (A-C) and Taar1 KO mice (D-F). Data are presented as mean + s.e.m. n=13 WT and 11 Taar1 KO mice. The partial TAAR1 agonist RO5263397 increases wakefulness as well as suppresses REM sleep in WT mice (G-L). Total time in wake (G, J), NREM (H, K) and REM sleep (I, L) was summed for 3 h following dosing with RO5263397 (0.1-1.0 mg/kg, cool colors), caffeine (Caf, 10 mg/kg, black) or vehicle (Veh, gray) at zeitgeber time (ZT) 6 in WT (G-I) and Taar1 KO mice (J-L). Data are presented as mean + s.e.m. n=12 WT and 8 Taar1 KO mice. Two-way mixed-factor ANOVA and post hoc Bonferroni t tests: * P<0.05 vs Veh; + P<0.05 vs WT
As a comparison to RO5256390, Figures 1G-L present the effects of the partial agonist RO5263397 on sleep/wake in WT and Taar1 KO mice (38). In contrast to the full agonist RO5256390, RO5263397 (0.3 and 1.0 mg/kg) significantly increased wake time (Figure 1G; interaction effect: F4,72=4.882, P=0.002) and decreased NREM time compared to Veh (Figure 1H; interaction effect: F4,72=4.470, P=0.003) and also powerfully suppressed REM sleep at all doses in WT mice (Figure 1I; interaction effect: F4,72=5.373, P<0.001). These effects were absent in Taar1 KO mice (Figure 1J-L). By contrast, Caf significantly enhanced wake (Figure 1G,J) while suppressing NREM sleep (Figure 1H,K) in both WT and Taar1 KO mice. Caf also suppressed REM sleep in WT mice but, surprisingly, not in KO mice (Figure 1I,L). Thus, both RO5263397 and RO5256390 suppressed REM sleep in WT mice but RO5263397 also promoted wakefulness and reduced NREM sleep. None of these effects were observed in Taar1 KO mice, demonstrating that both REM-suppressing and wake-promoting effects are mediated via TAAR1.
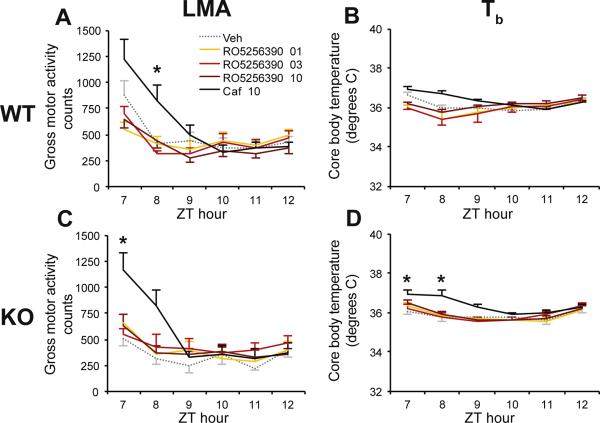
Since RO5263397 reduced Tb without affecting LMA (38), we assessed the effects of RO5256390 on these parameters. Similar to RO5263397, RO5256390 had no effect on LMA in either WT (Figure 2A) or Taar1 KO (Figure 2C) mice, unlike Caf which increased LMA in both strains. Whereas RO5263397 had a hypothermic effect in WT mice, RO5256390 had no such effect in either WT or Taar1 KO mice (Figure 2B,D). Thus, RO5256390 and RO5263397 have similar effects on REM sleep but have different effects on wakefulness, NREM and Tb.
Figure 2.
The TAAR1 agonist RO5256390 had no effects on locomotor activity (LMA; A, C) or core body temperature (Tb; B, D) in WT or Taar1 KO mice. Compound RO5256390 (1-10 mg/kg, warm colors), caffeine (Caf, 10 mg/kg, black) and vehicle (Veh, gray dots) were administered at zeitgeber time (ZT) 6. Two-way repeated-measures ANOVA and post hoc Bonferroni t tests: * P<0.05 vs. Veh. Data are mean±s.e.m.
Protocol 2: Efficacy of full and partial TAAR1 agonists in mouse narcolepsy models
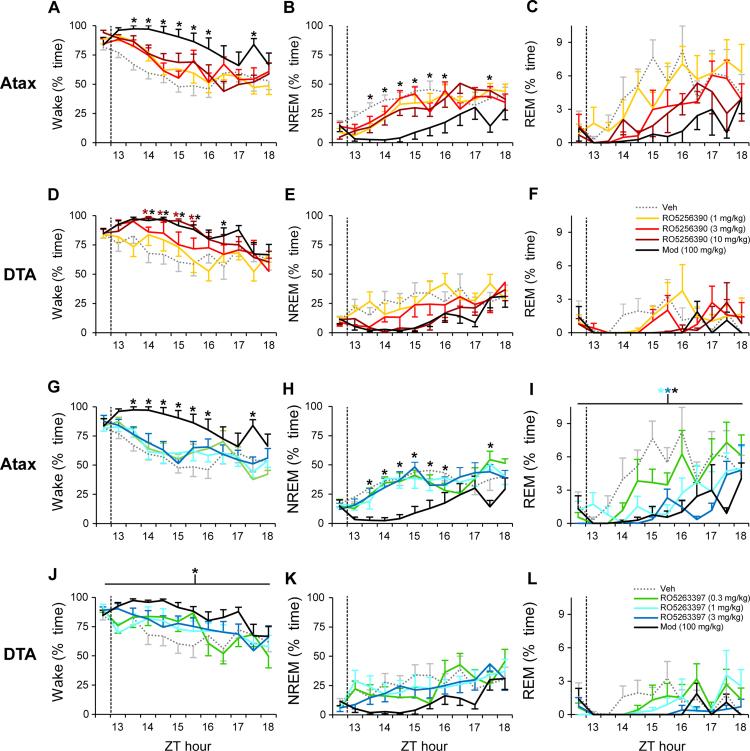
To assess the potential of TAAR1 agonists to promote wakefulness and ameliorate cataplexy, arousal states were assessed in Atax and DTA mice during the first half of the active phase (ZT12-18). In Atax mice that were pretreated with a non-sedating dose of Alm (30 mg/kg) (41, 42), only the positive control Mod increased wakefulness (Figure 3A; F44,308=1.46, P<0.04) and decreased NREM sleep (Figure 3B; F44,308=1.5, P<0.03). However, in DTA Dox(−) mice, both the TAAR1 agonist RO5256390 (10 mg/kg) and Mod increased wakefulness (Figure 3D; F44,308=1.48, P<0.03). In contrast to WT mice treated at ZT6 (Figure 1C), REM sleep was not significantly affected by RO5256390 in either mouse narcolepsy model when treated at ZT12 (Figure 3C,F), a time of day when REM sleep is very low.
Figure 3.
During the dark phase, the TAAR1 agonist RO5256390 at 10 mg/kg increased wakefulness in DTA mice (n=8; D) while RO5263397 (3397) suppressed REM sleep in orexin/ataxin-3 (Atax, n=8; I) mice. Compounds RO5256390 (1-10 mg/kg; A-F, warm colors), 3397 (0.3-3 mg/kg; G-L, cool colors), modafinil (Mod, 100 mg/kg, black) and vehicle (Veh, gray dots) were administered at zeitgeber time (ZT) 12.5 (dashed vertical line) in Atax mice (A-C, G-I) after pretreatment with almorexant (30 mg/kg, p.o.) at lights off (ZT12), and in DTA Dox(−) mice after Hcrt neuron degeneration (D-F, J-L). Modafinil also increased wakefulness in both narcoleptic models (A, D) and suppressed NREM sleep in Atax mice (B). Two-way repeated-measures ANOVA and post hoc Bonferroni t tests: * P<0.05 vs. Veh. Data are mean±s.e.m.
In Atax mice, Mod also increased wakefulness (F44,308=1.52, P<0.03) and decreased NREM sleep (F44,308=1.59, P<0.02) in the RO5263397 dataset (Figure 3G-H). By contrast, RO5263397 did not significantly increase wakefulness nor reduce NREM sleep when Atax mice were treated at ZT12 (Figure 3G-H). Similarly, Mod increased wakefulness (F4,308=3.58, P<0.02) and showed a trend towards decreased NREM sleep (F4,308=2.69, P=0.052) in DTA Dox(−) mice, whereas RO5263397 did not affect wakefulness or NREM sleep at any dose tested at this time of day (Figure 3J-K). REM sleep was significantly suppressed by both Mod and RO5263397 in Alm-pretreated Atax mice (Figure 3I; F4,308=10.94, P<0.001), and trended towards a decrease in DTA Dox(−) mice (Figure 3L; F44,308=1.36, P=0.07). Cumulative REM sleep for 5.5 h post-dosing in Alm-pretreated Atax mice was suppressed by Mod and by RO5263397 at 1 and 3 mg/kg (F4,28=10.9, P<0.001). REM sleep returned to, or exceeded, baseline levels by 3-5.5 h post dosing in both models.
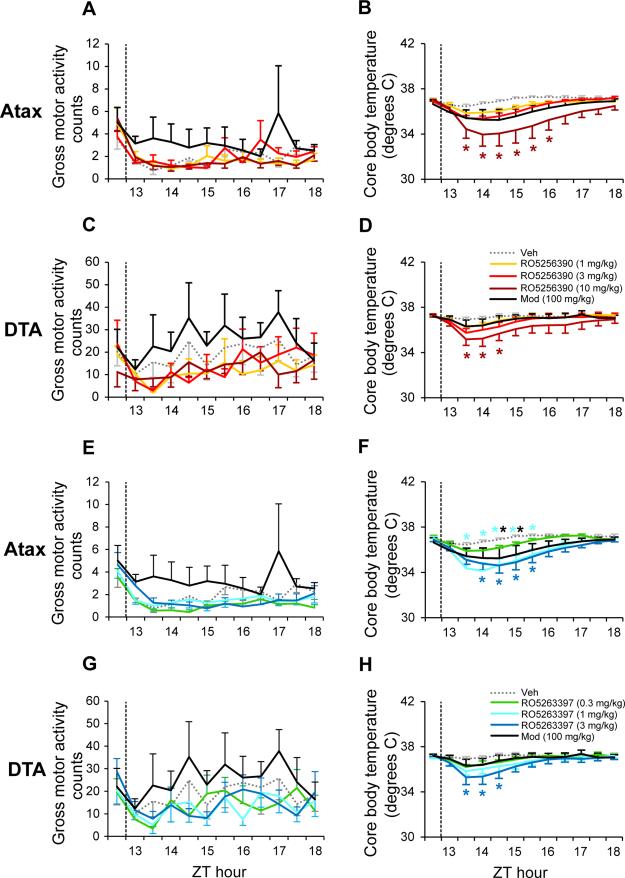
Consistent with our observations in WT mice (Figure 2 and (38)), there were no significant effects of RO5256390, RO5263397 or Mod on gross motor activity in either narcoleptic model (Figure 4A,C,E,G). Since RO5263397 reduced Tb in WT mice (38), we assessed the effects of both compounds on Tb in the mouse narcolepsy models. RO5256390 reduced Tb at the highest dose tested in both Atax (Figure 4B; F44,308=2.43, P<0.001) and DTA (Figure 4D; F44,308=1.98, P<0.001) mice. Similarly, RO5263397 reduced Tb in both Atax (Figure 4F; F44,308=2.53, P<0.001) and DTA (Figure 4H; F44,308=2.34, P<0.001) mice. Mod slightly decreased Tb in Atax, but not DTA mice.
Figure 4.
TAAR1 agonists RO5256390 and RO5263397 (3397) decreased core body temperature without altering gross motor activity in orexin/ataxin-3 (Atax, n=8) and orexin/tTA; TetO diphtheria toxin (DTA, n=8) mice. Compounds RO5256390 (1-10 mg/kg, A-D), 3397 (0.3-3 mg/kg, E-H), modafinil (Mod, 100 mg/kg, black) and vehicle (Veh, gray dots) were administered at zeitgeber time (ZT) 12.5 (dashed vertical line) in Atax mice (A, B, E, F), after p.o. pretreatment with almorexant (30 mg/kg, p.o.) at lights off (ZT12), and in DTA Dox(−) mice after Hcrt neuron degeneration (C, D, G, H). Two-way repeated-measures ANOVA and post hoc Bonferroni t tests: * P<0.05 vs. Veh. Data are mean±s.e.m.
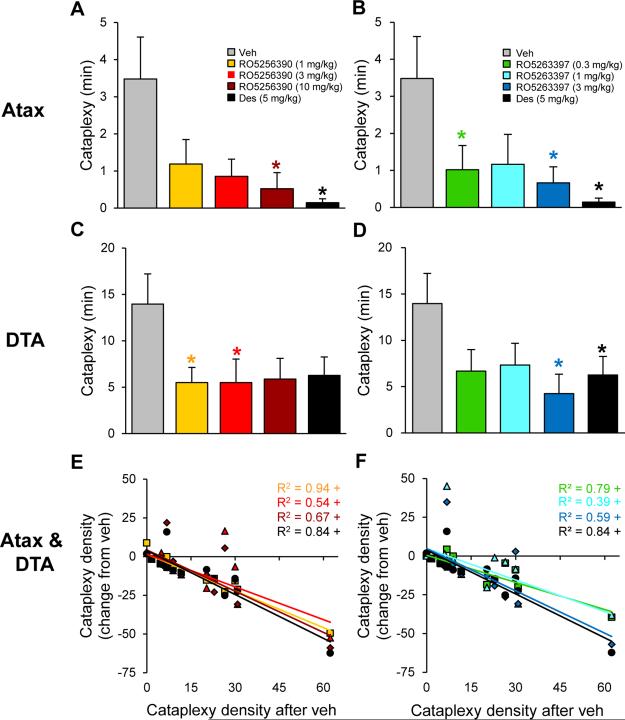
Cataplexy was assessed over the 5.5 h post-dosing at ZT12.5 with the TAAR1 agonists RO5256390 (1-10 mg/kg) and RO5263397 (0.3-3 mg/kg) vs. the known anticataplectic Des (5 mg/kg). Compared to Alm-pretreated Atax mice, DTA Dox(−) mice exhibited a 4-fold increase in the amount of time spent in cataplexy during the vehicle condition (Figure 5A,B vs. C,D). In Atax mice that were pretreated with a non-sedating dose of Alm (30 mg/kg), both RO5256390 and RO5263397 decreased time spent in cataplexy (Figure 5A-B; Friedman RM-ANOVA on Ranks:χ2(4)=12.0, P<0.02; one-way RM-ANOVA: F4,28=3.97, P<0.02, respectively). Similarly, both RO5256390 and RO5263397 decreased cataplexy in DTA Dox(−) mice (Figure 5C-D; one-way RMANOVA: F4,28=5.95, P<0.001 and F4,28=3.33, P<0.03, respectively).
Figure 5.
TAAR1 agonists RO5256390 and RO5263397 decreased cataplexy in orexin/ataxin-3 (Atax, n=8) and orexin/tTA; TetO diphtheria toxin (DTA, n=8) mice. Compounds RO5256390 (1-10 mg/kg; A, C, E), RO5263397 (0.3-3 mg/kg; B, D, F), desipramine (Des, 5 mg/kg; black) and vehicle (Veh; gray) were administered at zeitgeber time (ZT) 12.5 in Atax mice (A, B, E, F), after pretreatment with almorexant (30 mg/kg, p.o.) at lights off (ZT12), and in DTA Dox(−) mice after Hcrt neuron degeneration (C-F). Time in cataplexy was reduced after RO5256390 (10 mg/kg) and Des in Atax mice (A) and after RO5256390 (1, 3 mg/kg) in DTA mice (C). Time spent in cataplexy decreased during the 5.5 h following RO5263397 (0.3 and 3 mg/kg) and Des in Atax mice (B) and after RO5263397 (3 mg/kg) in DTA mice (D). Data are mean±s.e.m., Friedman RMANOVA on Ranks and post hoc Dunnett's Method (Atax) and one-way repeated-measures ANOVA and post hoc Bonferroni t tests (DTA): * P<0.05 vs. Veh. Cataplexy density (number of cataplexy bouts per min awake) after Veh vs. following RO5256390 (E), RO5263397 (F), or Des (E, F), plotted as the change from Veh, indicated a dose-related therapeutic response to TAAR1 agonists (data pooled from both mouse models). Pearson product-moment correlation: + P<0.01.
Because cataplexy occurrence varies widely between narcolepsy models (e.g., Veh groups in Figure 5A-D) as well as among mice of the same genotype (46), data from Alm-pretreated Atax mice and DTA Dox(−) mice were pooled to assess correlations between cataplexy density (number of cataplexy bouts per minutes awake) after Veh vs. the therapeutic change from Veh post-dosing with RO5263397 (0.3-3 mg/kg), RO5256390 (1-10 mg/kg) and Des (5 mg/kg) (Figure 5E-F). Pearson product-moment correlations revealed that, as basal cataplexy levels increased (measured as cataplexy density after Veh treatment), cataplexy density after drug treatment decreased for RO5263397 (0.3, 1, 3 mg/kg) (r(14)=−0.89, −0.62, −0.77, respectively, all P<0.01), RO5256390 (1, 3, 10 mg/kg) (r(14)=−0.97, −0.74, −0.82, respectively, all P<0.01) and Des (5 mg/kg) (r(14)=−0.92, P<0.01).
DISCUSSION
Narcolepsy is a disorder of REM sleep and cataplexy is its pathognomonic symptom. In the present study, two TAAR1 agonists showed a therapeutic benefit in reduction of cataplexy in two different mouse models of narcolepsy. The TAAR1 partial agonist RO5263397 reduced cataplexy in both mouse narcolepsy models (Figure 5B,D,F) and suppressed REM sleep in the Atax narcolepsy model (Figure 3I,L) as well as in WT mice (Figure 1I). By contrast, RO5256390 had a therapeutic benefit on cataplexy (Figure 5A,C,E) and reduced REM sleep in WT mice (Figure 1C) but its suppression of REM sleep did not reach statistical significance in either of the narcolepsy models (Figure 3C,F) when tested at a time of day when REM sleep levels were low. Whereas the studies in WT mice in Protocol 1 were conducted mid-rest phase to assess a potential wake-promoting effect of these compounds, the narcolepsy studies in Protocol 2 were conducted at the start of the active phase when cataplexy levels are high. It is possible that more consistent results on REM sleep would be obtained if the 3 strains of mice were tested at the same time of day.
Partial vs. Full Agonism at TAAR1 and Effects on Sleep/Wake
Our previous studies in rat showed that the TAAR1 partial agonists RO5203648 and RO5263397 dose-dependently increased wakefulness and reduced both NREM and REM sleep whereas RO5256390, a TAAR1 full agonist in the rat, had no effect (36, 37). Neither the partial nor full agonists affected LMA or Tb in the rat (36, 37). The full agonist RO5256390 reduced the firing of VTA DA neurons in vitro whereas the partial agonists RO5263397 and RO5203648 increased the discharge rate of these cells; these effects were absent in Taar1 KO mice (36, 37). Since the full agonist RO5166017 also inhibited the firing of these neurons (27) as did RO5256390 whereas the TAAR1 selective antagonist EPPTB (26) increased the firing frequency of these cells as did RO5263397 and RO5203648, we proposed that the partial agonists had some antagonistic activity that blocked constitutive TAAR1 tone. In rat, monkey and human, RO5256390 is clearly a full agonist but, in mice, the IA of RO5256390 in mice is considerably lower (79%), although still higher than the 59% value for the partial agonist RO5263397 determined in mice (37). Thus, it is not surprising that RO5256390 had some physiological effects in mice that are consistent with partial agonist activity and that such effects were greater in mice treated with RO5263397. For example, although both compounds decreased REM sleep in WT mice (Figure 1C,I) and reduced cataplexy in both narcolepsy models (Figure 5), these effects were more robust in mice treated with RO5263397.
In addition, RO5263397 has some physiological effects that were not evident in mice treated with RO5256390. For example, RO5263397 increased wake and decreased both NREM sleep (Figure 1G,H) and Tb (38) in WT mice whereas RO5256390 did not and RO5263397 also reduced REM sleep in Atax mice (Figure 3I,L) whereas RO5256390 was only weakly effective in this regard (Figure 3C,F). These differences could be due to the degree of TAAR1 partial agonism, or might be due to the >6-fold increased oral bioavailability and nearly twice the T½ of RO5263397 vs. RO5256390 (37).
Relationship Between Therapeutic Efficacy and Body Temperature
The ideal narcolepsy therapeutic would be effective in treating both EDS and cataplexy that are characteristic of this disorder. Curiously, although RO5263397 clearly increased wakefulness in WT mice, it was surprisingly ineffective in this regard at the doses tested in the two mouse narcolepsy models (Figure 3). As pointed out above, the assessments in WT mice vs. the narcolepsy models were conducted at different times of day. However, it should also be noted that RO5263397 had a significant hypothermic effect in WT mice and in both narcolepsy models that was not observed in rats. Thus, it is unclear whether the wake-promoting effect of RO5263397 was a secondary consequence of the decline in Tb, but the fact that RO5263397 was wake-promoting in rats in the absence of a change in Tb argues against this possibility. Similarly, although both RO5256390 and RO5263397 reduced cataplexy and decreased Tb in the mouse narcolepsy models, the doses were not always aligned. For example, only RO5256390 at 10 mg/kg reduced Tb in DTA mice (Figure 4D) but this dose did not significantly affect cataplexy (Figure 5C) whereas lower doses of RO5256390 reduced cataplexy without affecting Tb. Similarly, RO5263397 at 1 mg/kg reduced Tb in Atax mice but did not significantly affect cataplexy (Figure 5B).
Relationship Between Therapeutic Efficacy and Inhibition of REM Sleep
Suppression of REM sleep is a characteristic of many anticataplectic medications (46). Although both RO5263397 and RO5256390 were effective in reducing cataplexy, RO5263397 was more effective in reducing REM sleep in the Atax narcolepsy model (Figure 3I). It should be noted that basal REM sleep amounts in the DTA mice are very low at the time of day chosen for this experiment (Figure 3), possibly contributing to a floor effect for REM suppression in these mice. Both compounds reduced REM sleep in WT mice (Figure 1) but, again, RO5263397 had the stronger effect. Together, these results suggest that the stronger partial TAAR1 agonism, the more effective the therapeutic efficacy as an anticataplectic medication. Presumably, this increased efficacy reflects a greater TAAR1 antagonism and blockade of endogenous TAAR1 tone, and suggests that blood brain barrier-penetrable TAAR1 antagonists (currently unavailable) would be effective anticataplectic therapeutics. Since TAAR1 is thought to be a negative regulator of DA release (23), TAAR1 antagonism should result in increased firing of VTA DA neurons (26) and greater DA release. In this regard, it is of interest that the beneficial effects of amphetamine and modafinil, two of the major therapeutics for the treatment of wakefulness in narcolepsy, are mediated by inhibition of DA reuptake and presynaptic activation of DA neurotransmission. However, unlike amphetamine or modafinil, TAAR1 partial agonism does not increase motor activity; indeed, both RO5263397 and RO5256390 were shown to decrease hyperactivity induced by psychostimulants (37). The anticataplectic mechanism of action of TAAR1 partial agonists may be related to blockade of endogenous TAAR1 tone in the DRN and the subsequent increase in serotonergic tone, as restoration of excitatory Hcrt signaling in this region has been shown to reduce cataplexy (48).
Conclusions
EDS and cataplexy are the most problematic symptoms for narcolepsy patients. Since narcolepsy is due to loss of the Hcrt neurons by a mechanism that is yet to be fully understood, hypocretin/orexin replacement would be the ideal therapy. A small molecule hypocretin/orexin agonist has recently been described (49) but its utility as a drug is unclear at the present time. Current narcolepsy therapeutics are thus symptomatic rather than disease-modifying and, with the exception of GHB, no drug is approved for treatment of both EDS and cataplexy. As a controlled substance, use of GHB is problematic for patients. Consequently, the data presented here demonstrating the wake-promoting activity of RO5263397 in WT mice and RO5256390 in DTA mice and that both compounds reduce cataplexy in mouse narcolepsy models suggest that TAAR1 partial agonism may be a promising therapeutic pathway for the treatment of this disorder of REM sleep.
Supplementary Material
ACKNOWLEDGEMENTS
Research supported by NIH R01 NS082876, R21 NS083639 and R21 NS085757 to T.S.K. We thank Professor Akihiro Yamanaka of Nagoya University for the orexin/tTA; TetO diphtheria toxin A fragment)/Yamanaka (DTA) mice. We also thank Drs. A. Harmeier, J.-L. Moreau and J. Wettstein for valuable scientific input and Jeremiah Palmerston and Kelsie Bogyo for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Drs. Black, Schwartz and Ms. Chen reported no biomedical financial interests or potential conflicts of interest. Dr. Hoener is an employee of F. Hoffmann-La Roche Ltd. Over the past 2 years, Dr. Kilduff has served as a consultant for NIH, the Japanese Society for the Promotion of Science, Merck Pharmaceuticals and Pfizer; made paid educational presentations for the benefit of APSS, LLC and the Physician's Postgraduate Press; and received research support from F. Hoffmann-LaRoche, Ltd., Sunovion Pharmaceuticals, Inc. and Inscopix, Inc.
REFERENCES
- 1.Burgess CR, Scammell TE. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci. 2012;32:12305–12311. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal T, Moore R,Y, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 5.Gerashchenko D, Murillo-Rodriguez E, Lin L, Xu M, Hallett L, Nishino S, et al. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Exp Neurol. 2003;184:1010–1016. doi: 10.1016/S0014-4886(03)00388-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Lin L, Kaur S, Thankachan S, Blanco-Centurion C, Yanagisawa M, et al. The development of hypocretin (orexin) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience. 2007;148:34–43. doi: 10.1016/j.neuroscience.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 9.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 10.Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, et al. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsycataplexy in the rat. J Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 12.Tabuchi S, Tsunematsu T, Black SW, Tominaga M, Maruyama M, Takagi K, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34:6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess CR, Tse G, Gillis L, Peever JH. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33:1295–1304. doi: 10.1093/sleep/33.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilleminault C, Cao MT. Narcolepsy: Diagnosis and management. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. Elsevier Saunders; St. Louis, Missouri: 2011. pp. 957–968. [Google Scholar]
- 15.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 17.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 18.Mignot E. Narcolepsy: Pathophysiology and Genetic Predisposition. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. Elsevier Saunders; St. Louis, Missouri: 2011. pp. 938–956. [Google Scholar]
- 19.Bosch OG, Quednow BB, Seifritz E, Wetter TC. Reconsidering GHB: orphan drug or new model antidepressant? J Psychopharmacol. 2012;26:618–628. doi: 10.1177/0269881111421975. [DOI] [PubMed] [Google Scholar]
- 20.Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2012;16:431–443. doi: 10.1016/j.smrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 24.Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, et al. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 25.Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB, et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37:2580–2592. doi: 10.1038/npp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci U S A. 2009;106:20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, et al. TAAR1 modulates cortical glutamate NMDA receptor function. Neuropsychopharmacology. 2015;40:2217–2227. doi: 10.1038/npp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Grandy DK. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol Ther. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 32.Kok SW, Roelfsema F, Overeem S, Lammers GJ, Frolich M, Meinders AE, et al. Altered setting of the pituitary-thyroid ensemble in hypocretin-deficient narcoleptic men. Am J Physiol Endocrinol Metab. 2005;288:E892–899. doi: 10.1152/ajpendo.00327.2004. [DOI] [PubMed] [Google Scholar]
- 33.Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- 34.Davis BA. Biogenic amines and their metabolites in body fluids of normal, psychiatric and neurological subjects. J Chromatogr. 1989;466:89–218. doi: 10.1016/s0021-9673(01)84617-3. [DOI] [PubMed] [Google Scholar]
- 35.Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol. 2009;76:229–235. doi: 10.1124/mol.109.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012;72:934–942. doi: 10.1016/j.biopsych.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18:543–556. doi: 10.1038/mp.2012.57. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MD, Black SW, Fisher SP, Palmerston JB, Morairty SR, Hoener MC, et al. Trace amine-associated receptor 1 regulates wakefulness and EEG spectral composition. Neuropsychopharmacology. doi: 10.1038/npp.2016.216. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher SP, Schwartz MD, Black SW, Thomas AM, Chen T-M, Miller MA, et al. Quantitative EEG analysis provides an early-stage indicator of disease onset and progression in the zQ175 knock-in mouse model of Huntington's disease. Sleep. 2016;39:379–391. doi: 10.5665/sleep.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher SP, Wurts Black SW, Schwartz MD, Wilk AJ, Liu H, Chen T-M, et al. Longitudinal analysis of the sleep phenotype and EEG signatures in the R6/2 model of Huntington's disease. Brain. 2013;136:2159–2172. doi: 10.1093/brain/awt132. [DOI] [PubMed] [Google Scholar]
- 41.Black SW, Morairty SR, Fisher SP, Chen TM, Warrier DR, Kilduff TS. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36:325–336. doi: 10.5665/sleep.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black SW, Morairty SR, Chen TM, Leung AK, Wisor JP, Yamanaka A, et al. GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy. J Neurosci. 2014;34:6485–6494. doi: 10.1523/JNEUROSCI.0080-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 44.Koberstein R, Fischll W, Clozel M, Aissaou H, Weller T. In: Substituted 1,2,3,4-tetrahydroisoquinoline derivatives. WIPO, editor. WIPO: Actelion Pharm. LTD.; 2005. [Google Scholar]
- 45.Willie JT, Renthal W, Chemelli RM, Miller MS, Scammell TE, Yanagisawa M, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–995. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Black SW, Yamanaka A, Kilduff TS. Challenges in the development of therapeutics for narcolepsy. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, et al. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J Med Chem. 2015;58:7931–7937. doi: 10.1021/acs.jmedchem.5b00988. [DOI] [PubMed] [Google Scholar]
- 50.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.