Abstract
Objective
To provide family physicians with an understanding of the epidemiology, clinical features, diagnosis, and management of hidradenitis suppurativa (HS).
Sources of information
A PubMed literature search was performed using the MeSH term hidradenitis suppurativa.
Main message
Hidradenitis suppurativa is a chronic, recurrent, and debilitating skin condition. It is an inflammatory disorder of the follicular epithelium, but secondary bacterial infection can often occur. The diagnosis is made clinically based on typical lesions (nodules, abscesses, sinus tracts), locations (skin folds), and nature of relapses and chronicity. Multiple comorbidities are associated with HS, including obesity, metabolic syndrome, inflammatory bowel disease, and spondyloarthropathy. Although the lack of curative therapy and the recurrent nature makes HS treatment challenging, there are effective symptomatic management options.
Conclusion
Family physicians should be suspicious of HS in patients presenting with recurrent skin abscesses at the skin folds. Family physicians play an important role in early diagnosis, initiation of treatment, and referral to a dermatologist before HS progresses to debilitating end-stage disease.
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, recurrent, and debilitating inflammatory skin condition.1,2 It is a disorder of the follicular epithelium and commonly occurs in the axillae, inframammary folds, and groin.1,3 Early lesions of HS mimic other skin conditions and thus are often misdiagnosed as recurrent furunculosis or boils. The delay in HS diagnosis can be 12 years or longer.4 Prompt recognition and initiation of treatment can reduce the risk of HS progression to debilitating end-stage disease. The psychosocial effect of HS is devastating because of the associated pain, malodorous discharge, and scarring. In fact, HS is associated with greater impairment of quality of life and professional activity than other chronic skin conditions such as psoriasis and atopic dermatitis.3,5
Case description
A 45-year-old obese male patient presented with skin abscesses at the axillae and groin that had been recurring since his late 20s, and some of these lesions developed into sinus tracts over time. He also had a long-standing history of acne-like lesions on his upper back and waistline. A diagnosis of HS was made. He was treated with clindamycin and rifampin for 2 months, tetracycline for a year, isotretinoin for 9 months, and acitretin for 6 months. Unfortunately, he continued to have several relapses of HS and had severe scarring at the axillae.
Sources of information
Using the MeSH term hidradenitis suppurativa, we performed a PubMed literature search for randomized controlled trials (RCTs), systematic reviews, observational studies, case series, and case reports from the earliest possible date to February 2015. We only included articles published in English. We also manually searched the reference lists of relevant articles retrieved.
Main message
How common is HS?
The prevalence of HS is about 1% to 4% in Europe but lower in North America.6–8 Hidradenitis suppurativa is more common in females, with a female-to-male ratio of 4:1.8 The age of onset is usually after puberty and before the age of 40, peaking in the second and third decades of life.9 Although HS in prepubertal children is rare, the earliest reported age of onset is 6 years old.10
What causes HS?
The cause of HS is unclear but likely multifactorial. Genetics play a role, as up to 40% of patients with HS have a positive family history, but there has been no monozygotic concordance study for HS.11 An autosomal dominant mode of inheritance in HS is rare but has been reported.12 The gene locus responsible for HS is unknown, but the γ-secretase mutation has been associated with HS.13
Several exogenous factors are associated with HS. Hormones play a role given its postpubertal and premenopausal pattern of onset. Specifically, androgen-containing medications precipitate or worsen HS, and antiandrogenic agents are used to treat HS.10 In addition, obesity can aggravate the disease, likely owing to the increased mechanical stress on the intertriginous skin.3 Obesity is also more commonly seen in HS, but the correlation between body mass index and HS severity is controversial.7,9 Finally, the association between cigarette smoking and HS is well documented, with a higher incidence of HS in smokers than non-smokers.7,14,15
Although HS was traditionally viewed as a disease of the sweat glands, current understanding suggests it is a disorder of the follicular epithelium resulting from repetitive mechanical stress in genetically susceptible individuals.2,3 Specifically, HS begins with follicular plugging due to the aforementioned exogenous factors. The occluded pilosebaceous unit then initiates inflammation and abscess formation. Subsequent follicular rupture promotes the formation of sinus tracts, which can be secondarily infected.3,16
How does HS present?
Hidradenitis suppurativa typically presents with inflammatory nodules, abscesses, comedones, sinus tracts, or scarring.1,3 It has an insidious onset, starting with mild discomfort, erythema, burning, pruritus, and hyperhidrosis. It progresses to form tender or deep-seated nodules, which expand and coalesce to form large painful abscesses. The rupture of these abscesses releases malodorous and purulent discharge. Recurrent or persistent HS results in the formation of double-ended comedones, sinus tracts, and scarring.3,17
Hidradenitis suppurativa is commonly seen in intertriginous skin including the axillae, perineum, and inframammary and inguinal folds, usually with a bilateral distribution (Figure 1). As the disease advances, areas exposed to repetitive mechanical stress, such as the nape of the neck, trunk, and waistband area can also be affected (Figure 2).3
Figure 1.
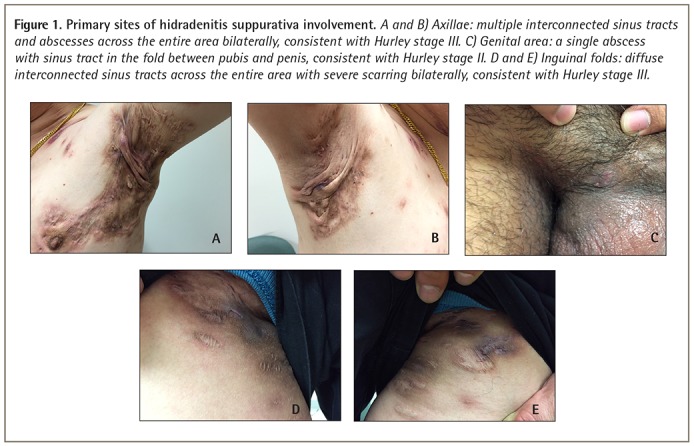
Primary sites of hidradenitis suppurativa involvement. A and B) Axillae: multiple interconnected sinus tracts and abscesses across the entire area bilaterally, consistent with Hurley stage III. C) Genital area: a single abscess with sinus tract in the fold between pubis and penis, consistent with Hurley stage II. D and E) Inguinal folds: diffuse interconnected sinus tracts across the entire area with severe scarring bilaterally, consistent with Hurley stage III.
Figure 2.
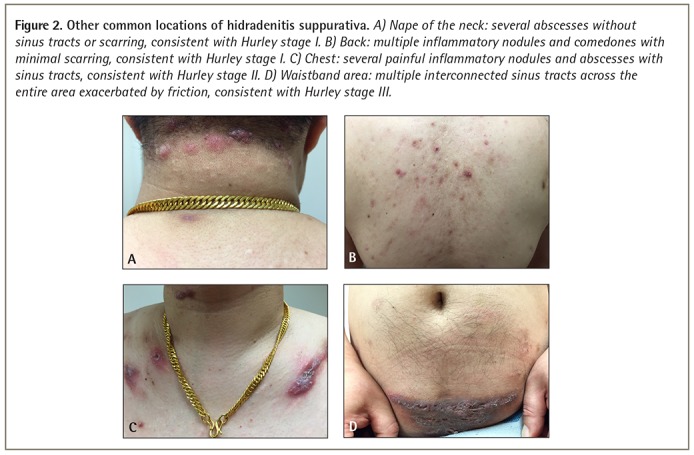
Other common locations of hidradenitis suppurativa. A) Nape of the neck: several abscesses without sinus tracts or scarring, consistent with Hurley stage I. B) Back: multiple inflammatory nodules and comedones with minimal scarring, consistent with Hurley stage I. C) Chest: several painful inflammatory nodules and abscesses with sinus tracts, consistent with Hurley stage II. D) Waistband area: multiple interconnected sinus tracts across the entire area exacerbated by friction, consistent with Hurley stage III.
Complications of HS are distressing. Locally, HS can cause scarring with associated restricted limb mobility, strictures or fistulas at the anus and urethra from chronic inflammation, and disfigurement.17 Rarely, squamous cell carcinoma at the site of HS has also been reported.18 Systemically, HS with serious infection can present with fever and septicemia. Anemia is also associated with HS.19 Finally, HS is associated with multiple comorbidities, including obesity, metabolic syndrome, inflammatory bowel disease, and spondyloarthropathy.20,21
How is HS diagnosed?
Hidradenitis suppurativa is a clinical diagnosis and remains diagnostically challenging. It is often misdiagnosed as boils or furunculosis on initial presentation and can take 12 years or longer from onset of symptoms to HS diagnosis.4 It is important to note that HS is an inflammatory disease; however, secondary bacterial infection is common. The differential diagnoses of HS are listed in Box 1.3 As a component of the follicular occlusion tetrad, HS is also known to be associated with the other components of the tetrad, which are dissecting cellulitis, acne conglobata, and pilonidal cyst (Figure 3).3
Box 1. Differential diagnoses of HS.
Differential diagnoses of HS include the following:
Carbuncle
Furuncle
Epidermoid or dermoid cyst
Erysipelas
Lymphogranuloma venereum
Granuloma inguinale
Tuberculosis
Follicular occlusion tetrad
HS—hidradenitis suppurativa.
Data from Alikhan et al.3
Figure 3.
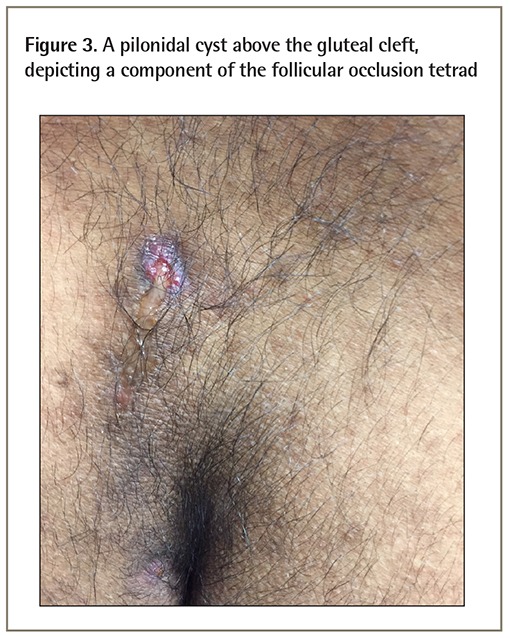
A pilonidal cyst above the gluteal cleft, depicting a component of the follicular occlusion tetrad
The 3 important clinical features that support a diagnosis of HS are typical lesions, typical locations, and relapses and chronicity (Box 2).1,21 Hence, a constellation of recurrent inflammatory nodules, sinus tracts, and scarring in intertriginous areas strongly suggests HS.1 Other elements to be elicited on history include family history of HS, initial onset of symptoms, and gastrointestinal symptoms given the association with Crohn disease.20 Physical examination should assess the extent and severity of HS, and characterize the lesions. The Hurley staging system, as described in Table 1, is commonly used for assessing HS severity.3,21,22
Box 2. Clinical diagnosis of HS.
The 3 important clinical features that support a diagnosis of HS are as follows:
Typical lesions including nodules, abscesses, sinus tracts, and scarring
Typical locations including axillae, inframammary folds, groin, and perineum
Relapses and chronicity (> 2 times in 6 months)
HS—hidradenitis suppurativa.
Table 1.
Recommendations for HS treatment based on disease severity guided by the Hurley clinical staging system
| HURLEY CLINICAL STAGING SYSTEM | CHARACTERISTICS | RECOMMENDED TREATMENT | ||
|---|---|---|---|---|
|
| ||||
| NONMEDiCAL | PHARMACOLOGIC | SURGICAL | ||
| Stage I (mild) | Single or multiple abscesses without sinus tracts and scarring | The following steps should be considered regardless of the severity of HS (grade F, level III):
|
First-line treatments:
Second-line treatments:
|
Incision and drainage (not encouraged) |
| Stage II (moderate) | Recurrent abscesses with sinus tracts and scarring | First-line treatments:
Second-line treatments:
|
If multiple medical therapies have failed, refer to a plastic surgeon for excisions | |
| Stage III (severe) | Diffuse or multiple interconnected sinus tracts and abscesses across the entire area | Refer to a dermatologist who might initiate the following:
|
If multiple medical therapies have failed, refer to a plastic surgeon for excisions | |
Investigations should be considered when dealing with complications of HS. The initial workup might include measuring complete blood count, routine chemistries, and inflammatory markers. In certain cases, suspected perianal HS should be biopsied to rule out malignancy and Crohn disease. Skin biopsy is rarely indicated unless the diagnosis is uncertain or one needs to rule out other disorders, especially lesions suggestive of squamous cell carcinoma. Wound cultures can be considered in lesions with evidence of secondary infection.1,21
How should HS be managed?
Although HS is difficult to treat and no cure exists for this condition, there are effective interventions available to control the disease and improve symptoms. Goals of HS treatment include preventing new lesions, treating newly formed lesions early and effectively, and removing existing nodules and sinus tracts. The general approach to HS includes nonmedical interventions, topical and systemic medications, and surgery. A summary of recommended interventions based on the disease severity according to the Hurley staging is shown in Table 1.3,21,22 Referral to a dermatologist should be considered in patients with refractory HS or Hurley stage II and III HS.
Nonmedical therapy: General measures should be initiated in all patients regardless of the disease severity. Lifestyle modifications include smoking cessation, weight loss, and eating a healthy diet. Reducing mechanical stress on skin by wearing loose cotton clothing is also important.3 Additional measures include warm compress, topical antiseptics, and antibacterial soap. Although the efficacy of general measures in managing HS has not been formally evaluated, the associated benefits are illustrated in case studies.1,23
In addition, given the considerable psychological effects associated with HS, it is important to reassure patients that HS is not caused by poor hygiene nor is it contagious. Patients should further be informed that recurrence is common and that the disease cannot be cured with current treatment. Finally, the associated pain in HS should be appropriately addressed.3,21
Medical therapy: Many medications used to treat HS have limited efficacy and are associated with a high recurrence rate, as reported in case series and reports, RCTs, and retrospective studies.22 Hence, therapies with good evidence will be discussed here.21,22,24 The general medical approach is stepwise, starting with topical therapy for mild HS and systemic therapy for moderate to severe HS.
Topical resorcinol: Resorcinol is a chemical peeling agent with keratolytic and anti-inflammatory properties. Its use in HS was evaluated in a case series consisting of 12 women with Hurley stage I or II HS. Topical 15% resorcinol was applied directly to inflammatory nodules and shown to reduce pain and promote healing.25 Current European HS treatment guidelines recommend applying it twice daily during flare-ups and once daily during maintenance treatment.21
-
Antibiotics: Topical clindamycin (1% solution or gel twice daily for 3 months) is often used as a first-line therapy for mild or localized HS, with good safety and tolerability. It can decrease the number of mild inflammatory lesions and treat or prevent secondary infection. It is as effective as oral tetracycline, as shown in a double-blind RCT consisting of 46 patients with Hurley stage I and mild stage II HS.26
Oral antibiotics are often considered in refractory or widespread HS. As antibiotics are used for their anti-inflammatory effects, longer courses lasting 2 to 3 months are required to achieve disease control. Although there is no first-line choice, 500 mg of tetracycline twice daily for 4 months is the only oral antibiotic studied in an RCT.26 Recurrence is common after antibiotic cessation.26
Combination therapy of oral clindamycin (300 mg twice daily) and rifampin (600 mg once daily) for 10 weeks can be considered in patients who fail to respond to the aforementioned antibiotics. It was shown to have great efficacy and prolonged remission in a retrospective cohort study consisting of 116 patients with severe HS.27 Similar results were seen in other smaller retrospective studies, as well.28,29
Hormonal therapy: Given the involvement of androgen in the development of HS, antiandrogenic therapy has been explored and shown to be effective. Both cyproterone acetate (100 mg daily) and norgestrel-containing oral contraceptive pills for 6 months improved HS with similar efficacy, as shown in a double-blind controlled crossover trial consisting of 24 women with Hurley stage II or III HS.30,31 Finasteride (5 mg daily) is also an effective drug that has been used in pediatric patients and in adults of both sexes, as shown in some case series.10,32
Oral retinoids: The antiproliferative and immunomodulatory effects of oral retinoids can control the inflammatory nature of HS. Use of acitretin for 9 to 12 months resulted in lesion resolution, pain relief, and persistent improvement even after discontinuing the therapy, as shown in a retrospective cohort study consisting of 12 patients with severe and recalcitrant HS.33 However, it is not recommended in women of child-bearing age, as they should not conceive for 3 years even after treatment cessation. On the other hand, although isotretinoin has a shorter half-life and women can try to conceive 1 month after therapy, it has limited therapeutic effects in moderate to severe HS, as shown in a retrospective cohort study consisting of 68 patients with various stages of HS.34
Immunosuppressive therapy: As HS is thought to be due to a dysregulation of the innate and adaptive immune system, immunosuppressive agents are sometimes used to treat HS. Intralesional corticosteroid injection with 5 to 10 mg/mL of triamcinolone acetonide has been used as an adjunctive therapy to accelerate the resolution of early painful inflammatory nodules.1,21 It should be avoided if secondary bacterial infection is present. A short course of oral prednisone with rapid tapering can be used to reduce acute inflammation.21 Cyclosporine can be used in refractory HS, resulting in moderate to marked improvement, as shown in case studies; however, the duration of treatment is often limited by its side effects.35
Biologics: Biologic agents with anti–tumour necrosis factor-α properties can be considered in patients who fail to respond to conventional medical therapy. Both infliximab and adalimumab are efficacious in moderate to severe HS, as demonstrated in case series, retrospective studies, and RCTs.36–39 Adalimumab has been recently approved by the US Food and Drug Administration, as well as the European Medicines Agency, for the treatment of moderate to severe HS.
Surgery: Surgery allows excision of discrete lesions and scar revision, and thus is effective for managing isolated HS lesions.3,21,40 However, good evidence is lacking on the appropriate timing of surgery and its associated outcomes when compared with medical therapies. The current standard approach is to surgically manage patients who fail to respond or who have partial response to multiple medical therapies or those with intractable Hurley stage III HS. Both general measures and medical therapies should be continued postoperatively to prevent HS recurrence. Surgery, however, is not considered curative and carries a high recurrence rate.
Revisiting the case
This 45-year-old male patient had the classic presentation of recalcitrant, Hurley stage III HS. Since he began using the treatment regimen consisting of adalimumab, 5% topical benzoyl peroxide, and 1% clindamycin more than 6 years ago, great improvement has been noted. He also had 2 surgeries to remove sinus tracts.
Conclusion
Hidradenitis suppurativa is a chronic inflammatory skin condition associated with considerable psychosocial effects. Recognition of typical lesions in typical locations with a recurrent nature allows for early diagnosis. General measures including lifestyle modifications, education, and counseling should be offered to all patients with HS. Medical management should follow a stepwise approach depending on the severity of HS. Surgical management should be considered the last resort, after medical treatment is optimized.
EDITOR’S KEY POINTS
Hidradenitis suppurativa (HS) is a chronic, recurrent, and debilitating inflammatory skin condition. It is a disorder of the follicular epithelium and commonly occurs in the axillae, inframammary folds, and groin. The psychosocial effect of HS is devastating because of the associated pain, malodorous discharge, and scarring.
Early lesions of HS mimic other skin conditions and thus are often misdiagnosed as recurrent furunculosis or boils. The delay in HS diagnosis can be 12 years or longer. Prompt recognition and initiation of treatment can reduce the risk of HS progression to debilitating end-stage disease.
Hidradenitis suppurativa is not curable, but effective interventions are available. In particular, biologic agents with anti–tumour necrosis factor-α properties can now be offered to patients with moderate to severe HS who fail to respond to conventional therapy.
Footnotes
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de février 2017 à la page e86.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–64. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44(7):535–40. doi: 10.1111/j.1365-4632.2004.02536.x. [DOI] [PubMed] [Google Scholar]
- 3.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539–61. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 4.Mebazaa A, Ben Hadid R, Cheikh Rouhou R, Trojjet S, El Euch D, Mokni M, et al. Hidradenitis suppurativa: a disease with male predominance in Tunisia. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(4):165–72. [PubMed] [Google Scholar]
- 5.Matusiak Ł, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol. 2010;62(4):706–8. 708.e1. doi: 10.1016/j.jaad.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35(2 Pt 1):191–4. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 7.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68(3):412–9. doi: 10.1016/j.jaad.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Canoui-Poitrine F, Le Thuaut A, Revuz JE, Viallette C, Gabison G, Poli F, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133(6):1506–11. doi: 10.1038/jid.2012.472. Epub 2012 Dec 13. [DOI] [PubMed] [Google Scholar]
- 10.Randhawa HK, Hamilton J, Pope E. Finasteride for the treatment of hidradenitis suppurativa in children and adolescents. JAMA Dermatol. 2013;149(6):732–5. doi: 10.1001/jamadermatol.2013.2874. [DOI] [PubMed] [Google Scholar]
- 11.Von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14(5):389–92. doi: 10.1046/j.1468-3083.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ali FM, Ratnamala U, Mehta TY, Naveed M, Al-Ali MT, Al-Khaja N, et al. Hidradenitis suppurativa (or acne inversa) with autosomal dominant inheritance is not linked to chromosome 1p21.1–1q25.3 region. Exp Dermatol. 2010;19(9):851–3. doi: 10.1111/j.1600-0625.2010.01088.x. [DOI] [PubMed] [Google Scholar]
- 13.Pink AE, Simpson MA, Desai N, Trembath RC, Barker JN. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol. 2013;133(3):601–7. doi: 10.1038/jid.2012.372. Epub 2012 Oct 25. [DOI] [PubMed] [Google Scholar]
- 14.Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–9. doi: 10.1111/j.1365-2133.2009.09198.x. Epub 2009 Apr 29. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133(1):97–103. doi: 10.1038/jid.2012.255. Epub 2012 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012;21(10):735–9. doi: 10.1111/j.1600-0625.2012.01552.x. Epub 2012 Aug 7. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther. 2004;17(1):50–4. doi: 10.1111/j.1396-0296.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 18.Dufresne RG, Jr, Ratz JL, Bergfeld WF, Roenigk RK. Squamous cell carcinoma arising from the follicular occlusion triad. J Am Acad Dermatol. 1996;35(3 Pt 1):475–7. doi: 10.1016/s0190-9622(96)90632-5. [DOI] [PubMed] [Google Scholar]
- 19.Tennant F, Jr, Bergeron JR, Stone OJ, Mullins JF. Anemia associated with hidradenitis suppurativa. Arch Dermatol. 1968;98(2):138–40. [PubMed] [Google Scholar]
- 20.Van der Zee HH, de Winter K, van der Woude CJ, Prens EP. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br J Dermatol. 2014;171(3):673–5. doi: 10.1111/bjd.13002. Epub 2014 Jul 22. [DOI] [PubMed] [Google Scholar]
- 21.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–44. doi: 10.1111/jdv.12966. Epub 2015 Jan 30. [DOI] [PubMed] [Google Scholar]
- 22.Alhusayen R, Shear NH. Pharmacologic interventions for hidradenitis suppurativa: what does the evidence say? Am J Clin Dermatol. 2012;13(5):283–91. doi: 10.2165/11631880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28(4):779–93. doi: 10.1016/j.det.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev. 2015;10:CD010081. doi: 10.1002/14651858.CD010081.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boer J, Jemec GB. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35(1):36–40. doi: 10.1111/j.1365-2230.2009.03377.x. Epub 2009 Jun 22. [DOI] [PubMed] [Google Scholar]
- 26.Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39(6):971–4. doi: 10.1016/s0190-9622(98)70272-5. [DOI] [PubMed] [Google Scholar]
- 27.Gener G, Canoui-Poitrine F, Revuz JE, Faye O, Poli F, Gabison G, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219(2):148–54. doi: 10.1159/000228334. Epub 2009 Jul 8. [DOI] [PubMed] [Google Scholar]
- 28.Van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219(2):143–7. doi: 10.1159/000228337. Epub 2009 Jul 8. [DOI] [PubMed] [Google Scholar]
- 29.Mendonça CO, Griffiths CE. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154(5):977–8. doi: 10.1111/j.1365-2133.2006.07155.x. [DOI] [PubMed] [Google Scholar]
- 30.Sawers RS, Randall VA, Ebling FJ. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproterone acetate) and oestrogen therapy. Br J Dermatol. 1986;115(3):269–74. doi: 10.1111/j.1365-2133.1986.tb05741.x. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer PS, Dawber RP, Gales MA, Moore RA. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115(3):263–8. doi: 10.1111/j.1365-2133.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 32.Joseph MA, Jayaseelan E, Ganapathi B, Stephen J. Hidradenitis suppurativa treated with finasteride. J Dermatolog Treat. 2005;16(2):75–8. doi: 10.1080/09546630510031403. [DOI] [PubMed] [Google Scholar]
- 33.Boer J, Nazary M. Long-term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer? Br J Dermatol. 2011;164(1):170–5. doi: 10.1111/j.1365-2133.2010.10071.x. [DOI] [PubMed] [Google Scholar]
- 34.Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40(1):73–6. doi: 10.1016/s0190-9622(99)70530-x. [DOI] [PubMed] [Google Scholar]
- 35.Rose RF, Goodfield MJ, Clark SM. Treatment of recalcitrant hidradenitis suppurativa with oral ciclosporin. Clin Exp Dermatol. 2006;31(1):154–5. doi: 10.1111/j.1365-2230.2005.01983.x. [DOI] [PubMed] [Google Scholar]
- 36.Delage M, Samimi M, Atlan M, Machet L, Lorette G, Maruani A. Efficacy of infliximab for hidradenitis suppurativa: assessment of clinical and biological inflammatory markers. Acta Derm Venereol. 2011;91(2):169–71. doi: 10.2340/00015555-1025. [DOI] [PubMed] [Google Scholar]
- 37.Lesage C, Adnot-Desanlis L, Perceau G, Bonnet M, Palot JP, Bernard P, et al. Efficacy and tolerance of prolonged infliximab treatment of moderate-to-severe forms of hidradenitis suppurativa. Eur J Dermatol. 2012;22(5):640–4. doi: 10.1684/ejd.2012.1795. [DOI] [PubMed] [Google Scholar]
- 38.Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GB. A double-blind placebo-controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol. 2011;165(2):391–8. doi: 10.1111/j.1365-2133.2011.10339.x. Epub 2011 Jun 30. [DOI] [PubMed] [Google Scholar]
- 39.Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–55. doi: 10.7326/0003-4819-157-12-201212180-00004. [DOI] [PubMed] [Google Scholar]
- 40.Van Hattem S, Spoo JR, Horváth B, Jonkman MF, Leeman FW. Surgical treatment of sinuses by deroofing in hidradenitis suppurativa. Dermatol Surg. 2012;38(3):494–7. doi: 10.1111/j.1524-4725.2011.02255.x. Epub 2011 Dec 30. [DOI] [PubMed] [Google Scholar]


