Abstract
Aims
Insulin resistance (IR) correlates with mitochondrial dysfunction, free fatty acids (FFAs), and intramyocellular lipid (IMCL) in adults with type 2 diabetes (T2D). We hypothesized that muscle IR would relate to similar factors in T2D youth.
Methods
Participants included 17 youth with T2D, 23 normal weight controls (LCs), and 26 obese controls (OBs) of similar pubertal stage and activity level.
Results
T2D and OB groups were of similar BMI. T2D youth were significantly more IR and had higher calf IMCL and serum FFA concentrations during hyperinsulinemia. ADP time constant (ADPTC), a blood-flow dependent mitochondrial function measure, was slowed and oxidative phosphorylation rates lower in T2D. In multiple linear regression of the entire cohort, lack of FFA suppression and longer ADPTC, but not IMCL or HbA1c, were independently associated with IR.
Conclusion
We found that elevated FFAs and mitochondrial dysfunction are early abnormalities in relatively well-controlled youth with T2D.
Further, post-exercise oxidative metabolism appears affected by reduced blood flow, and is not solely an inherent mitochondrial defect. Thus, lowering FFAs and improving mitochondrial function and blood flow may be potential treatment targets in youth with T2D.
Keywords: Type 2 diabetes, Adolescents, Insulin resistance, Mitochondrial function, Free fatty acids
1. Introduction
Type 2 diabetes (T2D) was initially thought to be a disease exclusive to adults, however the incidence of T2D has risen in youth in parallel with the increasing prevalence of obesity among children (Dabelea et al., 2007). The onset of T2D during adolescence is associated with early development of diabetes-associated co-morbidities such as cardiovascular disease, nonalcoholic fatty liver disease and renal disease (Bjornstad et al., 2014; Kelsey, Forster, Van Pelt, Reusch, & Nadeau, 2014; Levitt Katz et al., 2015; Nadeau, Klingensmith, & Zeitler, 2005; Nadeau et al., 2009). Initial studies indicate that these youth are now experiencing increased morbidities and even mortality as early as the third decade of life, highlighting the urgency in understanding differences between youth-onset and adult-onset T2D (Kelsey, Zaepfel, Bjornstad, & Nadeau, 2014).
In both youth and adults, T2D is characterized by insulin resistance (IR) plus pancreatic beta-cell insufficiency. Many of the diabetes co-morbidities are related to IR (Cree-Green, Triolo, & Nadeau, 2013; Nadeau et al., 2009; Shulman, 2014). Of importance, it is not clear if IR in youth and adults is related to similar factors. For example, IR in youth with T2D is at least partially and uniquely related to the hormonal milieu characteristic of puberty (Argov, De Stefano, & Arnold, 1996; Cree-Green et al., 2013). Moreover, the currently available methods to improve IR have little impact on glucose control in youth, as compared to more positive results with both lifestyle modification and metformin therapy in adults (Levy-Marchal et al., 2010; Zeitler et al., 2012). Therefore, further study of pediatric IR is needed to develop future additional prevention and treatment options for youth at risk for developing diabetes.
The etiology of IR in adults with T2D is complex, and incompletely understood. Previous studies suggest that excess or ectopic lipids, including serum free fatty acids (FFAs), intramyocellular lipid (IMCL), intraheptic lipid and lipid subspecies such as diacyglycerol may be related to IR (D'Adamo & Caprio, 2011; De Feyter et al., 2008; Shulman, 2014). Additionally, decreased mitochondrial substrate oxidation may be associated with the development of IR. The simplified potential explanation of this relationship is that mitochondria have decreased oxidation and clearance of FFAs, leading to increased IMCL and diacylglycerol, or via increased production of reactive oxygen species (Cree-Green et al., 2013). Mitochondrial oxidative dysfunction has been documented in adults with T2D as well as in the adult lean offspring of people with T2D, who are at risk for the development of diabetes (Befroy et al., 2007; Shulman, 2014). However, mitochondrial function has not been well studied in youth with T2D.
In addition to alterations in mitochondrial metabolism and intracellular storage of FFA, alterations in blood flow may affect mitochondrial oxidative function in T2D. Oxidative phosphorylation requires a constant supply of intracellular oxygen. Patients with peripheral arterial disease have impaired oxygenation of peripheral tissue, and were found to have abnormal mitochondrial respiration in skeletal muscle (Esterhammer et al., 2008; Schocke, Esterhammer, & Greiner, 2008). Further, venous occlusion of the limb in healthy subjects has been shown to produce similar mitochondrial findings in healthy volunteers, demonstrating the importance of acute changes in oxygen delivery to mitochondrial function (Argov et al., 1996). Youth with T2D have increased rates of CVD and CVD risk markers and decreased limb blood flow (Nadeau et al., 2009). However, it is unknown if this is severe enough to affect oxygen delivery to the mitochondria.
Our goal was to assess IR and its potential contributors, namely mitochondrial function, FFA and IMCL concentrations in youth with T2D, as compared to BMI-similar obese non-diabetic youth, and normal weight non-diabetic youth. The primary aim of the study was to determine if obese youth with T2D had impaired mitochondrial function that related to IR, as seen in adults. Our secondary aim was to evaluate fat metabolism in terms of FFA availability during hyperinsuinemia and IMCL concentrations. The last aim was to utilize non-invasive measures to assess post-exercise mitochondrial oxidative function (some of which are blood flow dependent) to begin to determine if blood flow abnormalities were a component of mitochondrial dysfunction in pediatric T2D.
2. Materials and methods
2.1. Participants
66 participants were recruited from pediatric clinics at the Children's Hospital Colorado and the Barbara Davis Center for Childhood Diabetes for a prospective, cross-sectional study from 2009 to 2014. This is a clinically similar but distinct cohort of youth from those previously described (Nadeau et al., 2009). Volunteers included non-obese (body mass index (BMI) <85th percentile for age) control youth without diabetes and obese youth (BMI ≥95th percentile for age) with and without T2D. All volunteers were sedentary (defined as less than 3 h per week of any exercise, verified by standardized 3-day activity recall, and 7-day accelerometer recording, Actigraph, Pensacola, FL), had achieved Tanner Stage 3 or above in puberty (as assessed by physical exam by a pediatric endocrinologist, KJN or MCG). Youth with T2D had a hemoglobin A1C (HbA1C) of <12% (108 mmol/mol). Anemia was ruled out in all subjects prior to study, and obese and lean control volunteers underwent a standard 2 h OGTT and were excluded if dysglycemia was present (fasted blood sugar >100 mg/dL or 2 h >140 mg/dL). Exclusionary medications included antihypertensives, lipid lowering agents, oral steroids, atypical antipsychotics and any diabetes medication other than metformin or insulin. T2D was defined by American Diabetes Association criteria and the absence of glutamic acid decarboxylase, islet cell or insulin autoantibodies or secondary causes of diabetes. This study was approved by the University of Colorado Anschutz Medical Campus Institutional Review Board and the Children's Hospital of Colorado Scientific Advisory Review Committee. Parental informed consent and participant assent were obtained from all participants less than 18 years old and participant consent from those aged 18 years and above.
2.2. Overall study design
Participants underwent a screening visit to ensure eligibility and then a study visit with an overnight stay. Participants consumed 3 days of an isocaloric, weight maintenance diet (55% carbohydrate, 15% protein, 30% fat) provided by the CTRC nutrition services and refrained from any physical activity 3 days prior to study. During these 3 days, participants with T2D also refrained from taking metformin, checked blood glucose concentrations at least four times a day, and continued any insulin with the last long acting insulin given 24 h prior to evening admission. Youth with a fasting glucose >200 mg/dl, random glucose >300 mg/dl or acute illness in the previous 2 weeks were rescheduled. Menstruating females were studied in the follicular phase, i.e. the first 14 days following the start of the last menstrual period.
Upon arrival, fasting exercise/imaging studies were performed which included magnetic resonance imaging (MRI) of the calf for maximal cross-sectional area (MCSA), proton magnetic resonance spectroscopy (1H MRS) to measure IMCL content, and in-MRI exercise testing with 31phosphorus MRS (31P MRS). Body composition was measured with dual-energy X-ray absorptiometry (DEXA) scan (Hologic, Waltham, MA) (Nadeau et al., 2010). Blood glucose was checked in youth with T2D immediately prior to the MRI.
2.3. Measure of insulin sensitivity
All participants consumed an isocaloric study dinner and then fasted overnight on the inpatient Clinical/Translation Research Center (CTRC). Youth with diabetes received an overnight insulin infusion to normalize glycemia (goal glucose ≈ 100 mg/dl). In the AM, a fasting hyperinsulinemic euglycemic clamp (80 mU/m2 min insulin) was performed to measure insulin sensitivity, similar to our previously described methods (Nadeau et al., 2010). Briefly, serum glucose concentrations were maintained at approximately 95 mg/dL based on blood samples every 5 min, and analyzed with a YSI (Yellow Springs Instrument, OH). Glucose infusion rate (GIR) was expressed as milligrams dextrose infused per kilogram fat-free mass from the DEXA scan, corrected for serum blood glucose.
2.4. Magnetic resonance imaging and spectroscopy
2.4.1. Imaging acquisition
Imaging and spectroscopy were performed on a General Electric (GE) 3 T magnet with HDx MRI (GE, Milwaukee, WI) running Version 15M4 software equipped with the GE multi-nuclear spectroscopy accessory of hardware and research software and a custom 1H/31P leg coil (Clinical MR Solutions, Brookfield, WI) (Cree-Green et al., 2014). The coil is a concentric probe made of an inner coil 9 cm in diameter (for 31P) and a 13 cm outer coil tuned to 1H frequency for scout imaging and shimming. Cross-sectional area for the calf was measured as previously described (Cree-Green et al., 2014).
2.4.2. Spectroscopy
Rates of mitochondrial phosphorylation were assessed by 31P MRS performed at 51.70 MHz with the 1H/31P coil. The machine was auto-shimmed with 1H, and then a 31P scan was performed for resting baseline measurements (long TR of 15,000 ms, flip angle of 135° and 32 scans) to measure a fully relaxed spectrum. The 31P exercise scan was then performed under partially saturated conditions (TR 1000 ms, flip angle 135, 2048 pts). IMCL and EMCL were measured via 1H MRS, as previously described (Nadeau et al., 2010).
2.4.3. 31P MRS exercise protocol
Strength testing to determine maximal volitional contraction (MVC) was done on a custom-built MR-compatible plantar flexion device with force measurement capability and force during the exercise perturbation recorded as previously described (Cree-Green et al., 2014; Larson-Meyer, Newcomer, Hunter, Hetherington, & Weinsier, 2000).
The 31P MRS exercise protocol consisted of measurements during rest for 90 s, isometric plantar flexion exercise for 90 s at 45% and 70% MVC, and recovery for 5 min post-exercise. We selected a 90 s isometric exercise bout as this perturbation has been extensively modeled and utilized for assessing both aerobic and anaerobic processes (Sirikul, Hunter, Larson-Meyer, Desmond, & Newcomer, 2007). Force was monitored continuously throughout the exercise, with verbal feedback provided to the subject to help keep the force measurements within the target goal. The average force applied was recorded in kg. All participants were able to complete the exercise for 90 s at or near target force.
2.4.4. Spectroscopy analysis
Peak positions and areas of interest [phosphocreatine (PCr), inorganic free phosphate (Pi), β-ATP(3 peaks), α-ATP(2 peaks), γ-ATP(2 peaks), and phosphomonoester] were determined by time domain fitting using jMRUi (Klose, 1990; van den Boogaart, 1997) utilizing AMARES (A Method of Accurate, Robust and Efficient Spectral fitting), a nonlinear least-square-fitting algorithm using previously built prior knowledge files (Rico-Sanz et al., 1999). All exercise spectra were corrected for saturation using the fully relaxed spectra for that day. The jMRUi data were used to calculate metabolic variables as previously described (Newcomer & Boska, 1997). Calculations included the rates of oxidative phosphorylation (OxPhos) following exercise, creatine kinase (CK) reaction, initial PCr synthesis (VPcr), anaerobic glycolysis (AnGly) and QMAX, the apparent mitochondrial capacity, i.e. maximal oxidative ATP production rate, as previously described (Cree-Green, Newcomer, Brown et al., 2015; Cree-Green et al., 2014). ADP, PCr and Pi time constants were calculated via regression analyses with Sigmaplot (Systat Software, Inc., San Jose, CA). Time constant measures are blood flow dependent, as they include the entire recovery period which at 20–40 s allows for ample time for all oxygen delivery following the cessation of exercise. QMAX is thus also blood-flow dependent as ADPTC is in the calculation. AnGly, OxPhos, and CK are all based only on substrate availability at the time of cessation of exercise, as they only include the first 10 s of data post exercise, which is not enough time for blood flow related reperfusion and delivery to the mitochondria. QMAX is a calculation of maximal mitochondrial function. Therefore, these endpoints were also chosen in order to distinguish the effect of blood-flow on mitochondrial function.
1H MRS data were analyzed as previously described (Nadeau et al., 2010). IMCL concentrations, obtained by reference to the unsuppressed water peak, are reported in institutional units.
2.5. Statistical analysis
The distribution of all variables was examined via a Shapiro–Wilk test and results presented as mean ± SD of the mean or median (25%, 75%) as appropriate. Group comparisons were made using one way ANOVA or Kruskal–Wallis for non-normally distributed variables. The association between GIR and covariates: HbA1c, BMI Z-score, IMCL, and FFA concentrations during hyperinsulinemia and the three primary mitochondrial outcomes from the 70% exercise were examined (ADP time constant, Qmax, and OxPhos) using a Spearman correlation. As the mitochondrial measurements are interrelated, a linear regression model of GIR vs. each of the three primary mitochondrial outcomes (ADP time constant, QMAX, and OxPhos) was then performed, including only those covariates with a correlation significantly different from zero. P-values <0.05 were considered statistically significant. All statistical analyses were performed with SigmaStat Software, Version 11.2 (Systat Software, Inc., San Jose, CA.)
3. Results
Of the 66 adolescents enrolled, 17 had T2D and 49 were non-diabetic controls (26 OCs, 23 LCs). Participant demographics are shown in Table 1. Groups were of similar sex, age and pubertal distributions. All but 1 participant were Tanner stage 4 or 5 with no significant difference in Tanner stage between groups. Participants with diabetes had a median HbA1c of 6.9%, and a diabetes duration of <2 years. BMI was similar between the T2D and OC groups. Of note, hs-CRP and platelets were different between groups, and significantly highest in the youth with T2D; other markers of inflammation were not significantly different. Fasting lipids were significantly different between the groups, and most abnormal in the youth with T2D. The insulin clamps were similar between the groups in terms of serum insulin concentrations and achieved glucose serum concentrations. Suppression of FFAs during the clamp in response tohyperinsulinemia (consistent with adipose IR) was different between the groups (p = 0.007) and most abnormal in the T2D group as was the GIR, consistent with muscle IR. IMCL concentrations were different between groups, and highest in the group with T2D (Table 1, p = 0.04).
Table 1.
Participant characteristics and metabolic outcomes.
| Lean Control (N = 23) | Obese Control (N = 26) | T2D (N = 17) | ANOVA P-value | * = LC vs. T2D + = LC vs. OC ** = OC vs. T2D |
|
|---|---|---|---|---|---|
| Gender (M/F) | 6/17 | 4/22 | 3/14 | – | |
| Age (years) | 15.0 (13.0, 17.0) | 14.0 (13.0, 16.3) | 15.0 (13.0, 16.5) | 0.643 | |
| Race n (%) | |||||
| White | 13 (56.5%) | 9 (34.6%) | 3(17.6%) | ||
| Hispanic | 4 (17.4%) | 11 (42.3%) | 8 (47.1%) | ||
| Black | 6 (26.1%) | 7 (26.9%) | 6 (35.3%) | ||
| Other (more than 1) | 1 (4.3%) | 1 (3.8%) | 1 (5.9%) | ||
| BMI (kg/m2) | 20.1 (18.9, 22.4) | 31.8 (29.7, 34.4) | 33.1 (28.3, 38.9) | <0.001 | *,+ |
| Waist/Hip Ratio | 0.80 ± 0.01* | 0.92 ± 0.02* | 0.96 ± 0.02* | <0.001 | *,+ |
| Intramyocellular lipid (arbitrary units) | 1896 ± 339 | 3211 ± 456 | 3649 ± 553 | 0.04 | *,+ |
| HbA1c (%) | 5.3 (5.1, 5.4) | 5.4 (5.2, 5.5) | 6.9 (5.7, 8.4) | <0.001 | *, ** |
| Inflammatory Markers | |||||
| AST (U/L) | 31 (25, 42) | 34 (29, 49) | 29 (24, 39) | 0.237 | |
| ALT (U/L) | 27 (20, 34) | 33 (26, 37) | 23 (17, 36) | 0.137 | |
| hsCRP (mg/L) | 0.2 (0.1, 0.8) | 1.4 (0.4, 2.3) | 2.1 (1.0, 4.9) | <0.001 | *,+ |
| Myeloperoxidase (mg/L) | 362 ± 62 | 519 ± 39 | 487 ± 55 | 0.172 | |
| WBC (1 K cell/μg) | 6.1 (4.5, 7.1) | 6.7 (5.2, 7.7) | 7.1 (5.8, 8.1) | 0.199 | |
| Platelets (1 K cell/μg) | 241 (231, 290) | 252 (222, 287) | 293 (264, 339) | 0.022 | *,** |
| Serum Lipids | |||||
| Total cholesterol (mg/dL) | 147 ± 6 | 156 ± 7 | 146 ± 8 | 0.527 | |
| Triglycerides (mg/dL) | 74 (58, 93) | 100 (87, 19) | 124(83, 273) | 0.002 | *,** |
| HDL (mg/dL) | 46 ± 2* | 40 ± 2* | 33 ± 2* | 0.001 | *,+,** |
| LDL (mg/dL) | 87 ± 6 | 75 ± 6 | 98 ± 6 | 0.021 | ** |
| Clamp Measurements | |||||
| FFA end clamp (mmol/L) | 21.0 (17.0, 29.0) | 35.0 (24.3, 48.0) | 66.0 (31.5, 153.0) | 0.007 | * |
| Insulin end clamp (mmol/L) | 94.5 (93.4,100.2) | 93.4 (91.6, 96.3) | 94.9 (90.1, 101.1) | 0.499 | |
| Blood Glucose end clamp (mg/dL) | 95 (93, 100) | 93 (92, 96) | 95 (90, 101) | 0.429 | |
| Glucose infusion rate (mg/kg FFM/min) | 18.5 ± 0.99* | 14.4 ± 1.3* | 9.1 ± 1.5* | <0.001 | *,** |
| Glucose infusion rate (mg/kg/min) | 13.0 ± 0.6* | 7.9 ± 0.8* | 4.3 ± 0.7* | <0.001 | *,+,** |
Values are reported as N (%), mean ± SD, or median (min, max). P-values are from One Way Analysis of Variance or Kruskal–Wallis, as appropriate.
Group means of raw and calculated data from 31P MRS for 70% exercise are shown in Fig. 1. The peak ADP concentration during exercise was lower in the group with T2D than LCs (Fig. 1A), and thus free phosphate concentrations were also lower in T2D youth (Fig. 1C). However, youth with T2D had similar perturbations in PCr (Fig. 1B) and pH (Fig. 1D). The three groups worked equally hard during exercise, as force generated per area was similar between groups (Table 2). The tracings of force throughout the exercise bouts are shown in Fig. 2. Youth with T2D generated a similar isometric force relative to the size of their calf muscle for both levels of exertion, indicating equal relative workloads, and the mean force over the entire 70% exercise bout was identical between groups (Cree-Green et al., 2014).
Table 2.
Markers of muscle metabolism from 70% and 45% force.
| 70% Maximal Force | Lean Control (N = 23) | Obese Control (N = 26) | T2D (N = 17) | ANOVA p-value | * = LC vs. T2D + =LC vs. OC ** = OC vs. T2D |
|---|---|---|---|---|---|
| Force/area (kg/cm2) | 0.007 (0.0058, 0.0098) | 0.007 (0.0063, 0.008) | 0.005 (0.0047, 0.0068) | 0.006 | *, ** |
| Anaerobic Glycolysis (mmol/L/s) | 0.28 (0.13, 0.49) | 0.23 (0.17, 0.62) | 0.31 (0.16, 0.55) | 0.810 | |
| Creatine Kinase ATP production (mmol/L/s) | 0.06 (0.03, 0.11) | 0.09 (0.04, 0.14) | 0.08 (0.03, 0.17) | 0.387 | |
| Oxidative Phosphorylation (mmol/L/s) | 0.18 (0.13, 0.26) | 0.18 (0.12, 0.23) | 0.11 (0.06, 0.18) | 0.014 | * |
| Total ATP production (mmol/L) | 0.60 (0.06, 0.65) | 0.52 (0.28, 0.80) | 0.51 (0.35, 0.90) | 0.813 | |
| ADP TC (s) | 18.5 (15.4, 22.6) | 21.3 (18.0, 23.5) | 23.1 (19.1, 27.1) | 0.034 | * |
| PCr TC (s) | 29.4 (24.6, 35.4) | 29.1 (25.7, 33.8) | 28.1 (23.9, 51.3) | 0.798 | |
| VPCr (mmol/s) | 0.29 (0.21, 0.44) | 0.27 (0.17, 0.40) | 0.18 (0.06, 0.25) | 0.019 | |
| QMax (mmol/s) | 0.484(0.413, 0.637) | 0.513(0.378, 0.602) | 0.342(0.182, 0.557) | 0.177 | |
| Mitochondrial Efficiency | 0.107(0, 0.136) | 0.149(0.104, 0.258) | 0.0831(0.060, 0.189) | 0.010 | + |
| 45% Maximal Force | |||||
| Force/area (kg/cm2) | 0.0063 ± 0.0006 | 0.0065 ± 0.0019 | 0.0044 ± 0.0005 | 0.092 | |
| Anaerobic Glycolysis (mmol/L/s) | 0.270 (0.163, 0.383) | 0.360 (0.078, 0.580) | 0.360 (0.116, 0.760) | 0.863 | |
| Creatine Kinase ATP production (mmol/L/s) | 0.05 (0.03, 0.12) | 0.04 (0.01, 0.08) | 0.05 (0.03, 0.07) | 0.124 | |
| OxPhos (mmol/L/s) | 0.168 (0.104, 0.213) | 0.078 (0.018, 0.142) | 0.095 (0.071, 0.167) | 0.085 | |
| Total ATP production (mmol/L) | 0.07 ± 0.02 | 0.19 ± 0.0 | 0.13 ± 0.04 | 0.121 | |
| ADP TC (s) | 15.3 (13.9, 18.3) | 24.1 (19.2, 27.5) | 22.7 (18.1, 33.5) | 0.018 | * |
| PCr TC (s) | 21.8 (17.8, 26.4) | 25.7 (22.6, 31.7) | 26.9 (21.1.9, 33.8) | 0.174 | |
| VPCr (mmol/s) | 0.26 ± 0.04 | 0.14 ± 0.02 | 0.13 ± 0.03 | 0.024 | * |
| QMax (mmol/s) | 0.588 ± 0.083 | 0.379 ± 0.034 | 0.320 ± 0.056 | 0.016 | * |
Mitochondrial metabolite measurements and calculations are shown following 90 s of 70% and 45% maximal volitional force calf exercise. Significance of p < 0.05 is denoted, with post-hoc testing identifying which groups were different from each other. * = LC vs. T2D; + = LC vs. OC; ** = OC vs. T2D.
Despite the lower ADP concentrations during 70% exercise, ADPTC was different between groups and longest in T2D (T2D 23.1(19.1, 27.0) s vs. OC 21.2 (18.1, 23.5) vs. LC 18.5(15.3, 22.6); p = 0.03), with a longer ADPTC indicating slower mitochondrial rates of conversion of ADP to ATP. The youth with T2D also had a lower rate of oxidative phosphorylation (T2D 0.11(0.06, 0.18) mmol/L/s vs. OC 0.18 (0.12, 0.23) vs. LC 0.18 (0.13, 0.26); p = 0.01). However, there was no difference in QMAX, (T2D 0.34(0.18, 0.56) mmol/L/s vs. OC 0.51 (0.38, 0.60) vs. LC 0.48(0.41, 0.64); p = 0.18). Rates of the creatine kinase reaction were also similar between groups. Results from 45% exercise in a subset of subjects were similar in terms of ADP TC (T2D 22.7 (18.1, 33.5) s vs. OC 24.1 (19.2, 27.5) vs. LC 15.3 (13.8, 18.3); p = 0.01) with trends in oxidative phosphorylation (T2D 0.09 (0.07, 0.18) mmol/L/s vs. OC 0.08 (0.02, 0.14) vs. LC 0.17(0.10, 0.21); p = 0.08). The substrate curves during 45% exercise are similar but lower than the 70%, and not shown.
Results from regression analyses for the predictors of GIR are shown in Table 3. Variables that were significant in the initial Spearman's correlation analysis were FFA following hyperinsulinemia (end FFA), HbA1c and the primary mitochondrial endpoints (OxPhos, QMAX and ADPTC from 70% maximal exercise). In Model 1, GIR correlated independently with ADPTC and FFA, but not HbA1c. In Model 2, GIR correlated independently with FFA but not OxPhos or HbA1c. In Model 3, GIR independently related to QMAX and FFA, but not HbA1c (Table 3).
Table 3.
Multiple regression of GIR relationship to mitochondrial outcomes.
| Covariate | Parameter estimate | S.E. of parameter estimate | P-value |
|---|---|---|---|
| Model 1 (ADPTC) | |||
| ADP TC | −0.272 | 0.102 | 0.014 |
| HbA1c | −1.132 | 0.664 | 0.103 |
| FFA | −0.0908 | 0.0259 | 0.002 |
| Model 2 (OxPhos) | |||
| OxPhos | 0.207 | 8.940 | 0.981 |
| HbA1c | −1.266 | 0.752 | 0.106 |
| FFA | −0.0944 | 0.0249 | <0.001 |
| Model 3 (QMAX) | |||
| QMAX | 7.211 | 1.726 | <0.001 |
| HbA1c | −0.775 | 0.582 | 0.197 |
| FFA | −0.0797 | 0.0229 | 0.002 |
Linear regression outcomes and significance for GIR as related to either ADP time constant, Oxidative Phosphorylation and QMAX are shown, with FFA and HbA1C in the models.
4. Discussion
We examined the relationship between IR and post-exercise mitochondrial oxidative function, serum FFA, IMCL and hyperglycemia in youth with T2D, as compared to both lean and obese youth without diabetes. We found that adolescents with T2D have decreased muscle mitochondrial function and IR when compared to obese and lean youth without diabetes of similar age, pubertal stage, level of habitual physical activity, and for obese controls, similar BMI. Specifically, youth with T2D had a slower rate of conversion of ADP back to ATP following cessation of near-maximal isometric exercise, consistent with defects in oxidative phosphorylation. Additionally, youth with T2D had failure of suppression of FFA in response to high dose insulin, elevated IMCL and hyperglycemia. Whole body IR correlated independently only with elevated FFAs and muscle mitochondrial dysfunction. Therefore, FFAs and muscle mitochondrial dysfunction, but not IMCL or hyperglycemia, appear related to IR in pediatric T2D. Finally, impaired mitochondrial function may be partially related to alterations in blood flow, as measures requiring oxygen delivery were worse.
Post-exercise muscle mitochondrial function, represented by a slowed ADPTC and lower QMAX, was independently related to IR in youth with T2D. While no other mitochondrial data from youth with T2D have previously been reported, other groups have examined mitochondrial function in adults, with conflicting results (Antonetti, Reynet, & Kahn, 1995; Bajpeyi et al., 2011; Cree-Green, Newcomer, Brown et al., 2015; De Feyter et al., 2008; Schrauwen-Hinderling et al., 2007). Many groups have found a decrease in mitochondrial function, markers or proteins in adults with T2D (Shulman, 2014). However, mitochondrial dysfunction is not always present in T2D (De Feyter et al., 2008), and Martin, Morrison, Konstantopoulos, and McGee (2014) found that the relationship between the two is extremely complex and may differ by cell type. Acquired mitochondrial dysfunction may predispose to IR, as the elderly and individuals at risk for diabetes have mitochondrial dysfunction (Befroy et al., 2007; Shulman, 2014). Further, the association between mitochondrial function and IR has been validated in other patient populations including burn trauma, aging, type 1 diabetes and polycystic ovarian syndrome (Cree-Green, Newcomer, Coe et al., 2015; Cree-Green et al., 2013; DeLany et al., 2014; Levitt Katz et al., 2015). Our cross-sectional design does not permit differentiation of which abnormality, IR or mitochondrial dysfunction, comes first; our data indicate that both abnormalities can occur at a young age, early in T2D and can be seen following both mild and submaximal exercise.
Elevations in FFA during hyperinsulinemia were associated with IR in youth with T2D, both in this cohort and in a previous study (Kelsey, Forster et al., 2014). Supporting this finding is the work of Shulman (2014) in which rodents fed high-fat diets to increase serum FFA or given intravenous FFA infusions showed transient increases in muscle diacylglycerol (DAG) content which indirectly led to the inhibition of muscle insulin signaling. In addition, acute and chronic reductions of FFA via Acipimox have been shown to improve both mitochondrial function and whole body insulin sensitivity in adults with T2D (Bajaj et al., 2005; Daniele et al., 2014). Further, Daniele et al. (2013) demonstrated that reductions of plasma FFA concentrations improved mitochondrial-mediated ATP synthesis in skeletal muscle tissue, as well as insulin sensitivity. In contrast, a recent study found that whereas IR was improved with Acipomoxin obese controls and adults with T2D, mitochondrial function only improved in the controls, not in the adults with T2D (Phielix, Jelenik, Nowotny, Szendroedi, & Roden, 2014). Further investigation is needed on interventions that lower FFAs in order to improve mitochondrial function and/or IR in youth with T2D.
IMCL was different between groups, yet there was no relationship between GIR and IMCL, confirming our findings in a different, smaller cohort of youth with T2D (Nadeau et al., 2009). We have also demonstrated IR and mitochondrial dysfunction, unrelated to IMCL in adolescents with type 1 diabetes (Cree-Green, Newcomer, Brown et al., 2015). Similarly, in a study of normal weight control adults, mitochondrial function and peripheral IR were related, but no relationship was found with IMCL (DeLany et al., 2014). IMCL is a reliable measure of muscle lipid concentration and it can be used for muscle energy, as seen in caloric restriction or endurance exercise, or as an ectopic lipid disposal site as in obesity. Our findings support that IMCL may be related to fuel stores and availability, not directly related to mitochondrial oxidative function or IR.
Glycemia was unrelated to mitochondrial function and IR in relatively well-controlled T2D youth (mean HbA1c 6.9%). This finding is supported by studies in adults with and without diabetes (Antonetti et al., 1995; Cree et al., 2008; Rabol et al., 2009). In particular, Rabol et al. (2009) found that adults with diabetes and HbA1c in a range similar to that of our patient population experienced mitochondrial dysfunction independent of hyperglycemia. We also found that in youth with type 1 diabetes, HbA1c was not related to IR or mitochondrial function (Cree-Green, Newcomer, Brown et al., 2015). Therefore, when glucose levels are kept near target clinical glucose ranges, IR and mitochondrial function do not correlate with hyperglycemia.
Our results indicate that in youth with T2D, mitochondria themselves appear impaired, in addition to indicating that there are alterations in oxygen delivery. Methods utilizing 31P MRS are an ideal approach to assess the role of blood flow in skeletal muscle mitochondrial function in adolescents with T2D (Wu et al., 2012). Not only is MRS non-invasive, unlike previous muscle biopsy-based methods, but it also allows an in vivo assessment of mitochondrial function. Such in vivo methods avoid the perturbations that occur when concentrations and activities of enzymes in oxidative phosphorylation and the TCA cycle are measured after removing muscle tissue from its natural state and blood supply. Both calculations of ADP TC and QMAX include the entire recovery period following exercise, which is influenced by post-exercise blood flow (Sirikul et al., 2007). The abnormal blood flow-dependent mitochondrial measures in our volunteers with diabetes suggest that altered blood flow may contribute to their mitochondrial dysfunction. In support of this concept, Pedersen, Baekgaard, and Quistorff (2009) showed that mitochondrial function is impaired in adults with only diabetes and in adults with only peripheral arterial disease, and was worst in those with both diabetes and peripheral arterial disease. Moreover, we have previously shown evidence of vascular dysfunction in youth and adults with T2D (Bjornstad et al., 2014; Levitt Katz et al., 2015; Nadeau et al., 2005, 2009). Therefore, further study of the role of blood flow and delivery in T2D and its impact on mitochondrial function is indicated.
There are several strengths to our study. We were careful to choose obese controls with a BMI similar to youth with T2D to ensure control for adiposity. We also controlled acute and habitual physical activity, pubertal stage and menstrual cycle, which may all impact IR and mitochondrial function. We similarly provided a study diet to control for potential differences in home diet between groups, and withdrew metformin and controlled glycemia overnight in the inpatient setting so that all groups had fasting labs and the insulin clamp began with similar blood sugar levels. While subject burden limited us from an insulin infusion prior to mitochondrial measures, we did assess blood sugar prior to MRS and found it to be unrelated to mitochondrial endpoints. In addition, while muscle biopsy was considered, it is more difficult in youth, and our in vivo methods most closely reflect the normal physiologic state. However, our study does have some limitations, including the relatively small sample size, lack of longitudinal data and lack of direct blood flow measurements.
5. Conclusions
In summary, we found that youth with T2D have impaired mitochondrial function and increased concentrations of FFA during hyperinsulinemia, both of which independently relate to IR. Further, decreased mitochondrial function may also relate to impaired blood flow. As future therapeutics are developed to directly improve insulin sensitivity, those that involve mitochondrial function, FFA and improving vascular function should be strongly considered for youth with T2D.
Fig. 1.
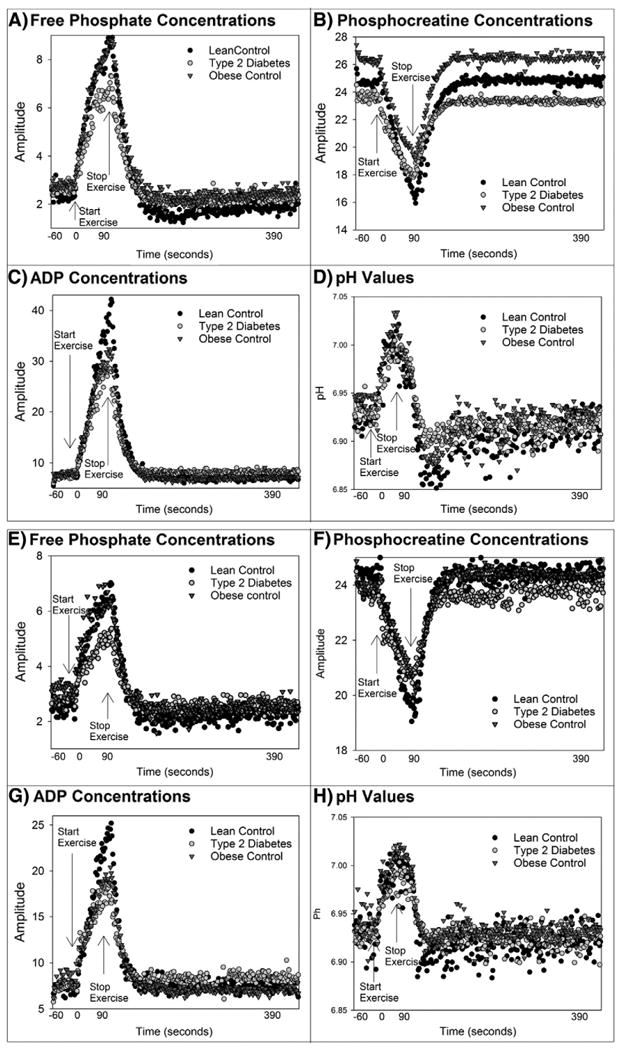
Average muscle metabolite concentrations before, during, and following exercise. Metabolite concentrations before, during and after 70% exercise are shown: A) shows inorganic phosphate, B) phosphocreatine concentrations, C) ADP D) intracellular pH. Metabolite concentrations before during and after 45% exercise are shown in: E) inorganic phosphate, F) phosphocreatine concentrations, G) ADP H) intracellular pH.
Fig. 2.
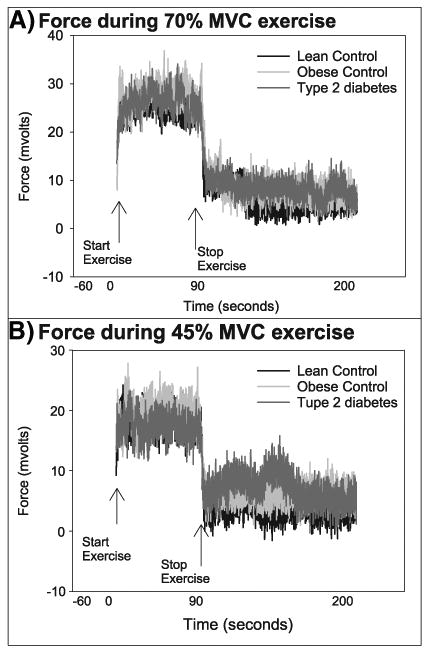
Plantar flexion force before, during, and after 70% and 45% exercise. Plantar flexion force of the soleus and gastrocnemius muscles before, during, and after 70% and 45% exercise.
Acknowledgments
Funding: KJN: NIH/NCRR K23 RR020038-05, NIH/NIDDK 1R56DK088971-01; Juvenile Diabetes Research Foundation JDRF5-2008-291, UCD Women's Health Research Pilot, ADA Career Development Award 7-11-CD-08. MCG: Fellowship in Pediatric Diabetes T32 DK063687; Thrasher Pediatric Research Foundation; CCTSI Co-Mentored Pilot Grant TL1 RR025778. UCD Center for Women's Health Research, AHA 13CRP14120015, Pediatric Endocrine Society Fellowship, BIRCWH K12HD057022. JEBR: VA Merit, Denver Research Institute, 5P01HL014985; Center for Women's Health Research. INSTITUTION: Adult GCRC NIH Grant #M01-RR00051, Pediatric CTRC NIH Grant #MO1 RR00069, NIH/NCRR Colorado CTSI Grant UL1 RR025780.
The authors would like to thank the participants and their families for participating. Also, Deb Singel for her MRI expertise.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- Antonetti DA, Reynet C, Kahn CR. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. The Journal of Clinical Investigation. 1995;95:1383–1388. doi: 10.1172/JCI117790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argov Z, De Stefano N, Arnold DL. ADP recovery after a brief ischemic exercise in normal and diseased human muscle—a 31P MRS study. NMR in Biomedicine. 1996;9:165–172. doi: 10.1002/(SICI)1099-1492(199606)9:4<165::AID-NBM408>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54:3148–3153. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Smith SR. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2011;96:1160–1168. doi: 10.1210/jc.2010-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, Nadeau KJ. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care. 2014;37:3033–3039. doi: 10.2337/dc14-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Green JM, Wolfe RR. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. American Journal of Surgery. 2008;196:234–239. doi: 10.1016/j.amjsurg.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015a;64:383–392. doi: 10.2337/db14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Newcomer BR, Brown M, Hull A, West AD, Singel D, Nadeau KJ. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Medicine and Science in Sports and Exercise. 2014 doi: 10.1249/MSS.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, Nadeau KJ. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. American Journal of Physiology. Endocrinology and Metabolism. 2015b;308:E726–E733. doi: 10.1152/ajpendo.00619.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Current Diabetes Reports. 2013;13:81–88. doi: 10.1007/s11892-012-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- D'Adamo E, Caprio S. Type 2 diabetes in youth: Epidemiology and pathophysiology. Diabetes Care. 2011;34(Suppl. 2):S161–S165. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele G, Eldor R, Merovci A, Clarke GD, Xiong J, Tripathy D, Defronzo RA. Chronic reduction of plasma FFA improves mitochondrial function and whole body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes. 2014;63(8):2812–2820. doi: 10.2337/db13-1130. http://dx.doi.org/10.2337/db13-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele G, Eldor R, Merovci A, Clarke GD, Xiong J, Tripathy D, DeFronzo RA. Chronic reduction of plasma free fatty acid improves mitochondrial function and whole-body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes. 2014;63:2812–2820. doi: 10.2337/db13-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJ, Prompers JJ. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. The FASEB Journal. 2008;22:3947–3955. doi: 10.1096/fj.08-112318. [DOI] [PubMed] [Google Scholar]
- DeLany JP, Dube JJ, Standley RA, Distefano G, Goodpaster BH, Stefanovic-Racic M, Toledo FG. Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the absence of obesity. The Journal of Clinical Endocrinology and Metabolism. 2014;99:4307–4314. doi: 10.1210/jc.2014-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterhammer R, Schocke M, Gorny O, Posch L, Messner H, Jaschke W, Greiner A. Phosphocreatine kinetics in the calf muscle of patients with bilateral symptomatic peripheral arterial disease during exhaustive incremental exercise. Molecular Imaging and Biology: MIB: The Official Publication of the Academy of Molecular Imaging. 2008;10:30–39. doi: 10.1007/s11307-007-0118-z. [DOI] [PubMed] [Google Scholar]
- Kelsey MM, Forster JE, Van Pelt RE, Reusch JE, Nadeau KJ. Adipose tissue insulin resistance in adolescents with and without type 2 diabetes. Pediatric Obesity. 2014a;9:373–380. doi: 10.1111/j.2047-6310.2013.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014b;60:222–228. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magnetic Resonance in Medicine. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier RL. 31P MRS measurement of mitochondrial function in skeletal muscle: Reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR in Biomedicine. 2000;13:14–27. doi: 10.1002/(sici)1099-1492(200002)13:1<14::aid-nbm605>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Levitt Katz L, Gidding SS, Bacha F, Hirst K, McKay S, Pyle L, Lima JA. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatric Diabetes. 2015;16:39–47. doi: 10.1111/pedi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, Chiarelli F. Insulin resistance in children: Consensus, perspective, and future directions. The Journal of Clinical Endocrinology and Metabolism. 2010;95:5189–5198. doi: 10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SD, Morrison S, Konstantopoulos N, McGee SL. Mitochondrial dysfunction has divergent, cell type-dependent effects on insulin action. Molecular Metabolism. 2014;3:408–418. doi: 10.1016/j.molmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. Journal of Pediatric Gastroenterology and Nutrition. 2005;41:94–98. doi: 10.1097/01.mpg.0000164698.03164.e5. [DOI] [PubMed] [Google Scholar]
- Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Reusch JEB. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. Journal of Clinical Endocrinology & Metabolism. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. The Journal of Clinical Endocrinology and Metabolism. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle & Nerve. 1997;20:336–346. doi: 10.1002/(SICI)1097-4598(199703)20:3<336::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Pedersen BL, Baekgaard N, Quistorff B. Muscle mitochondrial function in patients with type 2 diabetes mellitus and peripheral arterial disease: Implications in vascular surgery. European Journal of Vascular and Endovascular Surgery: The Official journal of the European Society for Vascular Surgery. 2009;38:356–364. doi: 10.1016/j.ejvs.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Phielix E, Jelenik T, Nowotny P, Szendroedi J, Roden M. Reduction of non-esterified fatty acids improves insulin sensitivity and lowers oxidative stress, but fails to restore oxidative capacity in type 2 diabetes: A randomised clinical trial. Diabetologia. 2014;57:572–581. doi: 10.1007/s00125-013-3127-2. [DOI] [PubMed] [Google Scholar]
- Rabol R, Hojberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2009;94:1372–1378. doi: 10.1210/jc.2008-1475. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. Journal of Applied Physiology. 1999;87:2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- Schocke M, Esterhammer R, Greiner A. High-energy phosphate metabolism in the exercising muscle of patients with peripheral arterial disease. VASA Zeitschrift fur Gefasskrankheiten Journal for Vascular Diseases. 2008;37:199–210. doi: 10.1024/0301-1526.37.3.199. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiomet-abolic disease. The New England Journal of Medicine. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- Sirikul B, Hunter GR, Larson-Meyer DE, Desmond R, Newcomer BR. Relationship between metabolic function and skeletal muscle fatigue during a 90 s maximal isometric contraction. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition et Metabolisme. 2007;32:394–399. doi: 10.1139/H06-117. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A. A user's guide to the magnetic resonance user Interface software package. Delft: Delft Technical University Press; 1997. MRUI MANUAL V. 96.3. [Google Scholar]
- Wu FY, Tu HJ, Qin B, Chen T, Xu HF, Qi J, Wang DH. Value of dynamic (3)(1)P magnetic resonance spectroscopy technique in in vivo assessment of the skeletal muscle mitochondrial function in type 2 diabetes. Chinese Medical Journal. 2012;125:281–286. [PubMed] [Google Scholar]
- Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England Journal of Medicine. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]


