Abstract
Background
To evaluate the efficacy and safety of Danggui Buxue Decoction for renal anemia when combined with western medicine treatment of anemia.
Methods
Electronic searching Medline, Embase, Web of Science, Cochrane Library, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), WanFang data, Chinese Sci-tech periodical full-text database (VIP). Randomized controlled trials reported results of efficacy and safety of Danggui Buxue Decoction in combination with western medicine treatment of anemia for renal anemia. The “risk of bias assessment tool (Version 5.1.0)” of Cochrane Handbook was applied to assess the quality of included trials and RevMan 5.3 software was used for data analysis.
Results
A total of 111 studies was retrieved, seven studies including 460 cases were included, the methodological quality of included trials was poor. The result of meta-analysis demonstrated that there was no difference in hemoglobin (Hb) [weighted mean differences (WMD) =−8.75, 95% confidence interval (CI): (−18.64, 1.13), P=0.08], whereas the subgroup analysis showed the difference was significant when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1 [WMD =−16.27, 95% CI: (−28.73, −3.80), P=0.01], increase of Hb was more effective in experimental group than control group and the difference was not significant when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5≠1 [WMD =−0.57, 95% CI: (−4.52, 3.39), P=0.78]. There were significant differences in red blood cell (RBC) [WMD =−0.49, 95% CI: (−0.69, −0.28), P<0.00001], hematocrit (HCT) [WMD =−1.92, 95% CI: (−3.15, −0.69), P=0.002] and clinical efficacy [odd ratio (OR) =0.30, 95% CI: (0.13, 0.69), P=0.004] between Danggui Buxue Decoction combination group and control group, the experimental group was better than control group. There was no adverse event reported in the experimental group.
Conclusions
Danggui Buxue Decoction in combination with conventional western medicine (CWM) for renal anemia might be superior to CWM alone and there was no adverse event in the experimental group, it might be more effective when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1. However, the quality of included studies was not high, and less attention was paid to the safety, high quality randomized controlled trials are needed to further confirm the findings.
Keywords: Danggui Buxue Decoction, combine traditional Chinese and western medicine, renal anemia, systematic review, meta-analysis
Introduction
Anemia in chronic kidney disease (CKD) is caused by reducing production of the erythropoietin (EPO) in the kidney and shortening the red cell survival (1). Anemia appears with the decline of renal function of CKD caused by multiple causes (2). Anemia could occur in the early CKD, and it’s a very common phenomenon in the 5th stage of CKD. At present, iron supplements, EPO and blood transfusion therapy are applied to the treatment of CKD (3). Danggui Buxue Decoction, a widely used traditional Chinese medicine, was created by Dongyuan Li during the Jin dynasty, the ratio of Radix Astragali to Radix Angelicae Sinensis is 5:1 (4). Danggui Buxue Decoction was recommended as reinforcement “Qi” (energy) and blood tonic, used to treat anemia in clinic (5). The serious adverse reactions caused by EPO included pure red cell aplasia, hypertension, hyperglycemia, and so on (6), meanwhile, the cost is expensive; excess iron supplements could cause chronic iron overload.
Thus, we conducted this meta-analysis aiming to assess the efficacy and safety of Danggui Buxue Decoction for renal anemia when combined with western treatment of anemia.
Methods
Inclusion criteria
(I) Type of study: randomized controlled trial (RCT), application of blinding or not is available. (II) Patients: diagnosed with renal anemia, chronic renal failure meets the diagnostic criteria of Clinical Practice Guidelines for Chronic Kidney Disease and Dialysis (7) and Minutes of the Symposium of Classification and Treatment of Primary Glomerular Disease and Diagnostic Criteria (8). There are no limitations for the age, gender, and primary disease. (III) Intervention: experimental group: on the basis of Danggui Buxue Decoction (DBD), according to syndrome differentiation prescribes medication, meanwhile, in combination with conventional western medicine (CWM), Western medicine treatment of anemia meets the criteria of Diagnosis and Treatment of Renal Anemia with Chinese Expert Consensus (Revision 2014) (3) (including iron supplements, EPO and blood transfusion therapy), and can be combined with folic acid and vitamin B12 (9); control group: CWM only. Control the blood pressure and glucose, regulate fluid and electrolyte balance and conduct hemodialysis depending on the specific situation in the experimental group and the control group. The course of treatment is not less than 4 weeks. (IV) Outcomes: the primary outcome is hemoglobin (Hb) (g/L); the secondary outcome is red blood cell (RBC) (1012/L), hematocrit (HCT) (%), clinical efficacy, creatinine (SCr, µmol/L), urea nitrogen (BUN, mmol/L), adverse events.
Exclusion criteria
Combined with other therapies, for example, enema, acupuncture, etc.
Search strategy
Two authors electronic searched Medline, Embase, Web of Science, Cochrane Library, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), WanFang data, Chinese sci-tech periodical full-text database (VIP) by the method of combining subject headings with free-text terms from their inception through February 2016. The search terms used in this search were as follows: “Danggui Buxue”, “renal anemia”, “chronic renal failure accompanied by anemia”. Take CBM for example, #1 Danggui Buxue, #2 anemia, #3 #1 and #2.
Document screening and quality assessment
According to inclusion criteria and exclusion criteria, two authors filtered documents independently, differences further confirmed by a third party. The individual quality of included studies was assessed based on the “risk of bias assessment tool (Version 5.1.0)” of Cochrane Handbook, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. RevMan 5.3 software was applied to perform risk of bias graph and risk of bias summary.
Data extraction
General information of the eligible studies including name of the first author, baseline, sample volume, gender, age, intervention, course of treatment, outcomes, adverse events.
Statistical analysis
RevMan 5.3 provided by the Cochrane Collaboration was used to perform the meta-analysis. Z-test analysis and I2 test were applied to evaluate the overall heterogeneity of included studies in the meta-analysis. The estimated outcomes of included trials were calculated with the random effect model if P<0.05, I2>50%, otherwise the fixed effect model was used. The pooled odd ratio (OR) was calculated with 95% confidence interval (CI) for dichotomous data. The weighted mean differences (WMD) were calculated with 95% CI for continuous data. Subgroup analysis and sensitivity analysis were conducted to evaluate the robustness of results when heterogeneity was present.
Results
Search results
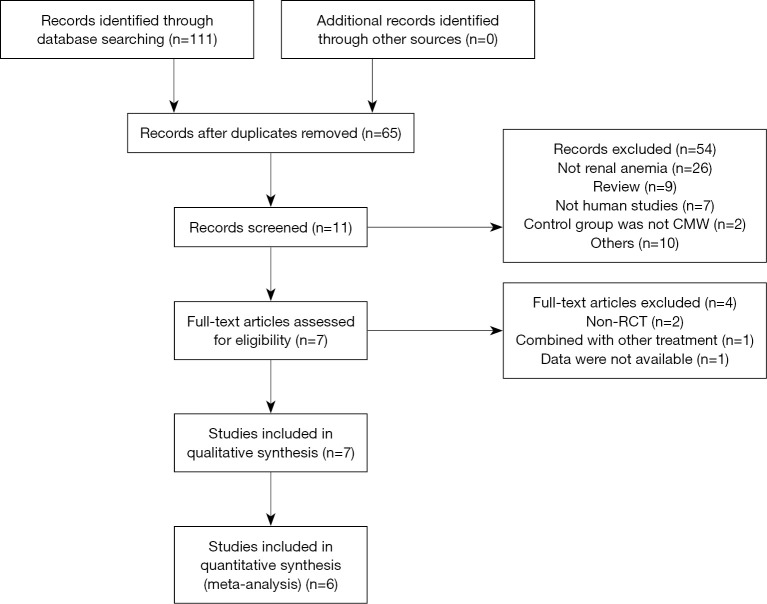
Medline (n=6), Embase (n=2), Web of Science (n=3), Cochrane Library (n=0), CBM (n=29), CNKI (n=32), WanFang data (n=28), VIP (n=11) were searched, a total of 111 studies were retrieved. According to inclusion criteria and exclusion criteria, seven studies were included in the final analysis. The process of selection of the eligible studies was illustrated in Figure 1.
Figure 1.
Flow diagram of searching for eligible studies.
Characteristics of included studies
A summary of the baseline characteristics of included studies was showed in Table 1. There were seven RCTs (N=460) in this systematic review. All RCTs were conducted in China and all studies published in Chinese. The duration of treatment ranged from four weeks to twelve weeks. Formula composition of included studies was presented in Table 2.
Table 1. Characteristics of included studies.
| Studies | Baseline | N (T/C) | Gender (M/F) | Age (Y) | Intervention | Duration (weeks) | Outcomes | Adverse events | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | ||||||
| Wang, 2015 (10) | Comparable | 30/30 | 15/15 | 16/14 | 61.2±2.4 | 61.3±2.4 | DBD + CWM | CWM | 6 | a, b, c, | 2 cases of cutaneous pruritus and 1 case of palpitation in the control group |
| Gao, 2014 (11) | Comparable | 34/34 | 19/15 | 16/18 | 54 | 51 | DBD + CWM | CWM | 12 | a, b, c, d | NA |
| Zhao, 2014 (12) | NA | 20/20 | NA | NA | NA | NA | DBD + CWM | CWM | 8 | a, c, d | NA |
| Xu, 2013 (13) | Comparable | 40/40 | 25/15 | 23/17 | 42.15 | 41.35 | DBD + CWM | CWM | 4 | d | NA |
| Li, 2013 (14) | Comparable | 43/43 | 22/21 | 24/19 | 43.2 | 41.5 | DBD + CWM | CWM | 8 | d | NA |
| Yang, 2013 (15) | Comparable | 38/38 | 42/43 | 47.2±4.9 | DBD + CWM | CWM | 8 | a, b, c | NA | ||
| Tong, 2003 (16) | NA | 32/18 | 17/15 | 10/8 | NA | NA | DBD + CWM | CWM | 8 | a, b, c, e, f | NA |
Outcomes: a, Hb; b, RBC; c, HCT; d, clinical efficacy; e, SCr; f, BUN. T, treatment; C, control; M, male; F, female; age, average age; Y, year; CWM, conventional western medicine; DBD, Danggui Buxue Decoction; NA, not available.
Table 2. Formula composition of included studies.
| Studies | Formula composition |
|---|---|
| Wang, 2015 (10) | Radix Astragali (Huangqi) 30 g, Radix Angelicae Sinensis (Danggui) 6 g, Glabrous Greenbrier Rhizome (Tufuling) 30 g, Motherwort Herb (Yimucao) 30 g, Suberect Spatholobus Stem (Jixueteng) 30 g, Mulberry Fruit (Sangshen) 15 g, Fleeceflower Root (Heshouwu) 15 g, Rhubarb (Shengdahuang) 9 g |
| Gao, 2014 (11) | Radix Astragali (Huangqi) 10 g, Radix Angelicae Sinensis (Danggui) 10 g |
| Zhao, 2014 (12) | Radix Astragali (Huangqi) 30 g, Radix Angelicae Sinensis (Danggui) 6 g, Prepared Rehmannia Root (Shudihuang) 10 g, Tangshen (Dangshen) 10 g, White Atractylodes Rhizome (Baizhu) 10 g, Poria (Fuling) 10 g, Liquorice Root (Gancao) 6 g |
| Xu, 2013 (13) | Radix Astragali (Huangqi) 50 g, Radix Angelicae Sinensis (Danggui) 10 g, Sichuan Lovage Rhizome (Chuanxiong) 10 g, Ass Hide Glue (Ejiao) 10 g, Dried Tangerine Peel (Chenpi) 10 g, Suberect Spatholobus Stem (Jixueteng) 30 g |
| Li, 2013 (14) | Radix Astragali (Huangqi), Radix Angelicae Sinensis (Danggui), Epimedium Herb (Xianlingpi), Suberect Spatholobus Stem (Jixueteng), White Atractylodes Rhizome (Baizhu), Poria (Fuling), the ratio of Radix Astragali (Huangqi) to Radix Angelicae Sinensis (Danggui) was 5:1 |
| Yang, 2013 (15) | Radix Astragali (Huangqi) 30 g, Radix Angelicae Sinensis (Danggui) 6 g, Tangshen (Dangsheng) 15 g, Poria (Fuling) 20 g, White Atractylodes Rhizome (Baizhu) 15 g, Common Yam Rhizome (Shanyao) 20 g, Lotus Seed (Lianzirou) 6 g, Hyacinth Bean (Baibiandou) 15 g, Coix Seed (Yiyiren) 20 g, Epimedium Herb (Yinyanghuo) 10 g, Ass Hide Glue (Ejiao) 10 g, Villous Amomum Fruit (Sharen) 6 g, Platycodon Root (Jiegeng) 6 g, Liquorice Root (Gancao) 6 g |
| Tong, 2003 (16) | Radix Astragali (Huangqi) 30 g, Radix Angelicae Sinensis (Danggui) 15 g, Debark Peony Root (Baishao) 15 g |
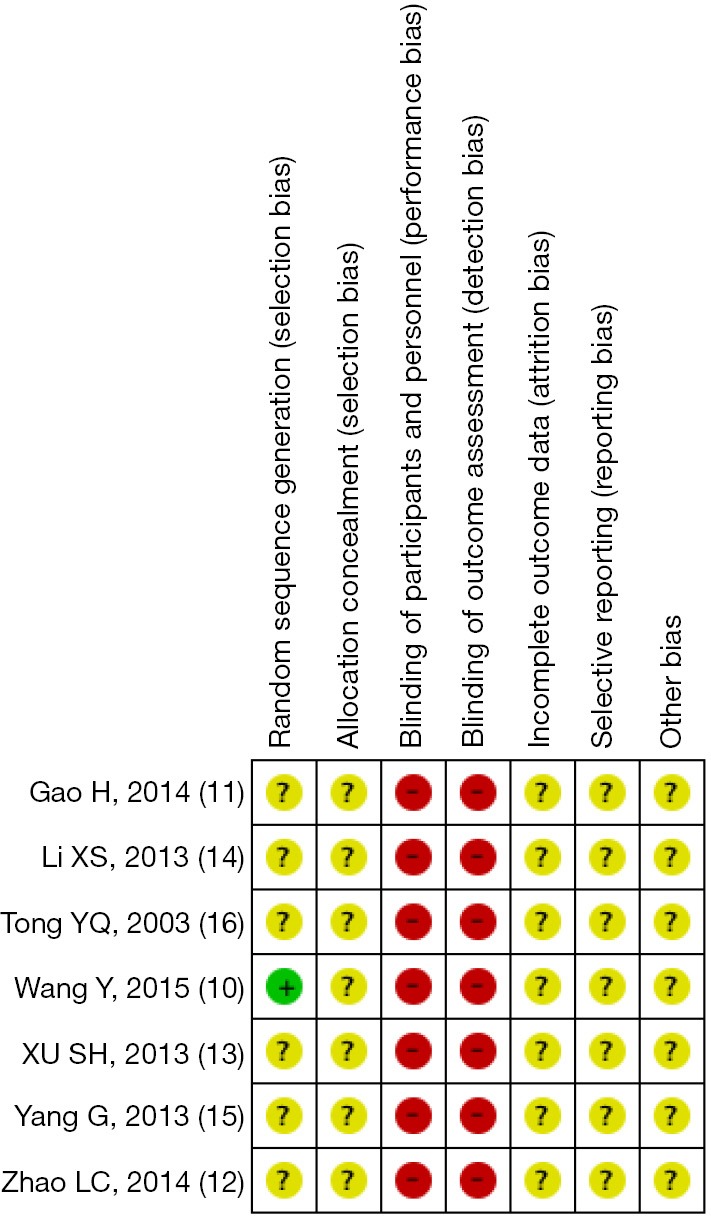
Risk of bias assessment
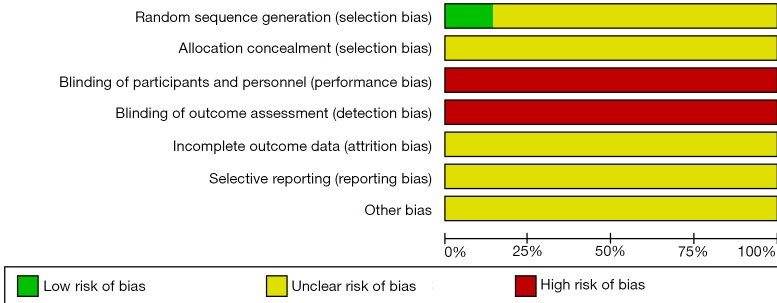
The overall quality of included studies was poor. The random number table was used for randomization in only one study (10), the remaining six trials simply mentioned “randomization”, specific methods hadn’t been reported. None of the studies mentioned the allocation concealment and blindness. There was neither any information about incomplete outcome data, selective reporting and other bias. The risk bias assessment of the methodological quality is shown in Figures 2,3.
Figure 2.
Risk of bias graph.
Figure 3.
Result of meta-analysis
Six studies (10,11,13-16) included for meta-analysis, one study (12) included for descriptive analysis.
Hb
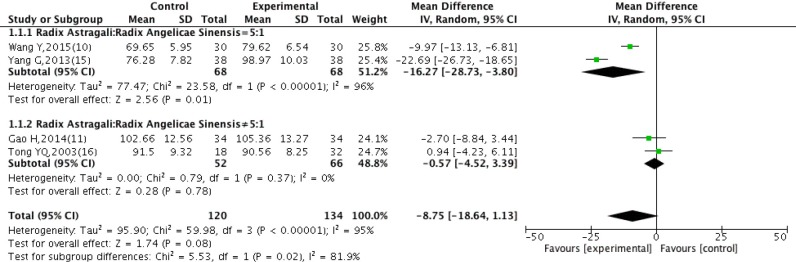
Four studies (10,11,15,16) included in this analysis provided the data of Hb. There was statistical heterogeneity (P<0.00001, I2=95%), the random effect model was used for meta-analysis. There was no statistically significant difference in Hb between experimental group and control group [WMD =−8.75, 95% CI: (−18.64, 1.13), P=0.08], whereas the subgroup analysis showed the difference was significant when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1 [WMD =−16.27, 95% CI: (−28.73, −3.80), P=0.01], increase of Hb was more effective in the experimental group than the control group. The difference was not significant when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5≠1 [WMD =−0.57, 95% CI: (−4.52, 3.39), P=0.78] (Figure 4).
Figure 4.
Subgroup analysis of Hb after treatment with DBD and CWM. Hb, hemoglobin; DBD, Danggui Buxue Decoction; CWM, conventional western medicine.
RBC
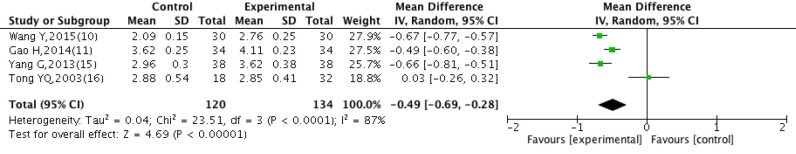
Four studies (10,11,15,16) reported data on the changes of RBC. These data were not found to be homogeneous (P<0.0001, I2=87%), the random effect model was applied in this meta-analysis. As shown in Figure 5, Danggui Buxue Decoction combined with CWM had an advantage of increasing the RBC compared with control group [WMD =−0.49, 95% CI: (−0.69, −0.28), P<0.00001].
Figure 5.
Forest plot comparison of RBC after treatment with DBD and CWM. RBC, red blood cell; DBD, Danggui Buxue Decoction; CWM, conventional western medicine.
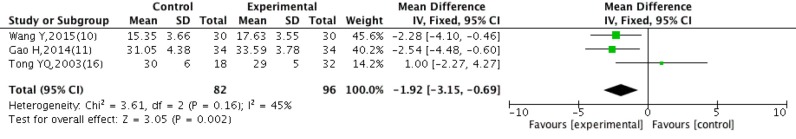
HCT
There were four studies (10,11,15,16) included in the meta-analysis on improvement of HCT. There was significant heterogeneity among data from the included studies (P<0.00001, I2=93%). Sensitivity analysis were conducted to evaluate the robustness of the result, there was no statistically significant heterogeneity among three (10,11,16) studies (P=0.16, I2=45%) except Yang (15), the fixed effect model was applied (Figure 6). Experimental group exhibited significant improvement of HCT, compared with control group [WMD =−1.92, 95% CI: (−3.15, −0.69), P=0.002].
Figure 6.
Forest plot sensitivity analysis of HCT after treatment with DBD and CWM. HCT, hematocrit; DBD, Danggui Buxue Decoction; CWM, conventional western medicine.
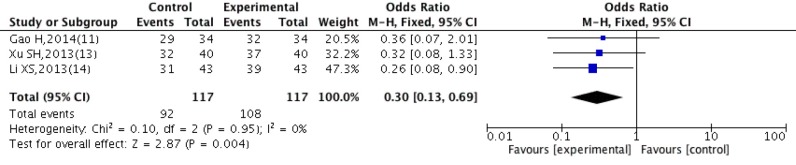
Clinical efficacy
Three studies (11,13,14) evaluated the clinical efficacy. There was no statistically significant heterogeneity among three studies (P=0.95, I2=0%), justifying the fixed effect model. As illustrated in Figure 7, result of clinical efficacy of the experimental group was significantly better than the control group [OR =0.30, 95% CI: (0.13, 0.69), P=0.004].
Figure 7.
Forest plot comparison of clinical efficacy after treatment with DBD and CWM. DBD, Danggui Buxue Decoction; CWM, conventional western medicine.
Other analysis results
Only one study (16) reported the data of SCr and BUN, the result revealed that SCr and BUN decreased significantly in the experimental group compared with the control group.
Only one (12) of eligible studies reported outcomes with mean ± standard of difference value of the result before and after treatment. There was statistically significant difference between experimental group and control group (P<0.05), experimental group was more effective than control group in terms of increasing RBC, Hb and HCT.
Adverse events
There were no adverse events reported in the experimental group. One (10) of included studies mentioned two cases of cutaneous pruritus and one case of palpitation in the control group.
Discussion
Danggui Buxue Decoction could not only withstand significantly decreation of blood cells by immune-mediated, but also stimulate on the growth of bone marrow colony cell and increase the weight of hemopoietic progenitor of bone marrow (17). In animal experiments, the ability of Danggui Buxue Decoction was to promote hematopoiesis and proliferative actions on hematopoietic progenitor cells, researchers have reported constituents related to various Danggui Buxue Decoction activities (18). The improvement of modified Danggui Buxue Decoction on blood deficiency might be related to increase of the expression of EPO and granulocyte-macrophage gene and protein (19). One of positive regulators for Danggui Buxue Decoction is Radix Angelicae Sinensis-derived ferulic acid, which enhanced the solubilities of active ingredients derived from astragali radix, therefore increased the biological efficacies of Danggui Buxue Decoction (20). Radix Angelicae Sinensis significantly enhanced the recovery of platelets, other blood cells and their progenitor cells, as well as the formation of colony forming unit (21). Modern pharmacological experiments showed Danggui Buxue Decoction not only promoted hematopoietic function, also had a role in immune regulation and liver protection (22), and attenuated renal lesion (23). Astragaloside IV could attenuate the apoptosis of renal tubular epithelial cells induced by transforming growth factor-β1 (TGF-β1) (24). Radix Astragali and Radix Angelicae Sinensis alleviated renal fibrosis by inhibiting the expression of TGF-β1/connective tissue growth factor mRNA (25), astragaloside IV and ferulic acid also can alleviate renal tubulointerstitial fibrosis, associated with not only inhibition of tubular epithelial-mesenchymal transdifferentiation and fibroblast activation, but also an increase in NO production in the kidney (26).
Assessment of methodological quality
Selection bias can be prevented by randomization, experimental design should follow the randomization and select the correct method. Six of included studies mentioned “randomization”, but did not report the specific methods, random number table was used in only one study (10).
Allocation concealment can also avoid selection bias, however, included studies didn’t describe allocation concealment.
Performance bias and measurement bias might occur if the intervention and assignment known by participators. Seven studies didn’t refer to blinding. In terms of appearance and taste, there were significant differences between traditional Chinese medicine decoction and western medicine, blinding is sometimes difficult to implement.
There was neither any information about selective reporting nor incomplete outcome reporting. Other bias could not be determined. There were high risks of selection and performance biases, therefore the overall quality of included studies was low.
Efficiency
The result of meta-analysis demonstrated that there was no difference in Hb [WMD =−8.75, 95% CI: (−18.64, 1.13), P=0.08], whereas the subgroup analysis showed the difference was significant when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1 [WMD =−16.27, 95% CI: (−28.73, −3.80), P=0.01], increase of Hb was more effective in the experimental group than the control group. Compared with other ratios, higher contents of ferulic acid and Astragaloside IV in Danggui Buxue Decoction when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1 (27). One (11) of two studies (11,16) with the ratio of Radix Astragali to Radix Angelicae Sinensis was 5≠1 adjusted the dosage of EPO, the other study reduced the dosage of EPO when combined with Danggui Buxue Decoction. No matter what the ratio of Radix Astragali to Radix Angelicae Sinensis was, Danggui Buxue Decoction combined with CWM could attenuate renal lesion, it might be more effective when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1. Traditional Chinese medicine can instead of EPO partially, reduced the dosage of EPO (28). Combination of Danggui Buxue Decoction and CWM was more effective in improving the symptoms and RBC, HCT. Danggui Buxue Decoction also has a role in kidney protection.
Due to poor methodological quality, the beneficial effect and safety of Danggui Buxue Decoction combined with CWM needed to be confirmed further.
Safety
Only one study (10) of included studies reported adverse events in the control group, the safety of Danggui Buxue Decoction combined with CWM was supposed to be high, whereas, the result was limited by low-quality studies.
Limitations
Several limitations were existed in this meta-analysis. Firstly, only five (10,11,13-15) of the seven studies mentioned the comparable baselines. Secondly, only seven studies were included and the sample sizes were small, the power of test might be influenced. Thirdly, the sample sizes of included studies was inconsistent, increasing the risk of heterogeneity. Fourthly, we included articles published in English or Chinese only, this might have increased the selection bias.
Conclusions
Danggui Buxue Decoction in combination with CWM for renal anemia might be superior to CWM alone. It might be more effective when the ratio of Radix Astragali to Radix Angelicae Sinensis was 5:1 and there was no adverse event in the experimental group. Future clinical trials on evaluating the efficacy and safety of Danggui Buxue Decoction should be designed more rigorously.
Acknowledgements
Thank Evidence-Based Clinical Club, EBC.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81303151), Beijing Nova Program (No. xxjh2015A093 and No. 1511000003150125), and Subjective Selected Subjects Program of China Academy of Chinese Medical Sciences (No. ZZ0708105).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tsagalis G. Renal anemia: a nephrologist's view. Hippokratia 2011;15:39-43. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HY, Li XM, Zhao MH, et al. editors. Nephrology. 3rd edn. Beijing: People's Medical Publishing House, 2008. [Google Scholar]
- 3.Renal Physicians Association Branch of China Consensus Group of Experts on the Diagnosis and Treatment of Renal Anemia Diagnosis and Treatment of Renal Anemia with Chinese Expert Consensus (Revision 2014). Chinese Journal of Nephrology 2014;30:712-5. [Google Scholar]
- 4.Chan PH, Zhang WL, Cheung CY, et al. Quality Control of Danggui Buxue Tang, a Traditional Chinese Medicine Decoction, by (1)H-NMR Metabolic Profiling. Evid Based Complement Alternat Med 2014;2014:567893. [DOI] [PMC free article] [PubMed]
- 5.Wang P, Liang YZ. Chemical composition and inhibitory effect on hepatic fibrosis of Danggui Buxue Decoction. Fitoterapia 2010;81:793-8. 10.1016/j.fitote.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Qian ZG, Li G. Literature Analysis of Adverse Drug Reactions induced by Recombinant Human Erythropoietin in 109 Case Reports. Pharmaceutical Care and Research 2014;14:45-8. 10.5428/pcar20140115 [DOI] [Google Scholar]
- 7.Wang HY, Wang M, Zuo L, et al. editors. Clinical Practice Guidelines for Chronic Kidney Disease and Dialysis. Beijing: People's Medical Publishing House, 2003. [Google Scholar]
- 8.Chinese Journal of Internal Medicine editorial board of the Professional group of Nephrology. Minutes of the Symposium of Classification and Treatment of Primary Glomerular Disease and Diagnostic Criteria. Chinese Journal of Internal Medicine 1993;32:131. [Google Scholar]
- 9.Chen YP, Yu XQ. editors. Nephrology. 2th ed. Beijing: People's Medical Publishing House, 2015. [Google Scholar]
- 10.Wang Y. Clinical Research on Treating 30 Cases of Anemia Accompanied with Chronic Renal Failure with the Danggui Buxue Decoction. Clinical Journal of Chinese Medicine 2015;7:80-1. [Google Scholar]
- 11.Gao H, Yuan J, Cheng XH. Danggui Buxue Decoction Combined with Recombinant Human Erythropoietin. Henan Traditional Chinese Medicine 2014;34:549-50. [Google Scholar]
- 12.Zhao LC. Jiawei Danggui Buxue Decoction in Treating 20 Cases of Renal Anemia in Type of Deficiency of Both Qi and Blood. Henan Traditional Chinese Medicine 2014;31:2049. [Google Scholar]
- 13.Xu SH. Danggui Buxue Decoction and Recombinant Erythropoietin in the Treatment of 40 Cases of Renal Anemia. Zhejiang Journal of Traditional Chinese Medicine 2013;48:414. [Google Scholar]
- 14.Li XS, Tang Y. Clinical Effect Observation of Supplemented Dangguibuxue Decoction Combined with Erythropoietin in Treatment of Renal Anemia. China Modern Medicine 2013;20:108-9. [Google Scholar]
- 15.Yang G. Danggui Buxue Decoction Combined with Shenlingbaishu Powder Curative Effect Observation on Treatment of Renal Anemia. Chinese Journal of Ethnomedicine and Ethnopharmacy 2013;22:88. [Google Scholar]
- 16.Tong YQ, Wang HF. Clinical Observation of Jiawei Danggui Buxue Decoction on Treatment of Renal Anemia. Chinese Journal of Integrated Traditional and Western Medicine 2003;23:140. [Google Scholar]
- 17.Yang X, Huang CG, Du SY, et al. Effect of Danggui Buxue Tang on immune-mediated aplastic anemia bone marrow proliferation mice. Phytomedicine 2014;21:640-6. 10.1016/j.phymed.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Chin YW, Heo TH. Erythropoietic Agents From Natural Sources. Altern Ther Health Med 2013;19:54-60. [PubMed] [Google Scholar]
- 19.Yao N, Huang XJ, Du TL, et al. Effect of Modified Dangguibuxuetang on the Expression of EPO and GM-CSF in Blood Deficiency Mice. Journal of Guangdong Pharmaceutical University 2013;29:177-80. [Google Scholar]
- 20.Zheng KY, Zhang ZX, Du CY, et al. Ferulic acid enhances the chemical and biological properties of astragali radix: a stimulator for danggui buxue tang, an ancient Chinese herbal decoction. Planta Med 2014;80:159-64. 10.1055/s-0033-1360314 [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Li J, Meng FY, et al. Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement Altern Med 2010;10:79. 10.1186/1472-6882-10-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou XM, Zhou X, Li YM. Research Progress of Danggui Buxue Decoction. World Chinese Medicine 2013,6:705-7. [Google Scholar]
- 23.Dong Q, Chen MC. Chemical Composition and Pharmacological Research Progress of Angelica. Asia-Pacific Traditional Medicine 2016;12:32-4. [Google Scholar]
- 24.Tian L, Xu WJ, Zhang Z, et al. Effects of Astragaloside IV on the apoptosis of renal tubular epithelial cells induced by transforming growth factor-β1. Chinese Journal of Nephrology Dialysis & Transplantion 2015;24:139-44. [Google Scholar]
- 25.Ye TS, Yao Q, Zhang YW. Experimental Study of Angelica and Astragalus on Rat Renal Fibrosis Drug Expression on Regulation of TGF-β1 /CTGFmRNA. Hubei Journal of Traditional Chinese Medicine 2016;38:1-4. [Google Scholar]
- 26.Meng LQ, Tang JW, Wang Y, et al. Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br J Pharmacol 2011;162:1805-18. 10.1111/j.1476-5381.2011.01206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei CH. Effect of Different ratio of Radix Astragali to Radix Angelicae Sinensis in Dang Gui Bu Xue Decoction on Chemical Components. Sichuan Journal of Traditional Chinese Medicine 2015;33:51-3. [Google Scholar]
- 28.Liu MX, Zhang S, Li Q. Research Progress of traditional Chinese and Western Medicine Treatment of renal anemia. Chinese Journal of Integrated Traditional and Western Nephrology 2015;16:552-3. [Google Scholar]