Abstract
This study proposed a rapid method to quantify the colonization rate of arbuscular mycorrhizal fungi (AMF) in plant roots. The method involved the use of an image analysis software (WinRHIZO Pro). The colonization rate is defined as the ratio of the fungal body to the plant root area in a micrograph. Three seedlings of Chengiopanax sciadophylloides, a woody species that accumulates radiocesium, were collected from a secondary forest in the Yamakiya district of Kawamata, Fukushima Prefecture during May–September 2014. The colonization of AMF structures was examined under a light microscope, and the percentage of colonization was determined using the WinRHIZO method. The superiority of the new method was verified by comparing with a modified grid-line intersect method. The colonization of AMF was confirmed in all the seedlings, and a significant coefficient of determination (R2 = 0.94) was found with both the methods. The results suggested that the WinRHIZO method is reliable for estimating the colonization of AMF in C. sciadophylloides.
Keywords: Image analysis, Microscopy, WinRHIZO, Woody plants
The behavior and accumulation of radiocesium (134Cs and 137Cs) in various ecosystems have been intensively investigated in Japan after the Fukushima Dai-ichi Nuclear Power Plant accident [1]. In forest ecosystems, the deciduous tree species Chengiopanax sciadophylloides, Japanese native species commonly called Koshiabura, has attracted research interest for its high Cs accumulating potential [2,3]. Sugiura et al. [2] suggested that microorganisms associated with the roots of C. sciadophylloides are involved in Cs accumulation, although the underlying mechanism remains unknown. Symbiotic arbuscular mycorrhizal fungi (AMF), which associate with diverse land plant taxa in various ecosystems [4,5,6], colonize the roots of C. sciadophylloides [7]. In addition, some reports have demonstrated the contribution of AMF in accumulating Cs in host plants [8,9,10]. To clarify the functional significance of AMF in accumulating radiocesium in C. sciadophylloides, the quantitative association between the Cs amount and the colonization rate of AMF in roots should be analyzed.
The conventional methods for estimating the colonization rate of AMF in roots are based on microscopic observations of the specific structure of AMF like arbuscules [11,12]. The grid-line intersect method by Giovannetti and Mosse [13] is most commonly used for evaluating AMF colonization [12]. The method can estimate the proportion of infected roots and their total length using a dissecting microscope and a Petri dish with grid lines on the bottom. The colonization rate can be estimated by counting the intersects of the grid lines and roots dispersed on the Petri dish. To date, this method has been widely accepted because of its simple protocol that involves the use of a dissecting microscope, low labor consumption, and provision of reliable datasets. However, the accuracy of the estimation depends on the skill and experience of the researchers. Thus, the estimated values can vary among researchers, making comparison between the results and those of published studies difficult. Although McGonigle et al. [14] invented a more objective and improved estimation method, i.e., the magnified intersections method, that uses a light microscope and counts each specific structure for AMF (arbuscules, vesicles, and hyphae), the labor-consuming factor persisted.
Therefore, for comprehensively understanding the processes of radioactive contamination and remediation and for objectively assessing the colonization rate of AMF, researchers should be experienced in conducting investigations on fungi and in other fields. To overcome the drawbacks of the quantitative analysis of AMF, we propose a new and objective method for estimating AMF using the image analysis software WinRHIZO Pro (Regent Instrument Inc., Quebec, Canada), called the WinRHIZO method.
MATERIALS AND METHODS
Sampling
Three seedlings of C. sciadophylloides (20–30 cm in height), which did not have any damage to their root systems, were carefully collected using a shovel from a secondary forest in Yamakiya district of Kawamata, Fukushima Prefecture, Japan (37°35′35.9″ N, 140°42′26.0′ E) during May–September 2014. These samples were collected in polyethylene zipper bags, preserved in a cooler, and transported to the laboratory.
Staining and observation of roots
The fine roots were stained according to the method described by Matsuda et al. [15]. The root system was washed with tap water to remove adhered soil and was further cleaned with distilled water in an ultrasonic bath (US CLEANER; Asone, Osaka, Japan). The roots with a diameter of 0.5–1 mm, used as observation samples, were soaked in 10% KOH solution and heated in an autoclave (KS-243; Tomy, Tokyo, Japan) at 121℃ for 25 min to remove the cell pigments of the roots. Furthermore, for better staining, the samples were soaked in 3.5% HCl solution at room temperature for around 12–24 hr. Next, the samples were stained with 0.05% trypan blue in lactoglycerol solution (lactic acid : glycerol : water = 1 : 1 : 1) in an autoclave at 121℃ for 15 min. To remove the excessive staining solution, the stained roots were stored in lactoglycerol for 1 wk.
Slides were prepared by squashing the stained root samples with a cover glass onto a glass slide. The specific AMF structures such as arbuscules, vesicles, and hyphae were observed using a light microscope (CX41LF; Olympus, Tokyo, Japan). For image analyses by using the WinRHIZO Pro software (Regent Instrument Inc.), microphotographs of stained roots (100×) were taken and saved as JPEG format.
Estimation of colonization rate
The colonization rate was estimated using the WinRHIZO Pro software, which evaluates the properties of the root system, including root length, surface area, and volume, and uses scanned images. Color analysis is an analytical function of WinRHIZO Pro software, in which the objects are quantified according to their color. Thus, we used the color analysis of the WinRHIZO Pro software to estimate AMF colonization using a micrograph of the stained roots. The criteria of the color analysis can be defined by the operator and saved as a cac format for further analyses. Thus, using the defined cac data, AMF analysis can be performed on the basis of previously defined criteria, when required. The analysis range can be changed from a whole picture to a selected part of the picture, and the undesired parts in the picture can be excluded. Using the color analysis, the colonization rate of AMF can be calculated as the ratio of the area of the stained fungal body to that of the roots within a picture because the color of the stained structure of AMF is different from that of the root body. The colonization rate of AMF was calculated using the following equation.
The reliability of the WinRHIZO method was verified by comparing it with the conventional grid-line intersect method [13]. The grid-line intersect method was performed for the same pictures, which were used for the WinRHIZO method. Grid lines were drawn on micrographs of stained roots using Photoshop CS6 (13.0.1, Adobe) (Fig. 1). Then, each number of the grid line intersections found on AMF or on the root body was counted, which was repeated twice to obtain an average value. To calculate the average value of the colonization rate, the following equation was used.
Fig. 1. Micrograph of a root fragment of Chengiopanax sciadophylloides seedling used in the modified grid-line intersect method.
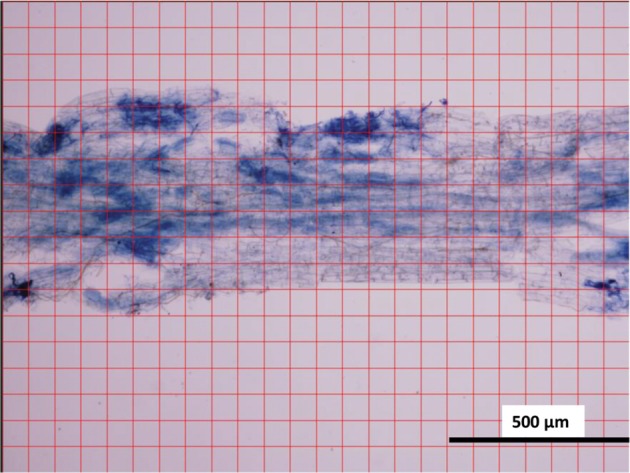
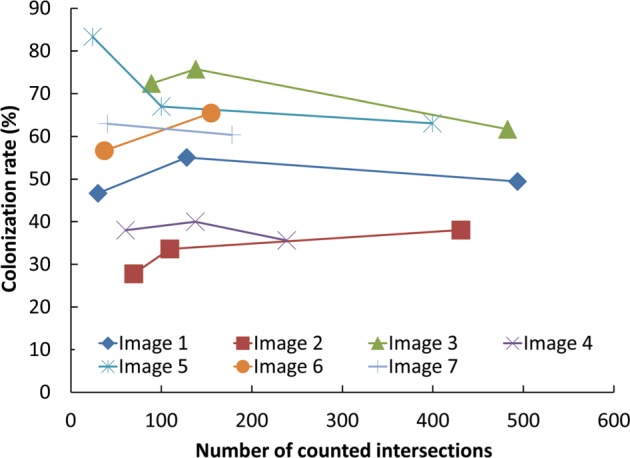
To optimize the count of grid line intersections, the actual grid sizes on the images in Photoshop CS varied from 5 to 20mm. We found that the grid size that provided 100–200 intersections on the roots was the most efficient in terms of accuracy and labor requirements (Fig. 2). The correspondence between the colonization rate of AMF by the WinRHIZO method and that by the grid-line intersect method for the same microphotographs of stained roots was investigated by regression analysis using R software ver. 3.2.3 [16].
Fig. 2. Change in the estimated colonization rate (%) of arbuscular mycorrhizal fungi in microscopic fields according to the number of counted intersect of grids.
RESULTS AND DISCUSSION
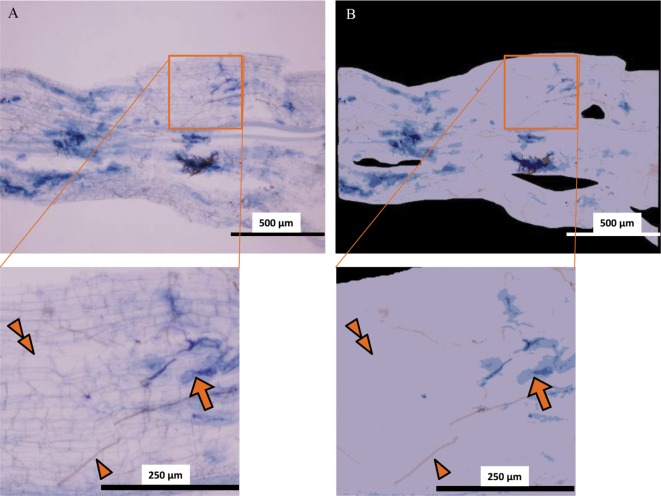
In all root samples of C. sciadophylloides, structures of AMF, i.e., Paris-type hyphal coils (Fig. 3) and vesicles (Fig. 4) were partially well stained with trypan blue [17]. Brownish fungal structures besides bluish-stained AMF structures were observed on the surface of the roots. These structures had hyphae with simple septa; and thus, the brownish fungal structures did not represent the AMF taxa. Therefore, the structures related to the AMF taxa were distinguished from those of the brownish fungal and plant tissues by the color analysis (Fig. 5).
Fig. 3. Paris-type hyphal coils (arrow) of arbuscular mycorrhizal fungi and brownish unknown fungal hyphae (arrowhead) observed in a root fragment of Chengiopanax sciadophylloides seedlings.
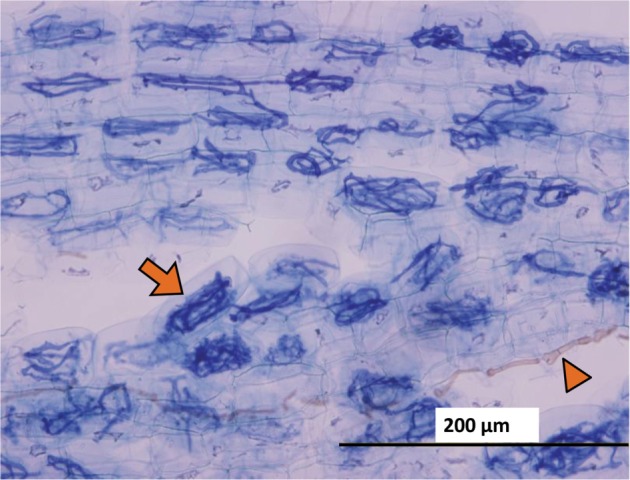
Fig. 4. Vesicle (circle) and hyphae (arrow) of arbuscular mycorrhizal fungi and brownish unknown species hyphae (arrowhead) observed in a root fragment of Chengiopanax sciadophylloides seedlings.
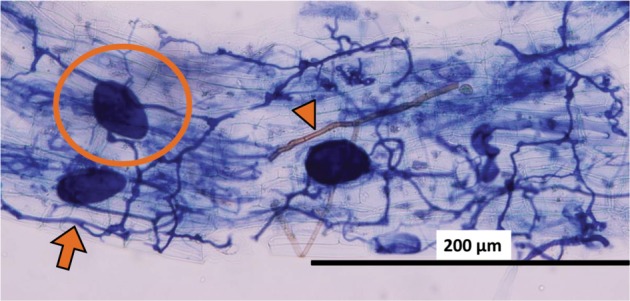
Fig. 5. An example of image analysis using the WinRHIZO Pro software for investigating arbuscular mycorrhizal fungi (AMF) in a root fragment of Chengiopanax sciadophylloides seedlings. A, Original micrograph; B, Image analyzed by the WinRHIZO Pro software (blue, AMF structures [arrows]; brown, brownish unknown species hyphae [arrowheads]; violet, plant tissue [double arrowheads]; and black, area not analyzed).
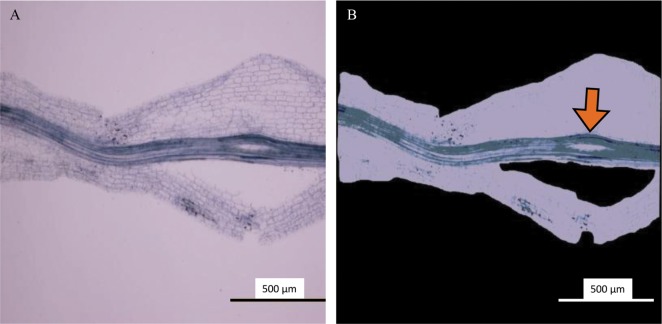
The colonization rates, estimated by the WinRHIZO method, were between 10.2% and 86.8% (Fig. 6). There was a significant positive correlation between the estimated colonization rate obtained using the WinRHIZO method and that obtained using the grid-line intersect method (n = 12, F-test, p < 0.05). The reliability of the WinRHIZO method was verified with a high correlation (R2 = 0.94), although the colonization rate estimated by the WinRHIZO method tended to be higher than that estimated by the grid-line intersect method (Fig. 6). This occurred because of the staining of the stele of the roots, which were also detected as AMF structures using the WinRHIZO method (Fig. 7). However, this overestimation could be corrected, because R2 and the slope of the regression line was almost 1 and the value derived from a stele appeared to be separated as an intercept (Fig. 6). This overestimation can be also eliminated by improving the staining techniques.
Fig. 6. Comparison of the colonization rates (%) of arbuscular mycorrhizal fungi in a root fragment of Chengiopanax sciadophylloides seedlings detected by the WinRHIZO method and the modified grid-line intersect method (n = 12). The t-test and F-test confirmed that differences in the slope, intercept, and coefficient of determination were statistically significant (*p < 0.05).
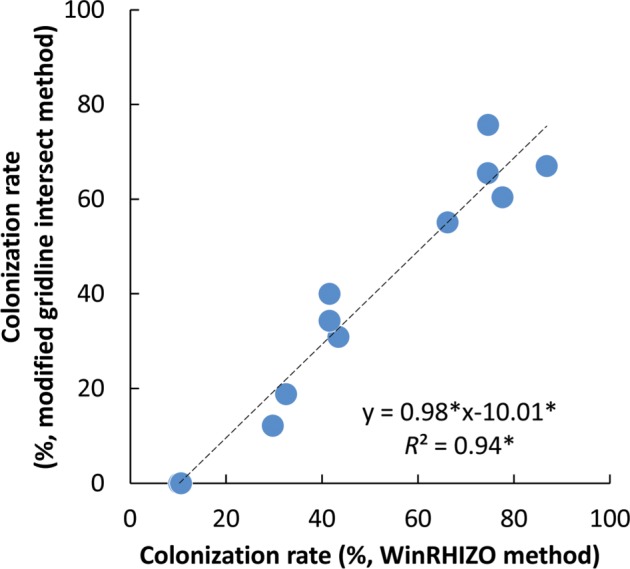
Fig. 7. A stele (arrow) detected as an arbuscular mycorrhizal fungal body in a root fragment of Chengiopanax sciadophylloides seedlings based on the findings of the WinRHIZO method. A, Original micrograph; B, Image analyzed by the WinRHIZO Pro software.
The WinRHIZO method provided estimates that were equally accurate to those obtained by the grid-line intersect method, and more importantly, its procedures were less labor consuming than those of the magnified intersection method. Moreover, the WinRHIZO method ensured a completely objective estimation of AMF colonization. Considering the large amount of woody roots distributed in heterogeneous forest soils, the proposed method enables us to examine more replications and a larger number of samples, which would provide an insight into the significance of AMF functioning in forest ecosystems. In addition, comparing results obtained by different researchers can be made easier by sharing a cac file of analytical settings. Therefore, the WinRHIZO method overcomes the drawbacks of conventional AMF estimation methods such as subjective judgement and labor-consuming procedure.
In conclusion, the WinRHIZO method objectively assesses symbiotic AMF structures automatically by using the same criteria and without the requirements of relevant skills of the observers and the labor intensity of the grid-line intersect method. Further studies and verification of our findings using various plant species will improve this method and establish it as a convenient and useful approach for evaluating AMF.
ACKNOWLEDGEMENTS
This research was financially supported by KAKENHI Grant Number 24110007.
References
- 1.Ministry of Health, Labour and Welfare. Levels of radioactive contaminants in foods tested in respective prefectures [Internet] Tokyo: Ministry of Health, Labour and Welfare; 2016. [cited 2016 Jul 22]. Available from: http://www.mhlw.go.jp/english/topics/2011eq/index_food_radioactive.html. [Google Scholar]
- 2.Sugiura Y, Kanasashi T, Ogata Y, Ozawa H, Takenaka C. Radiocesium accumulation properties of Chengiopanax sciadophylloides. J Environ Radioact. 2016;151(Pt 1):250–257. doi: 10.1016/j.jenvrad.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Yamaji K, Nagata S, Haruma T, Ohnuki T, Kozaki T, Watanabe N, Nanba K. Root endophytic bacteria of a 137Cs and Mn accumulator plant, Eleutherococcus sciadophylloides, increase 137Cs and Mn desorption in the soil. J Environ Radioact. 2016;153:112–119. doi: 10.1016/j.jenvrad.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Brundrett MC. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil. 2009;320:37–77. [Google Scholar]
- 5.van der Heijden MG, Martin FM, Selosse MA, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- 6.Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T, et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science. 2015;349:970–973. doi: 10.1126/science.aab1161. [DOI] [PubMed] [Google Scholar]
- 7.Yamato M, Iwase K. Community analysis of arbuscular mycorrhizal fungi in a warm-temperate deciduous broad-leaved forest and introduction of the fungal community into the seedlings of indigenous woody plants. Mycoscience. 2005;46:334–342. [Google Scholar]
- 8.de Boulois HD, Voets L, Delvaux B, Jakobsen I, Declerck S. Transport of radiocaesium by arbuscular mycorrhizal fungi to Medicago truncatula under in vitro conditions. Environ Microbiol. 2006;8:1926–1934. doi: 10.1111/j.1462-2920.2006.01070.x. [DOI] [PubMed] [Google Scholar]
- 9.Entry JA, Watrud LS, Reeves M. Accumulation of 137Cs and 90Sr from contaminated soil by three grass species inoculated with mycorrhizal fungi. Environ Pollut. 1999;104:449–457. [Google Scholar]
- 10.Rosén K, Weiliang Z, Mårtensson A. Arbuscular mycorrhizal fungi mediated uptake of 137Cs in leek and ryegrass. Sci Total Environ. 2005;338:283–290. doi: 10.1016/j.scitotenv.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Peterson RL, Massicotte HB, Melville LH. Mycorrhizas: anatomy and cell biology. Ottawa (ON): NRC Research Press; 2004. [Google Scholar]
- 12.Sun XG, Tang M. Comparison of four routinely used methods for assessing root colonization by arbuscular mycorrhizal fungi. Botany. 2012;90:1073–1083. [Google Scholar]
- 13.Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. [Google Scholar]
- 14.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Murahashi F, Kimoto M, Nakanishi K, Ito S. Arbuscular mycorrhizas on Athyrium yokoscense and A. niponicum grown at a lead-contaminated site. Mycoscience. 2005;46:261–264. [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing [Internet] Vienna: R Foundation for Statistical Computing; 2015. [cited 2016 Jul 22]. Available from: https://www.R-project.org/ [Google Scholar]
- 17.Smith FA, Smith SE. Structural diversity in (vesicular)-arbuscular mycorrhizal symbioses. New Phytol. 1997;137:373–388. doi: 10.1046/j.1469-8137.1997.00848.x. [DOI] [PubMed] [Google Scholar]