Abstract
Cordyceps militaris, known as Dong-Chong-Xia-Cao, produces the most cordycepin among Cordyceps species and can be cultured artificially. For these reasons, C. militaris is widely used as herb or functional food in the East Asia. In this study, we developed a new strain of C. militaris that produces higher cordycepin content than parent strains through mating-based sexual reproduction. Twenty parent strains were collected and identified as C. militaris based on internal trasncrived spacer and rDNA sequences. Seven single spores of MAT 1-1 idiomorph and five single spores of MAT 1-2 idiomorph were isolated from 12 parent strains. When 35 combinations were mated on the brown rice medium with the isolated single spores, eight combinations formed a stroma with a normal perithecia and confirmed mated strains. High pressure liquid chromatography analysis showed that mated strain KSP8 produced the most cordycepin in all the media among all the tested strains. This result showed due to genetic recombination occurring during the sexual reproduction of C. militaris. The development of C. militaris strain with increased cordycepin content by this approach can help not only to generate new C. militaris strains, but also to contribute to the health food or medicine industry.
Keywords: Cordycepin, Cordyceps militaris, Dong-Chong-Xia-Cao, MAT gene, Mating
Genus Cordyceps belonging to the division ascomycota, class sordariomycetes, order hypocreales, family clavicipitaceae and is entomopathogenic and endoparasitic fungi [1]. Cordyceps species are known as Dong-Chong-Xia-Cao in China because it is parasitic in the host's body in winter and forms stroma like grass after killing host and absorbing nutrients of host in summer. Cordyceps species mainly parasitize in arthropod from lepidopteran larva and pupae to imago and favors a warm and humid climate. Thus, hosts infected with Cordyceps species are predominantly observed in areas with hot and humid subtropical climate.
Many species of Cordyceps have been used as health food or medicine in China and South-East Asia as it contains abundant physiologically active substances [2,3]. Contrary to other Cordyceps species, Cordyceps militaris is readily available for artificial cultivation with higher production of cordycepin. The content of the bioactive substance in C. militaris is as high as bioactive substance in C. sinensis which produces many valuable bioactive components [4].
Chemical structure of cordycepin is very similiar to adenosine except that there is no hydroxyl group on carbon number 3. Because of this structural similarity, it inhibits polyadenylation of mRNA of cancer cell and thus exhibits anticancer effect [5]. In addition, the antithrombotic effect is indicated by lowering the concentration of Ca2+ and the anti-inflammatory effect is shown by lowering the production of inflammatory substances [6]. It exhibits various physiological activity effects as described above [7,8]. Also, as cordycepin has a half-life of about 1min and is rapidly decomposed by adenosine deaminase in the body, there are few side effects. Following these reasons, cordycepin is very valuable material. However, the genes involved in the cordycepin biosynthetic pathway are still unknown. It is only conjectured that biosynthesis pathway of cordycepin is similar to that of adenosine due to the structural similarity with adenosine. There is a method of chemically synthesizing cordycepin from adenosine and extracting codycepin from Cordyceps spp. However, it is not enough to meet demand for cordycepin. Thus, researches to increase the natural cordycepin content in C. militaris have been peformed through various methods such as breeding by mating or genetic manipulation and C. militaris with higher production of cordycepin is very valuable in the health food or medicine industry [9,10].
Breeding by mating is a method based on the sexual reproduction route of the fungus. C. militaris proliferates through not only asexual cycle but also sexual cycle producing ascospores. C. militaris is heterothallic and bipolar species in that two mating type loci consisting of opposite mating type idiomorph MAT1-1 and MAT1-2 exist discretely in two different single spores in the sexual reproductive pathway. Mating can initiate when two single spores with different MAT idiomorph meet.
MAT1-1 locus is consisted of MAT1-1-1 and MAT1-1-2 whereas MAT1-2 locus is consisted of MAT1-2-1 [11,12,13]. MAT idiomorph encodes transcription factor that regulates gene related to sexual reproduction [14]. Each of transcription factors by encoded MAT1-1 and MAT1-2 contains DNA binding motif. MAT1-1 is a transcription factor containing well-conserved α-domain as DNA binding motif. MAT1-2 is a transcription factor containing HMG-domain as DNA binding motif [15].
Many new C. militaris strains with high cordycepin content have been developed through gene mutation by irradiation or ultraviolet irradiation mutations [16,17]. In this study, we tried to construct new C. militaris strains with increased cordycepin by natural mating without mutation. C. militaris strains were mated with other strains to make new strains to produce more cordycepin than parent strains. Twelve strains of C. militaris were used to parent strains and mated each other after separating single spores from 12 C. militaris strains. The contents of cordycepin in all strains were analyzed through high pressure liquid chromatography (HPLC). As a result, we developed new C. militaris strains which produce which produced more cordycepin than the parent strains.
MATERIALS AND METHODS
Fungal strains and culture condition.
Twelve strains of C. militaris were used in this study (Table 1). Each strain was obtained from Korean Agricultural Culture Collection (KACC) or Systems Plant Microbiology Laboratory of Pusan National University (SPNU). All strains are mycelium state. Fungal strains were maintained on potato dextrose agar (PDA) at 25℃. For genomic DNA isolation from mycelium, mycelium was cultured for 7 days in potato dextrose broth (PDB) 50 mL in shaking-type photo bioreactor (PBR) (150 rpm) at 25℃ for 7 days under light.
Table 1. Cordyceps militaris strain used in this study.
KACC, Korean Agricultural Culture Collection, National Institute of Agricultural Science and Technology, Suwon, Korea; SPNU, System Plant Microbiology Lab. Of Pusan National University, Korea; EFCC, Entomopathogenic Fungal Culture Collection, Kangwon National University, Korea; MPNU, Mycological lab. Of Pusan National University, Korea.
PCR of rDNA region spanning the ITS1, ITS2, and 5.8S rRNA gene.
Genomic DNA of C. militaris was extracted from fresh mycelium by using a cetyltrimethy-lammonium bromide method [18]. The ITS4R and ITS5F primers were used to amplify rDNA region spanning the ITS1, ITS2, and 5.8S rRNA gene [19]. The reaction mixture for PCR was consisted of 10× Taq PCR buffer 2 µL, 1.6 µL dNTPs (2.5 mM stock), 1 µL primer 1 (10 pmol/µL), 1 µL primer 2 (10 pmol/µL), 0.1 µL Taq DNA polymerase (Takara, Tokyo, Japan), 1 µL dimethyl sulfoxide, and 50 ng/µL template. PCR was performed using Sure Cycler 8800 Thermal Cycler (Agilent Technologies, Santa Clara, CA, USA) under the following conditions: one cycle of 5 min at 96℃, followed by 30 cycles of 96℃ for 40 sec, 48℃ for 40 sec, and 72℃ for 40 sec, and finishing with extension at 72℃ for 10 min. The amplified PCR products was purified by PCR Purification kit (GeneAll Biotech, Seoul, Korea) and sequenced by 3730xl DNA analyzer (Macrogen, Seoul, Korea). Phylogenetic tree was created among 12 C. militaris strains including Cordyceps bassinia. All sequence were aligned by MUSCLE and curated by Gblocks. Phylogenetic tree was created by MEGA ver. 6.06 using the maximum liklihood. Bootstrap analysis was conducted with 2,000 replicates.
Single spore isolation and identification of MAT idiomorph.
For single ascospore isolation, spore drop/shooting method was performed. Stroma with perithecia was attached to inner side of petri dish containing 2% water agar (WA) at 25℃ under light for 5 days. After that, single spore separated germinated from stroma and then mycelium was cultured in PDB 50 mL in a 250-mL Erlenmeyer flask containing glass bead in shaking incubator conditioned with 150 rpm for 7 days at 25℃ and under light to facilitate growing conidia. Supernatant of culture medium was collected by centrifugation at 14,000×g for 10min and filtered through filter paper (ADVANTEC 2, Tokyo, Japan) to remove mycelium. After diluting supernatant having conidia serially in water up to 105, 200 µL of spore suspension was smeared in WA medium. WA plate were cultured at 25℃ under light for 5 days. To confirm MAT idiomorph of single spore, MAT-PCR was performed using MAT1-1-1 and MAT1-2-1 primer set to target MAT1-1-1 and MAT1-2-1 (MAT1-1-1-F, 5′-ATGGAACACAGATCGAGCGACAC-3′ and MAT1-1-1-R, 5′-ATATACCTTCGCGATCA TTGCCCAG-3′; MAT1-2-1-F, 5′-TGTTTTGTCGCGATGGTTCTGG-3′ and MAT1-2-1-R, 5′-CCTCTGGAGGTTCTGCATTCCA-3′) [20]. As MAT idiomorph of MPNU8001-1 and MPNU8001-2 were confirmed by MAT PCR and sequencing, MPNU8001-1 and MPNU8001-2 were used to control strain (Table 1). The PCR reaction mixture was same as described above. MAT type PCR was performed as follows: 95℃ 1 min, followed by 30 cycles of 95℃ for 30 sec, 58℃ for 30 sec, and 72℃ for 40 sec, and finishing with extension at 72℃ for 5 min.
Mating experiment and stroma induction.
Mated strain was induced to produce stroma in brown rice, silk worm pupae and PDB medium. Single spore inoculum was prepared in PDB. Inoculum was cultured in shaking-type PBR (120 rpm) at 25℃ for 7 days. Brown rice and silk worm pupae medium were made by mixing 40 g brown rice, 4 g silk worm pupae and 20 g brown rice, 40 g silk worm pupae in 64mL liquid medium (20 g sucrose, 20 g peptone, 1 g MgSO4·7H2O, 0.5 g KH2PO4, and distilled water 1 L) in polypropylene mushroom stroma bottle. Fifteen to 20 mL of inoculum set to 12 g/L in dry cell weight (DCW) and composed of two spores originated from different parent strains was inoculated in all media. Twelve parent strains were also induced to produce stroma in brown rice, silk worm pupae and PDB medium. Inoculum was prepared in PDB 50 mL by inoculating multi spore. Fifteen to 20 mL of multi spore inoculum set to 12 g/L in DCW was inoculated in all three media.
After incubated for 7 days under dark at 25℃ for promoting vegetative growth, all media was incubated at 20℃ for 50 days in growth chamber (JEIO TECH, Daejeon, Korea) under 12 hr light/dark cycle of 500–1,000 lux and high humidity (90%). All mated strains with stroma were photographed using a camera (Nikon, Tokyo, Japan).
Cordycepin analysis by HPLC.
When stroma was induced after 50 days, content of cordycepin was measured. Stroma and sclerotium of mated strains and parent strains in brown rice and silk worm pupae media were separated. In the case of mycelium in PDB, the amount of cordycepin was measured by separating extracellular cordycepin in culture medium and intracellular cordycepin in mycelium. Sample was freezedried and grinded into powder (diameter at approximate 50 meshes) together with liquid nitrogen except for PDB culture medium. all samples were heated at 110℃ for 1 hr in H2O. Sample was filtered using a syringe filter 0.22 µm (Chromdisc, Daegu, Korea).
HPLC analysis was performed on Shiseido Nanospace SI-2 HPLC (Shiseido, Tokyo, Japan) with Shiseido CAPCELL PAK C18 AQ Column (250 × 4.6 mm, 5 µm; Shiseido). Standard of cordycepin was purchased from Sigma Chemical Corp. (St. Louis, MO, USA) and injected five times from 7.8125–500 µg/mL to draw calibration. Detection was performed on the condition that composition ratio of the mobile phase was water : acetonitrile = 91 : 9 (v/v) and flow rate was 500 µL/mL at 30℃. Injection volume was 10 µL. Detection wavelength was 260 nm.
RESULTS
Identification and phylogenetic tree of 12 strains.
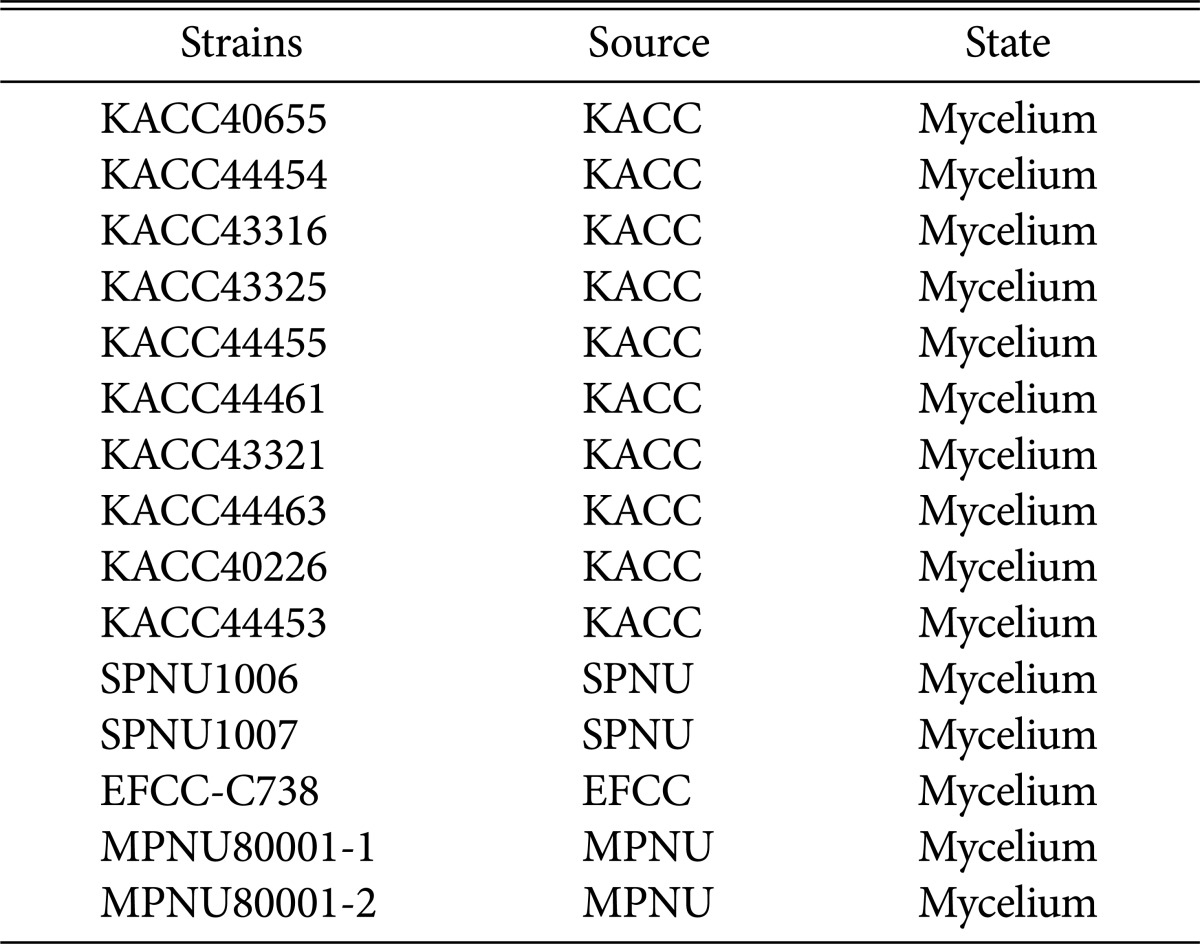
As mating is possible within same Cordyceps species, identification of 12 strains was performed by using internal transcribed spacer (ITS) sequences including 5.8S rRNA. ITS sequences including 5.8S rRNA of 12 C. militaris strains were successfully amplified with the ITS4F and ITS5R primers (Fig. 1A). Amplified target sequence was approximately 530–560 bp. As sequences of all strains have 98% identity or more with ITS sequence including 5.8S rRNA of C. militaris (AF153264) already registered on NCBI. ITS sequences including 5.8S rRNA of all strains were registered in NCBI. Phylogenetic tree was created with 12 strains of C. militaris and the other Cordyceps bassiana by MEGA 6.06 using maximum likelihood phylogenetic analysis (Fig. 1B). Phylogenetic tree among thirteen strains of C. militaris and Cordyceps bassiana demonstrated that all tested strains belong to C. militaris and are genetically far from C. bassinia.
Fig. 1. PCR and phylogenetic tree showing that relationships among Cordyceps spp. (C. takaomontana, C. japonica, and C. sinensis). Positive control was C. militaris (EFCC-C738). A, Internal transcribed spacer sequences including 5.8S rRNA was amplified from C. militaris strains; B, Phylogenetic tree based on maximum likelihood was created among Cordyceps spp. by MEGA 6.06 program. The number on the nodes correspond to the bootstrap percentages based on 2,000 pseudoreplicates.
Single spore isolation and identification of MAT idiomorph.
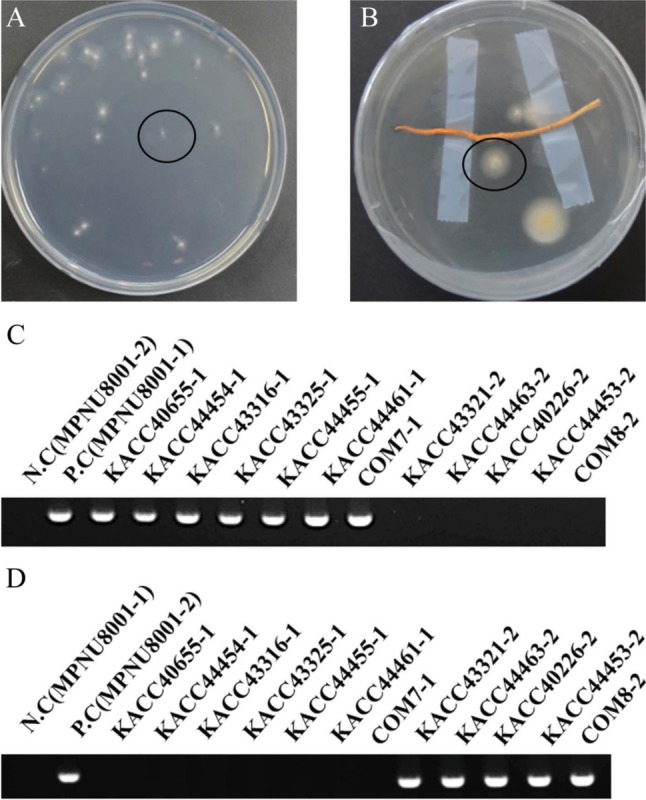
Single spores were isolated from 12 C. militaris strains. Isolated spores were germinated in WA after 5 days (Fig. 2A and 2B). As single spore isolation according to all strains was repeated five times, total (5 × 12 = 60) single spores of C. militaris were isolated. MAT PCR amplifying MAT1-1-1 and MAT1-2-1 partial gene resulted that MAT1-1-1 was 457 bp and MAT1-2-1 was 368 bp. MAT-PCR examination of 60 single spores indicated that seven single spores with MAT1-1 and five single spores with MAT1-2 were isolated (Fig. 2C and 2D). Name of single spore was determined by adding number of MAT idiomorph to parent strain name.
Fig. 2. Identification of MAT idiomorph in single spores isolated from Cordyceps militaris. A, Conidia suspension from mycelium was smeared and single spore marked circle was germinated in water agar (WA) plate after 5 days; B, Stroma with perithecia was attached to WA plate cover and single spores marked circle was germinated in WA plate after 5 days; C, MAT1-1-1 partial gene of MAT1-1 and MAT1-2-1 partial gene of MAT1-2 was amplified. The PCR products were electrophoresed on a 1.5% agarose gel. M, 100 bp DNA ladder molecular size marker. Positive control and negative control used single spores which MAT idiomorph were known by sequencing. MAT1-1-1 was successfully amplified in MAT1-1 idiomorph seven single spores. MAT1-1-1 was not amplified in MAT1-2 idiomorph five single spores; D, MAT1-2-1 was successfully amplified in MAT1-2 idiomorph five single spores. MAT1-2-1 was not amplified in MAT1-1 idiomorph seven single spores.
Mating and stroma induction of parent strain.
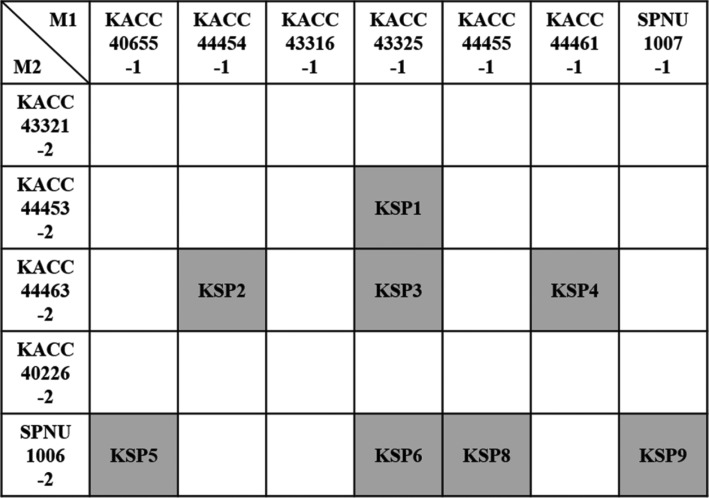
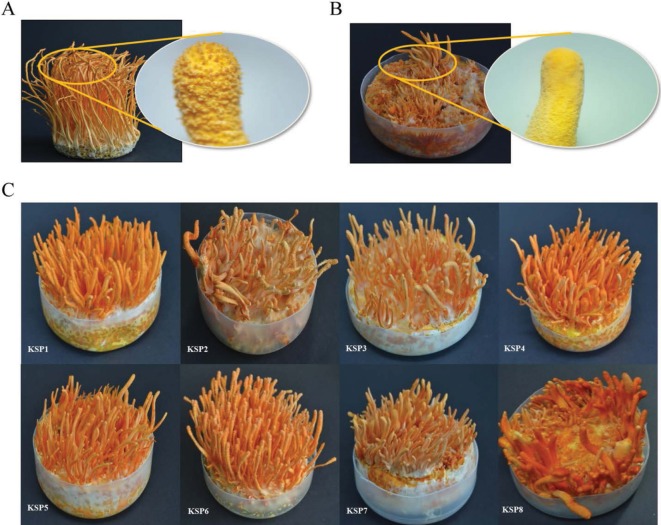
Mating was performed with 12 single spores composed of seven single spores with MAT1-1 and five single spores with MAT1-2 through induction of stroma in brown rice, silk worm pupae, and PDB medium. Mating of 35 combinations was performed through stroma induction in all media. Stroma was induced approximately after 50 days. Eight combinations of total 35 combinations were successfully mated, producing stroma with perithecia in brown rice medium. Newly mated strains were generated (Fig. 3). Some of combinations did not at all produce stroma and the others of combinations produce abnormal stroma without perithecia (Fig. 4A and 4B). Strains with abnormal stroma without perithecia were not mated, being fertile. Although there were mated strains having a normal stroma with perithecia in brown rice medium (KSP1, KSP2, KSP3, KSP4, KSP5, KSP6, KSP7, KSP7, and KSP8) (Fig. 4C), these strains could not produce normal stroma in silk worm pupa and PDB medium.
Fig. 3. Mating combinations based on separated 12 single spores in brown rice medium. Eight combinations are only mated. Mated strains were highlighted in gray and named.
Fig. 4. Eight mated Cordyceps militaris strains in brown rice medium. A, The stroma with perithecia in mated strain; B, The stroma without perithecia in non-mated strain; C, Eight mated Cordyceps militaris strains in this study.
Cordycepin content in brown rice medium.
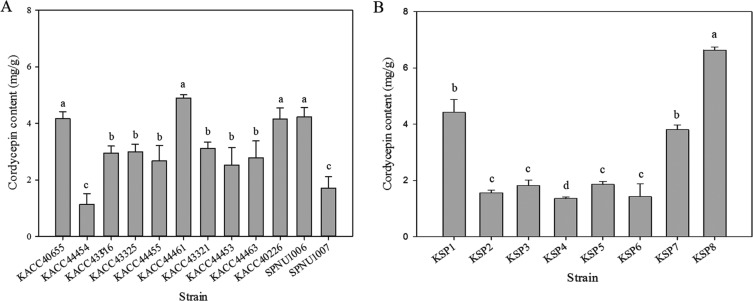
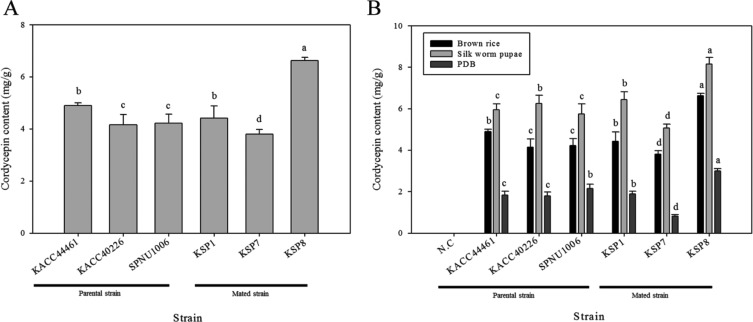
Cordycepin content of parent and mated strains cultivated in brown rice medium was measured. Retention time value of standard cordycepin was 12.23 min under an optimum chromatographic condition. The calibration curve was plotted with standard sample data which measured in triplicate from 7.8125 to 500 µg/mL. Estabilished standard curve equitation of cordycepin and R-squared were Y = 3.24E + 10X − 794,785.435 (R2 = 0.9998). The content of cordycepin in the twenty parent strains and eight mated strains was measured in triplicate. The cordycepin content per 1 g of DCW was calculated. Average cordycepin content of stroma and sclerotium was set to cordycepin content of each strain. The content of cordycepin in sclerotium was set to cordycepin content of strains in which stroma was not formed. Average content of cordycepin in 12 parent strains was 3.06 mg/g. Among top three strains of parental strains (KACC44461, KACC40226, and SPNU1006), KACC44461 produced the most cordycepin (4.89 mg/g), followed by SPNU1006 (4.22mg/g) (by ANOVA) (Fig. 5A). Average content of cordycepin in eight mated strains was 2.85 mg/g. Strain producing the most cordycepin content was KSP8 (6.63 mg/g), second-largest strain was KSP7 (3.8 mg/g) (by ANOVA) (Fig. 5B). Comparison of cordycepin content among top three mated strains and top three parent strains based on results of cordycepin content in all strains revealed that KSP8 produced the most cordycepin among top three mated strains and top three parent strains (by ANOVA) (Fig. 6A). KSP8 strain produced the most cordycepin. Therefore, KSP8 producing the most cordycepin among all strains was created by mating. Cordycepin content in KSP8 increased by 35% of cordycepin content in KACC44461 parent strain producing the most cordycepin in brown rice.
Fig. 5. Cordycepin content of all Cordyceps militaris strains in brown rice medium. Cordycepin content of 12 parent strains (A) and eight mated strains (B) in brown rice medium. Error bars indicate the standard deviation of three independent experiments. Different letters above the cordycepin content bar mean significant difference according to strain (p < 0.001, oneway ANOVA followed by Duncan's test).
Fig. 6. Comparison of cordycepin content between top three parent and mated strains. A, Cordycepin content of top three parent strains and mated strains in brown rice medium; B, Effect of medium on cordycepin production. Cordycepin content of top three mated and parental strains was indicated according to three media (brown rice, silk worm pupae, potato dextrose broth). Error bars indicate the standard deviation of cordycepin content. Different letters above the cordycepin content bar mean significant difference according to strain and indicated independently dependent on media (p < 0.001, one-way ANOVA followed by Duncan's test).
Effect of media on cordycepin production.
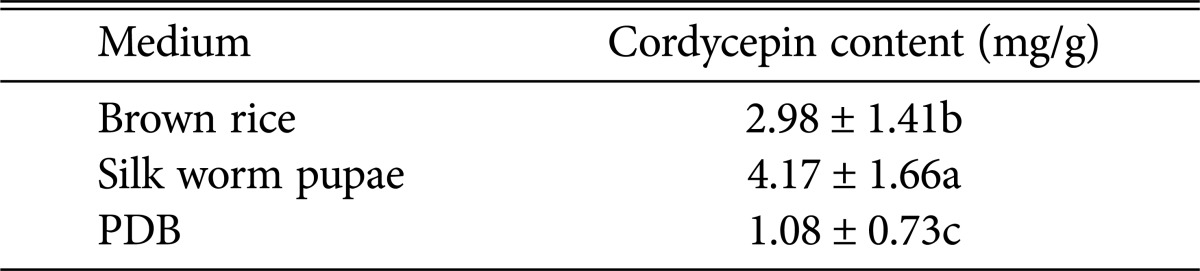
Cordycepin content of all strains cultivated in PDA and silk worm media were compared with cordycepin content of all strains cultivated in brown-rice medium. Cordycepin in PDB was measured separately mycelium and supernatant. Cordycepin content of top six strains among all strains was presented in Fig. 6B. Top six strains were determined by calculating average cordycepin content of all strains each cultivated in all media. Mated strain KSP8 produced more cordycepin than KACC44461 in all tested media. Additionally, result from cordycepin content according to media indicated that C. militaris strains produced the most cordycepin in silk worm pupae medium and the second highest cordycepin in brown rice medium regardless of strains, whereas C. militaris cultivated in PDB produced the least cordycepin (by ANOVA) (Table 2).
Table 2. Cordycepin content in different media.
PDB, potato dextrose broth.
F-value = 85.907, p = 0.000.
DISCUSSION
C. militaris is the most useful Cordyceps spp. as it contains not only many bioactive substances, but also the highest cordycepin content among Cordyceps spp. Cordycepin exhibits various physiological activity effects including antitumor, antiaging, antivirus, and antileukemi. In this study, new C. militaris strain was developed through mating two C. militaris strains. Combination of two C. militaris increases cordycepin productivity was not reported. Combination was randomly performed between two single spores of C. militaris and made a new C. militaris with many cordycepin.
Mating was performed by inoculating isolated single spores in brown rice media. Eight combinations successfully were mated among 35 combinations. The reason to get about 23% of mated strains is not only combination of different mating type single spores but also other factors that seem to be required to induce sexual cycle by mating. Reduction of fungal activity may be associated with reduction of gene expression related to stroma induction and sexual cycle [21,22]. It is possible that there are mated strains that did not produce stroma due to degeneracy. However, this could not be confirmed with the naked eye and there is no feature that forms a cramp like other mushrooms belonging to Basidiomycota.
Results of comparisions of cordycepin contents in all tested strains demonstrated that KSP8 produced the most cordycepin. It is probably that gene recombination caused by sexual reproduction. Mating of two single spores induce karyogamy and meiosis provoking recombination of gene related to cordycepin synthesis. Thus, cordycepin content in mated strain is not directly related with cordycepin content in parent strain. Production of cordycepin in different media showed cordycepin content in silk worm pupae medium was the highst among three media. Incidence of stroma in silk worm pupae medium was lower than in brown rice medium, though. This is presumably because the silk worm pupa media are not suitable for stroma formation [23,24].
New C. militaris strains, KSP8, producing the most cordycepin among 12 parent strains and eight mated strains, were made through mating. Industrial value of new C. militaris strains rise with the increase of cordycepin. This result implies that mating will be able to produce a new strain with higher production of cordycepin than known strains.
ACKNOWLEDGEMENTS
This research was supported by Research Fund year of 2017 through Youngsan University, Busan, South Korea
References
- 1.Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masuda M, Urabe E, Honda H, Sakurai A, Sakakibara M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2007;40:1199–1205. [Google Scholar]
- 3.Hur H. Chemical ingredients of Cordyceps militaris. Mycobiology. 2008;36:233–235. doi: 10.4489/MYCO.2008.36.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Wang J, Wang W, Zhang H, Zhang X, Han C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid Based Complement Alternat Med. 2015;2015:575063. doi: 10.1155/2015/575063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian X, Li Y, Shen Y, Li Q, Wang Q, Feng L. Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin. Oncol Lett. 2015;10:595–599. doi: 10.3892/ol.2015.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho HJ, Cho JY, Rhee MH, Lim CR, Park HJ. Cordycepin (3′-deoxyadenosine) inhibits human platelet aggregation induced by U46619, a TXA2 analogue. J Pharm Pharmacol. 2006;58:1677–1682. doi: 10.1211/jpp.58.12.0016. [DOI] [PubMed] [Google Scholar]
- 7.Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012;47:979–987. doi: 10.1016/j.exger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Müller WE, Weiler BE, Charubala R, Pfleiderer W, Leserman L, Sobol RW, Suhadolnik RJ, Schröder HC. Cordycepin analogues of 2′,5′-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry. 1991;30:2027–2033. doi: 10.1021/bi00222a004. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Cai G, He Y, Tong G. Separation of cordycepin from Cordyceps militaris fermentation supernatant using preparative HPLC and evaluation of its antibacterial activity as an NAD(+)-dependent DNA ligase inhibitor. Exp Ther Med. 2016;12:1812–1816. doi: 10.3892/etm.2016.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Q, Long L, Wu L, Zhang F, Wu S, Zhang W, Sun X. Evaluation of different agricultural wastes for the production of fruiting bodies and bioactive compounds by medicinal mushroom Cordyceps militaris. J Sci Food Agric. 2016 Oct 17; doi: 10.1002/jsfa.8097. [Epub] [DOI] [PubMed] [Google Scholar]
- 11.Zheng P, Xia Y, Xiao G, Xiong C, Hu X, Zhang S, Zheng H, Huang Y, Zhou Y, Wang S, et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011;12:R116. doi: 10.1186/gb-2011-12-11-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnol Bioprocess Eng. 2004;9:440–446. [Google Scholar]
- 13.Yokoyama E, Arakawa M, Yamagishi K, Hara A. Phylogenetic and structural analyses of the mating-type loci in Clavicipitaceae. FEMS Microbiol Lett. 2006;264:182–191. doi: 10.1111/j.1574-6968.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 14.Benkhali JA, Coppin E, Brun S, Peraza-Reyes L, Martin T, Dixelius C, Lazar N, van Tilbeurgh H, Debuchy R. A network of HMG-box transcription factors regulates sexual cycle in the fungus Podospora anserina. PLoS Genet. 2013;9:e1003642. doi: 10.1371/journal.pgen.1003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong W, Jing W, Nan L, Aiping F, MingJie C, DaPeng B. Distribution of mating-type genes in fruiting and non-fruiting forms of Cordyceps militaris. Shi Yong Jun Xue Bao. 2010;17:1–4. [Google Scholar]
- 16.Che Z, Wang Y, Zhou L, Tang C. Study on the breeding of a new variety of Cordyceps militaris by mutated with ultraviolet radiation. Food Ferment Ind. 2004;30:35–38. [Google Scholar]
- 17.Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett Appl Microbiol. 2008;47:534–538. doi: 10.1111/j.1472-765X.2008.02456.x. [DOI] [PubMed] [Google Scholar]
- 18.Doyle J. DNA protocols for plants. In: Hewitt GM, Johnston AW, Young JP, editors. Molecular techniques in taxonomy. Berlin: Springer; 1991. pp. 283–293. [Google Scholar]
- 19.Wang L, Zhang WM, Hu B, Chen YQ, Qu LH. Genetic variation of Cordyceps militaris and its allies based on phylogenetic analysis of rDNA ITS sequence data. Fungal Divers. 2008;31:147–155. [Google Scholar]
- 20.Tan Q, Cai T, Wei J, Feng A, Mao W, Bao D. Molecular identification of mating type genes in asexual spoeres of Cordyceps militalis; Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products; 2011 Oct 4-7; Arcachon, France. Paris: Institute National de la Recherche Agronomique (INRA); 2011. pp. 52–56. [Google Scholar]
- 21.Shrestha B, Park YJ, Han SK, Choi SK, Sung JM. Instability in in vitro fruiting of Cordyceps militaris. J Mushroom Sci Prod. 2004;2:140–144. [Google Scholar]
- 22.Butt TM, Wang C, Shah FA, Hall R. Degeneration of entomogenous fungi. In: Eilenberg J, Hokkanen HM, editors. An ecological and societal approach to biological control. Dordrecht: Springer; 2006. pp. 213–226. [Google Scholar]
- 23.Cho SM, Park HJ, Seo GS, Hong JD. Effect of medis composition on the cordycepin and content nutritional components of Cordyceps militaris. Kor J Mycol. 2009;37:161–166. [Google Scholar]
- 24.Wen TC, Kang JC, Hyde KD, Li GR, Kang C, Chen X. Phenotypic marking of Cordyceps militaris fruiting-bodies and their cordycepin production. Chiang Mai J Sci. 2014;41:846–857. [Google Scholar]